Abstract
Background
Although nitroglycerin has remained in clinical use since 1879 the mechanism by which it relaxes blood vessels to lower blood pressure remains incompletely understood. Nitroglycerin undergoes metabolism generating several reaction products, including oxidants, and this ‘bioactivation’ process is essential for vasodilation. Protein kinase G (PKG) mediates classical nitric oxide-dependent vasorelaxation, but the 1α isoform is also independently activated by oxidation involving interprotein disulfide formation within this homodimeric protein complex. We hypothesised that nitroglycerin-induced vasodilation is mediated by disulfide activation of PKG1α.
Methods and Results
Treating smooth muscle cells or isolated blood vessels with nitroglycerin caused PKG1α disulfide dimerization. PKG1α disulfide formation was increased in wild-type mouse aortae by in vivo nitroglycerin treatment, but this oxidation was lost as tolerance developed. To establish whether kinase oxidation underlies nitroglycerin-induced vasodilation in vivo we employed a Cys42Ser PKG1α knock-in mouse that cannot transduce oxidant signals as it doesn’t contain the vital redox-sensing thiol. This ‘redox-dead’ knock-in mouse was substantively deficient in hypotensive response to nitroglycerin compared to wild-type littermates as measured in vivo using radiotelemetry. Resistance blood vessels from knock-ins were markedly less sensitive to nitroglycerin-induced vasodilation (EC50=39.2±10.7μM) than wild-types (EC50=12.1±2.9μM). Furthermore, after ~24 hours of treatment wild-type controls stopped vasodilating to nitroglycerin and the vascular sensitivity to nitroglycerin was decreased, whereas this ‘tolerance’ phenomenon that routinely hampers the management of hypertensive patients was absent in knock-ins.
Conclusions
PKG1α disulfide formation is a significant mediator of nitroglycerin-induced vasodilation and tolerance to nitroglycerin is associated with loss of kinase oxidation.
Keywords: nitroglycerin, blood pressure, oxidation, signal transduction
Nitroglycerin generally referred to as glyceryl trinitrate (GTN) clinically, remains a treatment for unstable angina and hypertension.1 However, chronic treatment of patients with GTN is substantially limited by the development of tolerance, a condition in which the vasodilatory and blood pressure action of GTN is lost or higher doses of the drug are required 1. GTN is metabolised by smooth muscle cells to yield a molecular form that mediates vasodilation. This ‘bioactivation’ process yields nitric oxide (NO), S-nitrosothiols, inorganic nitrite and glycerol-1,2-dinitrate 2, as well as reactive oxygen species (ROS).3 GTN is bioactivated principally by mitochondrial aldehyde dehydrogenase (mtALDH),2, 4 but also by the cytosolic isoform,5 and this metabolic conversion is essential for its vasodilatory actions. It has been commonly assumed that the NO generated is responsible for GTN-induced vasodilation. In this scenario NO would bind to and activate soluble guanylate cyclase (sGC) to stimulate cyclic guanosine monophosphate (cGMP) production, which activates cGMP-dependent protein kinase (PKG). PKG then phosphorylates a number of target proteins resulting in smooth muscle relaxation and vasodilation.1 However, recent studies have provided evidence that NO does not mediate the relaxation of vessels to GTN. For example GTN relaxes vessels without elevating cellular NO levels,6 suggesting the classical NO-cGMP-PKG was not in operation and that another mechanism or bioactivation product was responsible for the vasodilation.
We have previously shown that PKG1α can be activated wholly-independently of the classical NO-cGMP pathway by thiol oxidants such as hydrogen peroxide (H2O2),7 or the nitrosothiol nitrosocysteine.8 PKG1α is a parallel-aligned homodimer held together by the electrostatic attraction of its N-terminal leucine zipper. This dimerization domain also contains two thiols (from Cys42 on each of the chains) which align directly opposite one another.9 Oxidants induce an interprotein disulfide between the two cysteines and this activates the kinase by increasing its affinity for substrates that results in their phosphorylation. Indeed, this oxidative activation of PKG1α is a major molecular mechanism by which oxidants relax blood vessels ex vivo, 7, 10-12 and in vivo.10
A logical possibility is that nitrosothiols generated during bioactivation recruits oxidative disulfide activation of PKG1α and this is a major mechanism underlying GTN-induced vasodilation. This possibility is supported by GTN-inducing protein S-nitrosylation in vivo,13, 14. S-nitrosylated protein thiols will readily react with a proximal thiol to generate a disulfide, a process we showed indeed occurs in PKG1α when cells are exposed to nitrosothiols.8 Furthermore, GTN bioactivation also promotes ROS formation, providing another potentially synergistic mechanism that could also drive oxidative activation of PKG1α.3, 15 Indeed, GTN-induced oxidative stress results in oxidation of ALDH in which a disulfide bond forms in its active site cysteine. Oxidised ALDH accumulates because the reducing equivalents required for its cyclical reduction (i.e. reduced lipoic acid and NADPH) become depleted as they are consumed during GTN bioactivation. This oxidation inactivates ALDH preventing GTN bioactivation and contributes to tolerance.16-18 In the absence of GTN bioactivation, which occurs during tolerance,1, 16, 17 nitrosothiols and ROS are then not generated; an anticipated consequence of which is the failure of PKG1α to oxidatively activate and consequently for vasodilation not to occur.
In this study we test the hypothesis that GTN-dependent vasodilation is mediated by oxidative activation of PKG1α. Our studies have been significantly aided by the use of a Cys42Ser PKG-1α knock-in (KI) mouse in which wild-type (WT) kinase has been systemically replaced by a “redox-dead” form in which the cysteinyl thiol has been replaced by a hydroxyl group. This mutation prevents oxidative disulfide activation of PKG1α whilst maintaining classical NO-cGMP-dependent stimulation. We demonstrate using radio-telemetric monitoring of blood pressure in vivo that KI are deficient in their hypotensive response to GTN compared to WT. Furthermore comparison of the dose-dependent relaxation of isolated blood vessels showed KIs were intrinsically less sensitive to GTN than WTs. Unlike their WT littermates, KI mice also fail to become tolerant, albeit the interpretation of this observation is complicated by their deficient response to GTN basally.
Methods
Cys42Ser redox-dead PKGIα knock-in mice
All procedures were performed in accordance with the Home Office Guidance on the Operation of the Animals (Scientific Procedures) Act 1986 in UK. Mice constitutively expressing PKGIα Cys42Ser were generated for us on a pure C57BL/6 background by TaconicArtemis. A targeting vector was constructed, which involved PCR amplification of the murine Prkg1, introducing the Cys42Ser mutation into exon 1a (which is specific for the alpha isoform) by site directed mutagenesis and inserting an FRT-flanked neomycin selection marker (to allow for selection of transfected embryonic stem (ES) cells) close to the mutation to favour homologous recombination. Then screening by southern blot was carried out to identify if homologous recombination had occurred followed by validation of the positive clones. ES cell transfection was then carried out followed by chimera generation. The chimeras were directly bred with an Flp deletor for the in vivo deletion of the selection marker. As the ES cells always go germline, chimeras can be directly bred to the deletor in order to obtain germline transmission and selection marker deletion at the same time.
