Abstract
Purpose
In randomized trials patients with resected nonmetastatic gastric cancer who received adjuvant chemotherapy and radiotherapy (chemoRT) had better survival than those who did not. We investigated the effectiveness of adjuvant chemoRT after gastric cancer resection in an elderly general population and its effects by stage.
Methods and Materials
We identified individuals in the Surveillance, Epidemiology, and End Results–Medicare database aged 65 years or older with Stage IB through Stage IV (M0) gastric cancer, from 1991 to 2002, who underwent gastric resection, using multivariate modeling to analyze predictors of chemoRT use and survival.
Results
Among 1,993 patients who received combined chemoRT or no adjuvant therapy after resection, having a later year of diagnosis, having a more advanced stage, being younger, being white, being married, and having fewer comorbidities were associated with combined treatment. Among 1,476 patients aged less than 85 years who survived more than 4 months, the 313 who received combined treatment had a lower mortality rate (hazard ratio, 0.83; 95% confidence interval, 0.71–0.98) than the 1,163 who received surgery alone. Adjuvant therapy significantly reduced the mortality rate for Stages III and IV (M0), trended toward improved survival for Stage II, and showed no benefit for Stage IB. We observed trends toward improved survival in all age categories except 80 to 85 years.
Conclusions
The association of combined adjuvant chemoRT with improved survival in an overall analysis of Stage IB through Stage IV (M0) resected gastric cancer is consistent with clinical trial results and suggests that, in an elderly population, adjuvant chemoradiotherapy is effective. However, our observational data suggest that adjuvant treatment may not be effective for Stage IB cancer, is possibly appropriate for Stage II, and shows significant survival benefits for Stages III and IV (M0) for those aged less than 80 years.
Keywords: Gastric cancer, Adjuvant therapy, Radiation therapy, SEER–Medicare, Elderly
INTRODUCTION
Despite declining incidence rates since the 1930s both in the United States and elsewhere, gastric cancer is still a major problem (1). It is the second-leading cause of cancer death worldwide (2) and a significant cause of cancer death in the United States, with 10,880 expected gastric cancer deaths in 2008 (3). Gastric resection is required in the curative treatment of this cancer; however, survival rates after complete resection for individuals with tumor that has penetrated the submucosa or that has metastasized to lymph nodes have been disappointing (4).
Since 2000, several randomized controlled trials have established survival benefits from the addition of chemotherapy with or without radiation therapy to surgical resection. The two largest and most influential of these trials were the U.S. Intergroup study (INT-0116), presented at the annual meeting of the American Society of Clinical Oncology in May 2000 (5) and published in 2001 (6), and the United Kingdom Medical Council MAGIC (Medical Research Council Adjuvant Gastric Infusional Chemotherapy) trial, published in 2006 (7). In INT-0116 overall survival was significantly worse in the surgery-only group (hazard ratio [HR], 1.35; 95% confidence interval [CI], 1.09–1.66) compared with those who received adjuvant chemoradiotherapy (5-fluorouracil [5-FU], leucovorin, and 4,500 cGy of radiation) after surgery (6). In the MAGIC trial overall survival was significantly worse in the surgery-only group compared with those who received three preoperative cycles and three postoperative cycles of epirubicin/cisplatin/5-FU (HR, 0.75; 95% CI, 0.60–0.93) (7). Although the results of the MAGIC trial have been accepted into practice throughout much of Europe and other parts of the world, adjuvant chemoradiotherapy according to INT-0116 is still the more common treatment in the United States.
Individuals aged 65 years or older comprise about two thirds of patients diagnosed each year with resectable gastric cancer (3). For various cancers, including colon and rectal cancer, it has been shown that elderly patients with good performance status can respond to and tolerate chemotherapy as well as younger patients (8–10). Nonetheless, these patients are less likely to receive appropriate therapy when compared with their younger counterparts (11–14). To our knowledge, little is known about the patterns of use and effectiveness of adjuvant chemoradiotherapy for gastric cancer among elderly patients.
In this study we investigate the use of adjuvant 5-FU–based chemotherapy and radiation therapy among patients in a general population aged 65 years or older with surgically resected Stage IB through Stage IV (M0) gastric cancer. In addition to identifying predictors of treatment, we assess the association of treatment with survival and explore whether the survival benefits vary as a function of tumor stage or age group.
METHODS AND MATERIALS
Data source
We used data from the linked Surveillance, Epidemiology, and End Results (SEER)–Medicare database, developed by Potosky et al. (15) in 1993. Files from the National Cancer Institute’s SEER cancer registry were linked with their Medicare claims files. The SEER database is a population-based cancer registry that covers approximately 9.5% of the U.S. population in 1991, 14% of the U.S. population from the years 1992 through 1999, and 26% from 2000 to present. It closely reflects the overall population of the United States in its demographics. It provides information on tumor histology, location, and extent of disease, as well as primary surgical and radiation treatment and survival. The Medicare files include claims for individuals aged 65 years or older and those with Social Security Disability Insurance benefits. The Medicare files contain extensive information on diagnosis and treatment.
