Abstract
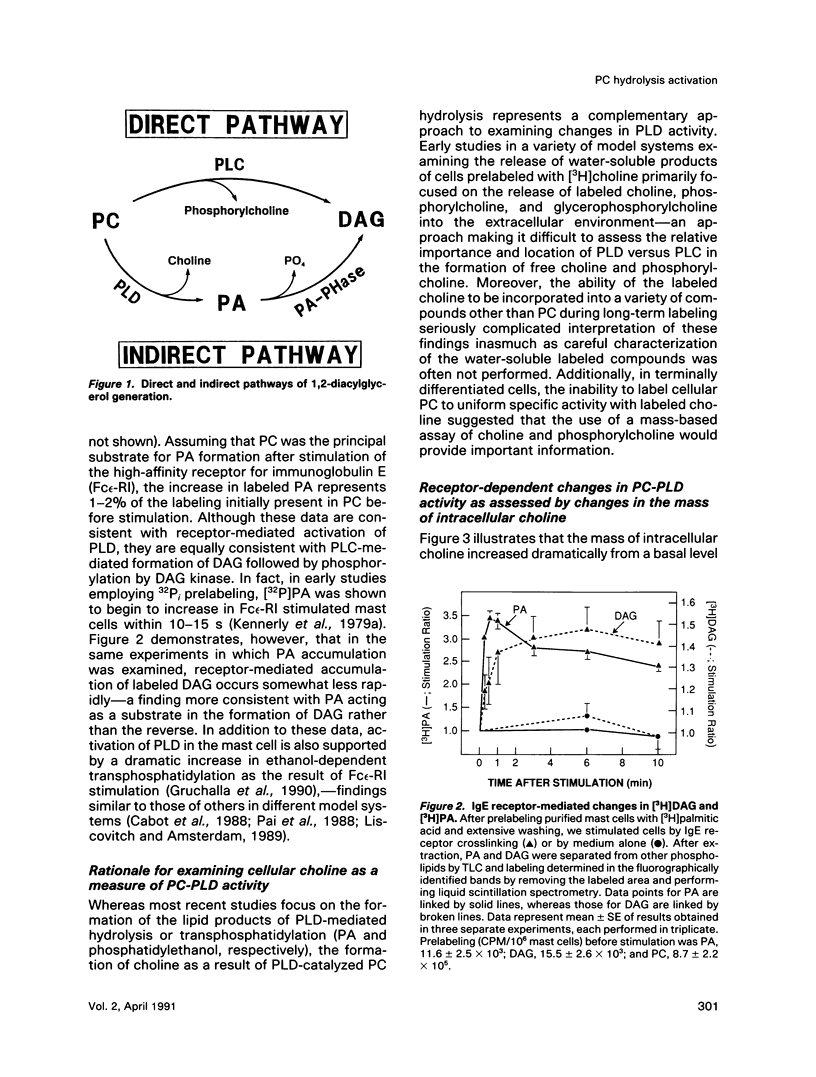
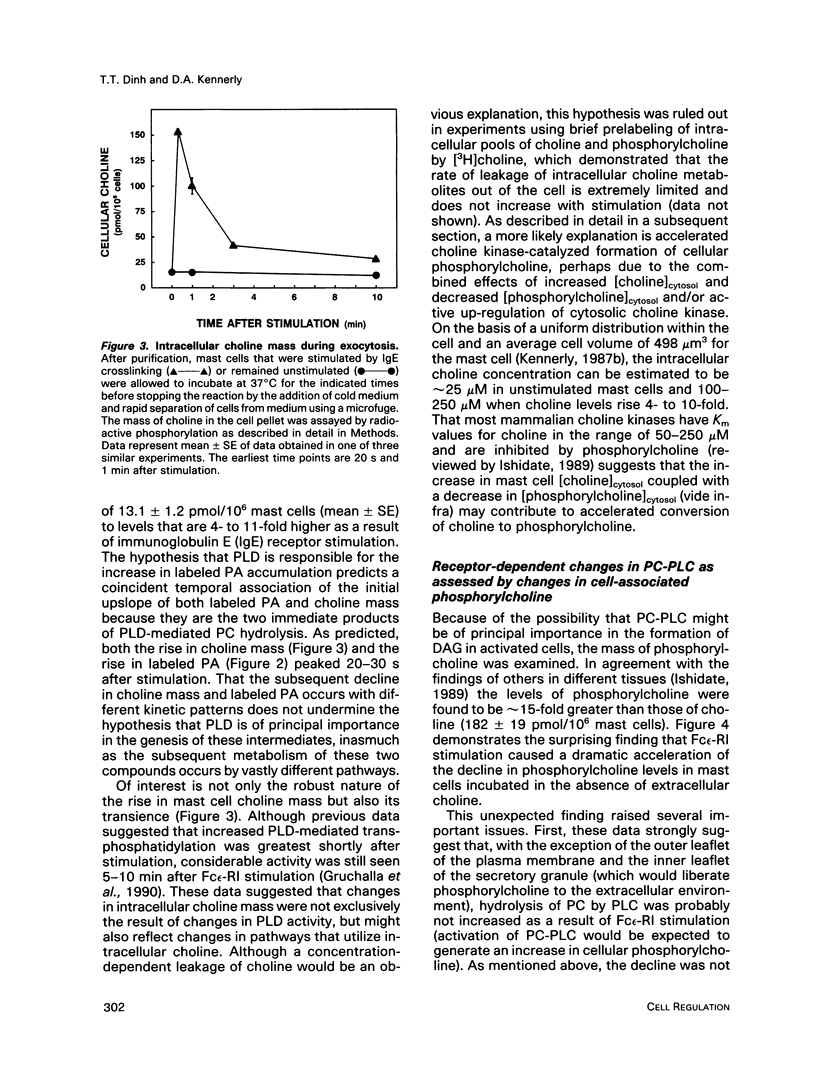
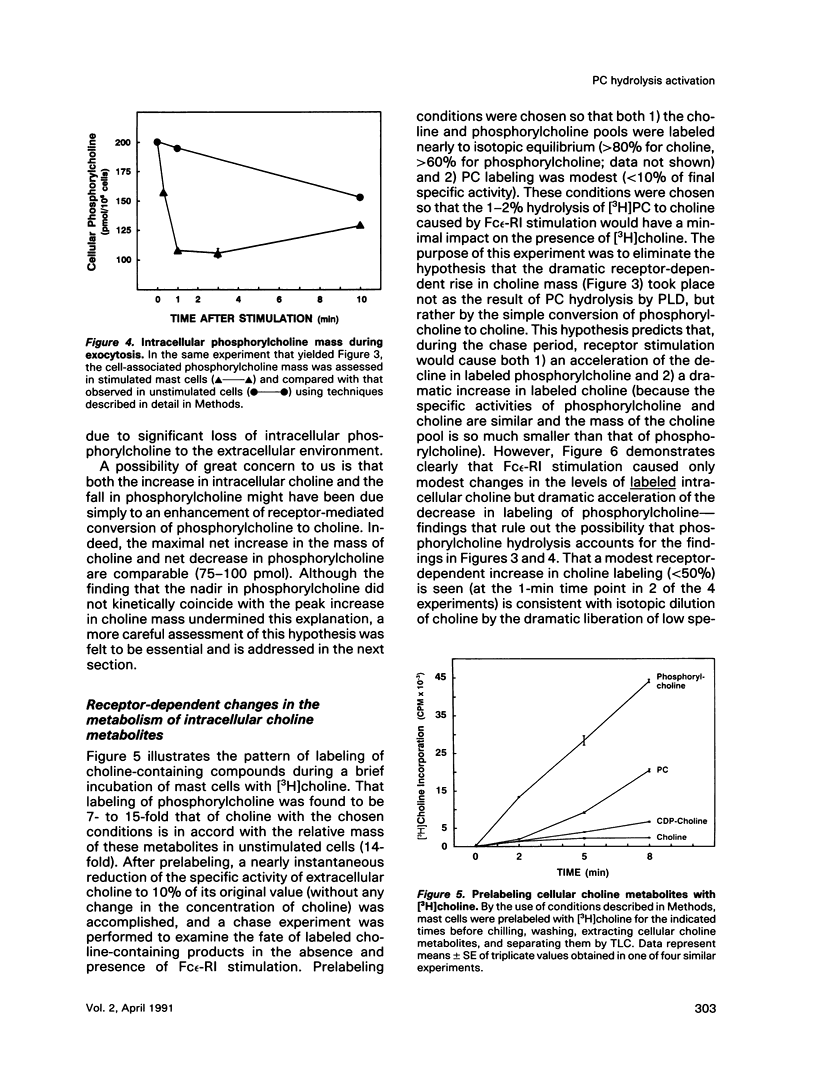
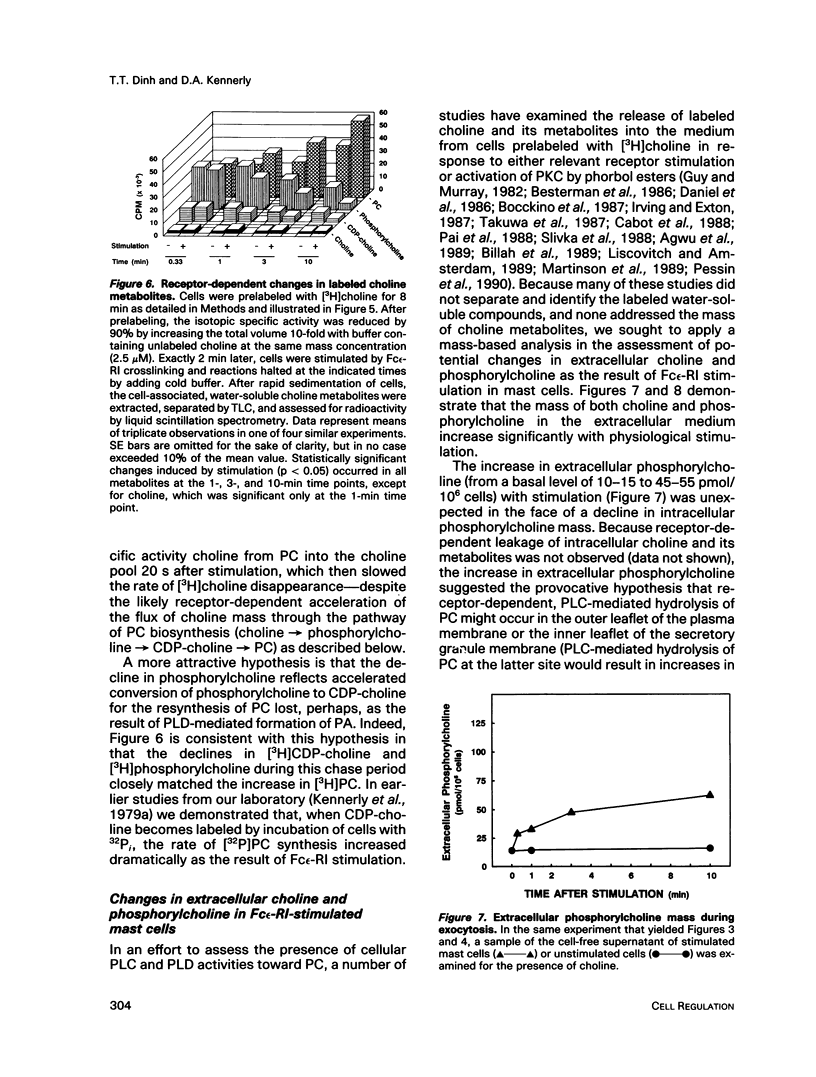
Enhancement of cellular phospholipase D (PLD)-1 and phospholipase C (PLC)-mediated hydrolysis of endogenous phosphatidylcholine (PC) during receptor-mediated cell activation has received increasing attention inasmuch as both enzymes can result in the formation of 1,2-diacylglycerol (DAG). The activities of PLD and PLC were examined in purified mast cells by quantitating the mass of the water-soluble hydrolysis products choline and phosphorylcholine, respectively. Using an assay based on choline kinase-mediated phosphorylation of choline that is capable of measuring choline and phosphorylcholine in the low picomole range, we quantitated the masses of both cell-associated and extracellular choline and phosphorylcholine. Activating mast cells by crosslinking its immunoglobulin E receptor (Fc epsilon-RI) resulted in an increase in cellular choline from 13.1 +/- 1.2 pmol/10(6) mast cells (mean +/- SE in unstimulated cells) to levels 5- to 10-fold higher, peaking 20 s after stimulation and rapidly returning toward baseline. The increase in cellular choline mass paralleled the increase in labeled phosphatidic acid accumulation detected in stimulated cells prelabeled with [3H]palmitic acid and preceded the increase in labeled DAG. Although intracellular phosphorylcholine levels were approximately 15-fold greater than choline in unstimulated cells (182 +/- 19 pmol/10(6) mast cells), stimulation resulted in a significant fall in phosphorylcholine levels shortly after stimulation. Pulse chase experiments demonstrated that the receptor-dependent increase in intracellular choline and the fall in phosphorylcholine were not due to hydrolysis of intracellular phosphorylcholine and suggested a