Abstract
Guiding the growth of a neurite by directing ~800 nm laser light to the leading edge of the neurite's growing region can be accomplished by controlling the position and direction in three dimensional space of a tapered optical fiber through which the light is projected. We control the position, angle and power of the laser beam to direct the growth of actin accumulations in neurites which affects their mobility.
OCIS codes: (170.0170) Medical optics and biotechnology; (060.2390) Fiber optics, infrared; (140.0140) Lasers and laser optics
1. Introduction
Much work has been done in directing neurite growth of cultured neurons with light projected from above or below the neurite. Instigating and regulating neurite growth with systems which either project the light through a microscope [1,2] (for example, laser from above or below), or use diffuse light from many directions [3].
It has been proposed previously that light can affect the growth of neural tissue [3–8]. It has been further proposed that guidance of neurite growth cones can be accomplished through the use of a micro-sized laser beam, where the width of the beam-spot is comparable to the width of a neurite. Experimenters have worked with various neuron and neuron-like cells to direct the outgrowth of neurites from the cell body and described conditions under which they have successfully and deterministically directed growth. Published experiments [2,7] have tended to follow this procedure: cells are cultured, the neurite to be controlled has already begun to naturally grow, ~800 nm laser light is directed (only) to the growing edge of the neurite at a fixed incident angle to the growth vector (usually perpendicular to a microscope stage), the light is directed to move as the neurite grows, with the growth of the neurite following the movement of the light. The phenomenon has been described as caused by the polymerization of actin [9–11] in the leading edge of the neurite’s growth cone.
We apply a laser beam though a tapered optical fiber positioned by a 3 axis micro-manipulator. The 3 axis micro-manipulator allows controlling the 3 dimensional position and angle of the laser beam. The experiment shows that guiding the growth of a neurite by directing ~800 nm laser light to the leading edge of a neurite’s growing region can be accomplished by controlling the position and direction in three dimensional space of a tapered optical fiber through which the light is projected. The laser illuminates cell neurites that are growing on a flat surface. The growth of neurites is affected by the radiation emission from the fiber taper as constrained by the surface that the neurite grows on and as affected by its initial (natural) growth vector. The growth of the neurite is affected by the light from the fiber taper, and can be made to grow in the direction of the fiber taper’s movement.
The experiment expands on two dimensional methods proposed by Anders, Higuchi, Ehrlicher, Wollman, and others [3–8].
2. Results
2.1. Summary
Of 50 actively growing illuminated neurites compared to a non-illuminated control set, all were affected by thickening agglomerations in the growth area indicating actin polymerization in the direction of illumination within 5 minutes when illuminated (according to the process described in Methods) with more than 4 mW/μm2 at 808 nm from a 1 μm fiber taper.
2.2. Trials
Experimental data was gathered in 10 trials with each trial comprising 10 cell culture dishes with a total of 6 different rat mothers [12] and two PC12 [13] cell sources. From each of 50 tested neurites, within about 3 minutes of illumination, agglomerations appeared in the growth cone. Of trials with both PC12 and rat pup cells all neurite growth cones which were observed for 10 minutes to be growing constantly at a speed of at least 0.1 μm were observed to have larger actin agglomerations in the neurite growth cone than a control set when subject to illumination from the fiber for at least 5 minutes.
Of 10 tests with randomly selected PC-12 and natural rat pup cells, 5 changed direction toward the illumination at least 45 degrees within 30 minutes after illumination, and 3 changed direction at least 90 degrees within 30 minutes (compared to a control set of 10 where none turned) as depicted in Figs. 1 and 2 .
Fig. 1.
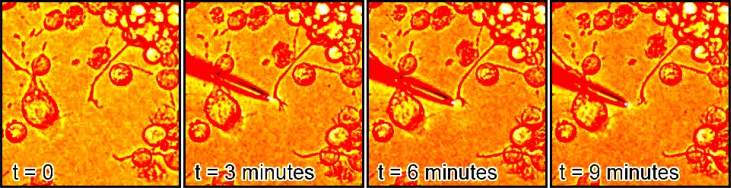
PC12 neurite making an almost 90 degree turn, image cropped.
Fig. 2.
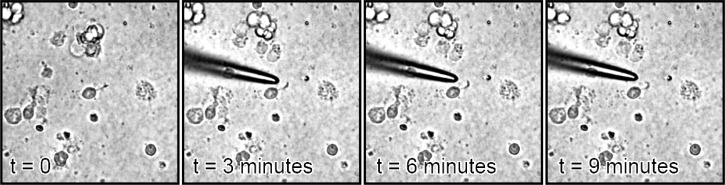
Rat neurite making a 180 degree turn, image contrast increased, cropped.
