Abstract
Exposure of civilian populations to radiation due to accident, war or terrorist act is an increasing concern. The lung is one of the more radiosensitive organs that may be affected in people receiving partial-body irradiation and radiation injury in lung is thought to be associated with the development of a prolonged inflammatory response. Here we examined how effectively damage to the lung can be mitigated by administration of drugs initiated at different times after radiation exposure and examined response in adolescent animals for comparison with the young adult animals that we had studied previously. We studied the mitigation efficacy of the isoflavone genistein (50 mg/kg) and the salen-Mn superoxide dismutase-catalase mimetic EUK-207 (8 mg/kg), both of which have been reported to scavenge reactive oxygen species and reduce activity of the NFkB pathway. The drugs were given by subcutaneous injection to 6- to 7-week-old Fisher rats daily starting either immediately or 2 weeks after irradiation with 12 Gy to the whole thorax. The treatment was stopped at 28 weeks post irradiation and the animals were assessed for levels of inflammatory cytokines, activated macrophages, oxidative damage and fibrosis at 48 weeks post irradiation. We demonstrated that both genistein and EUK-207 delayed and suppressed the increased breathing rate associated with pneumonitis. These agents also reduced levels of oxidative damage (50–100%), levels of TGF-β1 expression (75–100%), activated macrophages (20–60%) and fibrosis (60–80%). The adolescent rats developed pneumonitis earlier following irradiation of the lung than did the adult rats leading to greater severe morbidity requiring euthanasia (~37% in adolescents vs. ~10% in young adults) but the extent of the mitigation of the damage was similar or slightly greater.
INTRODUCTION
The recent earthquake and subsequent tsunami that inundated the Fukushima nuclear power plants, followed by radionuclide releases outside the crippled reactors have renewed awareness of such potential exposures (1). However, the appropriate management of persons exposed to significant doses of radiation due to accident, war or terrorist act is uncertain (2–7). Effects on the bone marrow may be treatable by bone marrow transplantation but the lung is also radiation sensitive and may become of significant concern in patients exposed to partial-body or nonuniform exposures. Appropriate strategies to mitigate or treat such exposures are currently very limited (8–12) and there is a serious need to develop effective radioprotectors and mitigators against radiation-induced tissue damage. The ideal such agents must be easy to administer, inexpensive and relatively nontoxic to normal cells (13, 14).
We and others have previously reported on the use of genistein and other antioxidants as potential protectors or mitigators of radiation-induced lung damage (15–18). In the current study, we extended our previous work (18) studying genistein as a mitigator by altering the administration from a dietary supplement to daily subcutaneous injection to avoid issues with intestinal absorption and reduced food intake. We compared genistein treatment to similar treatment with the antioxidant EUK-207, a salen-manganese superoxide dismutase (SOD)-catalase mimetic (19, 20) and examined how effectively damage to the lung can be mitigated by administration of the drugs when initiated at later times after radiation exposure. Both of these agents have been reported to scavenge reactive oxygen species and to reduce activity of the NFkB pathway (19–29) and are well-tolerated, suggesting low toxicity.
The current study was planned to examine effects on a younger age group than those examined previously, consistent with the overall goals of the Center for Medical Countermeasures against Radiation (CMCR) program (30). It was also designed to address a possible situation after a radiation accident where some exposed people may get rapid treatment while others may not get treatment for 1 or 2 weeks after the radiation exposure. Consequently, we used adolescent Fischer rats (31) and started the drug treatment at 0 or 2 weeks after irradiation for comparison to our previous studies that used young adult rats with treatment started at 1 week post irradiation. (10, 32). We terminated treatment with both agents at 28 weeks, but extended the time of analysis to 48 weeks to strengthen our previous findings that showed stopping the treatment at 28 weeks did not decrease the mitigating efficacy measured at late times. Both drugs had significant effects in mitigating radiation-induced lung damage. In some groups we crossed over the drug treatments at 14 weeks to test for possible drug resistance issues but we found no greater mitigation.