receptor-dependent increase in PC resynthesis. When the extracellular medium was examined for the presence of water-soluble products of PC hydrolysis, receptor-dependent increases in the mass of both choline and phosphorylcholine were observed. Labeling studies demonstrated that these extracellular increases were not the result of leakage of these compounds from the cytosol. Taken together, these data lend support for a quantitatively greater role for receptor-mediated PC-PLD compared with PC-PLC during activation of mast cells.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agwu D. E., McPhail L. C., Chabot M. C., Daniel L. W., Wykle R. L., McCall C. E. Choline-linked phosphoglycerides. A source of phosphatidic acid and diglycerides in stimulated neutrophils. J Biol Chem. 1989 Jan 25;264(3):1405–1413. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Duronio V., Cuatrecasas P. Rapid formation of diacylglycerol from phosphatidylcholine: a pathway for generation of a second messenger. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6785–6789. doi: 10.1073/pnas.83.18.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Pai J. K., Mullmann T. J., Egan R. W., Siegel M. I. Regulation of phospholipase D in HL-60 granulocytes. Activation by phorbol esters, diglyceride, and calcium ionophore via protein kinase- independent mechanisms. J Biol Chem. 1989 May 25;264(15):9069–9076. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Exton J. H. Stimulation of 1,2-diacylglycerol accumulation in hepatocytes by vasopressin, epinephrine, and angiotensin II. J Biol Chem. 1985 Nov 15;260(26):14201–14207. [PubMed] [Google Scholar]
- Bocckino S. B., Blackmore P. F., Wilson P. B., Exton J. H. Phosphatidate accumulation in hormone-treated hepatocytes via a phospholipase D mechanism. J Biol Chem. 1987 Nov 5;262(31):15309–15315. [PubMed] [Google Scholar]