3. Discussion
3.1. Effecting neurite guidance
We observe that the formation of agglomerated areas of actin in the growth cone affects the mobility of the growth cone. The growth of neurites is guided by polymerized agglomerations of actin [9] which are formed by laser illumination from the fiber taper as illustrated in comparison to control neurites Fig. 3 . Causing and controlling these agglomerated areas of polymerized actin influences the growth direction of the growth cone, causing it to turn or bifurcate.
Fig. 3.
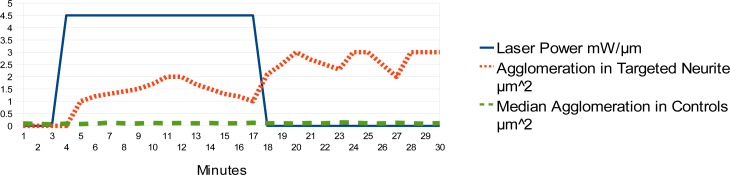
Tracking the surface area size of dark agglomerations of polymerized actin (tracked as pixel blobs) in the growth cone: their size is correlated with illumination from the fiber taper, compared with areas of polymerized actin in control neurites, median values.
3.2. Procedure and observations
In all cells tested, the edge of the neurite growth cone, where illuminated, was influenced to darken, thicken, and become immobile. This area is an accumulation of non-moving actin which has been polymerized [9,10] in the illuminated area. Within 30 to 200 seconds after illumination of a growing neurite with 808 nm light at higher than 4 mW per μm2, darkened and non-moving areas of collected actin formed in the growth cone of the neurite. These areas persisted as long as the illumination remained present. From long term observation [14] it can be observed that these areas are immobile, and hold the growth cone in place while other areas remain mobile. If it is not consistently illuminated with light, the collected actin will dissolve and the growing neurite will continue its growth motion.
Continuous repositioning of the taper is required to keep the growth in the ideal radiation band, which for our 1 μm fiber tip with 135 mW output requires a distance of 2 μm from tip to growth cone. Positioning was manual and positioning errors were corrected within a few seconds. Outside this band the neurite was not quickly directable or was too quickly thickened by the radiation. When illumination stronger than about 45 mW/μm2 covered the entire neurite growth area, neurite growth stopped within 10 minutes in every instance, though the radiation necessary to stop growth varied with each neurite. We conjecture that the growth stops due to thickening of the growth area so that new material from the cell is blocked from flowing into the growth area. When illuminated with below ~1 mW the neurite growth cone did not thicken in the direction nearest the light source within 5 minutes.
3.3. Flexible and omnidirectional neurite guidance
Holding the fiber taper laser in a micro-manipulator allows light to be directed at different angles and moved spatially relative to the cell. This freedom could possibly allow the direction of neurite growth in 3 dimensional space, and in constrained spaces as in vivo. Growth of neurites from cultured neurons optically directed in 3 dimensions as has been examined in previous work [2], which suggests that fiber based control could be extended into 3 dimensions. We propose that adding optics to the fiber or finely shaping its tip will greatly extend its usefulness.
4. Methods
4.1. Trials
Trials were performed over 15 months at different facilities in two cities. Trials were conducted over periods of 3 to 5 days, 1 to 2 trials per month. Each culture sample was removed from the incubator for about 60 minutes, and each individual neuron test lasted about 40 minutes. Each batch of cells was cultured about 1 month apart from winter to spring of 2011 and winter to summer of 2012. In total, more than 200 cell cultures were prepared from two PC12 sources [13], and six different rat mothers [12]. Feeding media was DMEM supplemented with high glucose 10% horse serum and 5% fetal calf serum, penicillin (100 U/mL) and streptomycin (100 μg/mL). The pH was not corrected; cell cultures were discarded after about 60 minutes. Ambient air temperature was 25 degrees Celsius or lower in winter to 35 degrees in summer. Cell cultures were tested at these room temperatures as our long term research goal is to produce cell cultures that function at room temperature, not at body temperature.
Neurites plated on flat plastic sample dishes were observed for about 10 minutes. Those which were selected for testing were those that appeared to be growing, not changing direction, and had an obvious current direction of growth of at least 0.1 μm/minute. An optical fiber taper was then positioned at an angle perpendicular to the growth direction and 45 degrees from vertical. 808 nm light from a laser was directed through the fiber, emitting from the taper end. The fiber taper was not allowed to physically contact any part of the cell or culture surface.