Cultured cells
Rat aortic smooth muscle cells (A10) were grown on 12-well plates in an incubator at 37°C with a 95% O2:5% CO2 environment. Once confluent, A10 cells were treated with or without 100μM GTN for 4 hours. For some samples additional treatments of 100μM of GTN were added either at 2, 3 and 4 hours.
Isolated vessels
Mice were killed by pentobarbital overdose and vessels were isolated and cleaned from surrounding tissues and fat in an ice-cold Krebs solution. Thoracic aorta vascular rings (5 mm) or third order mesenteric vessels were incubated with 3-300 μM of nitroglycerin (GTN, 1mg/ml from Hameln Pharmaceuticals Ltd; Gloucester, UK) at 37°C with a 95% O2:5% CO2 environment, frozen and homogenized in liquid nitrogen. NADPH fluorescence (340 nm excitation; 450 nm emission) was measured in isolated aortae before and after GTN (1 μM, 5 min) treatment using FLUOstar microplate-reader (BMG Labtech GmbH, Ortenberg, Germany)
Immunoblotting
Immunoblotting for PKG1α disulfide dimer was carried out as before 7, utilizing maleimide (100mM) in preparation buffers to alkylate thiols and prevent thiol disulfide exchange. Antibodies used in these studies included: cGKIa (E-17, Santa Cruz) or cGKIa (ADI-KAP-PK005, Enzo Life Science), phosphoSer239-VASP (16C2, Millipore) and GAPDH (sc-20357, Santa-Cruz Biotechnology, Inc). HRP-linked secondary antibody (Dako) and ECL reagent (GE Healthcare) were used. Digitized immunoblots were quantitatively analyzed using Gel-Pro Analyzer 3.1. The percentage of PKG1α disulfide dimer was quantified from a total PKG1α protein expression.
In vivo mouse studies
Mean arterial pressure (MAP) and heart rate were assessed by telemetry in conscious mice as described before 10. Briefly, mice were anesthetised with isofluorane and TA11PA-C10 probe catheter (Data Science International, St Pauls, MN) was implanted into the aortic arch via the left carotid artery. Following one week recovery, mice were placed above the telemetric receivers and MAP was recorded every 5min. GTN treatment (50 g/liter, UNIKEM, Switzerland) was achieved with subcutaneous. implantation of ALZET osmotic mini-pumps (200 μg/hour; 1 μl/hour for 3 days; Cupertino, CA). Solvent propylene glycol served as a vehicle control. In some experiments auranofin was administered (20mg/kg, intraperitoneally daily) for 2 days prior to implantation of GTN mini-pumps
Small vessels myography
Thoracic aorta vascular rings and third order mesenteric vessels were mounted for isometric tension recordings in a tension myograph (Danish Myo Technology), stretched to the optimal pre-tension condition with DMT normalisation module and bathed in Krebs solution at 37°C with a 95% O2:5% CO2 environment. Vasodilator responses to auranofin (1 nM-10 μM), GTN (1nM-300μM), atrial natriuretic peptide (ANP, 300 pM-1 μM), 8-Br-cGMP (1-300 μM) or S-Nitroso-N-Acetyl-D,L-Penicillamine (SNAP, 1nM-300 μM) were assessed with endothelium-intact isolated mouse vessels, determining the plateau responses of pre-contracted by α-adrenoreceptor agonist phenylephrine (EC80) or thromboxane mimetic compound U46619 (EC80) vessels to either of drugs. In some experiments, auranofin (3 μM) or ODQ (20 μM) were added to the bath for 30 min before pre-constriction and drug dose-response.
Statistical analysis
Results are presented as mean±SEM. Differences between groups were assessed using RM ANOVA followed by a Bonferroni t-test. Differences were considered significant at the 95% confidence level. Analyses were performed with GraphPad Prism5.
Results
Nitroglycerin Induces PKG1α Disulfide Dimerization In Vitro
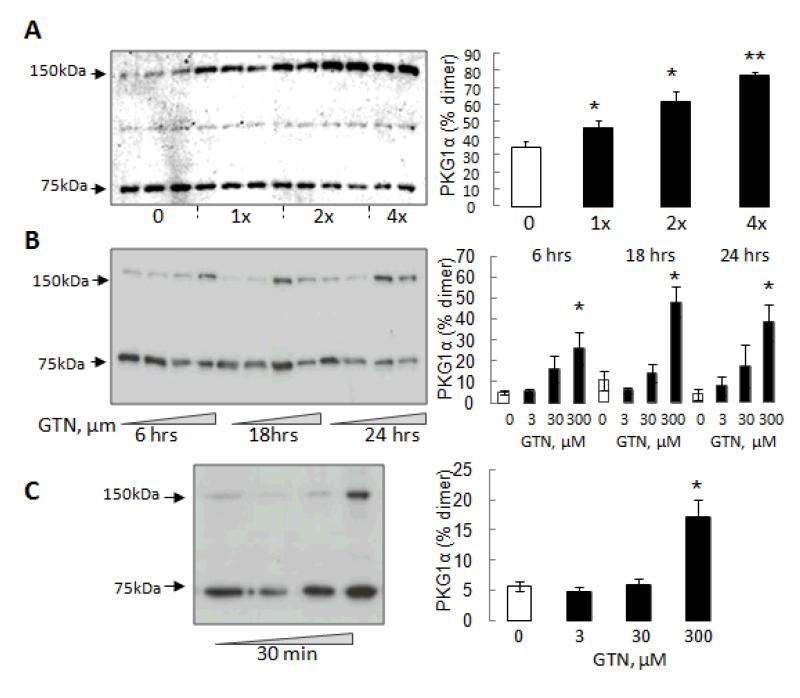
To evaluate the ability of GTN to induce PKG1α disulfide dimerization in vitro we used cultured smooth muscle cells, isolated mouse aortic rings and mesenteric vessels. A10 cells treated with 100μM GTN every hour for 4 hours showed a time-dependent increase in PKG1α disulfide dimerization (Fig. 1A). Mouse aortic rings then were exposed to increasing concentrations of GTN (3-300 μM) for 6, 18 or 24 hours which resulted in a dose-, but not time-, dependent increase in PKG1α disulfide dimer formation (Fig. 1B). Treatment of isolated mesenteric vessels with GTN (3-300 μM) resulted in PKG1α disulfide dimerization, albeit only at the highest concentration examined (Fig. 1C).
Figure 1.
