Key Points
Src signals are required for specific receptor and cytokine activation–induced rapid reorientation of lytic granules to the MTOC in NK cells.
Abstract
Natural killer (NK) cells participate in host defense by surveying for and ultimately killing virally infected or malignant target cells. NK cell cytotoxicity is a tightly regulated process that proceeds stepwise from adhesion and activation to the secretion of preformed lytic granule contents onto a diseased or stressed cell. We previously characterized rapid dynein-dependent lytic granule convergence to the microtubule-organizing center (MTOC) as an early, prerequisite step in NK cell cytotoxicity. Although multiple activating receptors can trigger granule convergence, the specific signal or signals responsible remained unknown. Using live cell confocal microscopy, NK cell lytic granule movement after NK cell activation was captured and measured. Using inhibitors of common early signaling mediators, we show that Src kinases are required for lytic granule convergence, but downstream signals that promote actin rearrangement, MTOC polarization, and calcium mobilization are not. Exposure to interleukin 2 was also sufficient to induce lytic granule convergence, which required noncanonical Src-dependent signaling. Thus, NK cell lytic granule convergence, prompted by specific receptor-mediated and soluble cytokine signals, depends on a directly downstream early Src kinase–dependent signal and emphasizes the importance of this step in readying NK cells for cytotoxicity.
Introduction
Natural killer (NK) cells are important in anticancer and antiviral response.1 NK cell killing occurs in a precise, stepwise fashion culminating in secretion of the contents of lysosome-related organelles, known as lytic granules, onto a stressed or infected target cell.2 NK cell activation occurs through engagement of germ-line–encoded receptors; immediately thereafter, NK cell F-actin accumulates at the site of contact to support an organized signaling and secretory platform termed the immunological synapse (IS). During synapse formation, lytic granules rapidly converge to a randomly located microtubule organizing center (MTOC) within minutes of target cell contact or activating receptor ligation.3 The lytic granules use dynein-dependent transport to reach the MTOC, but the process is independent of actin reorganization and microtubule dynamics.3 After convergence, the MTOC and associated lytic granules polarize to the IS, where granules pass through the actin network to fuse with the NK cell membrane and secrete their contents.4 Each of these intercellular events along the path to directed secretion is tightly regulated, allowing NK cells to precisely target their deadly effects to virally infected, stressed, or tumorigenic cells.
If a target cell is diseased, activating receptor engagement predominates the NK–target cell interaction, which triggers downstream signaling pathways.5-8 Activation of integrin leukocyte function–associated antigen-1 (LFA-1, composed of CD11a/CD18) leads to Vav1 activation,9 which in turn recruits F-actin to the IS.10,11 Similarly, phosphoinositide 3-kinase (PI3K) is activated, thus promoting mitogen-activated protein kinase kinase (MEK)/extracellular signal-regulated kinase (Erk) signaling and MTOC polarization.7,8 Activation of phospholipase C-γ (PLCγ) by ligated immunoreceptors stabilizes synapse formation and triggers intracellular release of calcium stores.12,13
If the target cell is healthy, however, inhibitory receptor engagement predominates the IS. Here, Killer cell immunoglobulin-like receptors (KIRs) recognize MHC-I and recruit Src homology domain containing-phosphatase 1 (SHP-1), which dephosphorylates key downstream proteins (including Vav1) to deactivate activation pathways and halt cytotoxic responses.14-16
NK cells are also influenced by cytokines. Interleukin-2 (IL-2) enhances NK cytotoxicity by promoting maturation, increasing activating receptor expression, and increasing cytokine production.17,18 Canonical IL-2 signaling requires specific Janus activated kinases (JAKs) and signal transducers and activators of transcription (STATs). IL-2 receptor signaling, however, also can proceed via a noncanonical pathway that links the Src homology-2 domain-containing transforming protein C with mitogen activated protein kinase.19,20
Src family kinase phosphorylation is common to NK cell signaling downstream of many activation receptors.9,14,21-23 As alluded to above, even cytokine signaling can induce Src kinase phosphorylation, which can serve to propagate function via maintaining active levels of JAK/STAT signaling.24
Lytic granule convergence to the MTOC is an early step in directed secretion for cytolysis. Convergence can potentially enable efficient delivery of large numbers of granules to the IS, thus promoting maximal cytotoxicity, preventing collateral damage to bystander cells, and priming NK cells for more efficient subsequent kills. Previously, we demonstrated that convergence is rapid, activation induced, dynein dependent, and independent of actin reorganization and microtubule dynamics.3 Although signaling involved in other aspects of NK cell cytotoxicity is well understood, no signaling molecules have been linked to the process of lytic granule convergence. Using specific inhibitors and cytokines, we herein define the signaling requirements for lytic granule convergence in NK cells. We found that lytic granule convergence follows adhesion, triggering receptor engagement, and IL-2 stimulation and is not inhibited by KIR. It requires Src family kinases but not PI3K, MEK, PLCγ, or canonical IL-2 signaling. We propose that lytic granule convergence is initiated very early after receptor engagement, lies directly downstream of Src kinase, and is a common feature of NK cell activation.
Materials and methods
Cell lines and ex vivo NK cells
The NK cell line YTS was retrovirally transduced to stably express green fluorescent protein (GFP)-α-tubulin or GFP-KIR2DL1.25 721.221 and K562 cells were used as susceptible target cells for YTS and ex vivo NK (eNK) cells, respectively.25 eNK cells were prepared from peripheral blood from healthy and LAD-1 donors using human NK cell isolation kits (Miltenyi Biotec) or RosetteSep NK (StemCell Technologies). 51Cr cytotoxicity assays, flow cytometry, and flow cytometric conjugation assays were performed as described previously.26
Live cell confocal microscopy
GFP-tubulin–expressing YTS were incubated with 5 μM LysoTracker Red DND-99 (Molecular Probes) for 30 minutes at 37°C, washed, and added to 721.221 cells that had been adhered to ΔT dishes (Bioptechs) precoated with antibodies against CD48 (BD).3 Conjugates were imaged in z-axis planes containing the MTOC using a Zeiss Z1 microscope with Yokogawa CSU10 spinning disc, 63× 1.43 NA objective, and solid-state 488- and 561-nm lasers. In experiments without target cells, LysoTracker-loaded NK cells were added to ΔT dishes precoated with antibodies against CD28 (BD) or CD11a (ATCC) and imaged every 10 seconds; in IL-2 experiments, ΔT dishes were uncoated, and IL-2 (Roche) was added 2 to 4 minutes after imaging began. Temperature was maintained at 37°C using ΔT dish and objective environmental control units (Bioptechs).
Fixed cell microscopy
Activation, fixation, permeabilization, and staining were performed as described25 with the following reagents used sequentially: (1) biotinylated monoclonal mouse-anti-tubulin (Invitrogen) or biotinylated control mouse IgG (BD); (2) streptavidin-Pacific Blue (Invitrogen); and (3) fluorescence in situ hybridization–conjugated mouse-anti-perforin clone ΔG9 or fluorescence in situ hybridization–conjugated control mouse IgG (BD) and 647-conjugated Phalloidin (Invitrogen). Slides were mounted with 0.15-mm coverslips (VWR Scientific) using ProLong AntiFade (Invitrogen).
Inhibitors and cytokines
YTS cells were preincubated with 25 μM PP2, 20 μM LY294002, 100 nM Wortmannin, 100 μM PD98059, 4 μM U73122 (all from Sigma), 1 μM ZM449829, or 20 μM AZM475271 (Tocris Bioscience) for 30 minutes and washed, and imaging was performed in the presence of inhibitors. IL-2 (Roche) was added at a concentration of 125 U/mL to YTS cells or 1000 U/mL to eNK cells, only after the time indicated; 100 ng/mL IL-10 (Serotec) was added to YTS cells at time zero. Western blot analysis for pSrc and Myosin II A was performed as described26 using a polyclonal antibody directed against phospho Src Y416 (Cell Signaling).
Image analysis
Images were analyzed using Volocity software (Perkin Elmer) and measured for fluorescence corresponding to the MTOC (GFP maximal intensity) or lytic granules (LysoTracker Red maximal intensity). Regions of positivity were selected based on 2 to 5 standard deviations above mean intensity. In time-lapse experiments, ≥1 image/min was used for quantitative analysis. Lytic granule convergence to the MTOC or to the centroid of all granules was measured as described.3 Presentation images were contrast enhanced uniformly.
Statistical analysis
The minimum number of cells evaluated was determined using sample size calculations based on preliminary data (α/β-error levels of 1%). Differences over time between inhibitor-treated and control-treated or between resting and IL-2–treated NK cells were evaluated using unpaired Mann-Whitney U tests. Individual means were compared using the Student t test. Differences were considered significant when P ≤ .05.