Sample selection
We identified all patients who had primary, invasive gastric adenocarcinoma (SEER histology codes 8010, 8020, 8021, 8140, 8142–8145, and 8480–8490), were aged 65 years or older, and for whom it was their first primary cancer, diagnosed from January 1, 1991, to December 31, 2002. We excluded individuals whose original reason for Medicare entitlement was not age, whose date of death in the SEER registry and Medicare files differed by more than 3 months, or whose reporting source of death was autopsy or death certificate. We also excluded those who were not eligible for Medicare Parts A and B or who were members of a health maintenance organization at some time from 12 months before diagnosis to 6 months after diagnosis. To include 65-year-old patients, we only required this for 2 months before diagnosis in addition to 6 months after diagnosis.
After the previously mentioned exclusions, 11,198 patients remained. Of these, we further required that patients have known American Joint Committee on Cancer Stage IB through Stage IV (M0) disease (n = 3,413). The INT-0116 trial used Stages IB through IV (M0), so we believed it would be reasonable to select these stages for ease of comparison to that landmark study.
In our effort to study patterns of care and survival in patients who received adjuvant therapy, we included only those patients who had undergone potentially curative resection. After also excluding those who had an unknown radiotherapy status or who had non-5-FU-based chemotherapy 2,333 patients remained.
These 2,333 patients were then categorized into 4 treatment groups: surgery alone, surgery/5-FU, surgery/radiation, and surgery/5-FU/radiation. The groups that received surgery/5-FU (n = 189), and surgery/radiation (n = 151) were considered to be too small and potentially biased for analysis in this observational study and were excluded. Thus our final sample consisted of 1,993 patients, of whom 1,671 had surgery alone and 322 had surgery/5-FU/radiation.
Treatment
To be eligible for this study, patients were required to have a surgery code in the Medicare files or SEER registry, or both. In the SEER registry site-specific surgery codes 20 through 70 were used, and in the Medicare files the Current Procedural Terminology codes 43620 through 43634 were used. The validity of using SEER–Medicare claims data for cancer-related surgery has been described previously (16).
Adjuvant 5-FU–based chemotherapy was identified with Medicare claims files by searching the Healthcare Common Procedure Coding System codes (level II code J9190). Patients who received 5-FU within 6 months of their date of diagnosis were classified as receiving adjuvant 5-FU treatment. The validity of using Medicare claims data for determining chemotherapy use has been described previously (17).
We also identified patients who received radiotherapy by use of SEER and Medicare data. To capture radiation treatment in the Medicare files, we used Current Procedural Terminology codes 77401 through 77499, excluding codes 77421 and 77470; those whose radiotherapy date was within 6 months after their date of diagnosis were classified as receiving adjuvant radiotherapy. Those whose radiation date preceded their surgery date were excluded. In the SEER registry, a variable identifies the timing of the radiation. Those noted as receiving radiation before surgery were excluded from the cohort; those with an unknown sequence were also excluded, provided that an appropriate sequence of surgery preceding radiation was not identified in the Medicare files. The validity of using SEER–Medicare claims data for radiation use has been described previously (18).
Socioeconomic status
Socioeconomic status (SES) was defined by use of three SEER variables: education, poverty, and income. These are determined at the census-tract level from the 2000 Census, as described previously (19). The variables were arbitrarily categorized into quintiles, ranging from highest SES to lowest SES. Some individuals had missing SES information and are noted as such.
Comorbid disease
We assessed the prevalence of comorbidities in the cohort by calculating Charlson indices for all individuals (20). This is an index that estimates the degree of comorbidity of the individual by determining the presence of ten conditions (stroke, dementia, renal failure, and so on) and that predicts, with high accuracy, the life expectancy of the individual. We constructed the indices in accordance with the new National Cancer Institute combined index, described by Klabunde et al. (21). Medicare hospital and physician claims data sources (22) were searched for International Classification of Diseases, Ninth Revision, Clinical Modification diagnostic codes from 12 months before to 1 month after the diagnosis of cancer (23). Because 65-year-old patients do not have 12 months of Medicare claims before the dates of their cancer diagnoses, we searched the months that were available to us.
Survival
Survival time was defined as the time from the cancer diagnosis date to the Medicare date of death. Our Medicare claims files had data on follow-up through December 31, 2005. Patients surviving past this date were censored (alive at the end of follow-up), and the time interval from their diagnosis date to the end of follow-up was used in the survival analysis.
Statistical analyses
All analyses were performed with SAS software, version 9.1 (SAS Institute, Cary, NC). Univariate associations between the treatment groups and various demographic and clinical factors were evaluated by use of chi-square tests. A logistic regression model was then created by use of treatment group (surgery alone vs. surgery/5-FU/radiation) as the dependent variable. Demographic factors, such as age, year of diagnosis, race/ethnicity, sex, marital status, residence, and SES, as well as clinical factors, such as comorbidity index, tumor subsite, American Joint Committee on Cancer overall stage, and tumor grade, were used as covariates (8, 9). Survival analysis was calculated by use of Cox proportional hazards modeling. Kaplan-Meier curves were generated to observe survival graphically.
RESULTS
We identified 1,993 patients in the SEER–Medicare database with Stage IB through Stage IV (M0) resected gastric adenocarcinoma diagnosed from January 1, 1991, to December 31, 2002. Their median age was 77 years (range, 65–103 years). Overall, the patients were mostly white (67.6%), resided in metropolitan areas (94.1%), were male (55.1%), and were married (56.7%) (Table 1). In addition, the patients in this cohort were more likely to have a comorbidity index of 1 or more (55.9%) and a higher-grade tumor (65.0%).
