Abstract
Restoration measures of deteriorated river ecosystems generally aim at increasing the spatial heterogeneity and connectivity of these systems in order to increase biodiversity and ecosystem stability. While this is believed to benefit overall ecological integrity, consequences of such restoration projects on biogeochemical processes per se (i.e. ecosystem functioning) in fluvial systems are rarely considered. We address these issues by evaluating the characteristics of surface water connection between side arms and the main river channel in a former braided river section and the role and degree of connectivity (i.e. duration of surface water connection) on the sediment biogeochemistry. We hypothesized that potential respiration and denitrification would be controlled by the degree of hydrological connectivity, which was increased after floodplain restoration. We measured potential microbial respiration (SIR) and denitrification (DEA) and compared a degraded floodplain section of the Danube River with a reconnected and restored floodplain in the same river section. Re-establishing surface water connection altered the controls on sediment microbial respiration and denitrification ultimately impacting potential microbial activities. Meta-variables were created to characterize the effects of hydrology, morphology, and the available carbon and nutrient pools on potential microbial processing. Mantel statistics and path analysis were performed and demonstrate a hierarchy where the effects of hydrology on the available substrates and microbial processing are mediated by the morphology of the floodplain. In addition, these processes are highest in the least connected sites. Surface water connection, mediated by morphology regulates the potential denitrification rate and the ratio of N2O to N2 emissions, demonstrating the effects of restoration in floodplain systems.
Key words: Floodplain restoration, Connectivity, Substrate induced respiration, Denitrification enzyme activity, Danube River, Path analysis
1. Introduction
At the catchment scale, rivers transport nutrients and organic matter from terrestrial and aquatic sources to coastal areas (Bennett et al., 2001; Seitzinger et al., 2002; Townsend-Small et al., 2005), produce and degrade organic matter during transport (del Giorgio and Pace, 2008; Hedges et al., 2000), and constitute an important element in the global carbon cycle (Cole et al., 2007; Battin et al., 2009). Riverine landscapes, where biological and physical activities (ex: primary production and sedimentation) occur, constitute biogeochemical hot spots, in particular for nitrogen cycling (Fischer et al., 2005; Forshay and Stanley, 2005; Hynes, 1975; McClain et al., 2003; Naiman and Decamps, 1997; Ren et al., 2000).
At the landscape scale, two fundamental principles regulate the cycling and transfer of carbon and nutrients in river ecosystems, particularly in large river floodplains (Pinay et al., 2002). The first principle relates to delivery patterns of carbon and nutrients into floodplain ecosystems. In floodplains of most large regulated rivers, inputs of sediment, nutrients, and organic matter occur primarily via surface flow (i.e. flooding), although groundwater transport and atmospheric deposition can also contribute high amounts of nutrients (Durisch-Kaiser et al., 2008; Tockner et al., 2000). River floodplains are recognized as important storage sites for sediments and associated nutrients mobilized from upstream catchments during floods (Forshay and Stanley, 2005; He and Walling, 1997). In addition to the magnitude, frequency and duration of floods, the transfer and storage of materials in floodplains is largely under the control of the surface water connectivity pattern within the riverine landscape (Brunet et al., 1994; Van der Lee et al., 2004; Burt and Pinay, 2005; Pinay et al., 2007). The second basic principle describes the geomorphological characteristics of floodplains which are defined, in this study, as the present morphology and the processes that shape it (ex. water–substrate contact, water–sediment interface). This is generally positively correlated to the efficiency of nutrient retention and use in river ecosystems, and these positive relationships can occur both in the main channel itself and in the riparian and floodplain zones (Jones and Holmes, 1996; Lefebvre et al., 2004; Pinay et al., 2009). Increasing the length or the duration of contact between water and substrates increases the biological use and thereby the total amount of nutrients cycled through the system (Sjodin et al., 1997), although this cycling capacity can be affected by the load itself (Mulholland et al., 2008). Similarly, the role of water levels, especially floods and flow pulses (Tockner et al., 2000), is important in determining the area available for water–substrate interactions. By changes in the frequency, duration, period of occurrence, and variability of water levels, the water regime or surface water connectivity can directly affect nitrogen cycling in alluvial sediments and the sediment–water interface by controlling the duration of oxic and anoxic phases and thereby altering nitrification and denitrification rates (Groffman and Tiedje, 1988; Hefting et al., 2004). These factors create a mosaic of geomorphologic features that influence the spatial pattern and successional development of riparian vegetation (Hein et al., 2005; Roberts and Ludwig, 1991; Salo et al., 1986) which in turn largely supports consumer biomass (Zeug and Winemiller, 2008).
River systems can be strongly affected by natural disturbances or anthropogenic perturbations, such as dams, drainage, dredging, deforestation of riparian zones, and embankments. The two previously mentioned principles can be used to understand the mechanisms of how anthropogenic changes alter the biogeochemistry of riparian and instream zones as well as their ability to mediate nutrient fluxes originating from upstream (Bernot and Dodds, 2005). In order to mitigate anthropogenic disturbance, river restoration and rehabilitation projects have been undertaken. Most projects have been aimed at increasing the spatial heterogeneity of these ecosystems in order to support higher habitat and biological diversity (Henry et al., 2002). Yet, a more integrated approach including restoration of vital ecological processes, such as nitrogen cycling and retention, is necessary to recognize the biogeochemical role of floodplains (Hein et al., 2004; Hohensinner et al., 2004; Pedroli et al., 2002). Although nitrogen dynamics in floodplains have been well studied (Spink et al., 1998; Steiger and Gurnell, 2003), restoration strategies specifically aimed at reducing nitrogen loads have been, until recently, limited to small streams (Cabezas and Comín, 2010; Craig et al., 2008). The effects of altered water regime on the nitrogen cycling of river systems have been demonstrated at local scales (Hedin, 1990; Hill et al., 2000; Pinay et al., 1995; Triska et al., 1993). The main challenge is now to evaluate the effects of these changes at larger landscape-level scales (Lamers et al., 2006).
The primary objective of this study was to determine how changes in the physical gradients (i.e. physical elements in the landscape) related to the water delivery and discharge regime can affect the nitrogen and carbon cycles in floodplain ecosystems. More specifically, the aim of our study is to determine how floodplain restoration, by increasing hydraulic exchange conditions between a large river main channel and its backwaters affects sediment and water quality as well as potential denitrification and respiration. We tested to what extent three major restoration variables can control sediment characteristics, water quality, and microbial activities: (i) the type of connection to the main river channel (degraded or reconnected), (ii) the average annual duration of connection and (iii) the water age prior to sampling.
In this study, we examined the role of hydrology and local geomorphology on potential microbial processing in sediments of two river side channels. A restored and a degraded side arm system along a 10-km floodplain section of the Danube River downstream of Vienna, Austria were examined in this study. The two selected systems differed by the type of connection to the main river channel – disconnected and restored via reconnection (Lobau and Orth, respectively). These two floodplain systems, while spatially close, vary greatly in their hydrology and geomorphology. Restoration via surface water reconnection changed the local conditions within the floodplain, by increasing substrate input and reducing water retention times in the system. These changes were hypothesized to increase the microbial processing occurring in a restored floodplain when compared to a degraded floodplain. Within these two floodplain systems we selected sites which differed by their average annual duration of connection and the water age before sampling. This gradient was selected in order to cover a representation of the different floodplain characteristics as they are influenced by hydrology (i.e. substrate availability, morphology, flow patterns). Potential microbial processing (substrate induced respiration and denitrification enzyme activity) was used to compare sites under controlled and unlimited nutrient conditions, and to furthermore assess the potential maximum rates of the in situ microbial community.
2. Materials and methods
2.1. Sites description
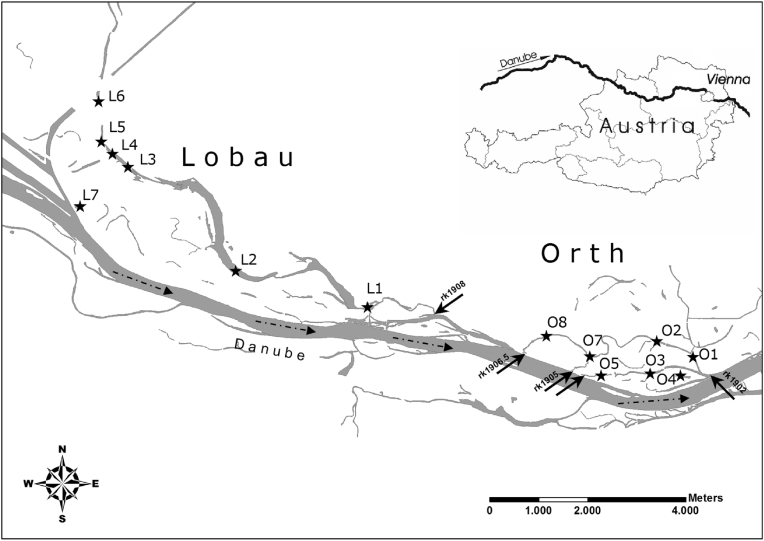
Two floodplain segments of the Danube River were studied: the Lower Lobau and Orth (Fig. 1). Both floodplains are located within the boundaries of the Alluvial Zone National Park, downstream the city of Vienna, Austria. In this area, the Danube River is a 9th order river with a drainage basin of 104,000 km2. The flow regime has an alpine character with variable and stochastic patterns (regulated low discharge: 915 m3 s−1, mean discharge: 1930 m3 s−1, annual flood discharge: 5300 m3 s−1, 30 year flood discharge: 9340 m3 s−1). Following the major regulation scheme in 1875, the Danube River was confined between flood protection dams, thus the main channel was disconnected from the adjacent floodplains (Chovanec et al., 2000). Restoration projects began in 1997 with the goal of reconnecting several floodplains to the main channel of the Danube (Hein et al., 2004; Schiemer et al., 1999).