Results
β2 integrin is required for lytic granule convergence in human NK cells
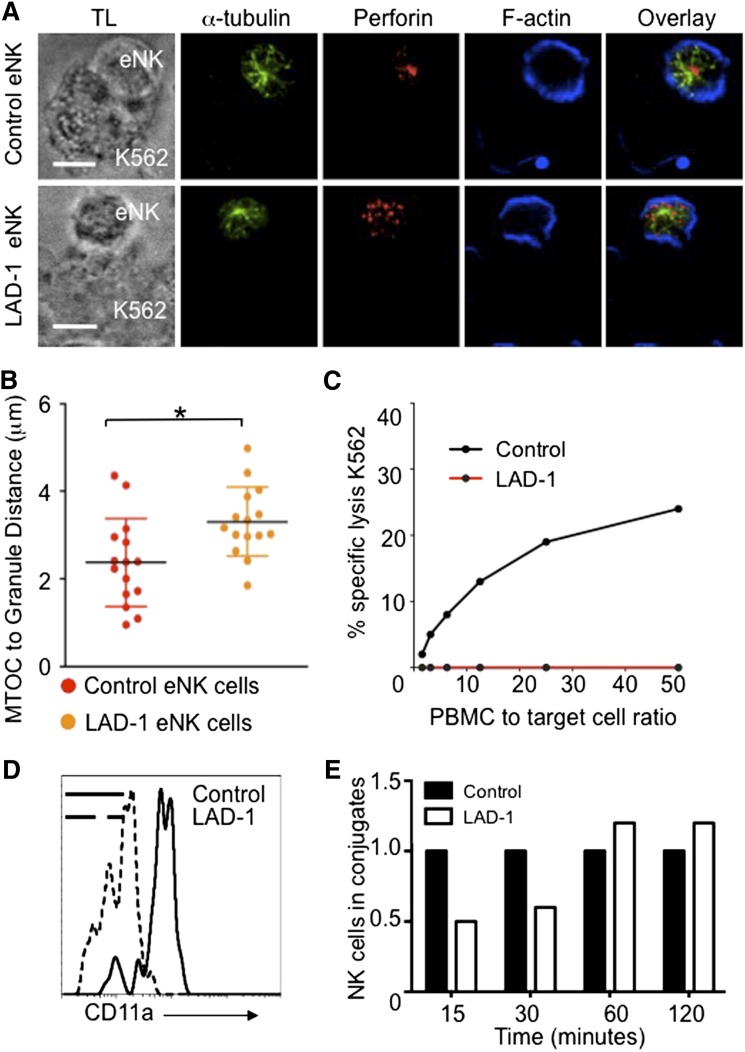
Lytic granule convergence to the MTOC is rapid and independent of many checkpoints in the process of IS maturation. In our previous studies, we noted that blocking LFA-1 with anti-CD11a antibody interrupted granule convergence.3 LFA-1 is required for optimal NK cell adhesion27 and, like granule convergence, certain signals downstream of LFA-1 are independent of F-actin reorganization.10 To confirm and extend the role of LFA-1, we examined granule convergence in leukocyte adhesion deficiency-1 (LAD-1) patients who lack the CD18 subunit of LFA-1.28 eNK cells from control and LAD-1 donors were incubated with K562 target cells, fixed, and then stained for lytic granules, the MTOC, and F-actin. LAD-1 eNK cells conjugated with target cells were detected. In fixed cell conjugates, lytic granules in control eNK cells converged to the MTOC. In contrast, LAD-1 eNK cell lytic granules failed to converge to the MTOC after target cell conjugation (Figure 1A-B). LAD-1 eNK cells had a profound defect in NK cell mediated cytotoxic function (Figure 1C), similar to that previously reported for LAD-1 peripheral blood mononuclear cells.27 CD11a expression on LAD-1 patient cells was greatly reduced, presumably because of loss of CD18 and resulting instability (Figure 1D).28 Despite an absence of LFA-1 expression on the cell surface, LAD-1 patient eNK formed conjugates with susceptible targets. These were delayed in formation but approached normal levels after 60 minutes in fluorescence-activated cell sorter–based conjugation assays (Figure 1E). These data confirm that signaling through the LFA-1 complex is necessary for lytic granule convergence in NK cells and suggest an additional NK cell impairment other than target cell conjugation in these rare patients.
Figure 1.
β2 integrin is necessary for lytic granule convergence to the MTOC in human ex vivo NK cells. All human samples were obtained after informed donor consent in accordance with the Declaration of Helsinki and were used with approval of the Institutional Internal Review Board for the Protection of Human Subjects of the Children’s Hospital of Philadelphia and/or National Institutes of Allergy and Infectious Diseases. (A) Confocal microscopy of fixed cells showing representative eNK cells from (top) a healthy donor and (bottom) a LAD-1 patient conjugated to susceptible K562 cells. After 30 minutes at 37°C on poly-L-lysine–coated glass slides (PolyPrep; Sigma-Aldrich), conjugates were fixed and stained with anti–α-tubulin (green), anti-perforin (red), and Phalloidin-F-actin (blue). TL, transmitted light. (B) Lytic granule distance from the MTOC in 15 cells; error bars show ±SD. Mean distance of lytic granules from the MTOC in LAD-1 eNK cells was significantly different from that in control eNK cells (*P < .05). (C) Standard 4-hour Cr51 cytotoxicity assay showing impaired NK cell lytic function by LAD-1 patient peripheral blood mononuclear cells (red) compared with healthy control (black). (D) Reduced CD11a expression on LAD-1 (dashed histogram) CD56+CD3− eNK cells as detected by flow cytometry compared with healthy donor control (solid histogram). (E) Conjugate formation between control (black) and LAD-1 (white) eNK cells as determined by fluorescence-activated cell sorter–based conjugation assays. Control values for each time point were normalized to 1.
Src kinase activity is required for human NK cell lytic granule convergence
In addition to LFA-1, activating receptors like CD28, but not nonspecific, nonactivating receptors like CD45, can promote convergence.3 We hypothesize that most NK cell activation signaling promotes lytic granule convergence as a way to prime and prepare NK cells for eventual directed secretion. Thus, we focused on signals downstream of NK cell activating receptors, prioritizing those involved in key stages of NK cell cytotoxicity.
Src family kinases are redundant tyrosine kinases responsible for phosphorylation of many NK cell receptors.9,21,22 The resulting downstream signaling is crucial for NK cell activation and includes Vav1 (required for IS actin accumulation10), PI3K (necessary for MTOC and granule polarization7,8), and PLCγ (required for degranulation29).
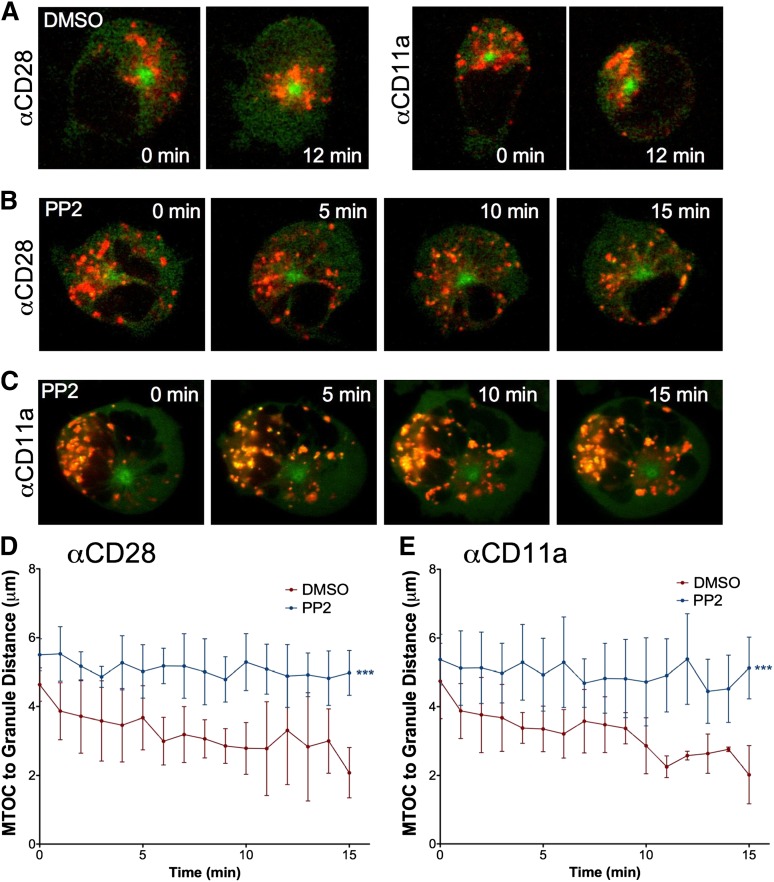
To determine whether blocking Src kinases, and thereby interrupting downstream NK cell activation signaling, would preclude lytic granule convergence, we pretreated GFP-α-tubulin–expressing YTS cells with the Src kinase inhibitor PP2. We used YTS cells as a human NK cell model system because of our prior validation of granule convergence in YTS relative to eNK cells.3 After inhibition, cells were loaded with LysoTracker, placed live into imaging chambers coated with antibody specific for the YTS triggering receptor CD28 or the CD11a subunit of LFA-1, and imaged. Compared with dimethylsulfoxide (DMSO)-treated control cells that showed normal lytic granule convergence after activation (supplemental Video 1 on the Blood website), PP2-treated cells failed to demonstrate lytic granule convergence after activation on anti-CD28– (Figure 2A-B) or anti-CD11a–coated (Figure 2A,C) surfaces (supplemental Video 2). In DMSO-treated control cells, the average distance of lytic granules from the MTOC after 15 minutes was approximately 2 vs 5 μm in PP2-treated cells (Figure 2D-E).