Table 1.
Demographic and clinical characteristics of patients with Stage IB through Stage IV (M0) resected gastric adenocarcinoma, by treatment, based on data from Surveillance, Epidemiology, and End Results–Medicare database, 1991 through 2002
| Characteristic | Surgery alone | Surgery + 5-FU + radiotherapy | Total | p Value* |
|---|---|---|---|---|
| No. of patients | 1,671 | 322 | 1,993 | |
| Demographic characteristics | ||||
| Age at diagnosis | <0.0001 | |||
| 65–69 y | 199 (11.9) | 108 (33.5) | 307 (15.4) | |
| 70–74 y | 336 (20.1) | 111 (34.5) | 447 (22.4) | |
| 75–79 y | 441 (26.4) | 74 (23.0) | 515 (25.8) | |
| 80–85 y | 467 (28.0) | 24 (7.5) | 491 (24.6) | |
| >85 y | 228 (13.6) | 5 (1.6) | 233 (11.7) | |
| Year of diagnosis | <0.0001 | |||
| 1991–1993 | 471 (28.2) | 40 (12.4) | 511 (25.6) | |
| 1994–1996 | 443 (26.5) | 68 (21.1) | 511 (25.6) | |
| 1997–1999 | 335 (20.1) | 25 (7.8) | 360 (18.1) | |
| 2000–2002 | 422 (25.3) | 189 (58.7) | 611 (30.7) | |
| Race/ethnicity | 0.03 | |||
| White | 1,114 (66.7) | 233 (72.4) | 1,347 (67.6) | |
| Black | 184 (11.0) | 18 (5.6) | 202 (10.1) | |
| Asian | 168 (10.1) | 34 (10.6) | 202 (10.1) | |
| Other/unknown | 205 (12.3) | 37 (11.5) | 242 (12.1) | |
| Sex | 0.01 | |||
| Male | 900 (53.9) | 199 (61.8) | 1,099 (55.1) | |
| Female | 771 (46.1) | 123 (38.2) | 894 (44.9) | |
| Marital status | <0.0001 | |||
| Unmarried | 747 (44.7) | 79 (24.5) | 826 (41.5) | |
| Married | 894 (53.5) | 236 (73.3) | 1,130 (56.7) | |
| Unknown | 30 (1.8) | 7 (2.2) | 37 (1.9) | |
| Residence | 0.04 | |||
| Metropolitan area | 1,581 (94.6) | 295 (91.6) | 1,876 (94.1) | |
| Non-metropolitan area | 90 (5.4) | 27 (8.4) | 117 (5.9) | |
| Socioeconomic status (quintile) | 0.2 | |||
| Missing | 43 (2.6) | 4 (1.2) | 47 (2.4) | |
| Lowest | 265 (15.9) | 38 (11.8) | 303 (15.2) | |
| Second | 306 (18.3) | 60 (18.6) | 366 (18.4) | |
| Third | 345 (20.7) | 75 (23.3) | 420 (21.1) | |
| Fourth | 358 (21.4) | 80 (24.8) | 438 (22.0) | |
| Highest | 354 (21.2) | 65 (20.2) | 419 (21.0) | |
| Clinical characteristics | ||||
| Comorbidity index | 0.005 | |||
| 0 | 714 (42.7) | 164 (50.9) | 878 (44.1) | |
| 1 | 485 (29.0) | 93 (28.9) | 578 (29.0) | |
| >1 | 472 (28.3) | 65 (20.2) | 537 (26.9) | |
| Subsite | 0.9 | |||
| Cardia/fundus | 301 (18.0) | 56 (17.4) | 357 (17.9) | |
| Body/antrum/pylorus | 719 (43.0) | 136 (42.2) | 781 (39.2) | |
| Other/unknown | 651 (39.0) | 130 (40.4) | 855 (42.9) | |
| Grade | 0.01 | |||
| Well or moderately differentiated | 545 (32.6) | 89 (27.6) | 634 (31.8) | |
| Poorly differentiated or undifferentiated | 1,066 (63.8) | 229 (71.1) | 1,295 (65.0) | |
| Unknown | 60 (3.6) | 4 (1.2) | 64 (3.2) | |
| T stage | 0.06 | |||
| T1 | 62 (3.7) | 18 (5.6) | 80 (4.0) | |
| T2 | 1,095 (65.5) | 188 (58.4) | 1,283 (64.4) | |
| T3 | 316 (18.9) | 75 (23.3) | 391 (19.6) | |
| T4 | 198 (11.9) | 41 (12.7) | 239 (12.0) | |
| N stage | <0.0001 | |||
| N0 | 641 (38.4) | 56 (17.4) | 697 (35.0) | |
| N1 | 735 (44.0) | 178 (55.3) | 913 (45.8) | |
| N2 | 239 (14.3) | 67 (20.8) | 306 (15.4) | |
| N3 | 56 (3.4) | 21 (6.5) | 77 (3.9) | |
| AJCC stage | <0.0001 | |||
| IB: T1N1, T2N0 | 543 (32.5) | 52 (16.2) | 595 (29.9) | |
| II: T1N2, T2N1, T3N0 | 564 (33.8) | 115 (35.7) | 679 (34.1) | |
| III: T2N2, T3N1, T4N0, T3N2 | 381 (22.8) | 107 (33.2) | 488 (24.5) | |
| IV (M0): T1–3N3, T4N1–3 | 183 (11.0) | 48 (14.9) | 231 (11.6) | |
Abbreviations: 5-FU = 5-fluorouracil; AJCC = American Joint Committee on Cancer.