Fig. 1.
Map of the Lobau (degraded) and Orth (restored) floodplains, located downstream from Vienna, Austria. Sampling sites are marked with stars and openings to the Danube River marked with arrows and their respective river km. Flow direction of the Danube River marked with dashed arrows.
The Lobau floodplain covers an area of approximately 23 km2. As no significant restoration measures have been undertaken within the Lobau floodplain, it is not integrated within riverine flow and in this study, considered as an altered and degraded floodplain. Aside from ground-surface water exchange and a controlled small water intake, the primary water exchange with the main channel takes place through an artificial 5 m wide breach in the flood levee in the Lobau's south-eastern end (Fig. 1). Positioned at river km 1908, the opening in the flood protection dam allows limited surface water connection between the main river and the Lobau at discharge above 1500 m3 s−1 (approximately 235 days year−1). As the floodplain is connected at a downstream opening, flood waters flow in an upstream direction into the side arms. When flood waters recede, the water discharges from the Lobau through the same opening back into the main channel of the Danube River. Due to the “bath tub” characteristic of the floodplain, flood waters move slowly into the backwater areas. The effective, active connections with sites in the floodplain to the Danube River have been significantly reduced, with highest connection occurring at the downstream portion of the floodplain. Three major retention structures with culverts prevent the side arms from becoming completely dry during low flow periods, resulting in shallow lake-like conditions. The riparian forests are dominated by hardwood forests and agricultural relics; natural floodplain vegetation covers only a minimal portion of the floodplain itself (Burger and Dogan-Bacher, 1999). Phragmites sp. is generally present at all sites along the terrestrial aquatic boundary. As a heavily used recreational area, the Lobau is managed and maintained to provide access for bicyclists as well as larger trucks throughout, which use the paved roads to transport materials.
In contrast, the reconnected and restored floodplain Orth, located downstream of the Lobau floodplain covering approximately 5.5 km2 (Fig. 1), is characterized by very diverse flow conditions. Some side arms in this system have through-flow conditions just above riverine summer mean flow (2230 m3 s−1), while others are connected only at much higher flow conditions. As part of the Danube River Restoration Project (Schiemer et al., 1999), most of the historical retention structures present in the Orth floodplain have been removed, increasing the side-arm discharge significantly, as well as the duration of surface water connection to the main channel, i.e. connection duration (Tritthart et al., 2009). The three openings with the same width and depth as the floodplain channels (one at river km 1906.5 and two at river km 1905) and one outlet (river km 1902) connect parts of this side-arm system to the main river at discharges of 4400 m3 s−1 (approximately 7 days year−1), 1500 m3 s−1 (approximately 235 days year−1), and less than 900 m3 s−1 (approximately 365 days year−1), respectively. The Orth floodplain is dominated by a channel-like system with high, steep, eroded banks. High amounts of gravel and woody debris are transported within the restored channels, creating dynamic gravel beds and log jams in the channels. Due to the restoration efforts, this floodplain is not actively managed for recreational purposes and is perceived as a “wild” floodplain. With the removal of the riverside embankments and controlled management, the Danube River is given the space to reshape the landscape in the Orth floodplain. Sites within both floodplains are not only connected during flooding situations, but during a wide range of discharge levels, as previously noted.
2.2. Hydrology
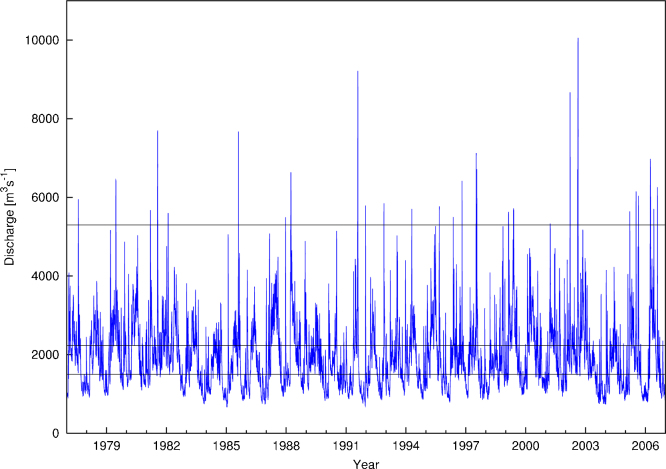
Based on the results of a hydrodynamic model in the Orth floodplain (Tritthart et al., 2009) and a simplified hydrostatic flooding model in the Lobau (Tritthart et al., 2011), a hydrological connectivity model was developed prior to field sampling. The current morphology of the floodplains was used in combination with a long-term hydrograph. A number of steady-state water surface calculations together with a long-term hydrograph (30 years) (Fig. 2) were used in order to estimate both statistically averaged and event-based connectivity parameters: duration of connection, duration of disconnection, and frequency of connection (Tritthart et al., 2009). The average duration of disconnection described the duration (days) between connection events. From this model, the water age of the surface water (in days) was calculated for each site at the time of sampling. The water age, which has been corrected for a minimum velocity of 0.2 m s−1 required for particles to pass through the system without settling, described the age of the water as it passed through the sampling point (Hein et al., 2004). To calculate water age in the Lobau where bidirectional flow occurred, it was important to consider the flow direction: the water age was assumed to be zero throughout the rising limb of the hydrograph; once the peak of the hydrograph passed and the flow reverses to outflow conditions, no nutrients from the river could enter the system on a surface pathway; thus the water age was calculated from that point onwards.
Fig. 2.
Thirty year (1977–2007) hydrograph for the Danube River. Horizontal lines represent connection discharges for the different connection points (upper line annual flood 5300 m3s−1, mid line summer mean low flow 2230 m3s−1, lower line 1500 m3s−1).
Data source: via donau and the Austrian Federal Ministry for Agriculture, Forestry, Environment and Water).
The two first variables, i.e. type of connection and average annual duration of connection, were used for side arm restoration schemes, as they were defined and assessed for the technical descriptions of the measures. The third variable, i.e. the water age, is defined as the retention time of the surface water in the side arm system.
2.3. Field sampling
Fourteen sites were selected in the side arms of the Lobau and Orth floodplains, using the connectivity model, described in Section 2.2 to encompass varying flowing and morphological characteristics. Ten sites were selected in 2006 and four additional sites were sampled in 2007 (Fig. 1). In both years, water and in-channel sediment sampling occurred during the growing seasons under periods of stable hydrological conditions (but not stagnant), when the Danube River was not experiencing a flood event. Triplicate sediment samples of 5–10 cm depth were taken randomly using a PVC corer (internal diameter 5 cm) in deep and shallow macrophyte-free areas within the floodplain side arm channel of each sampling site. Each triplicate sample was a homogenized mixture of 3–5 sediment cores from one location which were mixed to provide a representative sample of the sampling location. To estimate the amount of macrophytes and terrestrial leaf litter, sites were ranked on a 0–5 scale following Udy et al. (2006), with 0 when neither macrophytes nor leaf litter were present and 5 with 100% coverage within a 10 m2 area. Water samples were taken at the same time using 5 L containers. All samples were kept cool (<10 °C) while in transport back to the lab. Water and activity samples were analyzed within 24 h of sampling. Sediment samples were stored frozen at −20 °C until analyzed for their nutrient content. Electrical conductivity, dissolved oxygen (%), pH, and temperature of the surface water were measured using an HQ40d sonde (Hach Lange, Düsseldorf, Germany) at the time of sampling.