4.2. Measurement
Sequences of images were made for tracking of neurite growth edges. Pixel tracking was conducted from 2040x1536 pixel white visible light images.
Image processing with C++ and OpenCV (Intel Corporation) of time-sequence images recorded through the microscope was used to quantify the size of actin agglomerations. Images were recorded at 2040x1536 pixels, the image area near the growing neurite edge was defined as a region of interest and the rest of the image was discarded. On each image frame, edge detection was performed on the thresholded image to create closed, bounded entities (pixel blobs) from the pixels which were inside the upper and lower bounds of the greyscale value thresholds. The number of pixels inside the pixel blobs was counted. This number was compared to the measured size of the image to determine the surface areas.
Growth cone directional changes, were quantified by drawing a line from the base of the lamellipodium to the farthest edge of the growth cone and comparing lines drawn from subsequent images.
4.3. Fiber tapers
Tapers were created from 150 μm core multimode glass fibers with a divergence near 90 degrees. Best results were achieved with a fiber which tapered to 1 μm through a length of 1 cm, ending in a smooth, regular and un-chipped tip. Light loss at entry into the fiber is 15% + 1% loss from attenuation [15] in the fiber = 16%, 161 mW output from the laser is calculated to be about 135 mW exiting from the fiber taper. Output power was calibrated after the fiber against another known laser.
4.4. Beam characteristics
A 500 mW calibrated and temperature controlled LED laser emitting 808 nm light was directed through a two lens fiber coupler into our constructed fiber taper. The output of the fiber was calibrated to 135 mW output at the fiber tip by comparing photodiode measurements with another calibrated laser. We measured beam divergence in culture solution. The fiber was immersed in a cloudy culture solution and found to have a 90 degree divergence. The rapid drop-off of energy with distance makes the ideal region of influence a narrow band between 1 and 5 μm from the end of a taper with 90 degree divergence.
The energy and divergence of the beam can be approximately [16–18] modeled as a relationship where divergence div is 360°/90° = 4/1 = 4; r is distance to a 1 μm2 section of the target neurite. The model is diagrammed in Fig. 4 , showing v1, v3, v5, as increments of distance from the source of radiation at origin o0.
Fig. 4.
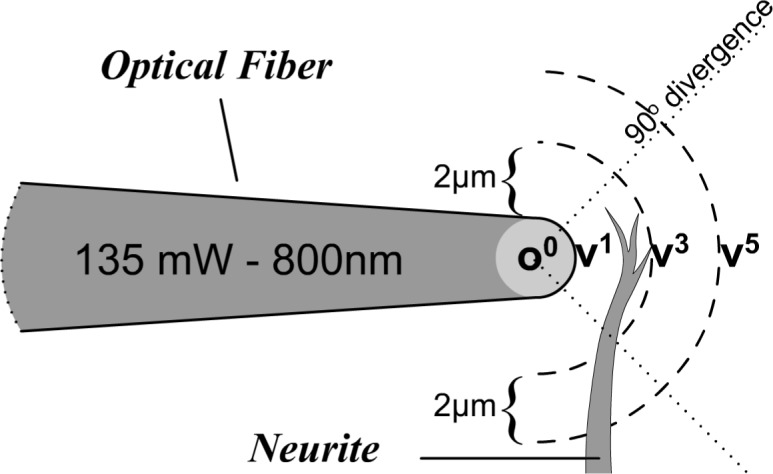
Through-the-microscope view (looking down), the diagram shows the energy of the illumination at points v1, v2, and v3. v1: r = 1 μm, 45 mW /μm2; v3: r = 3 μm, 4.8 mW/μm2; v5: r = 5 μm, 1.72 mW /μm2.
5. Conclusion
The experiment represents one approach among many currently existing methods (other promising tools might include micro-scale mirrors and light sources) which bring the origin of directionally controlled light very close to neurites to better deterministically direct naturally occurring neurite growth.
We propose that the results give evidence that micro-optical fiber tapers projecting light are useful in directing neurite growth. This could be significant because such micro-optical fibers are common with in vivo work. We have hope that in combination with microsurgery this form of neurite manipulation may be useful for restoring damaged nerves.
Acknowledgments
This work is supported by the National Natural Science Foundation of China (61273274, 60973061, 31271278, 31040044), Program for New Century Excellent Talents in University (NCET-10-0115) and General Administration of Sport of China (10B029), National Basic Research (973) Program of China (2011CB302203), and Ph.D. Programs Foundation of the Ministry of Education of China (20100009110004).