Values are based on chi-square tests comparing patients who received surgery alone with those who received surgery and chemoradiotherapy.
Table 2 shows the results of the logistic regression analysis for predictors of treatment. As shown, practice changed dramatically after 1999, after which 31% of the cohort was treated with combined chemoradiotherapy. More advanced stage was very strongly associated with receipt of the adjuvant treatment. Being younger, being white, being married, and having fewer comorbidities were each also associated with a greater likelihood of being treated with 5-FU and radiation.
Table 2.
Factors associated with increased odds of receiving surgery plus chemoradiotherapy (n = 322) compared with surgery alone (n = 1,671) on final adjusted multiple logistic regression analysis for patients with resected gastric adenocarcinoma, Stages IB through IV (M0), based on data from Surveillance, Epidemiology, and End Results–Medicare database, 1991 through 2002
| Characteristic | Odds ratio | 95% CI |
|---|---|---|
| Age at diagnosis | ||
| 65–69 y | 1.00 | Referent |
| 70–74 y | 0.62* | 0.43–0.89* |
| 75–79 y | 0.28* | 0.19–0.42* |
| 80–85 y | 0.08* | 0.05–0.14* |
| >85 y | 0.03* | 0.01–0.09* |
| Year of diagnosis | ||
| 1991–1999 | 1.00 | Referent |
| 2000–2002 | 5.55* | 4.15–7.42* |
| Race/ethnicity | ||
| White | 1.00 | Referent |
| Black | 0.44* | 0.25–0.80* |
| Asian | 0.66 | 0.42–1.05 |
| Other/unknown | 0.68 | 0.44–1.06 |
| Sex | ||
| Male | 1.00 | Referent |
| Female | 1.03 | 0.76–1.40 |
| Marital status | ||
| Unmarried | 1.00 | Referent |
| Married | 1.71* | 1.23–2.39* |
| Unknown | 1.81 | 0.70–4.73 |
| Residence | ||
| Metropolitan area | 1.00 | Referent |
| Non-metropolitan area | 1.45 | 0.82–2.55 |
| Socioeconomic status (quintile) | ||
| Missing | 0.92 | 0.29–2.90 |
| Lowest | 1.00 | Referent |
| Second | 1.14 | 0.68–1.90 |
| Third | 1.47 | 0.88–2.44 |
| Fourth | 1.65 | 0.99–2.74 |
| Highest | 1.12 | 0.66–1.89 |
| Comorbidity index | ||
| 0 | 1.00 | Referent |
| 1 | 0.84 | 0.61–1.17 |
| >1 | 0.61* | 0.43–0.88* |
| Subsite | ||
| Cardia/fundus | 1.00 | Referent |
| Body/antrum/pylorus | 1.47 | 0.98–2.21 |
| Other/unknown | 1.41 | 0.95–2.11 |
| Grade | ||
| Well or moderately differentiated | 1.00 | Referent |
| Poorly differentiated or undifferentiated | 1.00 | 0.73–1.38 |
| Unknown | 0.45 | 0.15–1.36 |
| AJCC stage | ||
| IB: T1N1, T2N0 | 1.00 | Referent |
| II: T1N2, T2N1, T3N0 | 2.28* | 1.54–3.35* |
| III: T2N2, T3N1, T4N0, T3N2 | 3.27* | 2.18–4.92* |
| IV (M0): T1–3N3, T4N1–3 | 2.89* | 1.77–4.72* |
Abbreviations: CI = confidence interval; AJCC = American Joint Committee on Cancer.
Significant values.
There were 339 patients who died less than 4 months after their date of diagnosis. Patients aged greater than 85 years, as well as patients who died less than 4 months after their date of diagnosis, were excluded from the initial cohort of 1,993 patients with Stage IB through Stage IV (M0) gastric cancer. Those who died less than 4 months after diagnosis were excluded because these patients, even if treated, would not have had time to derive survival benefit from the adjuvant treatment.