GTN induces PKG1α disulfide dimerization in in vitro and ex vivo preparations. (A) Time-dependent increase in PKG1α disulfide dimer after A10 smooth muscle cells were treated with 100μM GTN every hour for 4 hours. (B) Aortic rings were treated with GTN (3-300 μM) for 6, 18 or 24 hours. PKG1α disulfide increased in a dose-dependent manner at each of the time points. (C) Mesenteric vessels were treated with GTN (3-300 μM) for 30 minutes; the highest concentration being effective in inducing PKG1α disulfide.
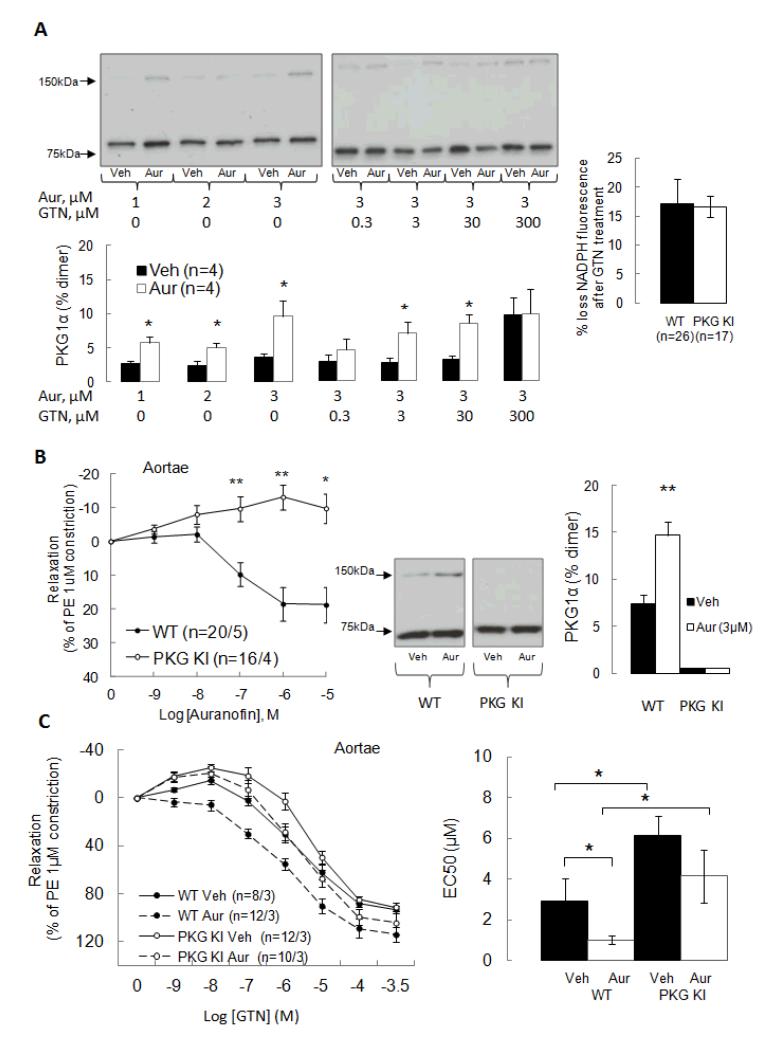
The GTN concentration required to induce a stable increase in disulfide PKG1α (i.e. above 100 μM) is not clinically relevant. However, redox cycling of oxidised PKG1α back to the reduced state is anticipated to occur and this would limit the accumulation of the disulfide product. To determine if disulfide PKG1α is indeed redox cycled we treated aortic rings with the thioredoxin reductase inhibitor auranofin (1-3 μM), which alone significantly increased oxidation of the kinase independently of GTN addition (Fig. 2A). Furthermore, when aortic rings were pre-incubated with auranofin (3 μM) it significantly potentiated GTN-induced disulfide formation (Fig. 2A). Consistent with disulfide PKG being redox recycled by the thioredoxin-thioredoxin reductase system, we observed loss of NADPH from aorta of both genotypes following GTN treatment (Fig. 2A).
Figure 2.
PKG1α disulfide dimerization increases with inhibition of thioredoxin-dependent redox-cycling using auranofin. (A) Aortic rings treated with auranofin alone (1-3μM) showed increased PKG1α disulfide dimer formation and this was potentiated by co-treatment with GTN. GTN-treatment of WT or KI aortae resulted in a loss of NADPH, consistent with it inducing oxidation of the kinase which is then recycled by the NADPH-consuming thioredoxin-thioredoxin reductase system. (B) Auranofin induced dose-dependent relaxation of aortic rings from WT, but not Cys42Ser PKG1α KI, mice. Western immunoblot analysis showed that auranofin treatment alone induces PKG disulfide formation in WT aortae, but failed to in KIs. (C) Auranofin sensitized WT, but not KI, aortae to GTN-induced relaxation.
This effect of auranofin was of particular note as it caused stable accumulation of disulfide PKG1α at much lower concentrations of GTN (i.e. 3 and 30 μM) than was achieved by GTN-alone which required 300 μM. These observations are again consistent with PKG1α oxidation occurring at low GTN concentrations but the reductive recycling limiting our ability to observe this on immunoblots. The implication is that GTN-induced vasodilation may occur through oxidative activation of PKG1α but the accumulation of substantive disulfide is prevented by reductive recycling. As PKG1α disulfide dimerization couples to vasodilation,7, 10-12 we reasoned that comparing the responses of WT and KI vessels would be illuminating regards the role of PKG1α oxidation. Indeed, there was a dose-dependent relaxation in WT aortic rings in response to auranofin (0.001 μM -10 μM) which was fully absent in PKG1α KI mice (Fig. 2B) consistent with the anticipated complete absence of disulfide dimerization basally or after auranofin (3 μM) treatment in aortic rings from KI mice (Fig. 2B). In addition, auranofin sensitized WT aortae to GTN-induced relaxation (EC50=0.9 ± 0.2 μM versus 2.9 ± 1.1 μM for vehicle-treated, Fig. 2C). In contrast auranofin failed to sensitize KI aortae to GTN-induced relaxation (EC50=4.2 ± 1.3 μM versus 6.1 ± 0.9 μM for vehicle-treated, Fig. 2C).