Figure 2.
Signaling for lytic granule convergence requires Src kinase activity. Time-lapse frames of lytic granule movement in YTS GFP-tubulin cells pretreated with (A) DMSO or PP2 on an (B) anti-CD28– or (C) an anti-CD11a–coated surface. In each image, confocal immunofluorescence in the plane of the MTOC is shown. Green, GFP-tubulin; red, LysoTracker-loaded acidified organelles. Zero minutes represents the time at which the NK cell appeared to contact the glass surface. Quantitative analyses of lytic granule distance from the MTOC of PP2- or DMSO-treated YTS GFP-tubulin cells on (D) anti-CD28– or (E) anti-CD11a–coated surfaces as a function of time as measured by mean MTOC to granule distance in 9 cells per condition; error bars show ±SD. Mean distance of lytic granules from the MTOC was significantly greater in PP2-treated NK cells than in DMSO-treated NK cells (***P < .001).
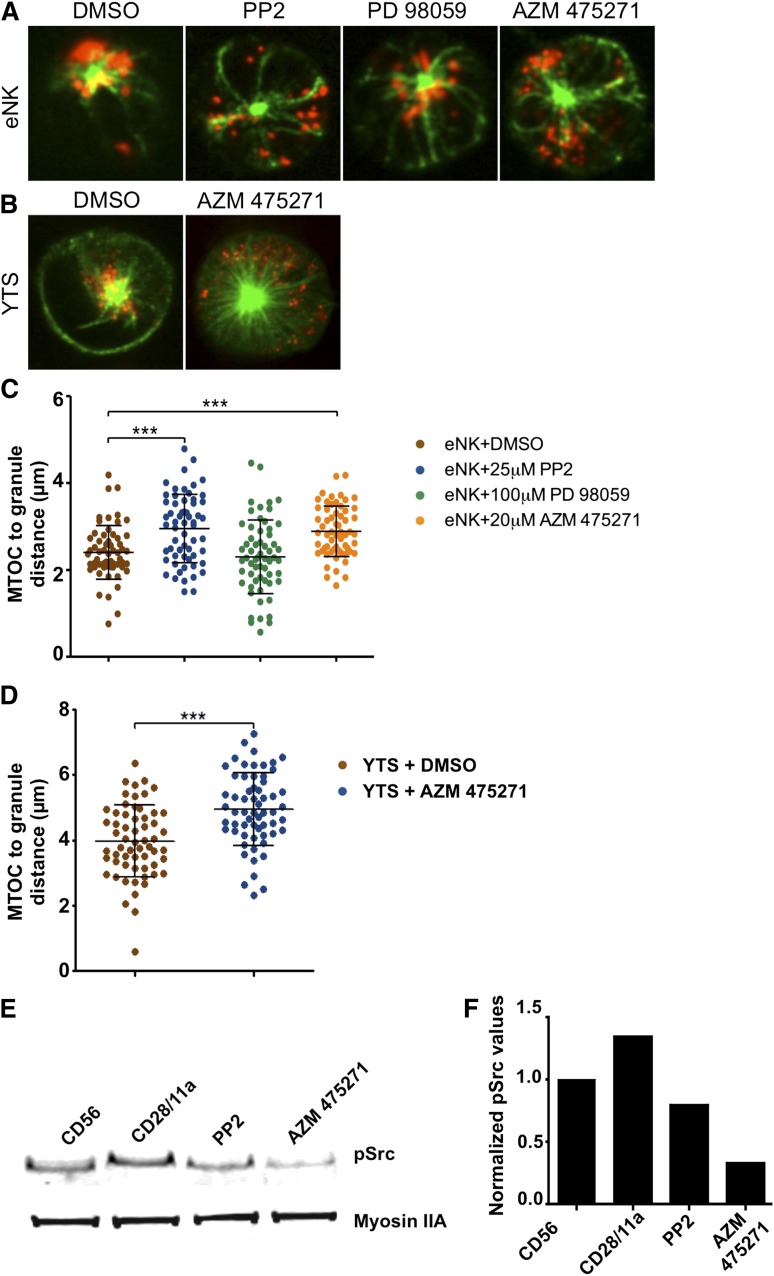
To ensure that the role for Src in granule convergence identified in YTS cells was also applicable to eNK cells, we performed parallel experiments after eNK cell activation with anti-CD18 and anti-NKp30. Compared with the DMSO control, PP2 blocked lytic granule convergence to the MTOC (Figure 3A,C). Because PP2 is a relatively promiscuous inhibitor, we also used the more specific Src kinase inhibitor AZM 47527130 and found that it prevents activation-induced lytic granule convergence similarly to PP2 in eNK cells (Figure 3A,C) and YTS cells (Figure 3B,D). Both PP2 and AZM 475271 were able to block activation-induced Src phosphorylation as determined using western blot analysis (Figure 3E-F). Thus, Src kinase activity is required for lytic granule convergence to the MTOC in NK cells.
Figure 3.
Specific inhibition of Src kinase activity prevents lytic granule convergence to the MTOC. Fixed cell confocal microscopy of (A) eNK cells pretreated with DMSO (vehicle control), PP2, PD 98059, or AZM 475271 and (B) YTS cells pretreated with DMSO or AZM 475271 for 30 minutes at 37°C before activation on an anti-NKp30/CD18–coated surface for eNK cells or anti-CD28/CD11a–coated surface for YTS cells for 15 minutes at 37°C, followed by 10 minutes of fixation at room temperature. In each image, confocal immunofluorescence in the plane of the MTOC is shown. Green, anti–α-tubulin detected by Pacific Blue-streptavidin; red, fluorescein isothiocyanate anti-perforin. Quantitative analyses of lytic granule distance from the MTOC are shown as a feature of inhibitor treatments in (C) eNK cells and (D) YTS cells. Data represent >20 cells per condition from independent experiments using 3 healthy donors for eNK cells and 3 independent experiments for YTS cells. Error bars show ±SD. Mean distance of lytic granules from the MTOC was significantly larger in eNK cells after PP2 and AZM 475271 (***P < .001) and in YTS cells after AZM 475271 II treatment (***P < .001). (E) Western blot analysis of YTS cells incubated on an anti-CD56–coated surface (left lane) or anti-CD28/CD11a–coated surfaces (right lanes). For the rightmost lanes, cells were pretreated with Src kinase inhibitors PP2 or AZM475271. The blot is representative of 4 independent repeats. (F) Quantitative analysis of the blot shown in panel E with pSrc values normalized to those of Myosin IIA, which was included as a loading control.