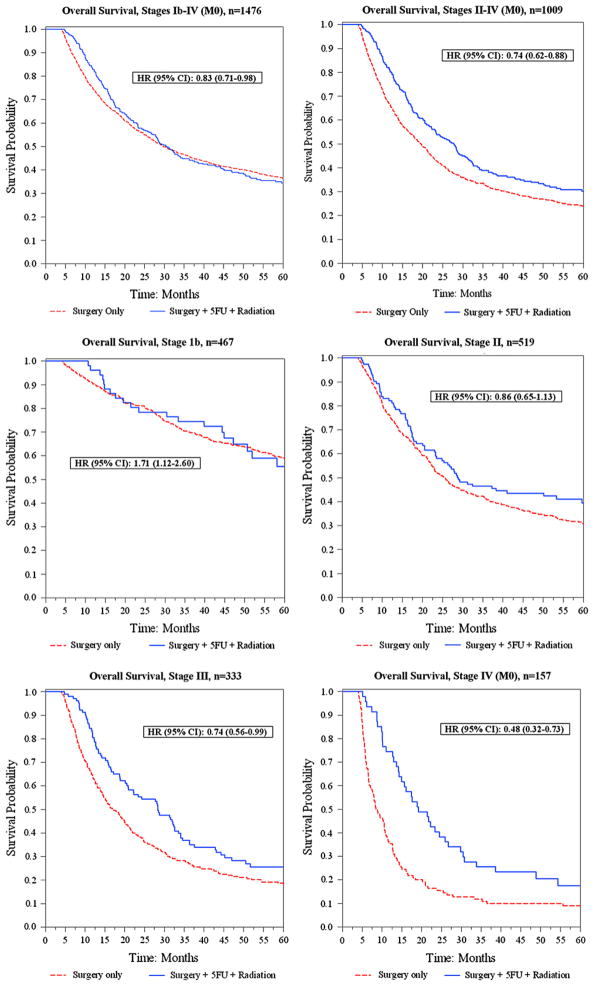
Of the remaining 1,476 Stage IB through Stage IV (M0) gastric cancer patients, 1,106 (75%) died during the follow-up period. The median number of unadjusted months to death for surgery alone vs. surgery/5-FU/radiation was 59 months vs. 47 months for Stage IB, 26 vs. 29 for Stage II, 17 vs. 28 for Stage III, and 9 vs. 19 for nonmetastatic Stage IV. In addition, unadjusted Kaplan-Meier curves were generated for each stage, to display the survival data graphically (Fig. 1). The 3-year overall survival rates for surgery alone vs. surgery/5-FU/radiation were 70.2% vs. 74.5% for Stage IB, 40.8% vs. 46.4% for Stage II, 27.4% vs. 36.9% for Stage III, and 10.9% vs. 25.5% for Stage IV (M0).
Fig. 1.
Unadjusted Kaplan-Meier curves, overall and by stage. Hazard ratios were generated from a multivariate Cox regression model adjusted for age, race, sex, comorbidity index, subsite, grade, American Joint Committee on Cancer stage, and treatment group. HR = hazard ratio; CI = confidence interval; 5FU = 5-fluorouracil.
The results of the Cox analysis for overall Stages IB through IV (M0), as well as the results of the model when patients with Stage 1B are removed, are shown in Table 3. We stratified the results by age and year of diagnosis as well. Combined surgery/5-FU/radiation was associated with an overall 17% reduced risk of death (HR, 0.83; 95% CI, 0.71–0.98). Stage IB patients who received adjuvant treatment had significantly worse outcomes than those undergoing surgery alone (HR, 1.71; 95% CI, 1.12–2.60), whereas Stage II patients who received treatment trended toward better outcomes, although this finding was not statistically significant (HR, 0.86; 95% CI, 0.65–1.13). Significant survival benefits were found with adjuvant treatment in Stage III patients (HR, 0.74; 95% CI, 0.56–0.99) and Stage IV (M0) patients (HR, 0.48; 95% CI, 0.32–0.73).
Table 3.
Cox proportional hazard ratios for overall mortality associated with demographic and clinical characteristics of patients with resected gastric cancer, who survived for 4 months or more after date of diagnosis, 1991 to 2002, ages 65 to 85 years (median age, 75 years)
| Variable | Overall survival | |||
|---|---|---|---|---|
| Stages IB–IV (M0) (n = 1,476) | Stages II–IV (M0) (n = 1,009) | |||
| HR | 95% CI | HR | 95% CI | |
| Age at diagnosis | ||||
| 65–69 y | 1.00 | Referent | 1.00 | Referent |
| 70–74 y | 1.17 | 0.97–1.42 | 1.10 | 0.89–1.37 |
| 75–79 y | 1.43* | 1.18–1.73* | 1.31* | 1.06–1.63* |
| 80–85 y | 1.66* | 1.36–2.02* | 1.38* | 1.10–1.74* |
| Race/ethnicity | ||||
| White | 1.00 | Referent | 1.00 | Referent |
| Black | 1.34* | 1.10–1.64* | 1.35* | 1.06–1.71* |
| Asian | 0.72* | 0.58–0.88* | 0.73* | 0.58–0.92* |
| Other/unknown | 1.23* | 1.03–1.47* | 1.33* | 1.09–1.63* |
| Sex | ||||
| Male | 1.00 | Referent | 1.00 | Referent |
| Female | 0.80* | 0.70–0.91* | 0.84* | 0.72–0.98* |
| Marital status | ||||
| Unmarried | 1.00 | Referent | 1.00 | Referent |
| Married | 0.82* | 0.72–0.94* | 0.84* | 0.71–0.98* |
| Unknown | 1.03 | 0.64–1.66 | 1.26 | 0.73–2.17 |
| Comorbidity index | ||||
| 0 | 1.00 | Referent | 1.00 | Referent |
| 1 | 1.16* | 1.01–1.34* | 1.14 | 0.97–1.34 |
| >1 | 1.23* | 1.06–1.43* | 1.17 | 0.99–1.39 |
| Subsite | ||||
| Cardia/fundus | 1.00 | Referent | 1.00 | Referent |
| Body/antrum/pylorus | 0.72* | 0.61–0.86* | 0.73* | 0.60–0.89* |
| Other/unknown | 0.79* | 0.67–0.94* | 0.81* | 0.66–0.98* |
| Grade | ||||
| Well or moderately differentiated | 1.00 | Referent | 1.00 | Referent |
| Poorly differentiated or undifferentiated | 1.17* | 1.03–1.33* | 1.27* | 1.08–1.49* |
| Unknown | 1.39 | 0.99–1.95 | 1.45 | 0.96–2.19 |
| AJCC stage | ||||
| IB | 1.00 | Referent | — | — |
| II | 1.96* | 1.68–2.30* | 1.00 | Referent |
| III | 2.83* | 2.39–3.36* | 1.42* | 1.21–1.65* |
| IV (M0) | 4.60* | 3.72–5.68* | 2.30* | 1.88–2.80* |
| Treatment group | ||||
| Surgery alone | 1.00 | Referent | 1.00 | Referent |
| Surgery + 5-FU + radiotherapy | 0.83* | 0.71–0.98* | 0.74* | 0.62–0.88* |
Abbreviations: HR = hazard ratio; CI = confidence interval; AJCC = American Joint Committee on Cancer; 5-FU = 5-fluorouracil.