PKG1α Oxidation Occurs in Aortae after In Vivo Nitroglycerin Treatment
Next we tried to assess the role of PKG1α disulfide activation on blood pressure lowering by GTN in vivo; again the Cys42Ser redox-dead PKGIα KI mouse which cannot form a disulfide in response to oxidants were employed. When establishing a model of chronic GTN treatment we initially tested administering it by subcutaneous injections as used by others.15 We envisaged that three injections per day (every 8 hours) would replicate sustained treatment and generate the anticipated classical blood pressure profile of an initial hypotension that eventually is lost as the tolerance phenomenon ensues. However the first injection of GTN (either 50 or 100 μg) caused an immediate increase in mean arterial pressure (MAP) and not the hypotension expected (Fig. S1A). This hypertensive response was likely due to a stress-induced catecholamine surge and occurred in both WT and KI. Essentially the same profile was observed again in both genotypes after the second GTN injection; however the 50 μg dose caused a statistically significant (p<0.05) greater hypotension in the WT than the KI. This differential effect between genotypes was observed again after the third injection, and was now also clearly prominent in the group treated with 100 μg GTN. It may be that with repeated handling the stress response of the mice declines due to a training effect, allowing the vasodilatory actions of GTN to occur as well as the anticipated differential response between the two genotypes to become apparent. When high doses of GTN (200μg) were trialled it caused a pronounced hypotension which was again less marked in the KI compared to WT, which is consistent with our hypothesis kinase oxidation contributes to vasodilation. However, this high dose also caused a marked bradicardia which at least in part would over-drive the hypertensive stress-response to result in a net hypotension (Fig. S1A, B). Nevertheless, despite the complexities caused by the stress response during the subcutaneous injection procedure, there is clear evidence that the GTN-induced hypotension is attenuated in KI compared to WT littermate controls in conscious free moving mice (Fig. S1 C, D). In complementary studies we also compared the effect of inhibiting the sGC-cGMP-NO-pathway with ODQ on GTN-induced hypotension in both genotypes in vivo. ODQ prevented the peak MAP decrease after treatment with 100 μg GTN in KI mice but not in WT (Fig. S1 E). This differential genotype response further supported a role for PKG oxidation in GTN-dependent vasodilation, but the observations and conclusions are again limited by the repeated stress response associated with the injections.
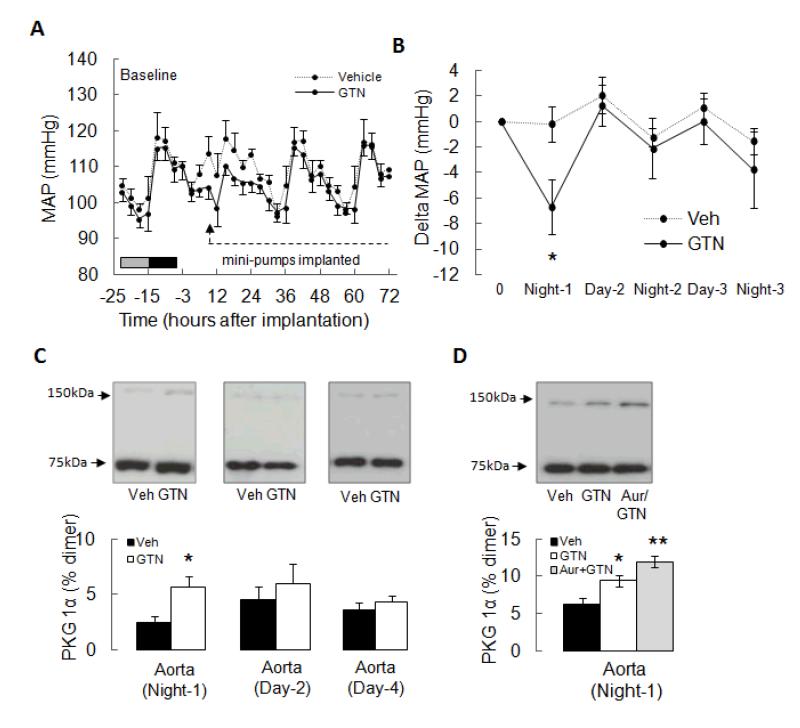
To overcome the limitations of administering GTN or vehicle by serial injections, we established a model employing an osmotic mini-pump to deliver the drug. Fig. 3A shows the MAP profile averaged every 3 hours for 24 hours before (baseline) and then 72 hours after GTN infusion was initiated. As mice are nocturnal they have higher blood pressure at night; it is therefore important to segregate the averaged day MAP from that at night. Another important consideration is the mild surgical pain causes a stress response on the day of mini-pump implantation which alone increases MAP. Thus implantation of mini-pumps delivering vehicle alone into C57BL/6 mice immediately increased their MAP and this was sustained for ~8 hours (Fig. 3A). MAP was also increased in the GTN group, but this was not as marked which is consistent with a counterbalancing vasodilatory action of the drug. To avoid confusion as a result of this stress response, which can be variable depending on the presence of GTN or vehicle, this initial period is omitted in subsequent graphs of the MAP profile. Fig. 3B is a further simplified trace, in which MAP has been 12-hour averaged and clearly demonstrates the anticipated hypotensive response in the GTN-treated group, but not the vehicle-treated controls. Furthermore, Fig. 3B also shows tolerance begins from the second day of continued GTN treatment. We assessed PKG1α disulfide formation in aorta at 12, 24 and 72 hours after vehicle control or GTN treatment. This analysis showed PKG1α disulfide dimerization was increased 2-fold after 12 hours of GTN-treatment, the time when the hypotensive response was maximal (Fig. 3C) and this was potentiated by co-treatment in vivo with auranofin (Fig. 3D). However, after 24 or 72 hours of continued GTN-treatment the disulfide PKG1α levels returned to basal and were no different than the levels in vehicle controls which did not promote kinase oxidation beyond basal at any time point.
Figure 3.
GTN lowers mean arterial pressure (MAP) and induces PKG1α disulfide dimerization in vivo in C57BL/6 mice. (A) GTN administered by osmotic mini-pumps shows a biphasic effect on MAP as measured by radiotelemetry.GTN initially lowered MAP before ‘tolerance’ ensued and the blood pressure returned to basal. (B) Comparison of the 12 hour-averaged delta change in MAP when C57BL/6 mice were administered GTN or vehicle. Presentation of the data in this simplified form clarifies the complex dataset shown in panel A, clearly showing GTN initially lowers MAP before returning to basal after 24 hours of continuous drug treatment (i.e. because tolerance develops). (C) PKG1α disulfide dimerization increased in aorta from C57BL/6 mice after 12 hours of GTN, but not vehicle, treatment. However after 24 or 72 hours of GTN-treatment, when tolerance was clearly present, PKG1α disulfide dimerization returned to basal. (D) GTN-treatment in vivo induced disulfide formation in aortae, and this was potentiated by co-treatment with auranofin.