2.4. Sediment and water characteristics
Dry weight of the soil samples was determined by oven-drying sediments at 70 °C to constant mass. Organic N and C concentration and isotope abundances were acidified (1 M HCl) to remove inorganic C and measured with an elemental analyzer (EA 1110, CE Instruments, Milan, Italy) connected to an isotope ratio mass spectrometry IRMS (DeltaPLUS, Finnigan MAT, Bremen, Germany). Dried sediments were size fractioned using a sieve tower. Sediment D50 was calculated from the sediment particulate size. Organic matter content of the sediment fractions was determined as weight loss by ignition (LOI %) of dry sediment at 450 °C for 4 h. Nitrogen concentrations in the sediment were analyzed for N–NH4+, N–NO3−, and N–NO2− using standard colorimetric methods (APHA, 1998) for a continuous flow analyzer (CFA, Systea Analytical Technology). Phosphorus fractions of inorganic P (HCl extraction), organic P (HNO3 combustion), and soluble reactive P (H2O extraction) were determined using a continuous flow analyzer (CFA, Systea Analytical Technology) (Ruban et al., 2001). From each site, a 50 ml water sample was taken and filtered through a GF/F (Whatman) filter to analyze P–PO4, N–NH4, N–NO3, and N–NO2 using a continuous flow analyzer (CFA, Systea Analytical Technology) and standard colorimetric methods (APHA, 1998).
2.5. Potential respiration and denitrification
Potential denitrification enzyme activity (DEA) was measured according to Smith and Tiedje (1979). Ten grams (fresh weight) subsets of sediment samples were weighed into 100 ml serum flasks, which were made anoxic by flushing the flask atmosphere with N2. The flask contents were incubated with 10% (v/v) acetylene to allow the accumulation of denitrified nitrogen as N2O, after adding 1 mg C g−1 sediment (added as glucose) and 0.2 mg N g−1 sediment (added as KNO3). Denitrification rates were calculated as the rate of N accumulated as N2O in the headspace after 4 h in dark at 25 °C and analyzed by gas chromatography with 63Ni electron capture detector (HP 5890II GC). DEA was also measured under the same conditions but without acetylene to determine the proportion of N denitrified as N2O during the assay and analyzed by gas chromatography to quantify N2O concentrations (Agilent 6890N, Santa Clara, U.S.A., connected to an automatic sample-injection system (DANIHSS 86.50, Headspace-sampler, Cologno Monzese, Italy).
Substrate induced respiration (SIR) was measured according to Beare et al. (1990) by incubating 10 g fresh weight of sediment with 2 mg glucose-C g−1 sediment in a 100 ml serum flask. SIR was calculated as the accumulation of CO2–C in the flask during incubation after 4 h incubation at 25 °C in the dark per gram of sediment (DW) and per hour, using the gas chromatograph Agilent 6890N.
2.6. Statistics
All measured processes and sediment characteristics (chemical and physical) were compared between floodplains using Mann Whitney U tests with the SPSS software package.
We considered local physical gradients, nutrient availability, and carbon availability as explanatory links between hydrology and ecosystem processes (i.e., potential respiration and potential denitrification). Each of these quantities can be regarded as a meta-variable which is described by a set of explicitly measured and correlated variables. In fact, we expressed each meta-variable as a matrix of pairwise dissimilarities between two sampling sites. This approach efficiently integrates information from the various variables needed to account for the complexity of our study system. We then used Mantel and partial Mantel (controlling for effects of hydrology) statistics to test for associations between these meta-variables. Further, we used causal modeling on dissimilarity matrices (i.e., path analysis based on Mantel statistics treated as correlation coefficients) to relate the various meta-variables in the hypothesized causal framework (Legendre and Legendre, 1998). All tests and path analysis were performed for both areas. Mantel statistics do not have to be large, i.e. close to 1 or −1, to be statistically significant. Significance of path coefficients was assessed by randomizing all involved matrices using 104 permutations, building randomized distributions for each path coefficient, and computing probabilities for observed path coefficients with the percentile method (Manly, 2006). All calculations were done in R 2.9. (R-Development-Core-Team, 2005), using the packages vegan (Oksanen et al., 2010) and sem (Fox et al., 2010).
3. Results
3.1. Hydrological and physical conditions
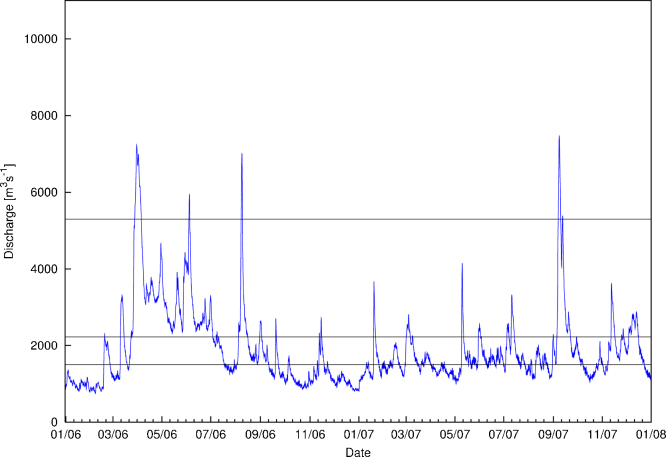
The two sampling years were hydrologically different (Fig. 3). In 2006, three flood events in April, June and August exceeded the level of an annual flood; of these, April and August floods were characterized by a statistical return period of 1 in 10 years, respectively. In 2007, however, the annual flood level was exceeded only once (in September) reaching a statistical return period of approximately 1 in 15 years. With this one annual flood event and average discharge, 2007 was similar to the long term discharge pattern (Fig. 2). In both the degraded (Lobau) and restored (Orth) side arms, the average connection (p = 0.068) was not significantly different between the two years (Table 1); however, the duration of disconnection was significantly shorter in the restored section (p < 0.05).
Fig. 3.
Hydrograph of the Danube River from January 2006 to January 2008, encompassing the study period. Discharges are presented as hourly mean values. Horizontal lines represent connection discharges for the different connection points (upper line annual flood 5300 m3s−1, mid line summer mean low flow 2230 m3s−1, lower line connection threshold for Lobau floodplain 1500 m3s−1) (Data source: via donau and the Austrian Federal Ministry for Agriculture, Forestry, Environment and Water).
Table 1.
Measured average and standard deviation (in parenthesis) of hydrological and morphological characteristics, sediment carbon content and quality and sediment and water nitrogen and phosphorus pools for the degraded (Lobau) and restored (Orth) floodplains N = 135.
| Floodplain | N | Duration connection (days) | Duration disconnection* (days) | Depth (mm) | Temperature* (°C) | pH* | Conductivity (μs cm−1) | Sediment D50* (mm) | DO* (mg l−1) | Leaf litter (rank) | Macrophytes* (rank) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Degraded | 78 | 10.31 (10.34) | 107.89 (211.71) | 1.52 (0.93) | 15 (5) | 7.5 (0.49) | 491 (65) | 0.32 (0.84) | 7.62 (2.5) | 3 (1.5) | 3 (1.4) |
| Restored | 57 | 10.05 (5.32) | 58.02 (80.72) | 1.02 (0.70) | 10.87 (4.32) | 7.70 (0.34) | 517 (130) | 0.30 (0.93) | 10.05 (2.74) | 3 (0.7) | 1 (1.1) |
| Floodplain | N | N–NO3* (mg kg dry sediment−1) | N–NO3* (μg L−1) | N–NO2 (mg kg dry sediment−1) | N–NO2* (μg L−1) | N–NH4* (mg kg dry sediment−1) | N–NH4* (μg L−1) | Ptot (mg kg dry sediment−1) | SRP (μg L−1) | SRP (mg kg dry sediment−1) | C:N* |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Degraded | 78 | 4.8 (7.4) | 299 (287.1) | 0.4 (0.7) | 3.7(2.8) | 208.2 (390.4) | 26.0 (15.2) | 773.1 (1174.6) | 6 (8.0) | 1.4 (2.0) | 10.4 (2.1) |
| Restored | 57 | 6.7 (4.5) | 766 (658.8) | 0.2 (0.2) | 8.0 (7.6) | 41.3 (56.1) | 69.0 (114.1) | 506.8 (285.8) | 5 (5.2) | 0.7 (0.5) | 17.3 (6.6) |
Significant differences (p < 0.05) between the two floodplains are marked with an asterisk.
3.2. Comparison of the side arm systems with different connection types
Sediment N and P pools varied between the two side arm systems (Table 1). Significant differences were observed between the two systems in the water column and sediment for N–NH4, N–NO3, and N–NO2 (water only). Concentrations of N–NO3, N–NO2, and N–NH4 in the sediment were higher in the Orth floodplain than in the Lobau floodplain (Table 1). The same trend was observed in the water column, except for N–NH4 which was significantly higher in the Lobau floodplain. Similarly, concentrations of SRP in the sediment were lower in the Orth side arms, although not significantly (Table 1). The sediment C:N ratio was significantly higher in the Orth floodplain (Table 1). Significantly lower water temperatures and higher dissolved oxygen concentrations were observed in the Orth side arms than in the Lobau (Table 1). The average grain size and mean surface water pH were significantly different between the two floodplain systems. No significant difference was observed for mean electrical conductivity between the systems.