References and links
- 1.Koch D., Betz T., Ehrlicher A., Gogler M., Stuhrmann B., Kas J., “Optical control of neuronal growth,” Proc. SPIE 5514, 428–436 (2004). 10.1117/12.559672 [DOI] [Google Scholar]
- 2.Graves C. E., McAllister R. G., Rosoff W. J., Urbach J. S., “Optical neuronal guidance in three-dimensional matrices,” J. Neurosci. Methods 179(2), 278–283 (2009). 10.1016/j.jneumeth.2009.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higuchi A., Kitamura H., Shishimine K., Konishi S., Yoon B. O., Hara M., “Visible light is able to regulate neurite outgrowth,” J. Biomater. Sci. Polym. Ed. 14(12), 1377–1388 (2003). 10.1163/156856203322599716 [DOI] [PubMed] [Google Scholar]
- 4.Anders J. J., Borke R. C., Woolery S. K., Van de Merwe W. P., “Low power laser irradiation alters the rate of regeneration of the rat facial nerve,” Lasers Surg. Med. 13(1), 72–82 (1993). 10.1002/lsm.1900130113 [DOI] [PubMed] [Google Scholar]
- 5.Higuchi A., Watanabe T., Matsubara Y., Matsuoka Y., Hayashi S., “Regulation of neurite outgrowth by intermittent irradiation of visible light,” J. Phys. Chem. B 109(21), 11033–11036 (2005). 10.1021/jp0508554 [DOI] [PubMed] [Google Scholar]
- 6.Higuchi A., Watanabe T., Noguchi Y., Chang Y., Chen W. Y., Matsuoka Y., “Visible light regulates neurite outgrowth of nerve cells,” Cytotechnology 54(3), 181–188 (2007). 10.1007/s10616-007-9087-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehrlicher A., Betz T., Stuhrmann B., Koch D., Milner V., Raizen M. G., Kas J., “Guiding neuronal growth with light,” Proc. Natl. Acad. Sci. U.S.A. 99(25), 16024–16028 (2002). 10.1073/pnas.252631899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wollman Y., Rochkind S., Simantov R., “Low power laser irradiation enhances migration and neurite sprouting of cultured rat embryonal brain cells,” Neurol. Res. 18(5), 467–470 (1996). [DOI] [PubMed] [Google Scholar]
- 9.Betz T., Koch D., Lim D., Käs J. A., “Stochastic actin polymerization and steady retrograde flow determine growth cone advancement,” Biophys. J. 96(12), 5130–5138 (2009). 10.1016/j.bpj.2009.03.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ehrlicher A., Betz T., Stuhrmann B., Gögler M., Koch D., Franze K., Lu Y., Käs J., “Optical neuronal guidance,” Methods Cell Biol. 83, 495–520 (2007). 10.1016/S0091-679X(07)83021-4 [DOI] [PubMed] [Google Scholar]
- 11.Vasioukhin V., Bauer C., Yin M., Fuchs E., “Directed actin polymerization is the driving force for epithelial cell-cell adhesion,” Cell 100(2), 209–219 (2000). 10.1016/S0092-8674(00)81559-7 [DOI] [PubMed] [Google Scholar]
- 12.Chinese Academy of Medical Sciences, Institute for Experimental Animal Research
- 13.Peking Union Medical Institute Cell-Bank. Wang F. atPeking Union Medical Institute
- 14.F. Jesse, Z. J. Miao, L. Zhao, Y. Chen, and Y. Y. Lv, “OSA2012-11 image sequences and data,” http://jesse.org/OSA2012-11/
- 15.Corning Incorporated, “Corning® ClearCurve® Multimode Optical Fiber Product information,” http://www.corning.com/assets/0/433/573/583/9F39014A-1475-4F63-A9A5-946B2DD39D69.pdf
- 16.Ciddor P. E., “Refractive index of air: new equations for the visible and near infrared,” Appl. Opt. 35(9), 1566–1573 (1996). 10.1364/AO.35.001566 [DOI] [PubMed] [Google Scholar]
- 17.Schiebener P., Straub J., Levelt Sengers J. M. H., Gallagher J. S., “Refractive index of water and steam as function of wavelength, temperature and density,” J. Phys. Chem. Ref. Data 19(3), 677–715 (1990). 10.1063/1.555859 [DOI] [Google Scholar]
- 18.Kitamura R., Pilon L., Jonasz M., “Optical constants of silica glass from extreme ultraviolet to far infrared at near room temperature,” Appl. Opt. 46(33), 8118–8133 (2007). 10.1364/AO.46.008118 [DOI] [PubMed] [Google Scholar]