METHODS
Animals
Female Fischer 344 rats (Harlan Teklad, Madison, WI) were used throughout the studies, housed in animal facilities accredited by the Canadian Council on Animal Care and treated in accordance with approved protocols. The rats were 6–7 weeks old, weighing 160–180 g at the start of the study. The rats were divided into 6 treatment groups each containing 8 rats and were treated with 12 Gy whole-thorax irradiation (LR), LR + PEG-400, LR + genistein started immediately after irradiation (within 1 h), LR + EUK-207 started immediately after irradiation, LR + genistein started at 2 weeks after irradiation and LR + EUK-207 started 2 weeks after irradiation. In the latter 2 groups, the drugs were crossed over at 14 weeks. All drug treatments were stopped at 28 weeks and the animals were held until 48 weeks after irradiation for sacrifice. There were 2 control groups, one (48-week control) also contained 8 rats and was held for 48 weeks until the end of the experiment, the other (6-week control) contained 4 rats that were sacrificed at the beginning of the experiment.
Drugs
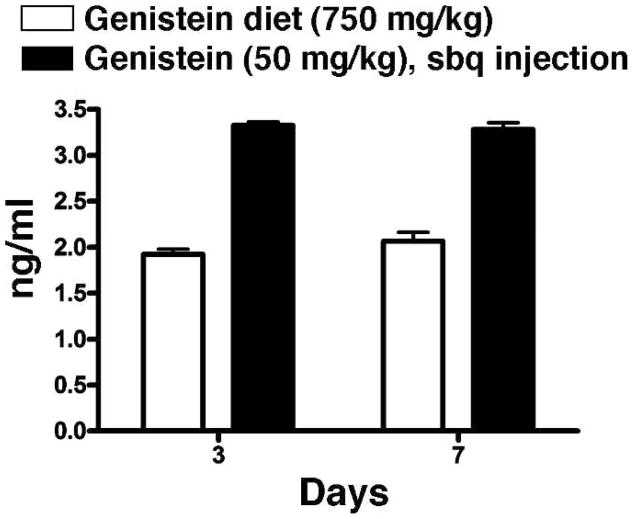
The isoflavone genistein (Sigma Aldrich, Canada) was dissolved in polyethylene glycol (PEG)-400 and given by daily subcutaneous injection (sc) at 50 mg/kg/day. This compound has been reported to scavenge reactive oxygen species (ROS) and to block the activation of NFkB by preventing phosphorylation of IkB, its binding partner (20–25). We used the AIN-76A diet (Harlan Teklad, Madison, WI), which contains no detectable phytoestrogens, during the whole 48 weeks of the experiment. Plasma levels of free genistein were measured in rats given 50 mg/kg/day on days 3 and 7 after initiation of the drug treatment by mass spectrometry as described previously (18). The plasma was sampled 12 h after sc injection of genistein on the relevant day. Results of the measurements are shown in Fig. 1 and compared to plasma levels in animals receiving a genistein diet (750 mg/kg) similar to that which we have used previously (18). The sc injection gave significantly higher levels of genistein than did the diet.
FIG. 1.
Analysis of free plasma genistein levels (ng/ml) using mass spectrometry at 3 and 7 days after initiation of treatment either in the diet (750 mg/kg diet) or by daily subcutaneous injection of 50 mg/kg dissolved in polyethylene glycol. Plasma was sampled 12 h after the daily subcutaneous injection.
The salen-manganese (SOD)-catalase mimetic, EUK-207, was custom-synthesized and characterized, as described previously (33). This compound is currently the best of the available compounds in this series in terms of activity and pharmacokinetics (34). The drug acts primarily as an antioxidant but also has activity against NFkB (19, 26–29). The animals received doses of 8 mg/kg/day by daily sc injection. It has previously been shown (34) that EUK-207 given by sc injection results in readily detectable plasma levels that persist for several hours. Such data, along with other efficacy and pharmacokinetics studies conducted with this and other salen Mn complexes (34), informed our choice of a daily sc injection regimen for this study.