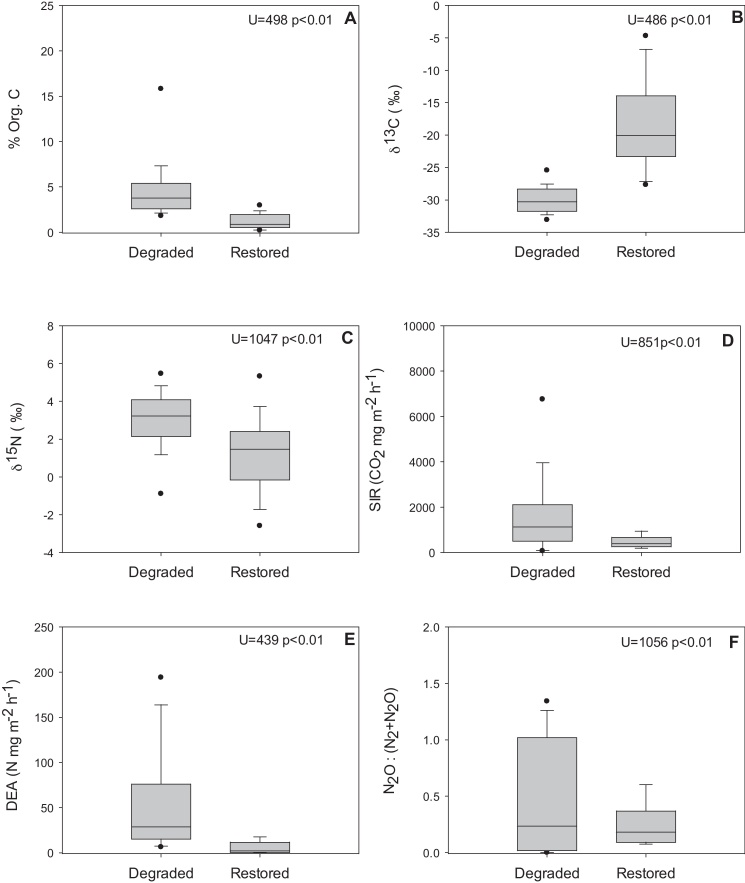
Parameters related to the quality of the sediment organic matter and microbial activities presented significant differences between the two floodplains types (Fig. 4). The percentage of organic matter (Fig. 4A) in the sediment was significantly higher in the degraded system, than in the restored floodplain. Organic matter content of the sediment did not only differ significantly between sites in terms of concentration, but also in terms of quality. Indeed, the δ13C of the organic matter content was significantly lower in the Lobau than in Orth (Fig. 4B), while the δ15N signature was significantly higher (Fig. 4C). In the degraded section, higher and more variable rates of SIR in the sediment were measured (mean 1678.5 mg CO2 m−2 h−1) compared to the restored system (mean 471.6 mg CO2 m−2 h−1) (Fig. 4D). Similarly, average rates of sediment DEA presented higher average rates and higher variance (F = 34.903, p < 0.01) in the degraded system (mean 48.70 mg N–N2O m−2 h−1) compared to those in the restored system (mean 6.23 mg N–N2O m−2 h−1) (Fig. 4E). The range of the ratios of DEA: was also larger in the degraded floodplain (mean 0.48) than in the restored floodplain (mean 0.26) (Fig. 4F).
Fig. 4.
Comparison of average percentage of sediment organic carbon content (A), δ13C (B), δ15N (C), SIR (D), DEA (E) and ratio of potential N2O to N2 emission (F) between the degraded system (n = 65) and the restored system (n = 34) floodplain systems. Box lines indicate upper and lower quartiles. Whiskers extend to the 95th and 5th percentiles. Mann Whitney U values between the sites and their significance are noted on each figure.
3.3. Influence of connectivity-related parameters
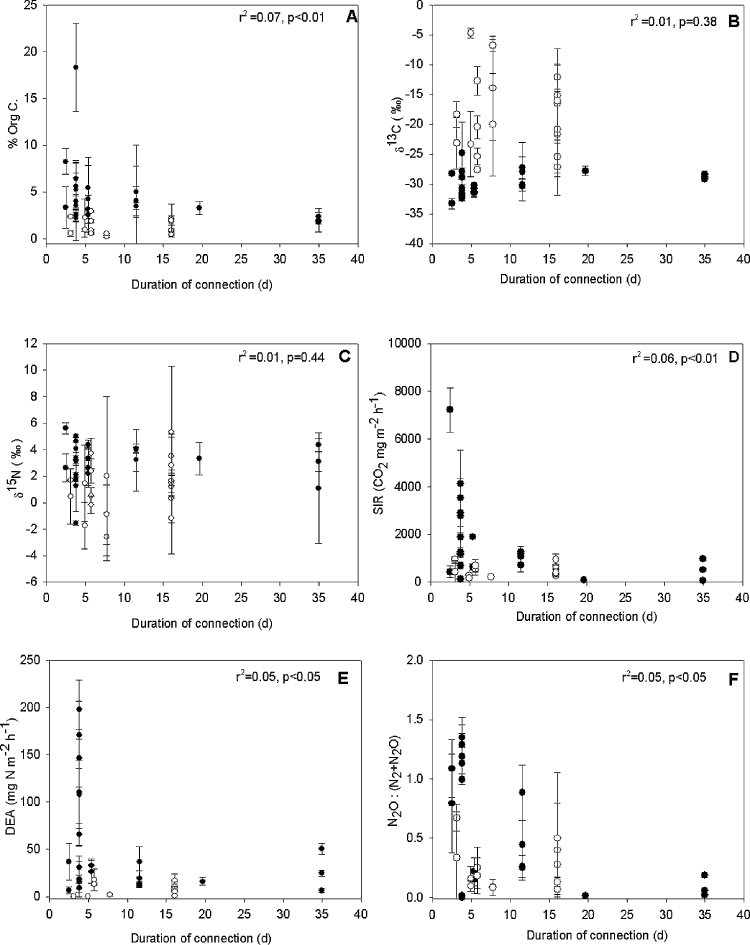
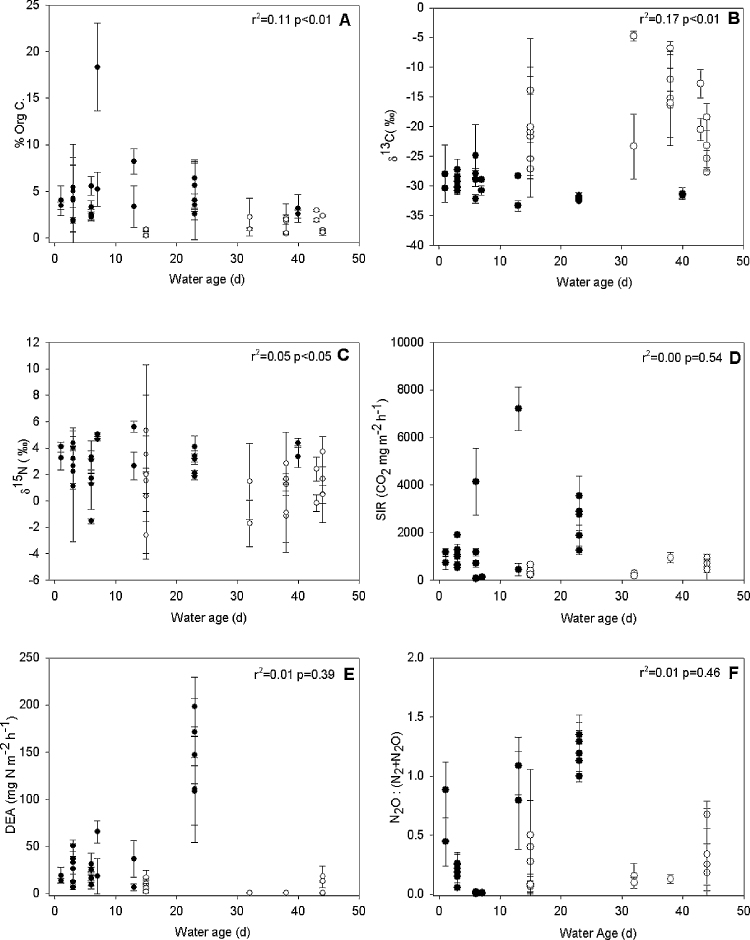
A weak, but significant trend was observed for sediment organic matter concentration, SIR, DEA, and DEA: decreasing as a function of average duration of connection (Fig. 5A, D–F), while δ13C and δ15N decreased slightly to an average of −25‰ and +2‰ respectively, although not significant (Fig. 5B and C). When considering these variables (sediment organic matter content, δ13C, δ15N, SIR, DEA, DEA: ) in relation to the water age the patterns change (Fig. 6). Although the same weakly decreasing trend for sediment organic matter content was observed with increasing water age, δ13C increased significantly, albeit weakly, with increasing water age and δ15N began to show a weakly significant decreasing trend (Fig. 6A–C). No significant relationships were found for the remaining variables (Fig. 6D–F).