Nitroglycerin Fails to Lower Blood Pressure in Redox-Dead Cys42Ser PKG1α Knock-in Mouse
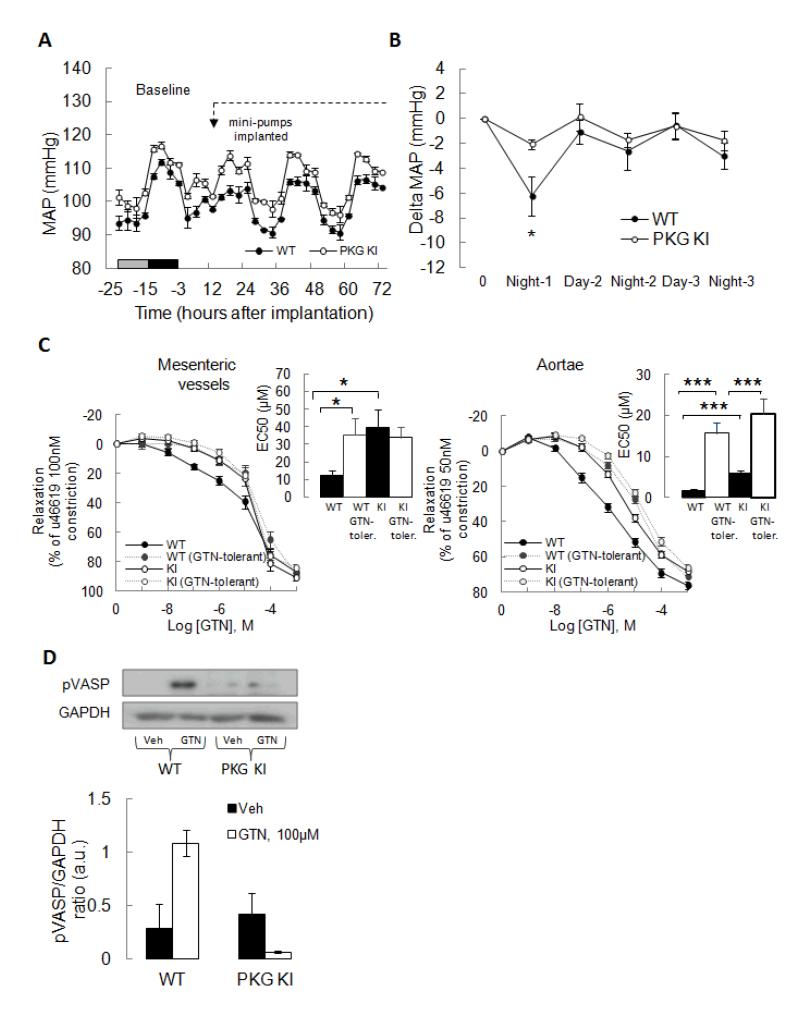
The GTN mini-pump delivery model was used again to compare the responses of the WT and KI mice. There was a clear and significant (p<0.05) hypotension in WT mice (Fig. 4A) and this was comparable to that observed with standard C57BL/6 mice described above (Fig. 3A-B). In contrast, the KI mice had a much attenuated response to GTN compared to WT littermates, with only a marginal decline in blood pressure after treatment (Figure 4B). Moreover, WT mice showed the tolerance profile expected and observed in standard C57BL/6 mice but this was fully abrogated in the KI. Albeit it is perhaps illogical to conclude tolerance was absent in KI when they did not initially vasodilate to the GTN intervention.
Figure 4.
KI mice are deficient in GTN-induced blood pressure-lowering compared to WT as measured in vivo by radiotelemetry. (A) The mean arterial pressure (MAP) of WT declined during GTN treatment by osmotic minipump whereas the KI failed to. (B) Comparison of the 12 hour-averaged delta change in MAP in both genotypes showed the WT, but not the KI, responded to GTN with a decline in MAP. However, after ~24 hours of GTN treatment the WT became tolerant and the MAP returned to basal to match the KI. (C) Dose-dependent relaxation of WT or KI mesenteries or aortae to GTN. Vessels were isolated from vehicle-treated control (i.e. non-tolerant) or GTN-tolerant mice from each genotype. Isolated mesenteric resistance blood vessels from KI were markedly less sensitive to GTN-induced vasodilation than WT. Mesenteric vessels from ‘tolerant’ WT mice were less sensitive to GTN (essentially matched the KI responsiveness). The responsiveness of KI mesenteries were not altered from basal by the tolerance protocol. The aortae of KI mice were also basally less responsive to GTN compared to WT. The tolerance protocol decreased the sensitivity of WT aortae to GTN, but this tolerance also occurred in KI. (D) GTN-treatment induced VASP phosphorylation in WT, but not KI, aortae.
In a separate cohort of WT and KI mice mini-pumps were used to compare the responses to GTN with a vehicle control over a 48 hour period. Once again we found vehicle infusion did not modulate MAP and that WT mice were the only genotype to show a hypotensive response to GTN before becoming tolerant. To establish if the differential responses of WT and KI mice to GTN were a result of intrinsic properties of their blood vessels, both mesenteries and aortae were isolated and analysed by myography. These measurements were made in vessels from both genotypes after 48 hours of vehicle or drug treatment, in which only WT mice administered GTN became tolerant. A GTN dose-response curve was constructed in U-46619 pre-constricted vessels and compared between genotypes treated with vehicle or GTN for 48 hours. Isolated mesenteric resistance blood vessels from KI were markedly less sensitive to GTN-induced vasodilation (EC50=39.2±10.7 μM) than WT (EC50=12.1±2.9 μM). Mesenteric vessels from ‘tolerant’ WT mice were less sensitive to GTN (EC50=35.2±9.4 μM), such that they essentially matched the KI responsiveness. The responsiveness of KI vessels were not altered from basal by the tolerance protocol (Fig. 4C). The aortae of KI mice were also basally less responsive to GTN (EC50=5.9±0.7μM) than the WT (EC50=1.6±0.4 μM) (Fig. 4C). The tolerance protocol decreased the sensitivity of WT aortae to GTN (EC50=15.6±2.6 μM), but in contrast to the observations with mesenteries, the KI vessel (EC50=20.3±3.8 μM) also clearly developed tolerance. We also compared the responses of aortae and mesenteric vessels to the PKG activators 8-Br-cGMP and ANP, observing no differences between WT and KIs (Fig. S2A, B). In addition, there was no difference in SNAP-induced vasorelaxation in mesenteric vessels of either genotype (Fig. S2C), and the relaxation induced by this NO-donor was completely blocked by ODQ. In contrast, ODQ did not fully prevent GTN-induced relaxation of wild-type vessel (Fig. S2C). Furthermore, ODQ-blockade of GTN-induced relaxation was more marked in knock-in compared to wild-type, which is consistent with the knock-in being unable to recruit vasodilation via PKG disulfide formation. Western immunoblot analysis (Figure 4D) showed that GTN induced VASP phosphorylation in WT vessels, whereas this did not occur in KI undergoing the same treatment.