Irradiation
An image guided micro-irradiator (CX-Rad-225, Precision X-ray Inc., North Branford, CT) was used for targeting and irradiating the thoracic cavity in each animal as described previously (18). The details of the unit and its calibration for these studies have been reported previously (18, 35). The dose rate at 225 kVp, 13 mA (HVL: 0.93 mm Cu, added filtration: 0.3 mm Cu) was estimated as 3.51 Gy/ min at the depth of 1.5 cm in solid water for the whole-thorax irradiation. Animals were first imaged and adjusted inside the jig for targeting the whole thorax inside lead surface collimators. The total imaging dose was estimated at less than 1 cGy. The animal then received a total of 12 Gy with anterior-posterior (a-p) and posterior-anterior (p-a) beams. The mid-plane of the animal was located at the iso-center. The radiation dose was chosen based on results from our previous studies as a dose required to induce measurable radiation damage (17, 18, 25).
Analyses of Treatment Response
To assess pneumonitis we measured the breathing frequency of the rats using a respiration rate monitor (Columbus Instruments, Columbus, OH) as described previously (18). At the end of the experiment, alpha-MEM media supplemented with antibiotics was perfused through the right ventricle of the hearts of deeply anesthetized (ketamineIxylazine) animals to remove as much blood in the lungs as possible. The lungs were removed and the lobes of the right lung were immediately frozen in liquid nitrogen and stored frozen until analysis for hydroxyproline content, soluble collagen and malondialdehyde (TBARs assay). In the majority of the animals, a volume (0.5–1.0 ml) of 10% buffered formalin was injected into the left lobe of the lung to expand the alveoli and the lobes were placed in 10% formalin for at least 48 h for fixation. The whole left lobe of the lungs was embedded in paraffin and sections 5 μm thick were cut and placed on slides in preparation for immunohistochemical staining by the research pathology laboratory in our facility. All of the lungs were process and stained at the same time. The primary antibodies used were activated macrophage marker ED-1 (MCA341, 1:100, AbD Serotec, Oxford, UK), cytokines IL-1α (sc-1254, 1:100, Santa Cruz Biotechnology Inc., Santa Cruz, CA), IL-1β (AAR15G, 1: 1000, AbD Serotec), IL-6 (sc-1265, 1:200, Santa Cruz Biotechnology Inc.), TNF-α (sc-1357, 1:200, Santa Cruz Biotechnology Inc.), TGF-β1 (MCA797, 1:50) (AbD Serotec), and for 8-hydroxy-2-deoxyguanosine (8-OHdG) (MOG-110P, 1:1000, JaICA, Shizuoka, Japan). Secondary antibodies included biotinylated anti-mouse immunoglobulin G, IgG (BA-1000, 1:200, Vector Laboratories), biotinylated anti-rabbit IgG (BA-1000, 1: 200, Vector Laboratories) and biotinylated anti-goat IgG (BA-5000, 1: 300, Vector Laboratories) for 30 min at room temperature.
Fibrosis was assessed by analyzing part of the right lung for hydroxyproline content using a colorimetric assay on papain-digested lung tissue (P3125) (Sigma-Aldrich Canada, Oakville, ON, Canada). Lung tissue (100 mg) was digested at 60°C for 48 h and then subjected to acid hydrolysis for 18 h at 110°C. Free hydroxyproline was released from protein and peptides into the solution, which was then neutralized. The hydroxyproline was oxidized into a pyrrole with chloramine T (857319, Sigma-Aldrich). This intermediate turned pink in color with the addition of Ehrlich’s Reagent (4-dimethylamino-benzaldehyde) (156477, Sigma-Aldrich). The samples were loaded into a 96-well microplate and the absorbance was measured at 560 nm using a plate reader. The concentrations of the samples were determined from a standard curve using cis-4-hydroxy-L-proline (H1637, Sigma-Aldrich). We separately assessed recently synthesized collagen using the Sircol collagen assay (Biocolor Ltd., Belfast, UK), which was performed following the manufacturer’s instructions. It is a colorimetric procedure that uses a dye reagent containing Sirius Red in picric acid, which specifically binds to soluble collagen. Part of the right lung (25 mg) was used for the analysis. A standard curve was derived from the kit and was used to determine the collagen content of samples.