- Cabot M. C., Welsh C. J., Cao H. T., Chabbott H. The phosphatidylcholine pathway of diacylglycerol formation stimulated by phorbol diesters occurs via phospholipase D activation. FEBS Lett. 1988 Jun 6;233(1):153–157. doi: 10.1016/0014-5793(88)81374-7. [DOI] [PubMed] [Google Scholar]
- Daniel L. W., Waite M., Wykle R. L. A novel mechanism of diglyceride formation. 12-O-tetradecanoylphorbol-13-acetate stimulates the cyclic breakdown and resynthesis of phosphatidylcholine. J Biol Chem. 1986 Jul 15;261(20):9128–9132. [PubMed] [Google Scholar]
- Das S., Rand R. P. Diacylglycerol causes major structural transitions in phospholipid bilayer membranes. Biochem Biophys Res Commun. 1984 Oct 30;124(2):491–496. doi: 10.1016/0006-291x(84)91580-8. [DOI] [PubMed] [Google Scholar]
- Exton J. H. Signaling through phosphatidylcholine breakdown. J Biol Chem. 1990 Jan 5;265(1):1–4. [PubMed] [Google Scholar]
- Goth A., Adams H. R., Knoohuizen M. Phosphatidylserine: selective enhancer of histamine release. Science. 1971 Sep 10;173(4001):1034–1035. doi: 10.1126/science.173.4001.1034. [DOI] [PubMed] [Google Scholar]
- Gruchalla R. S., Dinh T. T., Kennerly D. A. An indirect pathway of receptor-mediated 1,2-diacylglycerol formation in mast cells. I. IgE receptor-mediated activation of phospholipase D. J Immunol. 1990 Mar 15;144(6):2334–2342. [PubMed] [Google Scholar]
- Guy G. R., Murray A. W. Tumor promoter stimulation of phosphatidylcholine turnover in HeLa cells. Cancer Res. 1982 May;42(5):1980–1985. [PubMed] [Google Scholar]
- Hattori H., Kanfer J. N. Synaptosomal phospholipase D potential role in providing choline for acetylcholine synthesis. J Neurochem. 1985 Nov;45(5):1578–1584. doi: 10.1111/j.1471-4159.1985.tb07229.x. [DOI] [PubMed] [Google Scholar]
- Irving H. R., Exton J. H. Phosphatidylcholine breakdown in rat liver plasma membranes. Roles of guanine nucleotides and P2-purinergic agonists. J Biol Chem. 1987 Mar 15;262(8):3440–3443. [PubMed] [Google Scholar]