Fig. 5.
Average percentage of sediment organic carbon content (A), δ13C (B), δ15N (C), SIR (D), DEA (E), and ratio of potential N2O to N2 emission (F) as a function of mean annual duration of connection with the main Danube River channel. Filled circles represent degraded sites (n = 65) while open circles represent restored sites (n = 34). The r2 and significance (p) for the linear function is noted on each figure.
Fig. 6.
Average percentage of sediment organic carbon content (A), δ13C (B), δ15N (C), SIR (D), DEA (E), and ratio of potential N2O to N2 emission (F) as a function of the water age in the study sites. Filled circles represent degraded sites (n = 65) while open circles represent restored sites (n = 34). The r2 and significance (p) for the linear function is noted on each figure.
3.4. Environmental control of potential microbial processing
The weak linear relationships between the singular connectivity parameters and sediment characteristics and potential microbial activity and the high co-correlation between individual variables led to the creation of multivariable matrices (Table 2). Most of the links in the suggested causal framework were described by multivariate datasets. Information content of these various complex datasets with heterogeneous as well as co-linear variables was condensed to a limited number of dissimilarity matrices by computing Euclidean distances between all sampling sites based on standardized variables selected to describe the meta-variables: Hydrology, Physical Gradients, carbon sources (Carbon), nutrient concentrations (Nutrients) and potential processes (Output) (DEA and SIR, respectively) (Table 2).
Table 2.
Meta-variable dissimilarity matrices and underlying variables (units in brackets). All matrices are Euclidean distance matrices calculated on standardized variables. DEA and : DEA were combined into a single output and SIR was calculated as a separate output.
| Physical | Hydrology | Nutrients | Carbon | Output | Output |
|---|---|---|---|---|---|
| Mean depth water body (m) | Duration of connection (days) | N pools in sediment and water (N–NO3−, N–NH4+, N–NO2−) (mg kg dry sediment−1 and mg l−1, respectively) | Organic material in sediment (%) | DEA (mg N m−2 h−1) | SIR (mg CO2 m−2 h−1) |
| Water temperature (°C) | Duration of disconnection (days) | P pools in sediment and water (PO4+, Ptot, SRP) (mg kg dry sediment−1 and mg l−1, respectively) | δ13C in sediment | Ratio :DEA | |
| Conductivity (μS/m) | Connection (days year−1) | C:N in sediment | Present macrophytes (rank) | ||
| Sediment size (D50) (mm) | Water age (days) | δ15N in sediment | Litter coverage (rank) | ||
| Dissolved oxygen (%) | |||||
| pH |
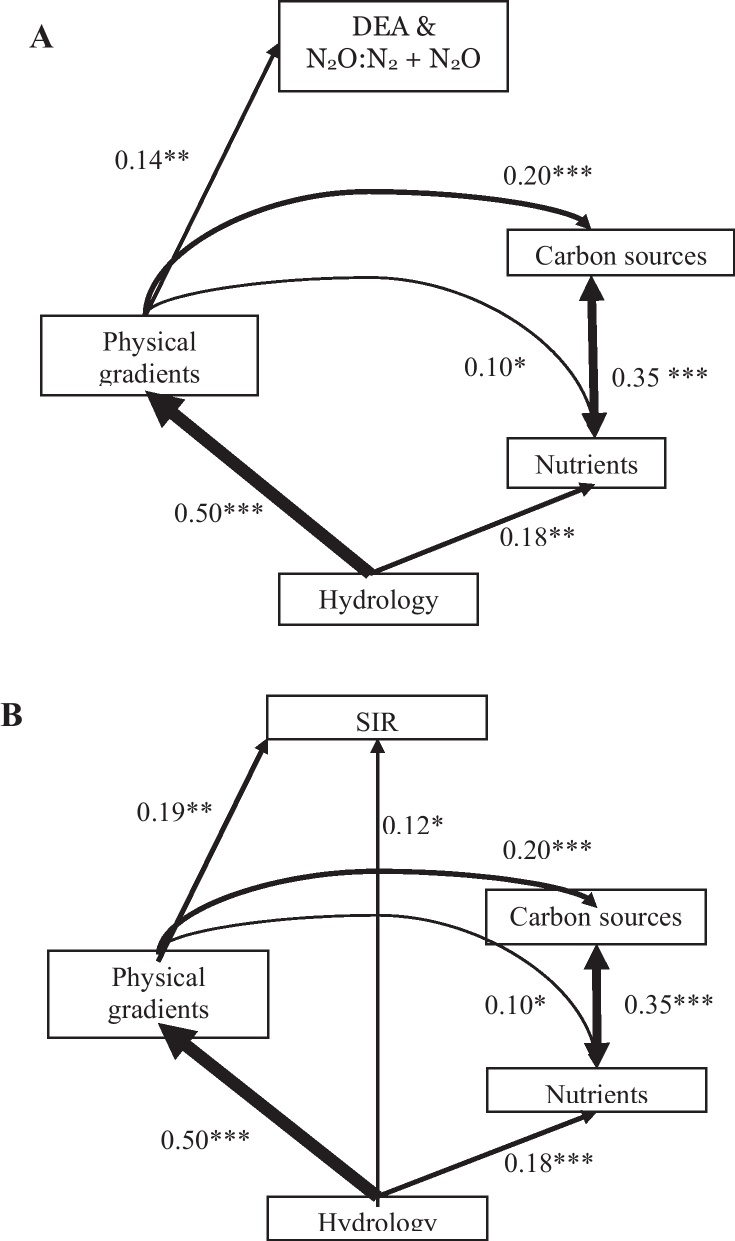
Mantel statistics could identify a direct effect of Hydrology on the Output (as DEA) (Mantel r = 0.079, P < 0.001, Fig. 4) and on SIR (Mantel r = 0.186, P < 0.001, Table 3) as well as on the physical characteristics of the floodplain (r = 0.503), the sediment N and P pools (r = 0.224) and the C sources (r = 0.141), all at P < 0.001 (Table 3). Partial Mantel tests controlling for the effect of hydrology showed a significant influence from the floodplain physical characteristic on the C sources (r = 0.170), SIR (r = 0.147) and DEA (r = 0.122), all at P < 0.001 (Table 3). Path analysis (Fig. 7) based on Mantel statistics computed among meta-variables suggested a strong influence of hydrology on physical gradients, which furthermore influence DEA and the ratio of :DEA. Both, nutrients and carbon were shown to be controlled by a similar, but weaker pathway, yet neither nutrients nor carbon participated in the determination of DEA. Path analysis based on Mantel statistics using SIR as the output variable (Table 3) suggested the same pathway of influence of hydrology on physical gradients which influenced SIR (Fig. 7B). However, a weaker direct link was calculated where hydrology directly influences SIR.
Table 3.
Associations between meta-variable dissimilarity matrices as expressed by Mantel statistics. As output variables either DEA and : DEA or SIR was used. Mantel (upper diagonal) and partial Mantel (lower diagonal) statistics (controlling for hydrology) presented, significant values printed bold, P-values not corrected for multiple testing.
| Physical gradients | Nutrients | Carbon | DEA | SIR | |
|---|---|---|---|---|---|
| Hydrology | 0.503P < 0.001 | 0.224P < 0.001 | 0.141P < 0.001 | 0.079P < 0.05 | 0.187P < 0.001 |
| Physical gradients | 0.085P < 0.001 | 0.217P < 0.01 | 0.145P < 0.001 | 0.219P < 0.001 | |
| Nutrients | 0.085 P = 0.05 | 0.398P < 0.001 | −0.054 P = 0.80 | −0.066 P = 0.89 | |
| Carbon | 0.170P < 0.01 | 0.380P < 0.001 | 0.055 P = 0.13 | −0.059 P = 0.91 | |
| DEA | 0.122P < 0.01 | −0.07 P = 0.91 | 0.04 P = 0.19 | ||
| SIR | 0.147P < 0.01 | −0.114 P = 0.99 | −0.088 P = 0.99 |
Fig. 7.
Path diagram depicting relationships among meta-variables described by dissimilarity matrices. Path coefficients are computed from Mantel statistics. Data of both floodplains Lobau and Orth were used simultaneously with A) DEA and N2O:N2 + N2O or B) SIR as the output meta-variable. For significant path coefficients, line width is proportional to the magnitude of the presented path coefficient. P values are presented as *p < 0.05, **p < 0.01, ***p < 0.001.