Discussion
This study reports the novel finding that the vasodilatory and blood pressure lowering effect of GTN is significantly mediated by oxidation of PKG1α. PKG1α oxidation involves interprotein disulfide bond formation which directly enables kinase catalytic activity independently of NO-induced cGMP elevation.7 This oxidative activation of PKG1α has been shown to be a major mechanism by which isolated blood vessels relax ex vivo to thiol-oxidants such as H2O2 or nitrosocysteine,7, 8, 10, 12 and also operates in human blood vessels.11 Furthermore, this mechanism has been shown to contribute in vivo, as KI mice that only express Cys42Ser mutant PKG1α that cannot be activated by oxidants are hypertensive compared to WT littermate controls.10
For there to be a differential effect on the two genotypes, GTN would logically have to induce an oxidative stress, which thereafter is sensed and transduced to vasodilation in the WT but not the KI in which a disulfide cannot form, so preventing activation by oxidants. Although GTN-induced vasodilation is often attributed to an NO donor function it is now well established there is significant additional complexity. For example GTN-induced vasodilation occurs without NO increasing,6 suggesting the classical pathway of activating PKG is not of primary importance. The vasodilatory actions of GTN require it to be metabolised, generally referred to as bioactivation. Although several enzymes may be capable of bioactivating GTN, ALDH is widely considered the predominant enzyme responsible.2, 4, 5 Bioactivation generates several end products including nitrosothiols which are capable of inducing protein disulfide formation. Previously we found that nitrocysteine can induce PKG1α oxidation 8, which logically would occurs via transient formation of an S-nitrosylation intermediate of one of the Cys42 thiols before reduction by the other Cys42 on the opposite chain in the kinase homodimer to yield a disulfide. The likelihood of such a mechanism is supported by studies showing GTN induces protein S-nitrosylation in vivo.13, 14 However, as S-nitrosylated PKG1α would be extremely short lived due to rapid reduction, demonstrating this intermediate occurs in vivo may be practically impossible using available technology such as gas phase chemiluminescence or the ascorbate-dependent biotin-switch method.19 Overall it is clear that there is a rationale molecular basis for hypothesising that GTN would induce oxidative activation of PKG1α. Furthermore we indeed observed the anticipated increased in kinase oxidation in cells, tissues and animals exposed to GTN. Although our observations strongly support a role for oxidative activation of PKG in GTN-dependent vasodilation, this conclusion is at odds with reports that relaxation by this nitrate is efficiently blocked by ODQ.20, 21 We have two potential explanations that may reconcile these seemingly opposing observations. Firstly, it is conceivable that as the concentration of GTN is elevated that it increasingly signals via sGC, becoming gradually more sensitive to ODQ. Although, Kleschyov et al found that GTN relaxed aortae without elevating cellular NO levels,6 as the nitrate concentration was increased NO became measurable. Secondly, although resistance vessels are the primary determinant of blood pressure, most studies investigate aortae. Here we assessed mesenteries and found WT and KI relax identically to SNAP and that this is fully abrogated by blockade of sGC with ODQ that in both genotypes. In notable contrast, ODQ did not fully abrogate the GTN-relaxation of wild-type mesenteries, consistent with it being able to relax by the kinase oxidation mechanism. Furthermore, ODQ-blockade of GTN-induced relaxation was more marked in knock-in compared to wild-type, which is anticipated as the knock-in cannot recruit vasodilation via PKG disulfide.
In addition to nitrosothiols there may be additional mechanisms that contribute to the oxidative activation of PKG1α, consistent with GTN-treatment being historically associated with oxidative stress.16 Indeed, thiol-based antioxidants such as N-acetylcysteine (NAC) have been suggested as a therapy to limit oxidative damage and perhaps also prevent GTN tolerance. However, as NAC might be expected to react with (and so scavenge) nitrosothiols or other oxidants generated during GTN bioactivation, we might anticipate such an intervention may actually be ineffective and limit GTN-induced blood pressure lowering due by attenuating PKG1α oxidation. Indeed, clinical trials have shown that NAC is ineffective at limiting GTN tolerance,22 as might be predicted from our new mechanistic understanding. Whilst it is evident that GTN can directly activate oxidase enzymes to induce oxidative stress, for example by uncoupling endothelial nitric oxide synthase to yield superoxide,3, 15 this can also occur indirectly as consequence of the bioactivation process. During bioactivation ALDH forms an oxidised disulfide product that is catalytically dead and cannot metabolise another GTN molecule. However, a reduced lipoic acid molecule thiol-disulfide exchanges with ALDH to regenerate the dehydrogenase so it can bioactivate another GTN molecule. This redox cycling reaction yields oxidised (disulfide) lipoic acid which may potentially react with other thiols to induce S-thiolation oxidation products. Oxidised lipoic acid is also reduced back to its thiol state by an NADPH-dependent reductase enzyme.16-18 Depletion of NADPH during chronic GTN-treatment would therefore be expected to broadly compromise cellular reducing systems. This indirectly induces an oxidative stress that may contribute to the accumulation of disulfide PKG1α and contribute to the phenotypic difference observed between WT and KI.
One enzyme that utilises substantive amounts of NADPH reducing equivalents is thioredoxin reductase, which reduces oxidised thioredoxin back to the reduced state. Reduced thioredoxin (Trx) generically reduces disulfide-oxidised proteins back to their reduced state,23 but can also de-nitrosylate proteins.24 Indeed, Trx was considered the prime candidate responsible for converting reducing disulfide PKG1α to basal, serving as an ‘off-switch’ to provide regulation of oxidant-induced kinase activity. To establish if Trx reduces oxidised PKG1α we undertook studies with the Trx reductase inhibitor auranofin, which alone was sufficient to induce disulfide kinase in aortic rings. However, auranofin induced oxidation of a relatively small proportion of total PKG, and it was important to consider if such as stoichiometry can be functionally significant. As PKG1α disulfide dimerization couples to vasodilation 7, 10-12, we reasoned that comparing the responses of WT and KI vessels would be illuminating regards the functional impact of the limited PKG1α oxidation induced by auranofin. These studies confirmed that although auranofin only maximally induced 5-10 % of total PKG1α to form disulfide that there was a prominent differential effect between genotypes, such that the WT relaxed in a dose-dependent manner and the KI did not relax at all. That only a small proportion of PKG1α had to be oxidatively activated for vasodilation to occur is consistent with our previous observation that an endothelium derived hyperpolarizing factor (EDHF) protocol that efficiently induced vasodilation only increased disulfide PKG from ~4 % to ~9-10 %. This essentially matches the increase observed with 3 μM auranofin that, clearly demonstrating only a small amount of disulfide-activated kinase is required to induce vasodilation. It is difficult to know how disulfide activation compares to the classical cGMP-activation in terms of the stoichiometry of activation required to achieve a comparable vasodilation. However, as oxidative PKG activation involves the lowering of the enzymes affinity for substrate, whereas cGMP increases the Vmax; 7 it is conceivable that disulfide formation requires a smaller proportion of the total kinase pool to be activated compared to the classical pathway to achieve vasodilation. It is noteworthy that gold-containing compounds such as auranofin,25 or sodium aurothiomalate,26 which are used to treat rheumatoid arthritis, include vasomotor-like reactions characterized by facial flushing and occasionally hypotension in so called nitritoid reactions.