Significant values.
Stratification by age at diagnosis was limited by small numbers, but all age categories trended toward improvement with adjuvant 5-FU/radiation, with the exception of patients aged 80 to 85 years. Finally, when stratified by year of diagnosis, both late (2000–2002) and early (1991–1999) groups showed or trended toward benefit, with those diagnosed after 1999 deriving more benefit.
DISCUSSION
Although the INT-0116 randomized controlled trial found an overall survival benefit for adjuvant chemoradiotherapy after resection in a study of 603 patients with Stage IB through Stage IV (M0) gastric cancer, the number of patients with Stage IB and Stage II disease was small, and the benefits in this subgroup remain uncertain. In our population-based study we had a sufficient number of subjects to investigate the impact of the treatment on these early-stage gastric cancer patients. We found that adjuvant chemoradiotherapy significantly improves overall survival for Stage III and Stage IV (M0) resected gastric cancer as compared with surgery alone after controlling for known prognostic factors. For patients with Stage II disease, there was a nonsignificant trend toward a survival benefit. Importantly, we found no survival benefit after adjuvant treatment for Stage IB disease and possibly worse survival.
Using a population-based sample, we found a benefit for chemoradiotherapy similar to that found by other investigators. For patients with Stage III gastric cancer, treatment was associated with an HR for death of 0.74 and a median survival that was 11.3 months longer than with surgery alone. Similarly, for Stage IV (M0) patients, treatment was associated with an HR for death of 0.48 and a median survival that was 10.6 months longer than with surgery alone. These results confirm the findings of a previous study by Coburn et al. (24) that assessed the survival benefits of radiation therapy in gastric cancer patients using the SEER database. They found an HR of 0.77 (95% CI, 0.63–0.95) for Stage III and an HR of 0.61 (95% CI, 0.53–0.70) for Stage IV (M0) with the use of adjuvant radiation therapy, presumably with chemotherapy in most instances.
Adjuvant 5-FU and radiation therapy for Stage IB through Stage IV (M0) resected gastric adenocarcinoma became the recommended form of treatment in May 2000 when Mac-Donald et al. (5) presented the results of the INT-0116 trial at the annual meeting of the American Society of Clinical Oncology. Subsequently, there was rapid adoption of that trial’s findings (25). Patients diagnosed from 2000 to 2002 were more than five times more likely to have received adjuvant therapy compared with those diagnosed from 1991 to 1999.
We found that black patients were half as likely to be treated with adjuvant chemoradiotherapy as compared with white patients (Table 2). Racial disparities in treatment have been shown for the treatment of multiple other cancer sites, such as nonmetastatic prostate, early-stage lung, breast, and colon cancer (8, 26–31). The reasons for these disparities are not always clear, but efforts to increase the rates of treatment among this population continue to be a critical area of research.
Other characteristics that predicted the receipt of adjuvant treatment included being married, having fewer comorbidities, and having a higher gastric cancer stage. It is well known that being married benefits health and longevity (32–34). Older patients were less likely to be treated with adjuvant therapy in our study. This has been shown for a number of different cancers, including colorectal, breast, and ovarian carcinomas (12, 13, 35, 36). There can be many valid reasons for not treating older cancer patients with adjuvant therapy. Older patients may be frailer, may be in poorer health, and may prefer treatment less often than younger patients. However, there may also be many older patients who would greatly benefit from treatment but do not receive it.
The majority of patients in the INT-0116 study had Stage III or Stage IV cancer (6). Overall, the authors reported an HR for death of 1.35 for surgery alone and an extra 9 months of survival for those in the treatment group. There are limitations to comparisons of our study with INT-0116. INT-0116 was conducted in the controlled environment of a randomized trial, whereas our study is an observational cohort study using an administrative database. In addition, INT-0116 included patients with tumors of the gastroesophageal junction whereas ours did not.