4. Discussion
4.1. Restored versus degraded connection to the main river channel
Restored surface water connection entailed fast flowing water (mean flowing velocities > 1 m s−1) in the side arms during floods with larger grain size sediment deposits, whereas the degraded and decoupled surface connection to the main channel river channel entailed gradual flooding with low flow velocity, thus depositing fine sediments in the downstream areas of the floodplain (Reckendorfer and Hein, 2006). Due to these differences in flow, restored connection led to lower water temperature and higher dissolved oxygen concentration (Table 1). The higher organic matter content measured in the degraded system (Lobau) (Fig. 4A) most probably originated from autogenic sources, i.e. the riparian forest and macrophytes present in the area. The importance of autogenic organic carbon in the degraded side arm system was supported by the lower δ13C value of organic matter in the sediments at Lobau sites with short connection periods (Fig. 4B). The higher δ13C values measured in restored sites together with their very high variability, independent of the average connection time (Fig. 5B), suggested that organic matter in these restored sites was mostly controlled by riverine transported organic matter and had potentially a more recalcitrant nature (Hein et al., 2003; Aspetsberger et al., 2002). Sustained higher potential microbial activity in the sediment was confirmed by higher SIR and DEA (Fig. 4D and E), similar to results presented from the restored Baraboo River floodplains (Orr et al., 2007). Higher observed SIR and DEA in degraded floodplain sediments implied that such systems could potentially remove more carbon and nitrogen. However, the actual rates may be substrate limited due to a lack of inputs from the Danube River – the main source of substrates. Similar patterns between geomorphological distributions and potential denitrification have been shown between riverine and backwater sites in the Upper Mississippi, where backwater areas exhibited higher DEA than riverine sites despite receiving less nitrate inputs from the Mississippi (Richardson et al., 2004).
A higher δ15N and total organic N (NO3 and NH4) in the sediments from the degraded floodplain (Fig. 4C) supports our result pointing to higher DEA in degraded systems. In the absence of organic pollution such as manure or waste water, the dominant process contributing to higher δ15N could be a consequence of faster N cycling and higher denitrification activity, which fractionates between the two N isotopes and preferentially removes the lighter isotope from the sediment. Since δ15N was not measured from NO3 or NH4 separately, it can only be considered as a mixture of present organic N pools. Positive shifts have been shown to represent higher nitrogen cycling in lacustrine and marine systems (Lehmann et al., 2004; Teranes and Bernasconi, 2000). This trend is only representative when comparing the two ecosystems as neither the duration of connection nor the water age can explain the observed patterns.
The average ratio of potential N2O to N2 emission (DEA: ) was similar in both systems; yet, in the degraded system larger variation of this ratio as well as higher rates of potential emissions (DEA) were measured. Therefore, the degraded system could support higher potential denitrification dominated by N2O emissions (Fig. 4). The domination of N2O in the degraded site could be due to the high NH4 concentrations measured in the sediment. When NO3 is limiting, the last step of denitrification (N2O → N2) will be limited as this is the most energy dependent step (Morley and Baggs, 2010). In systems where the microbial community is conditioned for low NO3 concentrations, incomplete denitrification may be the dominant pathway. This tendency towards incomplete denitrification has major implications for the greenhouse gas balance of the system. Higher rates of denitrification resulting in N2O production mean that during floods the degraded site is a source of N2O whereas the restored site would be able to transform the excess NO3 to N2, resulting in a net gain of ecosystem services. As the degraded floodplain is 23 km2, a reduction of N2O emissions following restoration would be of a considerable magnitude (Verhoeven et al., 2006).
4.2. Geomorphologic controls
A detailed analysis of the relationship between average annual duration of connection of the side arms to the sediment organic matter quantity and potential microbial activities revealed a decrease of these variables with an increase of connection, both in terms of average value and variability (Fig. 5). Interestingly, this pattern was stronger for the degraded side arm system (filled circles). In the restored side arm system (open circles), the percentage of organic carbon remained low regardless the average duration of connection. The high variability in percentage of organic carbon (Fig. 5A) at sites with short duration of connection in Lobau could be interpreted as stronger influence of local environmental conditions (e.g., the type and density of riparian and instream vegetation) at these backwater sites with prolonged periods of disconnection. The higher variability of organic matter quantity was associated with a higher variability of potential respiration (SIR), but not DEA, especially in mostly disconnected sites (less than 5 days of connection per year; Fig. 5D). High rates (SIR only) and variability of SIR and DEA were significantly related to the duration of connection (Fig. 5D and E). The differences in overall hydrology changed the sediment environmental conditions, which in turn could cause shifts in the microbial community composition (Gutknecht et al., 2006). This study could not determine whether this high variability of response in long term disconnected sites was the result of a genetically different microbial community or simply a difference in density.
Altering the flow patterns entering the side arms not only changed the physical area (morphology) of the system, but also changed the delivery patterns of carbon and nutrients. The inherent hydrologic and morphologic heterogeneity of the two floodplains makes it difficult to use singular linear relationships to describe large-scale controls on potential microbial processing. The path analysis revealed that Hydrology factors directly affected SIR and nutrient content in sediments (Fig. 7B). Yet, more importantly, the hydrology strongly influenced the side arm physical characteristics, which in turn, significantly controlled the available carbon and nutrient sources (Fig. 7A and B). This demonstrated that flood regime was not the only variable which controlled biogeochemical processing; the overall morphology of the floodplain system was influencing these biogeochemical processes, too. This supported Boyer et al. (2006) who argued that hydrological and physical characteristics were a major controlling factor in N cycling in aquatic systems. By restoring surface water connections, the river can change the morphology of the floodplain which will further influence the local substrate availability for respiration and denitrification (Amoros, 2001). Even though the path analysis did not point to a direct relationship between the available nutrient and carbon pools and DEA, there will be a change in the available substrates following restoration as suggested in the observed changes along the connectivity gradients presented (Figs. 4–6). The absence of this link in the path diagrams is most likely due to the method of measurement used. DEA and SIR are just estimates of the potential activity and are measured under saturated conditions, thus separating the in situ nutrient conditions.
Due to the network of factors influencing each other, our results did not single out one main variable that drives the link between morphology and microbial processing. Using one single parameter to explain a biogeochemical reaction that is the result of several variables underestimates the complexity and heterogeneity of floodplains and the effects of restoration. The absence of a clear relationship between sediment characteristics and potential microbial activities on the one hand, and the water age before sampling on the other hand (Figs. 6 and 7A), supports the idea that it is the combination of the type of connection and morphological characteristics which are the main drivers of sediment quality and consequently, microbial processes. Modeling of potential respiration at the same site suggested that sites of high activity were found in areas of lower connectivity if connected during higher discharges and areas of high water depth (Tritthart et al., 2011). The results suggest that the local morphology coupled with the hydrologic regime at the landscape scale create the conditions necessary for microbial processing. Restoration of floodplains via surface water reconnection would return the necessary substrate inputs to the system. Frequent and constant riverine connections could increase denitrification efficiency, as suggested by the reduced N2:N2O ratios in the restored floodplain. Alteration of vegetation patterns (ex. appearance of floating vegetation in less connected areas; increased leaf litter in gouged channels) caused by changes in hydrology and morphology may also drive sediment quality changes and ultimately impact the conditions for microbial processing.
5. Conclusion
Large river floodplain restorations often imply reconnection of preexisting side arms to the main channel by partial removal of embankments or levees. Most of these reconnection schemes are aimed at enhancing biodiversity by creating a more dynamic hydrological regime in the floodplain. In this study we evaluated the importance of restoring the connection of side arms to their main river channel (i.e. increasing annual average duration of connection and decreasing water age) on sediment biogeochemical characteristics and their effects on potential microbial activities. The path diagram illustrated a hierarchical structure that suggested that the morphology of a specific site mediates the influence of the main source water (riverine inputs) for DEA and the carbon and nutrient conditions in the sediment. By re-establishing surface water connection of a site, the controls on sediment microbial respiration and denitrification were changed, eventually impacting potential microbial activities. Floodplain restoration would result in a series of morphological changes (ex. temperature, dissolved oxygen, and macrophyte distribution) resulting in an increase of substrate availability and ultimately more efficient N and C cycling, with an overall reduction of potential N2O emissions. Further quantification of these links between the type and duration of connection between side arms and main channel, including the hyporheic zone, measurable at large scale, and microbial processes, measurable at micro-scale, should allow quantifying the effects of floodplain restoration on nutrient cycling in the river systems.
Acknowledgements
This project was funded by the Austrian Science Fund (FWF) project CANFLOOD (P19907-B17). We would like to acknowledge C. Hinterleitner for sediment and water nutrient analysis, M. Mair and M. Felkl for their help in the field, and E. Hall, M. Striebel and K. Tockner for their helpful comments on an earlier version of the manuscript.