- Kennerly D. A. Diacylglycerol metabolism in mast cells. Analysis of lipid metabolic pathways using molecular species analysis of intermediates. J Biol Chem. 1987 Dec 5;262(34):16305–16313. [PubMed] [Google Scholar]
- Kennerly D. A. Phosphatidylcholine is a quantitatively more important source of increased 1,2-diacylglycerol than is phosphatidylinositol in mast cells. J Immunol. 1990 May 15;144(10):3912–3919. [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Amsterdam A. Gonadotropin-releasing hormone activates phospholipase D in ovarian granulosa cells. Possible role in signal transduction. J Biol Chem. 1989 Jul 15;264(20):11762–11767. [PubMed] [Google Scholar]
- Martinson E. A., Goldstein D., Brown J. H. Muscarinic receptor activation of phosphatidylcholine hydrolysis. Relationship to phosphoinositide hydrolysis and diacylglycerol metabolism. J Biol Chem. 1989 Sep 5;264(25):14748–14754. [PubMed] [Google Scholar]
- Mufson R. A., Okin E., Weinstein I. B. Phorbol esters stimulate the rapid release of choline from prelabelled cells. Carcinogenesis. 1981;2(11):1095–1102. doi: 10.1093/carcin/2.11.1095. [DOI] [PubMed] [Google Scholar]
- Murray J. J., Dinh T. T., Truett A. P., 3rd, Kennerly D. A. Isolation and enzymic assay of choline and phosphocholine present in cell extracts with picomole sensitivity. Biochem J. 1990 Aug 15;270(1):63–68. doi: 10.1042/bj2700063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai J. K., Siegel M. I., Egan R. W., Billah M. M. Activation of phospholipase D by chemotactic peptide in HL-60 granulocytes. Biochem Biophys Res Commun. 1988 Jan 15;150(1):355–364. doi: 10.1016/0006-291x(88)90528-1. [DOI] [PubMed] [Google Scholar]