In contrast to the other studies, this study had a significant number of patients with Stage IB cancer; in fact, there were 595 cases, nearly 30% of the total cohort. The survival analysis in this study suggests that adjuvant chemoradiotherapy in the context of Stage IB disease did not improve patient survival, perhaps because this stage of disease is so treatable by surgery alone that the toxicity from treatment outweighs the benefit. However, these results should be interpreted with caution because this was an observational study. Thus it is possible that patients with early disease who underwent treatment may have unmeasured confounders that may bias the group toward a worse prognosis.
Because we found that Stage I patients did not benefit from adjuvant treatment, we conducted further survival analyses without including them in our sample. Patients with Stage II through Stage IV (M0) disease (n = 1,009) were stratified by age for analysis. We found that all the age strata benefitted or trended toward benefit from adjuvant therapy with chemoradiation, except patients aged 80 to 85 years. Despite these findings, elderly patients are not treated as often as younger patients, as shown in Table 2. Again, these findings need to be interpreted with caution because they are observational and treated patients may have an unmeasurable better performance status that accounts for their improved outcomes.
In addition to adjuvant chemoradiotherapy, there were other significant predictors of survival in the adjusted Cox model. Increasing age, black race, male sex, single marital status, higher comorbidity score, more proximal subsite, higher grade, and higher stage were all significant predictors of worse prognosis. These factors have been noted to behave similarly in other contexts as well.
There are various limitations to this study. Although we were able to gain a sense of the patients’ functional status by assigning a comorbidity score, we were unable to determine the nutritional or performance status of the patients. These variables were controlled in INT-0116 but could not be ascertained in our study. Perhaps most importantly, we did not have detailed surgical information on the patients. A major criticism of INT-0116 was that the surgical dissections were largely incomplete, with limited lymphadenectomies; some critics have questioned whether the observed poor results in the surgery-only group were the effects of inadequate surgical dissection. In our study the same question remains, as well as another one: it is unknown whether the patients in this study had positive surgical margins after surgery. Patients with positive surgical margins did not receive curative surgery, and thus their therapy would not have qualified as “adjuvant” therapy. This would not systematically bias the results for or against the null, but it is an important variable that is not controlled. Another limitation is that the doses of chemotherapy and radiation therapy were not standardized among patients in the treatment category; this is especially true for the years before 2000, when the regimen of MacDonald et al. (5) had not yet been reported.
We have shown, albeit in an observational study, that the survival benefits of adjuvant chemoradiotherapy after gastrectomy for nonmetastatic gastric cancer, as described in the INT-0116 trial, are still present when the therapy is used in the general population. Indeed, the population analyzed in our study is elderly, and hence this therapy is appropriate for patients in the age range of greater than 65 years. With the size of our sample, we were able to investigate important subgroups. Our analyses suggest that this adjuvant therapy may not confer survival benefits in patients with Stage IB disease and possibly not in those with Stage II disease whereas it is more clearly appropriate for Stage III and Stage IV (M0) gastric cancer. Further studies with large populations are needed to confirm these results before they can be recommended definitively as standard of care.
Acknowledgments
Dr. Neugut is the recipient of a grant from the American Cancer Society (RSGT-01-024-04-CPHPS). Dr. Hershman is the recipient of an American Society of Clinical Oncology Advanced Clinical Research Award. Mr. McBride was supported by an R25 Fellowship from the National Cancer Institute (CA094061) and a T32 Fellowship (ULI RR024156) from the National Center for Research Resources.
Footnotes
Conflict of interest: none.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006;24:2137–2150. doi: 10.1200/JCO.2005.05.2308. [DOI] [PubMed] [Google Scholar]
- 3.Ries LAG, Melbert D, Krapcho M, et al. SEER cancer statistics review, 1975–2005. Bethesda, MD: National Cancer Institute; 2008. [Google Scholar]
- 4.Hundahl SA, Phillips JL, Menck HR. The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the “different disease” hypothesis. Cancer. 2000;88:921–932. [PubMed] [Google Scholar]
- 5.MacDonald J, Smalley S, Benedetti J, et al. Postoperative combined radiation and chemotherapy improves disease-free survival (DFS) and overall survival (OS) in resected adenocarcinoma of the stomach and G.E. junction. Results of Intergroup Study INT-0116 (SWOG 9008) [Abstract] Proc Am Soc Clin Oncol. 2000;19:1a. [Google Scholar]
- 6.MacDonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. doi: 10.1056/NEJMoa055531. [DOI] [PubMed] [Google Scholar]