References
- Amoros C. The concept of habitat diversity between and within ecosystems applied to river side-arm restoration. Environ. Manage. 2001;28:805–817. doi: 10.1007/s002670010263. [DOI] [PubMed] [Google Scholar]
- APHA . 20th ed. American Public Health Association; Washington, DC, USA: 1998. Standard methods for the examination of water and wastewater. [Google Scholar]
- Aspetsberger F., Huber F., Kargl S., Scharinger B., Peduzzi P., Hein T. Particulate organic matter dynamics in a river floodplain system: impact of hydrological connectivity. Arch. Hydrobiol. 2002;156:23–42. [Google Scholar]
- Battin T.J., Luyssaert S., Kaplan L.A., Aufdenkampe A.K., Richter A., Tranvik L.J. The boundless carbon cycle. Nat. Geosci. 2009;2:598–600. [Google Scholar]
- Beare M.H., Neely C.L., Coleman D.C., Hargrove W.L. A substrate-induced respiration (sir) method for measurement of fungal and bacterial biomass on plant residues. Soil Biol. Biochem. 1990;22:585–594. [Google Scholar]
- Bennett E.M., Carpenter S.R., Caraco N.F. Human impact on erodable phosphorus and eutrophication. A global perspective. Bioscience. 2001;51:227–234. [Google Scholar]
- Bernot M.J., Dodds W.K. Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems. 2005;8:442–453. [Google Scholar]
- Boyer E.W., Alexander R.B., Parton W.J., Li C., Butterbach-Bahl K., Donner S.D., Skaggs R.W., Del Grosso S.J. Modeling denitrification in terrestrial and aquatic ecosystems at regional scales. Ecol. Appl. 2006;16:2123–2142. doi: 10.1890/1051-0761(2006)016[2123:mditaa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Brunet R.C., Pinay G., Gazelle F., Roques L. Role of the floodplain and riparian zone in suspended matter and nitrogen retention in the adour river, south-west France. Regulated Rivers. Res. Manage. 1994;9:55–63. [Google Scholar]
- Burger, H., Dogan-Bacher, H. 1999. Biotoptypenkartierung von Flächen außerhalb des Waldes im Nationalpark Donauauen aus Farbinfrarotbildern. Endbericht zur Luftbildinterpretation und Kartenerstellung. eds. Unveröffentlichte Studie i.A. des Bundesministerium für Umwelt, Jugend und Familie. Vienna.
- Burt T.P., Pinay G. Linking hydrology and biogeochemisty in complex landscapes. Prog. Phys. Geogr. 2005;29:297–316. [Google Scholar]
- Cabezas A., Comín F.A. Carbon and nitrogen accretion in the topsoil of the Middle Ebro River Floodplains (NE Spain). Implications for their ecological restoration. Ecol. Eng. 2010;36:640–652. [Google Scholar]
- Chovanec A., Schiemer F., Cabela A., Gressler S., Grötzer C., Pascher K., Raab R., Teufl H., Wimmer R. Constructed inshore zones as river corridors through urban areas – The Danube in Vienna: preliminary results. Regulat. Rivers: Res. Manage. 2000;16:175–187. [Google Scholar]
- Cole J.J., Prairie Y.T., Caraco N.F., McDowell W.H., Tranvik L.J., Streigl R.G., Duarte C.M., Kortelainen P., Downing J.A., Middelburg J.J., Melack J. Plumbing the global carbon cycle. Integrating inland waters into the terrestrial carbon budget. Ecosystems. 2007;10:171–184. [Google Scholar]
- Craig L.S., Palmer M.A., Richardson D.C., Filoso S., Bernhardt E.S., Bledsoe B.P., Doyle M.W., Groffman P.M., Hassett B.A., Kaushal S.S., Mayer P.M., Smith S.M., Wilcock P.R. Stream restoration strategies for reducing river nitrogen loads. Frontiers Environ. 2008;6:529–538. [Google Scholar]
- del Giorgio P., Pace M. Relative independence of organic carbon transport and processing in a large temperate river. The Hudson River as both pipe and reactor. Limnol. Oceanogr. 2008;53:185–197. [Google Scholar]
- Durisch-Kaiser E., Pavel A., Doberer A., Reutimann J., Balan S., Sobek S., Radan S., Wehrli B. Nutrient retention total N and P export, and greenhouse gas emission from the Danube Delta lakes. Geo-Eco-Marina. 2008;14:81–90. [Google Scholar]
- Fischer H., Kloep F., Wilzcek S., Pusch M. A river's liver–microbial processes within the hyporheic zone of a large lowland river. Biogeochemistry. 2005;76:349–371. [Google Scholar]
- Forshay K.J., Stanley E.H. Rapid nitrate loss and denitrification in a temperate river floodplain. Biogeochemistry. 2005;75:43–64. [Google Scholar]
- Fox, J., Kramer, A., Friendly, M., 2010. sem: Structural Equation Models. R package version 0. 9-20. http://CRAN.R-project.org/package=sem.
- Groffman P.M., Tiedje J.M. Denitrification hysteresis during wetting and drying cycles in soil. Soil Sci. Soc. Am. J. 1988;52:1626–1629. [Google Scholar]
- Gutknecht J., Goodman R., Balser T. Linking soil process and microbial ecology in freshwater wetland ecosystems. Plant Soil. 2006;289:17–34. [Google Scholar]
- He Q., Walling D.E. Spatial variability of the particle size composition of overbank floodplain deposits. Water Air Soil Poll. 1997;99:71–80. [Google Scholar]
- Hedges J.I., Mayorga E., Tsamakis E., McClain M.E., Aufdenkampe A., Quay P., Richey J.E., Benner R., Opsahl S., Black B., Pimentel T., Quintanilla J., Maurice L. Organic matter in Bolivian tributaries of the Amazon River. A comparison to the lower mainstream. Limnol. Oceanogr. 2000;45:1449–1466. [Google Scholar]
- Hedin L.O. Factors controlling sediment community respiration in woodland stream ecosystems. Oikos. 1990;57:94–105. [Google Scholar]
- Hefting M., Clement J.C., Dowrick D., Cosandey A.C., Bernal S., Cimpian C., Tatur A., Burt T.P., Pinay G. Water table elevation controls on soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry. 2004;67:113–134. [Google Scholar]
- Hein T., Baranyi C., Herndl G.J., Wanek W., Schiemer F. Allochthonous and autochthonous particulate organic matter in floodplains of the River Danube: the importance of hydrological connectivity. Freshwater Biol. 2003;48:220–232. [Google Scholar]
- Hein T., Baranyi C., Reckendorfer W., Schiemer F. The impact of surface water exchange on the nutrient and particle dynamics in side-arms along the River Danube, Austria. Sci. Total Environ. 2004;328:207–218. doi: 10.1016/j.scitotenv.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Hein T., Reckendorfer W., Thorp J.H., Schiemer F. The role of slackwater areas for biogeochemical processes in rehabilitated river corridors: examples from the Danube. Arch. Fur Hydrobiol. Suppl. Large Rivers. 2005;15:425–442. [Google Scholar]
- Henry C.P., Amoros C., Roset N. Restoration ecology of riverine wetlands. A 5-year post-operation survey on the Rhône River, France. Ecol. Eng. 2002;18:543–554. [Google Scholar]
- Hill A.R., Kevin J.D., Campagnolo S., Sanmugadas K. Subsurface denitrification in a forest riparian zone. Interactions between hydrology and supplies of nitrate and organic carbon. Biogeochemistry. 2000;51:193–223. [Google Scholar]
- Hohensinner S., Habersack H., Jungwirth M., Zauner G. Reconstruction of the characteristics of a natural alluvial river–floodplain system and hydromorphological changes following human modifications: the Danube River (1812–1991) River Res. Appl. 2004;20:25–41. [Google Scholar]
- Hynes H.B.N. The stream and it's valley. Verhandlungen Int. Verinigung Theor. Angewante Limonol. 1975;19:1–15. [Google Scholar]
- Jones J.B.J., Holmes R.M. Surface–subsurface interactions in stream ecosystems. Trends Ecol. Evol. 1996;11:239–242. doi: 10.1016/0169-5347(96)10013-6. [DOI] [PubMed] [Google Scholar]
- Lamers L.P.M., Loeb R., Antheunisse A.M., Miletto M., Lucassen E., Boxman A.W., Smolders A.J.P., Roelofs J.G.M. Biogeochemical constraints on the ecological rehabilitation of wetland vegetation in river floodplains. Hydrobiologia. 2006;565:165–186. [Google Scholar]
- Lehmann M.F., Bernasconi S.M., McKenzie J.A., Barbieri A., Simona M., Veronesi M. Seasonal variation of the (13C and (15N of particulate and dissolved carbon and nitrogen in Lake Lugano. Constraints on biogeochemical cycling in a eutrophic lake. Limnol. Oceanogr. 2004;49:415–429. [Google Scholar]
- Lefebvre S., Marmonier P., Pinay G. Stream regulation and nitrogen dynamics in sediment interstices: comparison of natural and straightened sectors of a third-order stream. River Res. Appl. 2004;20:499–512. [Google Scholar]
- Legendre P., Legendre L. 2nd ed. Elseviers Science B.V; Amsterdam, The Netherlands: 1998. Numerical Ecology. [Google Scholar]
- Manly B.F.J. 3rd ed. Chapmann & Hall; London, UK: 2006. Randomization, Bootstrap and Monte Carlo Methods in Biology. [Google Scholar]
- McClain M.E., Boyer E.W., Dent C.L., Gergel S.E., Grimm N.B., Groffman P.M., Hart S.C., Harvey J.W., Johnston C.A., Mayorga E., McDowell W.H., Pinay G. Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems. 2003;6:301–312. [Google Scholar]
- Morley N., Baggs E.M. Carbon and oxygen controls on N2O and N2 production during nitrate reduction. Soil Biol. Biochem. 2010;42:1864–1871. [Google Scholar]
- Mulholland P.J., Helton A.M., Poole G.C., Hall R.O., Hamilton S.K., Peterson B.J., Tank J.L., Ashkenas L.R., Cooper L.W., Dahm C.N., Dodds W.K., Findlay S.E.G., Gregory S.V., Grimm N.B., Johnson S.L., McDowell W.H., Meyer J.L., Valett H.M., Webster J.R., Arango C.P., Beaulieu J.J., Bernot M.J., Burgin A.J., Crenshaw C.L., Johnson L.T., Niederlehner B.R., O’Brien J.M., Potter J.D., Sheibley R.W., Sobota D.J., Thomas S.M. Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature. 2008;452:202–205. doi: 10.1038/nature06686. [DOI] [PubMed] [Google Scholar]
- Naiman R.J., Decamps H. The ecology of interfaces. Riparian zones. Annu. Rev. Ecol. Syst. 1997;28:621–658. [Google Scholar]
- Oksanen, J., Blanchet, F.G., Kindt, R., Legendre, P., O’Hara, R.B., Simpson, G.L. et al., 2010. vegan: Community Ecology Package. R package version 1. 17-2. http://CRAN.R-project.org/package=vegan.