Lytic granule convergence, but not NK cell cytotoxicity, occurs independently of PI3K, MEK, and PLCγ
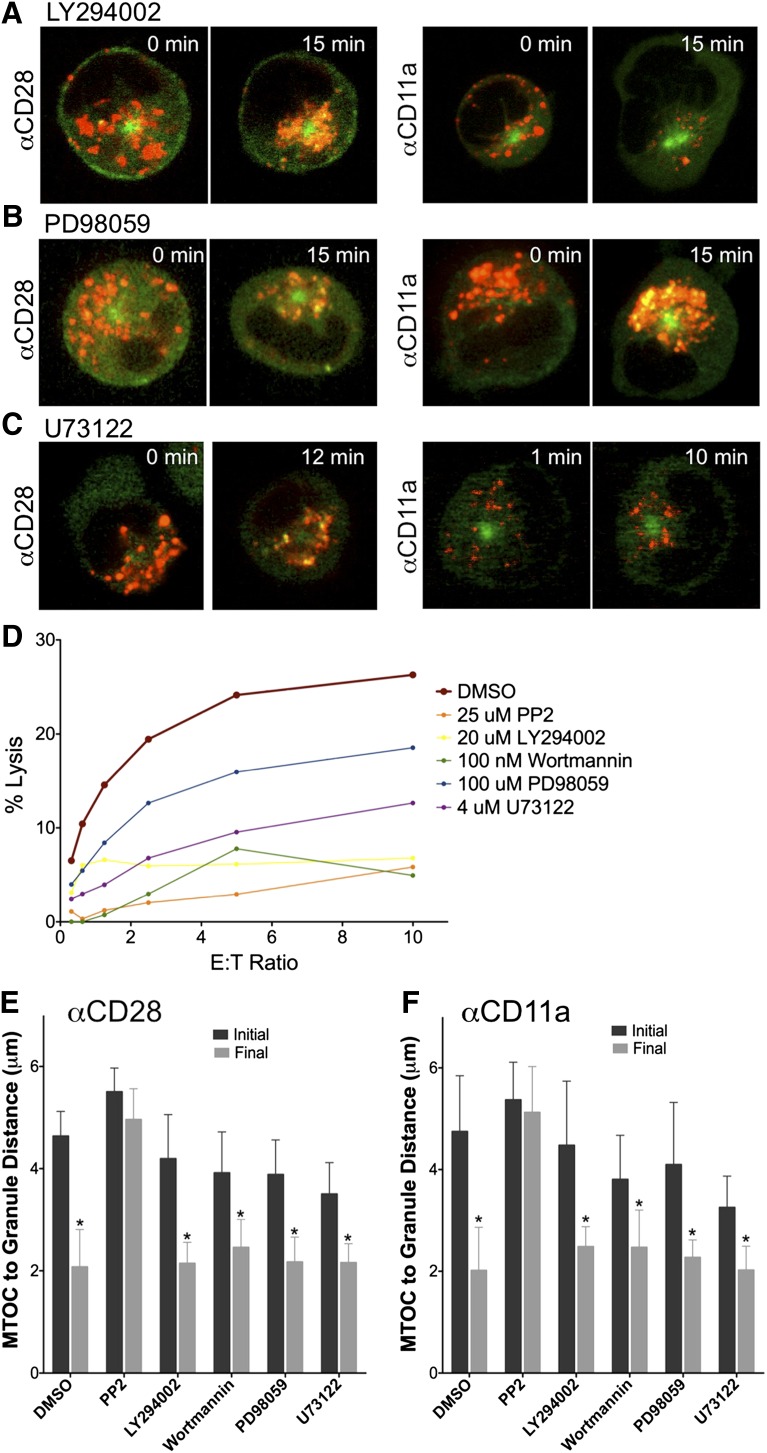
Downstream of triggering receptor engagement, PI3K, MEK, and PLCγ are all relatively early signaling mediators required for Erk activation and MTOC and granule polarization5,7,8 or maintenance of surface receptor levels and degranulation.29 We therefore questioned whether these signaling mediators are required for initiating transport of lytic granules to the MTOC before polarization occurs. Using LY294002 or Wortmannin to block PI3K (Figure 4A; supplemental Videos 3 and 4), PD98059 to block MEK (Figure 4B; supplemental Video 5), or U73122 to block PLCγ (Figure 4C; supplemental Video 6), we treated YTS GFP-tubulin cells and added them to anti-CD28– or anti-CD11a–coated imaging chambers. Lytic granule convergence still occurred in the presence of each inhibitor irrespective of the triggering receptor. Importantly, each of the inhibitors decreased NK cell cytotoxicity at the concentration used in the imaging experiments (Figure 4D). Because cytotoxicity was inhibited, lytic granule position relative to the MTOC was measured over time to detect any potentially subtle inhibitor effects on granule convergence. Compared with DMSO-treated control cells, no significant impact on convergence of inhibitors was identified irrespective of the triggering receptor engaged (Figure 4E-F). At the end of the assay, average granule distance from the MTOC after inhibitor treatment (other than PP2) was 2 to 2.5 μm and was not significantly different from controls. As an additional negative control, PD98059 was used to treat eNK cells before activation and similarly did not interfere with granule convergence (Figure 3A,C). Thus, we conclude that lytic granule convergence is independent of PI3K, MEK, and PLCγ signaling (even though they are required for MTOC polarization and/or cytotoxicity), because they are all presumably downstream or independent of the required Src signal.
Figure 4.
Lytic granule convergence depends on Src kinase but occurs independently of PI3K, MEK, and PLCγ activity. Time-lapse frames of lytic granule movement in YTS GFP-tubulin cells pretreated with (A) LY294002, (B) PD98059, or (C) U73122 on an anti-CD28– or an anti-CD11a–coated surface. In each image, confocal immunofluorescence in the plane of the MTOC is shown. Green, GFP-tubulin; red, LysoTracker-loaded acidified organelles. Zero minutes represents the time at which the NK cell appeared to contact the glass surface. (D) Cytotoxicity assay (4-hour 51Cr release) of YTS cells against 721.221 target cells showing loss of function after treatment with various inhibitors. AZM 475271 provided 75% inhibition at a 10:1 E:T ratio. (E-F) Quantitative analyses of lytic granule distance from the MTOC as a function of time (initial vs final) as measured by mean MTOC to granule distance in 9 to 10 cells per condition in DMSO-treated or inhibitor-treated YTS GFP-tubulin cells on (E) anti-CD28– or (F) anti-CD11a–coated surfaces. Error bars show ±SD. Excepting PP2, mean distance of lytic granules from the MTOC was not significantly different in any inhibitor-treated NK cells compared with DMSO-treated NK cells; distance of lytic granules from the MTOC was significantly greater at time point zero than at the end of the assay (*P < .05).
Lytic granule convergence occurs in NK cells forming an inhibitory synapse
When encountering a healthy NK cell, KIRs recognize MHC-I to restrain killing via recruitment of SHP-1, which can dephosphorylate Vav1 to interrupt actin reorganization.16 This prevents maturation of the IS and ultimately degranulation.
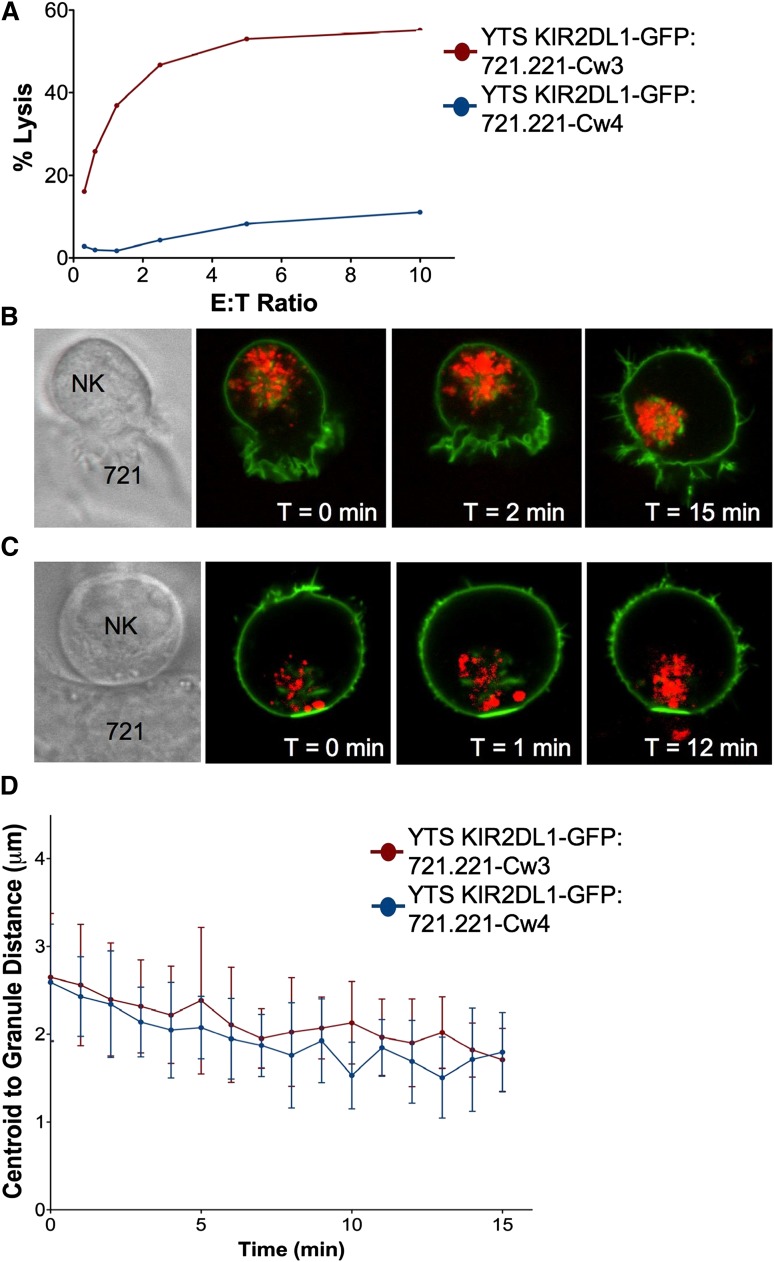
To study the effect of inhibitory synapse formation on granule convergence, we performed live cell confocal microscopy using YTS cells stably expressing KIR2DL1-GFP paired with 721.221 cells expressing either the cognate ligand HLA-Cw4 or the noncognate ligand HLA-Cw3. KIR2DL1-GFP cells can kill either parental or HLA-Cw3–expressing, but not HLA-Cw4–expressing, 721.221 cells (Figure 5A). YTS KIR2DL1-GFP cells form an activating synapse with 721.221 HLA-Cw3 cells with uniformly localized GFP around the NK cell surface and compacted LysoTracker Red-loaded lytic granules (Figure 5B; supplemental Video 7). In contrast, when YTS KIR2DL1-GFP cells conjugated to 721.221 HLA-Cw4 cells, GFP clustered at the synapse (Figure 5C; supplemental Video 8) as previously described.31 Interestingly, lytic granules still converged to central locations in these inhibitory conjugates. Whether an activating or inhibitory synapse was formed, lytic granules converged to within 2 μm of the centroid of the granules, and there was no significant difference in the kinetics of convergence (Figure 5D). Thus, inhibitory signaling, which can block Vav1 function, did not interfere with lytic granule convergence after target cell contact.