Clearly EDHF and auranofin couple to vasodilation despite only inducing ~9-10 % PKG1α oxidation. Thus although GTN at the time of maximal hypotension only induced ~6 % PKG1α oxidation in vivo this would also be adequate to account for the hypotensive response, as auranofin doses that cause comparable oxidation are adequate to relax WT but KI blood vessels ex vivo. It is also notable that basally WT mice have ~2-3 % disulfide PKG1α, whereas KI have 0 %, and this alone results in a MAP difference of ~10-15 mmHg 10. In addition, the ~6% PKG1α oxidation measured in vivo after GTN-treatment may be an underestimate due to redox recycling back to the reduced state. When increasing amounts GTN is applied or longer treatment durations are used it may be intuitive to expect proportionately more PKG1α to accumulate. However, as the rate of reduction can match or indeed be faster than the oxidation step, then minimal accumulation can occur despite significant flux through the disulfide-activated state. Increasing the blood vessel PKG1α disulfide content to ~6% was associated with 7 mmHg drop in MAP. We should be mindful that GTN was administered to healthy mice with ‘normal’ blood pressure. Clinically GTN would be given to hypertensive patients; should we have treated hypertensive mice then a much greater drop in MAP would likely been observed, but this would have added significant complexity.
To our knowledge, this is the first report showing that PKG1α oxidation significantly mediates GTN-induced vasodilation and blood pressure lowering. Our conclusions are robustly supported by studies with a redox-dead Cys42Ser KI mouse that essentially fails to become hypotensive in response to GTN-treatment. The response to GTN in vivo was studied using radiotelemetry, which despite now being the gold-standard for blood pressure measurements in mice, has been scarcely utilised in the study of tolerance 15. This technology overcomes many technical problems associated with other approaches, providing a robust and continuous readout of blood pressure in a model that replicates the clinical use of the drug and also clearly shows the tolerance phenomenon. Complementary studies comparing the vasorelaxation of vessels from the KI mice further corroborated our conclusions as they were significantly less responsive to GTN than WT preparations. Our observations are consistent with GTN lowering blood pressure by stimulating the same pathway as the endogenous vasodilating EDHF stimuli, which we previously showed operates significantly via PKG1α oxidation. Indeed, preparations taken from tolerant animals are deficient in the response to GTN- as well as an EDHF protocol.27 Our studies also provide some mechanistic insight to the tolerance phenomenon, but our conclusions are limited by the fact that KI do not respond to GTN. GTN initially caused PKG1α oxidation (at 12 hours) and also lowered MAP in WT mice. As the loss of GTN-induced MAP lowering was associated with a loss of PKG1α oxidation, we would suggest that tolerance occurs because of a failure to bioactivate GTN. Indeed, tolerance is associated with a loss of the bioactivation pathway.4, 16, 17 Loss of bioactivation would prevent the formation of oxidizing-species such as nitrosothiols and prevent disulfide activation of PKG1α, resulting in a failure of GTN to lower MAP (i.e. tolerance) which is what we observe.
Supplementary Material
Clinical Perspective.
Nitroglycerin, commonly referred to as glyceryl trinitrate (GTN), remains a treatment for unstable angina and hypertension after more than 130 years of clinical use. Despite this, the mechanism of action still is not fully understood. Here we present evidence that GTN induces the oxidation of protein kinase G (PKG) 1α such that it forms an interprotein disulfide bond, directly activating the kinase which couples to blood vessel dilation. This occurs independently of the classical mode of stimulating PKG in which nitric oxide stimulates the production of cGMP, which then binds to PKG to activate it. So although GTN is an organic nitrate, its mode of kinase activation at therapeutic concentrations is principally through oxidative activation and not cGMP binding. Prolonged (>24 hours) treatment with GTN commonly results in the well-recognised ‘tolerance’ phenomenon in which the vasodilatory and anti-anginal effects of GTN are lost, so hampering its clinical use. Our studies show tolerance to nitroglycerin is associated with loss of PKG oxidation. If pharmacological interventions can be identified that maintain GTN-induced PKG oxidation during chronic use, potentially this may counteract the development of tolerance.
Acknowledgments
Sources of Funding We would like to acknowledge support from the Medical Research Council, the British Heart Foundation, the Leducq Foundation and the Department of Health via the NIHR cBRC award to Guy’s & St Thomas’ NHS Foundation Trust.
Footnotes
Disclosures None
References
- 1.Munzel T, Daiber A, Mulsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97(7):618–628. doi: 10.1161/01.RES.0000184694.03262.6d. [DOI] [PubMed] [Google Scholar]
- 2.Chen Z, Stamler JS. Bioactivation of nitroglycerin by the mitochondrial aldehyde dehydrogenase. Trends Cardiovasc Med. 2006;16(8):259–265. doi: 10.1016/j.tcm.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Khong S, Andrews K, Huynh N, Venardos K, Aprico A, Michell D, Zarei M, Moe K, Dusting G, Kaye D, Chin-Dusting J. Arginase II inhibition prevents nitrate tolerance. Br J Pharmacol. 2012 doi: 10.1111/j.1476-5381.2012.01876.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, Foster MW, Zhang J, Mao L, Rockman HA, Kawamoto T, Kitagawa K, Nakayama KI, Hess DT, Stamler JS. An essential role for mitochondrial aldehyde dehydrogenase in nitroglycerin bioactivation. Proc Natl Acad Sci U S A. 2005;102(34):12159–12164. doi: 10.1073/pnas.0503723102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beretta M, Wolkart G, Schernthaner M, Griesberger M, Neubauer R, Schmidt K, Sacherer M, Heinzel FR, Kohlwein SD, Mayer B. Vascular bioactivation of nitroglycerin is catalyzed by cytosolic aldehyde dehydrogenase-2. Circ Res. 2012;110(3):385–393. doi: 10.1161/CIRCRESAHA.111.245837. [DOI] [PubMed] [Google Scholar]