- 8.Sundararajan V, Mitra N, Jacobson JS, et al. Survival associated with 5-fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med. 2002;136:349–357. doi: 10.7326/0003-4819-136-5-200203050-00007. [DOI] [PubMed] [Google Scholar]
- 9.Neugut AI, Fleischauer AT, Sundararajan V, et al. Use of adjuvant chemotherapy and radiation therapy for rectal cancer among the elderly: A population-based study. J Clin Oncol. 2002;20:2643–2650. doi: 10.1200/JCO.2002.08.062. [DOI] [PubMed] [Google Scholar]
- 10.Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345:1091–1097. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 11.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 12.Samet J, Hunt WC, Key C, et al. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–3390. [PubMed] [Google Scholar]
- 13.Greenfield S, Blanco DM, Elashoff RM, et al. Patterns of care related to age of breast cancer patients. JAMA. 1987;257:2766–2670. [PubMed] [Google Scholar]
- 14.Mor V, Masterson-Allen S, Goldberg RJ, et al. Relationship between age at diagnosis and treatments received by cancer patients. J Am Geriatr Soc. 1985;33:585–589. doi: 10.1111/j.1532-5415.1985.tb06313.x. [DOI] [PubMed] [Google Scholar]
- 15.Potosky AL, Riley GF, Lubitz JD, et al. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–748. [PubMed] [Google Scholar]
- 16.Cooper GS, Virnig B, Klabunde CN, et al. Use of SEER-Medicare data for measuring cancer surgery. Med Care. 2002;40:IV-43–IV-48. doi: 10.1097/00005650-200208001-00006. [DOI] [PubMed] [Google Scholar]
- 17.Warren JL, Harlan LC, Fahey A, et al. Utility of the SEER-Medicare data to identify chemotherapy use. Med Care. 2002;40:IV-55–IV-61. doi: 10.1097/01.MLR.0000020944.17670.D7. [DOI] [PubMed] [Google Scholar]
- 18.Virnig BA, Warren JL, Cooper GS, et al. Studying radiation therapy using SEER-Medicare-linked data. Med Care. 2002;40:IV-49–IV-54. doi: 10.1097/00005650-200208001-00007. [DOI] [PubMed] [Google Scholar]
- 19.Du XL, Fang S, Vernon SW, et al. Racial disparities and socioeconomic status in association with survival in a large population-based cohort of elderly patients with colon cancer. Cancer. 2007;110:660–669. doi: 10.1002/cncr.22826. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Klabunde CN, Legler JM, Warren JL, et al. A refined comorbidity measurement algorithm for claims-based studies of breast, prostate, colorectal, and lung cancer patients. Ann Epidemiol. 2007;17:584–590. doi: 10.1016/j.annepidem.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 22.Klabunde CN, Potosky AL, Legler JM, et al. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53:1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 23.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 24.Coburn NG, Govindarajan A, Law CH, et al. Stage-specific effect of adjuvant therapy following gastric cancer resection: A population-based analysis of 4,041 patients. Ann Surg Oncol. 2008;15:500–507. doi: 10.1245/s10434-007-9640-0. [DOI] [PubMed] [Google Scholar]
- 25.Coburn NG, Guller U, Baxter NN, et al. Adjuvant therapy for resected gastric cancer-rapid, yet incomplete adoption following results of intergroup 0116 trial. Int J Radiat Oncol Biol Phys. 2008;70:1073–1080. doi: 10.1016/j.ijrobp.2007.07.2378. [DOI] [PubMed] [Google Scholar]
- 26.Sundararajan V, Grann VR, Jacobson JS, et al. Variations in the use of adjuvant chemotherapy for node-positive colon cancer in the elderly: A population-based study. Cancer J. 2001;7:213–218. [PubMed] [Google Scholar]
- 27.Klabunde CN, Potosky AL, Harlan LC, et al. Trends and black/white differences in treatment for nonmetastatic prostate cancer. Med Care. 1998;36:1337–1348. doi: 10.1097/00005650-199809000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 29.Michalski TA, Nattinger AB. The influence of black race and socioeconomic status on the use of breast-conserving surgery for Medicare beneficiaries. Cancer. 1997;79:314–319. [PubMed] [Google Scholar]
- 30.Tropman SE, Hatzell T, Paskett E, et al. Colon cancer treatment in rural North and South Carolina. Cancer Detect Prev. 1999;23:428–434. doi: 10.1046/j.1525-1500.1999.99042.x. [DOI] [PubMed] [Google Scholar]
- 31.Bickell NA, McEvoy MD. Physicians’ reasons for failing to deliver effective breast cancer care: A framework for underuse. Med Care. 2003;41:442–446. doi: 10.1097/01.MLR.0000052978.49993.27. [DOI] [PubMed] [Google Scholar]
- 32.Osborne C, Ostir GV, Du X, et al. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 33.Johnson NJ, Backlund E, Sorlie PD, et al. Marital status and mortality: The national longitudinal mortality study. Ann Epidemiol. 2000;10:224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 34.Bloom JR, Stewart SL, Johnston M, et al. Sources of support and the physical and mental well-being of young women with breast cancer. Soc Sci Med. 2001;53:1513–1524. doi: 10.1016/s0277-9536(00)00440-8. [DOI] [PubMed] [Google Scholar]
- 35.Munoz KA, Harlan LC, Trimble EL. Patterns of care for women with ovarian cancer in the United States. J Clin Oncol. 1997;15:3408–3415. doi: 10.1200/JCO.1997.15.11.3408. [DOI] [PubMed] [Google Scholar]
- 36.Hightower RD, Nguyen HN, Averette HE, et al. National survey of ovarian carcinoma. IV: Patterns of care and related survival for older patients. Cancer. 1994;73:377–383. doi: 10.1002/1097-0142(19940115)73:2<377::aid-cncr2820730223>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]