Figure 5.
Lytic granule convergence occurs despite inhibitory synapse formation and is not reversed by KIR clustering. 721.221 cells expressing HLA-Cw3 or HLA-Cw4 were used as susceptible or nonsusceptible target cells for YTS GFP-KIR2DL1, respectively. (A) Four-hour 51Cr release cytotoxicity assay of YTS GFP-KIR2DL1 cells against 721-Cw3 (red) or 721-Cw4 (blue) target cells. Time-lapse frames of lytic granule movement in YTS GFP-KIR2DL1 cells conjugated to (B) 721-Cw3 or (C) 721-Cw4 cells. The leftmost panel shows transmitted light images of the conjugate pictured, and each confocal image demonstrates immunofluorescence in the plane of converged lytic granules; green, GFP-KIR2DL1; red, LysoTracker-loaded acidified organelles. T = 0 refers to the time that image acquisition began, which was between 1 and 5 minutes after YTS GFP-KIR2DL1 cells were added to the imaging chamber. (D) Quantitative analyses of lytic granule distance from the centroid of the granules as a function of time as measured by mean centroid to granule distance in 9 cells; error bars show ±SD. Mean distance of lytic granules from the centroid of the granules in YTS GFP-KIR2DL1:721.221-Cw4 conjugates was not significantly different from that in YTS GFP-KIR2DL1:721.221-Cw3 conjugates.
Soluble IL-2 promotes lytic granule convergence independently of JAK3 but not Src
IL-2 is a potent soluble NK cell stimulus,17,18 which canonically signals via JAK3. Ligation of the IL-2 receptor also promotes association of Src homology-2 domain-containing transforming protein C, which in turn activates the mitogen activated protein kinase and PI3K pathways to enable noncanonical signaling and further enhances NK cell activation.19,20,32 We wanted to determine whether IL-2 stimulation would be sufficient to induce lytic granule convergence as a way to potentially heighten the activation state of the NK cell.
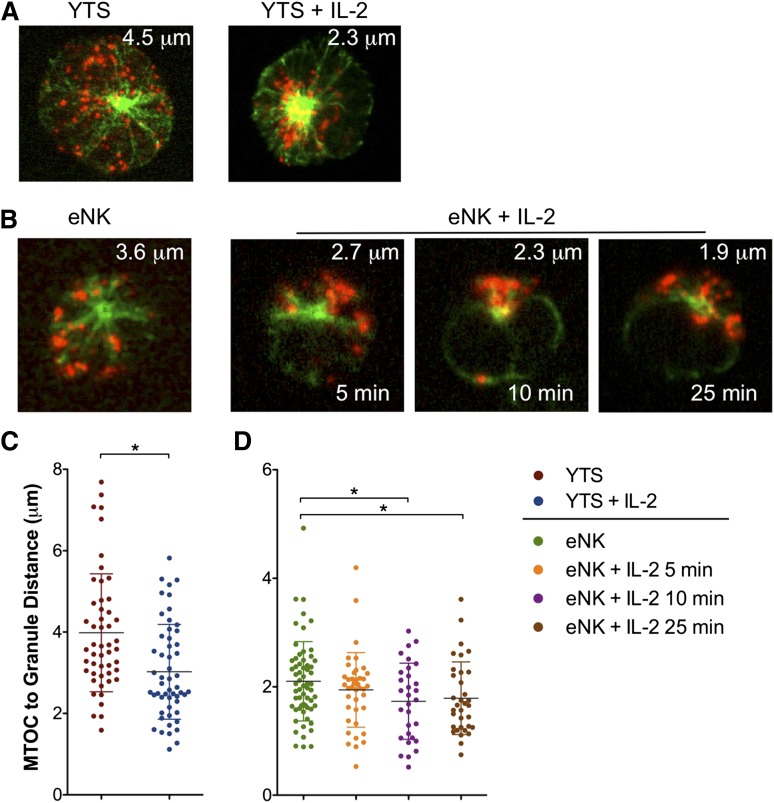
Thus, YTS cells were treated with IL-2, fixed, and stained for tubulin and perforin (Figure 6A). Lytic granules were diffuse in resting NK cells but markedly converged to the MTOC after IL-2 treatment. We confirmed this observation using eNK cells isolated from healthy donor blood (Figure 6B). To quantify the effect of IL-2, we measured average lytic granule distance from the MTOC and found a significant decrease in lytic granule distance from the MTOC in both YTS and eNK cells after IL-2 stimulation (Figure 6C-D).
Figure 6.
IL-2 promotes lytic granule convergence in NK cells. (A) Confocal immunofluorescent microscopy of fixed cells showing anti–α-tubulin (green) and anti-perforin (red) in (left) resting YTS cells or (right) YTS cells treated with IL-2 for 15 minutes or (B) resting eNK cells (leftmost) or eNK cells treated with IL-2 for 5, 10, or 25 minutes before fixation on poly-L-lysine–coated glass slides (PolyPrep; Sigma-Aldrich) for 5 minutes at 37°C. The biotinylated anti-tubulin mAb was detected with Pacific Blue-streptavidin; the anti-perforin antibody was directly fluorescein isothiocyanate conjugated. The measured mean MTOC to lytic granules in each image is provided as white text at the top. Quantitative analyses of lytic granule distance from the MTOC as a feature of IL-2 treatment in (C) YTS cells and (D) eNK cells. Data are representative of 2 separate experiments in each of which ≥30 cells were evaluated per condition. Error bars show ±SD. Mean distance of lytic granules from the MTOC was significantly less in YTS cells after IL-2 treatment (*P < .05) and in eNK cells after ≥10 minutes of IL-2 treatment (*P < .05 at 10 and 25 minutes).