- 6.Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, Schulz E, Sydow K, Fichtlscherer B, Mulsch A, Munzel T. Does nitric oxide mediate the vasodilator activity of nitroglycerin? Circ Res. 2003;93(9):e104–112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- 7.Burgoyne JR, Madhani M, Cuello F, Charles RL, Brennan JP, Schroder E, Browning DD, Eaton P. Cysteine redox sensor in PKGIa enables oxidant-induced activation. Science. 2007;317(5843):1393–1397. doi: 10.1126/science.1144318. [DOI] [PubMed] [Google Scholar]
- 8.Burgoyne JR, Eaton P. Transnitrosylating nitric oxide species directly activate type I protein kinase A, providing a novel adenylate cyclase-independent cross-talk to beta-adrenergic-like signaling. J Biol Chem. 2009;284(43):29260–29268. doi: 10.1074/jbc.M109.046722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma AK, Zhou GP, Kupferman J, Surks HK, Christensen EN, Chou JJ, Mendelsohn ME, Rigby AC. Probing the interaction between the coiled coil leucine zipper of cGMP-dependent protein kinase Ialpha and the C terminus of the myosin binding subunit of the myosin light chain phosphatase. J Biol Chem. 2008;283(47):32860–32869. doi: 10.1074/jbc.M804916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prysyazhna O, Rudyk O, Eaton P. Single atom substitution in mouse protein kinase G eliminates oxidant sensing to cause hypertension. Nat Med. 2012 doi: 10.1038/nm.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang DX, Borbouse L, Gebremedhin D, Mendoza SA, Zinkevich NS, Li R, Gutterman DD. H2O2-Induced Dilation in Human Coronary Arterioles: Role of Protein Kinase G Dimerization and Large-Conductance Ca2+-Activated K+ Channel Activation. Circ Res. 2012;110(3):471–480. doi: 10.1161/CIRCRESAHA.111.258871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Neo BH, Kandhi S, Wolin MS. Roles for soluble guanylate cyclase and a thiol oxidation-elicited subunit dimerization of protein kinase G in pulmonary artery relaxation to hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2010;299(4):H1235–1241. doi: 10.1152/ajpheart.00513.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ignarro LJ, Lippton H, Edwards JC, Baricos WH, Hyman AL, Kadowitz PJ, Gruetter CA. Mechanism of vascular smooth muscle relaxation by organic nitrates, nitrites, nitroprusside and nitric oxide: evidence for the involvement of S-nitrosothiols as active intermediates. J Pharmacol Exp Ther. 1981;218(3):739–749. [PubMed] [Google Scholar]
- 14.Sayed N, Kim DD, Fioramonti X, Iwahashi T, Duran WN, Beuve A. Nitroglycerin-induced S-nitrosylation and desensitization of soluble guanylyl cyclase contribute to nitrate tolerance. Circ Res. 2008;103(6):606–614. doi: 10.1161/CIRCRESAHA.108.175133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knorr M, Hausding M, Kroller-Schuhmacher S, Steven S, Oelze M, Heeren T, Scholz A, Gori T, Wenzel P, Schulz E, Daiber A, Munzel T. Nitroglycerin-induced endothelial dysfunction and tolerance involve adverse phosphorylation and S-Glutathionylation of endothelial nitric oxide synthase: beneficial effects of therapy with the AT1 receptor blocker telmisartan. Arterioscler Thromb Vasc Biol. 2011;31(10):2223–2231. doi: 10.1161/ATVBAHA.111.232058. [DOI] [PubMed] [Google Scholar]
- 16.Wenzel P, Hink U, Oelze M, Schuppan S, Schaeuble K, Schildknecht S, Ho KK, Weiner H, Bachschmid M, Munzel T, Daiber A. Role of reduced lipoic acid in the redox regulation of mitochondrial aldehyde dehydrogenase (ALDH-2) activity. Implications for mitochondrial oxidative stress and nitrate tolerance. J Biol Chem. 2007;282(1):792–799. doi: 10.1074/jbc.M606477200. [DOI] [PubMed] [Google Scholar]
- 17.Hink U, Daiber A, Kayhan N, Trischler J, Kraatz C, Oelze M, Mollnau H, Wenzel P, Vahl CF, Ho KK, Weiner H, Munzel T. Oxidative inhibition of the mitochondrial aldehyde dehydrogenase promotes nitroglycerin tolerance in human blood vessels. J Am Coll Cardiol. 2007;50(23):2226–2232. doi: 10.1016/j.jacc.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 18.Sydow K, Daiber A, Oelze M, Chen Z, August M, Wendt M, Ullrich V, Mulsch A, Schulz E, Keaney JF, Jr., Stamler JS, Munzel T. Central role of mitochondrial aldehyde dehydrogenase and reactive oxygen species in nitroglycerin tolerance and cross-tolerance. J Clin Invest. 2004;113(3):482–489. doi: 10.1172/JCI19267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgoyne JR, Eaton P. Contemporary techniques for detecting and identifying proteins susceptible to reversible thiol oxidation. Biochem Soc Trans. 2011;39(5):1260–1267. doi: 10.1042/BST0391260. [DOI] [PubMed] [Google Scholar]
- 20.Irvine JC, Favaloro JL, Widdop RE, Kemp-Harper BK. Nitroxyl anion donor, Angeli’s salt, does not develop tolerance in rat isolated aortae. Hypertension. 2007;49(4):885–892. doi: 10.1161/01.HYP.0000259328.04159.90. [DOI] [PubMed] [Google Scholar]
- 21.van der Zypp A, Majewski H. Effect of cGMP inhibitors on the actions of nitrodilators in rat aorta. Clin Exp Pharmacol Physiol. 1998;25(1):38–43. doi: 10.1111/j.1440-1681.1998.tb02141.x. [DOI] [PubMed] [Google Scholar]
- 22.Hogan JC, Lewis MJ, Henderson AH. N-acetylcysteine fails to attenuate haemodynamic tolerance to glyceryl trinitrate in healthy volunteers. Br J Clin Pharmacol. 1989;28(4):421–426. doi: 10.1111/j.1365-2125.1989.tb03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holmgren A, Lu J. Thioredoxin and thioredoxin reductase: current research with special reference to human disease. Biochem Biophys Res Commun. 2010;396(1):120–124. doi: 10.1016/j.bbrc.2010.03.083. [DOI] [PubMed] [Google Scholar]
- 24.Benhar M, Forrester MT, Hess DT, Stamler JS. Regulated protein denitrosylation by cytosolic and mitochondrial thioredoxins. Science. 2008;320(5879):1050–1054. doi: 10.1126/science.1158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Proudman S, Cleland LG. Auranofin-induced vasomotor reactions. Arthrithis and Rheumatism. 1992;35:1452–1454. doi: 10.1002/art.1780351208. [DOI] [PubMed] [Google Scholar]
- 26.Ho M, Pullar T. Vasomotor reactions with gold. Br J Rheumatol. 1997;36(2):154–156. doi: 10.1093/rheumatology/36.2.154. [DOI] [PubMed] [Google Scholar]
- 27.Kusama N, Kajikuri J, Yamamoto T, Watanabe Y, Suzuki Y, Katsuya H, Itoh T. Reduced hyperpolarization in endothelial cells of rabbit aortic valve following chronic nitroglycerine administration. Br J Pharmacol. 2005;146(4):487–497. doi: 10.1038/sj.bjp.0706363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.