- Orr C.H., Stanley E.H., Wilson K.A., Finlay J.C. Effects of restoration and reflooding on soil denitrification in a leveed midwestern floodplain. Ecol. Appl. 2007;17:2365–2376. doi: 10.1890/06-2113.1. [DOI] [PubMed] [Google Scholar]
- Pedroli B., de Blust G., van Looy K., van Rooij S. Setting targets in strategies for river restoration. Landscape Ecol. 2002;17:5–18. [Google Scholar]
- Pinay G., ClÉMent J.C., Naiman R.J. Basic principles and ecological consequences of changing water regimes on nitrogen cycling in fluvial systems. Environ. Manage. 2002;30:481–491. doi: 10.1007/s00267-002-2736-1. [DOI] [PubMed] [Google Scholar]
- Pinay G., Gumiero B., Tabacchi E., Gimenez O., Tabacchi-Planty A.M., Hefting M.M., Burt T.P., Black V.A., Nilsson C., Iordache V., Bureau F., Vought L., Petts G.E., Decamps H. Patterns of denitrification rates in European alluvial soils under various hydrological regimes. Freshwater Biol. 2007;52:252–266. [Google Scholar]
- Pinay G., O’Keefe T.C., Edwards R.T., Naiman R.J. Nitrate removal in the hyporheic zone of a salmon river in Alaska. River Res. Appl. 2009;25:367–375. [Google Scholar]
- Pinay G., Ruffinoni C., Fabre A. Nitrogen cycling in two riparian forest soils under different geomorphic conditions. Biogeochemistry. 1995;30:9–29. [Google Scholar]
- R-Development-Core-Team . R Foundation for Statistical Computing; Vienna, Austria: 2005. A Language and Environment for Statistical Computing.http://www.R-project.org [Google Scholar]
- Reckendorfer W., Hein T. Morphometrie, Hydrologie und Sedimentologie in der Unteren Lobau. Wissenschaftliche Reihe des Nationalpark Donau-Auen. 2006;4:1–46. http://www.donauauen.at/dateien/259_NPDA_04_2006_Reckendorfer_Sedimentologie_Untere_Lobau_.pdf2006. [Google Scholar]
- Ren T., Roy R., Knowles R. Production and consumption of nitric oxide by three methanotrophic bacteria. Appl. Environ. Microbiol. 2000;66:3891–3897. doi: 10.1128/aem.66.9.3891-3897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson W.S., Bartsch E., Monroe L., Cavanaugh E., Vingum J., Soballe L.D. Denitrification in the Upper Mississippi River: rates. Controls, and contribution to nitrate flux. Can. J. Fish Aquat. Sci. 2004;61:1102–1112. [Google Scholar]
- Roberts J., Ludwig J.A. Riparian vegetation along current-exposure gradients in floodplain wetlands of the River Murray, Australia. J. Ecol. 1991;79:117–127. [Google Scholar]
- Ruban V., López-Sánchez J.F., Pardo P., Rauret G., Muntau H., Quevauviller P. Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments – a synthesis of recent works. Anal. Bioanal. Chem. 2001;370:224–228. doi: 10.1007/s002160100753. [DOI] [PubMed] [Google Scholar]
- Salo J., Kalliola R., Hakkinen I., Makinen Y., Niemela P., Puhakka M., Coley P.D. River dynamics and the diversity of Amazon lowland forest. Nature. 1986;322:254–258. [Google Scholar]
- Schiemer F., Baumgartner C., Tockner K. Restoration of floodplain rivers: the Danube Restoration Project. Regulat. Rivers: Res. Manage. 1999;15:231–244. [Google Scholar]
- Seitzinger S., Kroeze C., Bouwman A., Caraco N., Dentener F., Styles R. Global patterns of dissolved inorganic and particulate nitrogen inputs to coastal systems. Recent conditions and future projections. Estuar. Coasts. 2002;25:640–655. [Google Scholar]
- Sjodin A.L., Lewis W.M., Saunders Iii J.F. Denitrification as a component of the nitrogen budget for a large plains river. Biogeochemistry. 1997;39:327–342. [Google Scholar]
- Smith M.S., Tiedje J.M. Phases of denitrification following oxygen depletion in soil. Soil Biol. Biochem. 1979;11:261–267. [Google Scholar]
- Spink A., Sparks R.E., Oorschot M.V., Verhoeven J.T.A. Nutrient dynamics of large river floodplains. Regulat. Rivers: Res. Manage. 1998;14:203–216. [Google Scholar]
- Steiger J., Gurnell A.M. Spatial hydrogeomorphological influences on sediment and nutrient deposition in riparian zones: observations from the Garonne River, France. Geomorphology. 2003;49:1–23. [Google Scholar]
- Teranes, J.L., Bernasconi, S.M. 2000. The record of nitrate utilization and productivity limited by (15N values in lake organic matter–A study of sediment trap and core sediments from Baldeggersee, Switzerland, 45, 801–813.
- Tockner K., Malard F., Ward J.V. An extension of the flood pulse concept. Hydrol. Process. 2000;14:2861–2883. [Google Scholar]
- Townsend-Small A., McClain M.E., Brandes J.A. Contributions of carbon and nitrogen from the Andes Mountains to the Amazon River. Evidence from an elevational gradient of soils, plants, and river material. Limnol. Oceanogr. 2005;50:672–685. [Google Scholar]
- Triska F.J., Duff J.H., Avanzino R.J. Patterns of hydrological exchange and nutrient transformation in the hyporheic zone of a gravel-bottom stream: examining terrestrial & aquatic linkages. Freshwater Biol. 1993;29:259–274. [Google Scholar]
- Tritthart M., Welti N., Bondar-Kunze E., Habersack H., Hein T. Modelling surface water connectivity for an improved understanding of carbon and nitrogen cycles in riparian zone. 7th ISE & 8th HIC Concepcion; Chile; 2009. [Google Scholar]
- Tritthart M., Welti N., Bondar-Kunze E., Pinay G., Hein T., Habersack H. Modelling highly variable environmental factors to assess potential microbial respiration in complex floodplain landscapes. Environ. Model. Softw. 2011;26:1097–1111. doi: 10.1016/j.envsoft.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Lee G.E.M., Venterink H.O., Asselman N.E.M. Nutrient retention in floodplains of the Rhine distributaries in the Netherlands. River Res. Appl. 2004;20:315–325. [Google Scholar]
- Verhoeven J.T.A., Arheimer B., Yin C.Q., Hefting M.M. Regional and global concerns over wetlands and water quality. Trends Ecol. Evol. 2006;21:96–103. doi: 10.1016/j.tree.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Udy J.W., Fellows C.S., Bartkow M.E., Bunn S.E., Clapcott J.E., Harch B.D. Measures of nutrient processes as indicators of stream ecosystem health. Hydrobiologia. 2006;572:89–102. [Google Scholar]
- Zeug S.C., Winemiller K.O. Evidence supporting the importance of terrestrial carbon in a large-river food web. Ecology. 2008;89:1733–1743. doi: 10.1890/07-1064.1. [DOI] [PubMed] [Google Scholar]