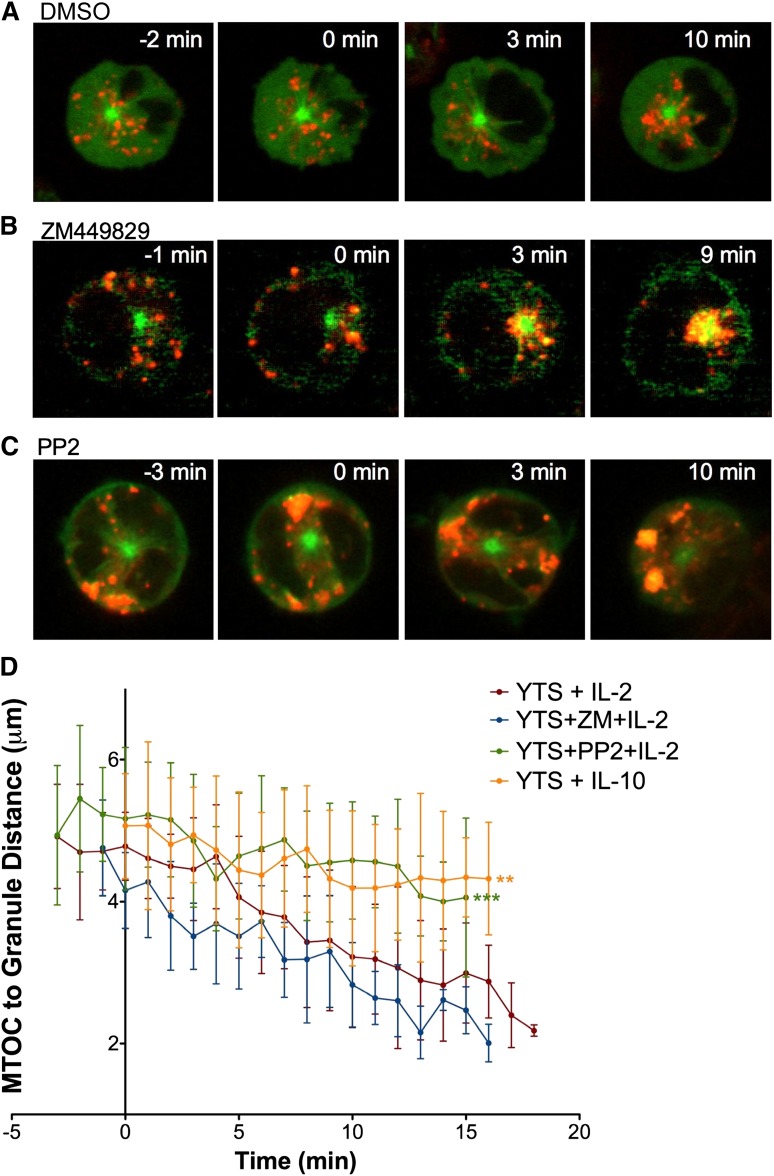
As IL-2–induced lytic granule convergence has not been previously studied, we further characterized this effect of IL-2 in living cells using glass-bottomed imaging chambers without any adhesive or antibody coating. After 2 to 4 minutes, IL-2 was added and granule traffic was observed (Figure 7A; supplemental Video 9). Addition of IL-2 decreased average granule distance from the MTOC (Figure 7D) comparably to that seen in NK cells triggered through an activating receptor or conjugated to a target cell. As an additional control for the addition of IL-2, parallel experiments were performed using IL-10 instead of IL-2, because IL-10, while also a cytokine, signals differently from IL-2 and is generally known for inhibitory effects relative to NK cell responses.33,34 Here lytic granule convergence was not observed over the >15 minutes after IL-10 addition (Figure 7D; supplemental Video 10). This demonstrates specificity to the IL-2 signal and that not all cytokines can induce NK cell granule convergence. It is important to reiterate that IL-2 is a soluble signal, thus distinguishing it from all of the surface receptor–, immobilized antibody–, or target cell contact–induced signals. Even though we had previously shown that CD45 cross-linking does not trigger convergence,3 IL-2 further illustrates that convergence is unlikely to represent an artifact of antibody-coated surfaces or physical NK cell deformation.
Figure 7.
Cytokine-induced lytic granule convergence does not occur through canonical signaling but depends on Src kinase activity. Time-lapse frames of lytic granule movement in YTS GFP-tubulin cells stimulated with IL-2 after (A) DMSO, (B) ZM449829, or (C) PP2 treatment. In each image, confocal immunofluorescence in the plane of the MTOC is shown. Green, GFP-tubulin; red, LysoTracker-loaded acidified organelles. Cells were imaged for 2 to 4 minutes before IL-2 was added. Time = 0 minutes represents the time at which IL-2 was added. (D) Quantitative analyses of lytic granule distance from the MTOC as a function of time as measured by mean MTOC to granule distance in 5 to 10 cells per condition; error bars show ±SD. Mean distance of lytic granules from the MTOC after IL-2 addition was not significantly different in ZM449829-treated NK cells compared with DMSO-treated NK cells but was significantly greater in PP2-treated NK cells (***P < .001). Mean distance of lytic granules from the MTOC after IL-10 addition was also significantly greater than after IL-2 addition (**P < .01).
To determine which IL-2–mediated signaling pathway contributes to lytic granule convergence, we first blocked canonical IL-2 signaling. ZM449829 potently and selectively inhibits JAK3 and completely blocks IL-2–induced STAT5 phosphorylation in NK cells.35 If canonical IL-2 signaling is required for IL-2–mediated lytic granule convergence, we would expect ZM449829 pretreatment to block lytic granule convergence. Thus, YTS GFP-tubulin cells were pretreated with ZM449829 and placed into plain glass imaging chambers to which IL-2 was added after 2 to 4 minutes (Figure 7B; supplemental Video 11). No defect in lytic granule convergence after JAK3 inhibition was observed as granules were ∼2 μm from the MTOC, which is the same as in uninhibited, IL-2–stimulated cells (Figure 7D). Furthermore, the kinetics of lytic granule convergence in inhibitor-treated cells tracked over time was also statistically indistinguishable from control (Figure 7D). Thus, canonical IL-2 signaling does not appear to be necessary for lytic granule convergence in IL-2–stimulated NK cells.
Because Src kinases can also function in IL-2 receptor signaling24 and because Src kinases are necessary for lytic granule convergence after activating receptor triggering, we asked whether this noncanonical pathway contributes to IL-2–mediated lytic granule convergence. We treated GFP-tubulin YTS cells with PP2 and observed them in plain glass imaging chambers for 2 to 4 minutes before addition of IL-2. Interestingly, lytic granules localized to the cell cortical plus ends of microtubules and did not move in a minus-ended direction to the MTOC (Figure 7C; supplemental Video 12). Granules remained ∼4.5 μm from the MTOC (comparable to PP2-treated cells on antibody-coated glass surfaces shown earlier), which was significantly greater than in uninhibited IL-2–stimulated cells (Figure 7D). Thus, PP2 specifically blocks the mechanism that drives lytic granules to the MTOC. In the case of IL-2 stimulation, as in activating receptor signaling, Src kinases are required for NK cell lytic granule convergence, and this suggests their role in preparing NK cells for precise killing.
Discussion
Lytic granule convergence is a rapid, early event in NK cell cytotoxicity. Although not all receptors trigger convergence, adhesion receptor engagement is sufficient.3 We extend this finding using NK cells from patients with naturally occurring human deficiency of β2 integrin (LAD-1) in which the distance of lytic granules from the MTOC was abnormal. LAD-1 patients lack LFA-1 integrin expression, resulting in reduced adherence to infected cells.28 Despite the engagement of target cells by LAD-1 NK cells, however, the convergence of lytic granules to the MTOC was reduced, and NK cell cytotoxic function was undetectable. Thus, LFA-1 is required for target cell–induced NK cell lytic granule convergence. Defective NK cell cytotoxicity in these rare patients was described many years ago, but the mechanism was always thought to be a lack of adherence to target cells. Although this is still likely to represent a major defect in LAD-1 NK cells, the role of defective lytic granule convergence is potentially important and warrants further consideration.
Immediately following NK cell receptor engagement, key tyrosines are phosphorylated by Src kinases,9 which recruit SH2 domain–containing signaling molecules that facilitate downstream signaling and eventual effector function.36 Our experiments show that inhibiting Src kinase function blocks lytic granule convergence. More work is necessary, however, to determine which specific Src family kinase enables lytic granule convergence. More than one may be involved, as they can serve redundant functions, and many have been implicated in the phosphorylation of NK cell activating and inhibitory receptors.21,22
Lytic granule convergence depends on receptor activation but not F-actin function or microtubule dynamics.3 Given the signals that follow actin organization at the IS in NK cells, this presents a limited range that are upstream and could be required for granule convergence. We therefore dissected signaling pathways downstream of receptor activation using specific inhibitors in an effort to discern the signaling prerequisites for convergence. We evaluated signals required for cytotoxicity and thus critical for NK cell activation. PI3K is required for NK cell MTOC polarization8 but was not needed for granule convergence. MEK, which interfaces with the PI3K signaling pathway and is also required for MTOC polarization,7 was similarly not necessary for granule convergence. Thus, the mechanism controlling lytic granule convergence is distinct from the PI3K pathway needed for MTOC polarization. This confirms that granule convergence and MTOC polarization are distinct events on the path to directed secretion of lytic granules and underscores that multiple regulatory checkpoints are involved in the precise control of NK cytotoxicity.