- Pessin M. S., Baldassare J. J., Raben D. M. Molecular species analysis of mitogen-stimulated 1,2-diglycerides in fibroblasts. Comparison of alpha-thrombin, epidermal growth factor, and platelet-derived growth factor. J Biol Chem. 1990 May 15;265(14):7959–7966. [PubMed] [Google Scholar]
- Siegel D. P., Banschbach J., Alford D., Ellens H., Lis L. J., Quinn P. J., Yeagle P. L., Bentz J. Physiological levels of diacylglycerols in phospholipid membranes induce membrane fusion and stabilize inverted phases. Biochemistry. 1989 May 2;28(9):3703–3709. doi: 10.1021/bi00435a012. [DOI] [PubMed] [Google Scholar]
- Slivka S. R., Meier K. E., Insel P. A. Alpha 1-adrenergic receptors promote phosphatidylcholine hydrolysis in MDCK-D1 cells. A mechanism for rapid activation of protein kinase C. J Biol Chem. 1988 Sep 5;263(25):12242–12246. [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Eisen S. A., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. II. Studies on the relationship between intracellular cyclic AMP concentrations and histamine release. J Immunol. 1975 May;114(5):1480–1485. [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Rasmussen H. A tumour promoter, 12-O-tetradecanoylphorbol 13-acetate, increases cellular 1,2-diacylglycerol content through a mechanism other than phosphoinositide hydrolysis in Swiss-mouse 3T3 fibroblasts. Biochem J. 1987 May 1;243(3):647–653. doi: 10.1042/bj2430647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance D. E., Trip E. M., Paddon H. B. Poliovirus increases phosphatidylcholine biosynthesis in HeLa cells by stimulation of the rate-limiting reaction catalyzed by CTP: phosphocholine cytidylyltransferase. J Biol Chem. 1980 Feb 10;255(3):1064–1069. [PubMed] [Google Scholar]
- Wright T. M., Rangan L. A., Shin H. S., Raben D. M. Kinetic analysis of 1,2-diacylglycerol mass levels in cultured fibroblasts. Comparison of stimulation by alpha-thrombin and epidermal growth factor. J Biol Chem. 1988 Jul 5;263(19):9374–9380. [PubMed] [Google Scholar]