Lipid peroxidation was assessed using a TBARS Assay to measure malondialdehyde (MDA) levels in the lung tissue. A part of the right lung (25 mg) of each rat was analyzed for MDA levels using a TBARS assay kit (Cayman Chemical Company, Ann Arbor, MI). The flash-frozen sample was thawed, sonicated for 15 s in RIPA buffer with protease inhibitors (Roche Applied Science, Laval, Quebec) and then centrifuged (1600g). The supernatant containing the lipid fraction was used for the assay. A standard curve was derived from the kit and used to determine the MDA content of the samples.
Image Analysis
Following staining, the slides were scanned using the ScanScope XT (Aperio Technologies, Vista, CA) and the whole lung section was analyzed using the Positive Pixel Algorithm, Aperio ImageScope (Aperio Technologies, Toronto, ON). The positive pixel count algorithm is used to quantify the amount of a specific stain present in a scanned slide image by assessing the number of positive pixels/ number of positive and negative pixels × 100 (% positivity). Air spaces were excluded. Details of this procedure have been reported previously (18). Sections were cut from the left lobe of the lung in the coronal plane. We analyzed one section from each surviving rat for each time point.
Statistical Analysis
Multiple linear regressions and Tukey’s method for the adjustment of least square means in multiple comparisons were used for analysis of the data sets. The radiation-only group was set as the primary comparison group for these analyses since the purpose of the study was to determine the efficacy of treatments relative to this group. A P value of less than 0.05 was considered as significantly different. Mixed modeling was used to examine time trends in the breathing rate data.
RESULTS
Breathing Rate Changes in Irradiated Rats
We measured breathing rate to compare the effects of genistein and EUK-207 on radiation-induced pneumonitis after whole-thorax irradiation (LR). We initiated treatment with genistein (50 mg/kg/day) or EUK-207 (8 mg/kg/day) by subcutaneous injection either immediately after irradiation or starting 2 weeks after irradiation. In the latter case we crossed over the drugs at 14 weeks post irradiation to address the possibility of developing drug resistance (Gen/ EUK or EUK/Gen). Even though both drugs are known to be antioxidants and suppress NFkB activation, any resistance mechanisms that might develop may not be identical at the molecular level. All of the treated groups had 8 rats per group and the drugs were withdrawn at 28 weeks post irradiation. The animals were monitored until euthanasia at 48 weeks post irradiation to assess late development of functional effects due to the irradiation. An extra radiation “control” group of rats received radiation plus the vehicle (polyethylene glycol-LR + PEG) used for dissolving the genistein prior to sc injection. EUK-207 is water-soluble and was injected in saline. There were two untreated control groups. One was euthanized at the time of irradiation (6-week control, 4 rats/group) and the other was maintained throughout the study until they were euthanized at 48 weeks post irradiation (48-week control, 8 rats/group).