In NK cells, calcium flux requires PLCγ37 and is required for lytic granule maturation, effective signaling, and degranulation.13 In our experiments, inhibiting PLCγ in NK cells had no effect on MTOC-directed lytic granule movement after NK cell activation. Thus, although PLCγ is essential for NK cell functions, it does not contribute to lytic granule traffic toward the MTOC.
We also examined lytic granule convergence in inhibitory synapses because much of activation signaling is specifically curtailed in this setting by the function of the inhibitory receptor. Interestingly, granule convergence in inhibitory synapses was comparable to that in activating synapses. Thus, downstream targets of KIR signaling, including SHP-1–mediated dephosphorylation of Vav1,16 appear to be dispensable for convergence. We had previously shown that lytic granule convergence does not require actin reorganization; the finding that convergence occurs in inhibitory synapses—a scenario where actin reorganization is blocked by Vav1 dephosphorylation—corroborates the conclusion that granule convergence is an early and actin-independent step.3
Our experiments suggest that the signaling requirement for lytic granule convergence lies downstream of the initial adhesion signal but upstream of signaling pathways as would be truncated by inhibitory receptor signaling or the specific inhibitors we used. Our use of Src inhibition, which abrogated lytic granule convergence, demonstrates that at least signals from this family of kinases are required. Thus, in the case of inhibitory receptors, the Src-mediated tyrosine phosphorylation of KIR intracellular domains that occurs upstream of SHP-1 recruitment may trigger lytic granule convergence itself,14 or it may be derivative from distinct initial activation signaling stemming from Src signaling from other receptors before SHP-1–mediated dephosphorylation has had its effect.38 In either case, the potential importance of Src kinase function on this preparatory NK cell activation step is distinguished.
IL-2 is a potent NK cell activating factor17,18,34,35 that may also help to further prime NK cells for cytotoxicity by promoting lytic granule convergence before target cell binding. This was specific to IL-2 and not a feature of simply adding cytokine to the cells, because IL-10, which signals differently from IL-2 and is generally appreciated for inhibitory effects,33,34 did not promote this function. This additionally substantiates that granule convergence is not simply a feature of cell deformation via target cell engagement or activating receptor cross-linking via antibody-coated surfaces.
Taken together, our results point to a direct effector mechanism for dynein-directed traffic of lytic granules downstream of Src kinase–mediated phosphorylation and upstream of independent of effectors such as PI3K, MEK, and PLCγ. One possibility is that Src kinases directly facilitate lytic granule convergence, although little evidence suggests Src kinases participate in cargo transport. We instead hypothesize that Src kinases are indirectly responsible for lytic granule traffic through an adaptor common to both activation receptor and cytokine signaling pathways. One candidate is the adaptor protein growth factor receptor-bound protein 2 (Grb2), a signaling mediator downstream of Src kinase phosphorylation but upstream of Vav1, PI3K, MEK, and PLCγ.39-43 Intriguingly, Grb2 also participates in IL-2 signaling.44 In immune cells, Grb2 is upstream or independent of PI3K,36 contributes to Erk signaling through the Ras pathway41 (also in noncanonical IL-2 signaling20), links Vav1 with activating receptors,36 and facilitates actin polymerization through interaction with WASp.43 Further, its interaction with PLCγ is disrupted in inhibitory signaling.37 Surprisingly, Grb2 has also been found to interact with the p150Glued subunit of dynactin,45 and in B cells, Grb2 is required for dynein-mediated transport of microclusters.46 These observations suggest that following receptor or cytokine activation, Grb2 could act as a Src-induced adaptor to facilitate interactions between lytic granules and dynein/dynactin. Future studies are needed to evaluate specific interactions between Grb2, dynactin, and lytic granules.
Our findings document for the first time the key signaling requirements for lytic granule convergence to the MTOC. They suggest specific and early signaling that drives microtubule-directed, dynein-dependent lytic granule traffic. Although lytic granule convergence is rapid, it likely represents one of the very first steps an NK cell takes to prepare and potentially prime for a cytotoxic event to enable the focused directed secretion of lytic granule contents.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) grant AI067946 (J.S.O.) and an NIH NIAID research supplement to promote diversity in health-related research (A.M.J.).
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M.J. performed the studies, analyzed data, and wrote the manuscript; H.-T.H., P.D., E.M.M., and P.P.B. performed studies and analyzed data; G.U. oversaw the participation and clinical management of the LAD-1 patient; and J.S.O. supervised the research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jordan S. Orange, Department of Pediatrics, Baylor College of Medicine, Center for Human Immunobiology, Texas Children’s Hospital, 1102 Bates Ave, Suite 330, Houston, TX 77030-2399; e-mail: orange@bcm.edu.
References
- 1.Sun JC, Lanier LL. NK cell development, homeostasis and function: parallels with CD8⁺ T cells. Nat Rev Immunol. 2011;11(10):645–657. doi: 10.1038/nri3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8(9):713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mentlik AN, Sanborn KB, Holzbaur EL, et al. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell. 2010;21(13):2241–2256. doi: 10.1091/mbc.E09-11-0930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rak GD, Mace EM, Banerjee PP, et al. Natural killer cell lytic granule secretion occurs through a pervasive actin network at the immune synapse. PLoS Biol. 2011;9(9):e1001151. doi: 10.1371/journal.pbio.1001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li C, Ge B, Nicotra M, et al. JNK MAP kinase activation is required for MTOC and granule polarization in NKG2D-mediated NK cell cytotoxicity. Proc Natl Acad Sci USA. 2008;105(8):3017–3022. doi: 10.1073/pnas.0712310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Trivedi PP, Ge B, et al. Many NK cell receptors activate ERK2 and JNK1 to trigger microtubule organizing center and granule polarization and cytotoxicity. Proc Natl Acad Sci USA. 2007;104(15):6329–6334. doi: 10.1073/pnas.0611655104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen X, Allan DS, Krzewski K, et al. CD28-stimulated ERK2 phosphorylation is required for polarization of the microtubule organizing center and granules in YTS NK cells. Proc Natl Acad Sci USA. 2006;103(27):10346–10351. doi: 10.1073/pnas.0604236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang K, Zhong B, Gilvary DL, et al. Pivotal role of phosphoinositide-3 kinase in regulation of cytotoxicity in natural killer cells. Nat Immunol. 2000;1(5):419–425. doi: 10.1038/80859. [DOI] [PubMed] [Google Scholar]
- 9.Watzl C, Long EO. Signal transduction during activation and inhibition of natural killer cells. Curr Protoc Immunol. 2010; Chapter 11: Unit 11.9B. doi: 10.1002/0471142735.im1109bs90. [DOI] [PMC free article] [PubMed]
- 10.Riteau B, Barber DF, Long EO. Vav1 phosphorylation is induced by beta2 integrin engagement on natural killer cells upstream of actin cytoskeleton and lipid raft reorganization. J Exp Med. 2003;198(3):469–474. doi: 10.1084/jem.20021995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sánchez-Martín L, Sánchez-Sánchez N, Gutiérrez-López MD, et al. Signaling through the leukocyte integrin LFA-1 in T cells induces a transient activation of Rac-1 that is regulated by Vav and PI3K/Akt-1. J Biol Chem. 2004;279(16):16194–16205. doi: 10.1074/jbc.M400905200. [DOI] [PubMed] [Google Scholar]
- 12.Bryceson YT, March ME, Ljunggren HG, et al. Synergy among receptors on resting NK cells for the activation of natural cytotoxicity and cytokine secretion. Blood. 2006;107(1):159–166. doi: 10.1182/blood-2005-04-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.March ME, Long EO. β2 integrin induces TCRζ-Syk-phospholipase C-γ phosphorylation and paxillin-dependent granule polarization in human NK cells. J Immunol. 2011;186(5):2998–3005. doi: 10.4049/jimmunol.1002438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Faure M, Barber DF, Takahashi SM, et al. Spontaneous clustering and tyrosine phosphorylation of NK cell inhibitory receptor induced by ligand binding. J Immunol. 2003;170(12):6107–6114. doi: 10.4049/jimmunol.170.12.6107. [DOI] [PubMed] [Google Scholar]
- 15.Cuevas B, Lu Y, Watt S, et al. SHP-1 regulates Lck-induced phosphatidylinositol 3-kinase phosphorylation and activity. J Biol Chem. 1999;274(39):27583–27589. doi: 10.1074/jbc.274.39.27583. [DOI] [PubMed] [Google Scholar]
- 16.Stebbins CC, Watzl C, Billadeau DD, et al. Vav1 dephosphorylation by the tyrosine phosphatase SHP-1 as a mechanism for inhibition of cellular cytotoxicity. Mol Cell Biol. 2003;23(17):6291–6299. doi: 10.1128/MCB.23.17.6291-6299.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonnema JD, Rivlin KA, Ting AT, et al. Cytokine-enhanced NK cell-mediated cytotoxicity. Positive modulatory effects of IL-2 and IL-12 on stimulus-dependent granule exocytosis. J Immunol. 1994;152(5):2098–2104. [PubMed] [Google Scholar]
- 18.Fehniger TA, Cooper MA, Nuovo GJ, et al. CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity. Blood. 2003;101(8):3052–3057. doi: 10.1182/blood-2002-09-2876. [DOI] [PubMed] [Google Scholar]
- 19.Merida I, Diez E, Gaulton GN. IL-2 binding activates a tyrosine-phosphorylated phosphatidylinositol-3-kinase. J Immunol. 1991;147(7):2202–2207. [PubMed] [Google Scholar]
- 20.Ravichandran KS, Burakoff SJ. The adapter protein Shc interacts with the interleukin-2 (IL-2) receptor upon IL-2 stimulation. J Biol Chem. 1994;269(3):1599–1602. [PubMed] [Google Scholar]
- 21.Mason LH, Willette-Brown J, Taylor LS, et al. Regulation of Ly49D/DAP12 signal transduction by Src-family kinases and CD45. J Immunol. 2006;176(11):6615–6623. doi: 10.4049/jimmunol.176.11.6615. [DOI] [PubMed] [Google Scholar]
- 22.Augugliaro R, Parolini S, Castriconi R, et al. Selective cross-talk among natural cytotoxicity receptors in human natural killer cells. Eur J Immunol. 2003;33(5):1235–1241. doi: 10.1002/eji.200323896. [DOI] [PubMed] [Google Scholar]
- 23.Billadeau DD, Upshaw JL, Schoon RA, et al. NKG2D-DAP10 triggers human NK cell-mediated killing via a Syk-independent regulatory pathway. Nat Immunol. 2003;4(6):557–564. doi: 10.1038/ni929. [DOI] [PubMed] [Google Scholar]
- 24.Shuh M, Morse BA, Heidecker G, et al. Association of SRC-related kinase Lyn with the interleukin-2 receptor and its role in maintaining constitutive phosphorylation of JAK/STAT in human T-cell leukemia virus type 1-transformed T cells. J Virol. 2011;85(9):4623–4627. doi: 10.1128/JVI.00839-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee PP, Pandey R, Zheng R, et al. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J Exp Med. 2007;204(10):2305–2320. doi: 10.1084/jem.20061893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grier JT, Forbes LR, Monaco-Shawver L, et al. Human immunodeficiency-causing mutation defines CD16 in spontaneous NK cell cytotoxicity. J Clin Invest. 2012;122(10):3769–3780. doi: 10.1172/JCI64837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohl S, Springer TA, Schmalstieg FC, et al. Defective natural killer cytotoxicity and polymorphonuclear leukocyte antibody-dependent cellular cytotoxicity in patients with LFA-1/OKM-1 deficiency. J Immunol. 1984;133(6):2972–2978. [PubMed] [Google Scholar]
- 28.Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. doi: 10.1146/annurev.me.38.020187.001135. [DOI] [PubMed] [Google Scholar]
- 29.Upshaw JL, Schoon RA, Dick CJ, et al. The isoforms of phospholipase C-gamma are differentially used by distinct human NK activating receptors. J Immunol. 2005;175(1):213–218. doi: 10.4049/jimmunol.175.1.213. [DOI] [PubMed] [Google Scholar]
- 30.Ischenko I, Guba M, Yezhelyev M, et al. Effect of Src kinase inhibition on metastasis and tumor angiogenesis in human pancreatic cancer. Angiogenesis. 2007;10(3):167–182. doi: 10.1007/s10456-007-9071-3. [DOI] [PubMed] [Google Scholar]
- 31.Davis DM, Chiu I, Fassett M, et al. The human natural killer cell immune synapse. Proc Natl Acad Sci USA. 1999;96(26):15062–15067. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnston JA, Bacon CM, Riedy MC, et al. Signaling by IL-2 and related cytokines: JAKs, STATs, and relationship to immunodeficiency. J Leukoc Biol. 1996;60(4):441–452. doi: 10.1002/jlb.60.4.441. [DOI] [PubMed] [Google Scholar]
- 33.Tripp CS, Wolf SF, Unanue ER. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA. 1993;90(8):3725–3729. doi: 10.1073/pnas.90.8.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spagnoli GC, Juretic A, Schultz-Thater E, et al. On the relative roles of interleukin-2 and interleukin-10 in the generation of lymphokine-activated killer cell activity. Cell Immunol. 1993;146(2):391–405. doi: 10.1006/cimm.1993.1035. [DOI] [PubMed] [Google Scholar]
- 35.Orange JS, Roy-Ghanta S, Mace EM, et al. IL-2 induces a WAVE2-dependent pathway for actin reorganization that enables WASp-independent human NK cell function. J Clin Invest. 2011;121(4):1535–1548. doi: 10.1172/JCI44862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upshaw JL, Arneson LN, Schoon RA, et al. NKG2D-mediated signaling requires a DAP10-bound Grb2-Vav1 intermediate and phosphatidylinositol-3-kinase in human natural killer cells. Nat Immunol. 2006;7(5):524–532. doi: 10.1038/ni1325. [DOI] [PubMed] [Google Scholar]
- 37.Valiante NM, Phillips JH, Lanier LL, et al. Killer cell inhibitory receptor recognition of human leukocyte antigen (HLA) class I blocks formation of a pp36/PLC-gamma signaling complex in human natural killer (NK) cells. J Exp Med. 1996;184(6):2243–2250. doi: 10.1084/jem.184.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schleinitz N, March ME, Long EO. Recruitment of activation receptors at inhibitory NK cell immune synapses. PLoS ONE. 2008;3(9):e3278. doi: 10.1371/journal.pone.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carlier MF, Nioche P, Broutin-L’Hermite I, et al. GRB2 links signaling to actin assembly by enhancing interaction of neural Wiskott-Aldrich syndrome protein (N-WASp) with actin-related protein (ARP2/3) complex. J Biol Chem. 2000;275(29):21946–21952. doi: 10.1074/jbc.M000687200. [DOI] [PubMed] [Google Scholar]
- 40.Graham DB, Cella M, Giurisato E, et al. Vav1 controls DAP10-mediated natural cytotoxicity by regulating actin and microtubule dynamics. J Immunol. 2006;177(4):2349–2355. doi: 10.4049/jimmunol.177.4.2349. [DOI] [PubMed] [Google Scholar]
- 41.Cussac D, Vidal M, Leprince C, et al. A Sos-derived peptidimer blocks the Ras signaling pathway by binding both Grb2 SH3 domains and displays antiproliferative activity. FASEB J. 1999;13(1):31–38. doi: 10.1096/fasebj.13.1.31. [DOI] [PubMed] [Google Scholar]
- 42.Okkenhaug K, Rottapel R. Grb2 forms an inducible protein complex with CD28 through a Src homology 3 domain-proline interaction. J Biol Chem. 1998;273(33):21194–21202. doi: 10.1074/jbc.273.33.21194. [DOI] [PubMed] [Google Scholar]
- 43.She HY, Rockow S, Tang J, et al. Wiskott-Aldrich syndrome protein is associated with the adapter protein Grb2 and the epidermal growth factor receptor in living cells. Mol Biol Cell. 1997;8(9):1709–1721. doi: 10.1091/mbc.8.9.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gadina M, Sudarshan C, O’Shea JJ. IL-2, but not IL-4 and other cytokines, induces phosphorylation of a 98-kDa protein associated with SHP-2, phosphatidylinositol 3′-kinase, and Grb2. J Immunol. 1999;162(4):2081–2086. [PubMed] [Google Scholar]
- 45.Sahni M, Zhou XM, Bakiri L, et al. Identification of a novel 135-kDa Grb2-binding protein in osteoclasts. J Biol Chem. 1996;271(51):33141–33147. doi: 10.1074/jbc.271.51.33141. [DOI] [PubMed] [Google Scholar]
- 46.Schnyder T, Castello A, Feest C, et al. B cell receptor-mediated antigen gathering requires ubiquitin ligase Cbl and adaptors Grb2 and Dok-3 to recruit dynein to the signaling microcluster. Immunity. 2011;34(6):905–918. doi: 10.1016/j.immuni.2011.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.