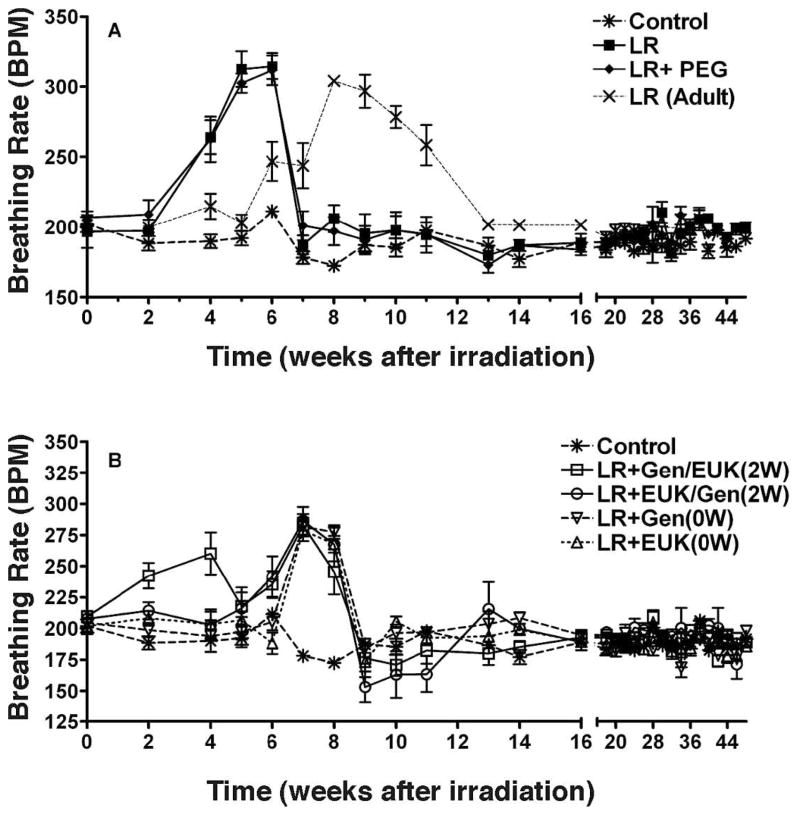
The rats were irradiated at 6–7 weeks of age (160–180 g) and we observed (Fig. 2A) an early sharp increase in breathing rate at 2–7 weeks in rats given LR alone or LR + PEG, with a peak of 310–315 BPM at 5–6 weeks. Data from our previous study (18) with young adult Fischer rats (12–14 weeks old) given identical radiation treatment is shown in Fig. 2 for comparison (LR-Adult) and demonstrates a later rise in the breathing rate indicating that the adolescent rats respond more rapidly to lung irradiation than adult rats. The groups treated with genistein and EUK-207 immediately after LR showed a significant (P < 0.05) shift and damping of the increased breathing rate peaks (280–285 at 7–8 weeks) compared to LR alone or LR + PEG with the increases occurring at 6–9 weeks post irradiation (Fig. 2B). The group given LR plus Gen/EUK starting at 2 weeks post irradiation demonstrated an earlier, but damped, increase in breathing rate at 2–4 weeks but it then returned to a profile similar to the other treated groups. The reason for this early increase is unclear. The breathing rate of all of the adolescent animals had returned to normal control levels by 9 weeks and remained at the same levels until 48 weeks after radiation. This return to normal levels did not occur until 13–15 weeks in the older animals in our previous study (18).
FIG. 2.
Breathing rate (mean breaths per minute) as a function of time after irradiation. Panels A and B: CON = no irradiation (soy-free diet); LR = whole lung irradiation (12 Gy); LR + PEG = whole lung irradiation (12 Gy) plus daily injections of polyethylene glycol (PEG); Gen = daily subcutaneous (sc) injections of 50 mg/kg genistein (dissolved in PEG); EUK = daily sc injections of EUK-207. The drug treatments were started 0 or 2 weeks post irradiation and terminated at 28 weeks. In two groups of rats, the drugs were crossed over at 14 weeks post irradiation (Gen/EUK or EUK/Gen). The rats were euthanized at 48 weeks. Also shown in panel A is data from our previous experiment (18) for adult rats given 12 Gy whole-lung irradiation, as indicated. Each point represents the mean (±SEM) for all rats available for analysis at different times.
Survival of Irradiated Rats
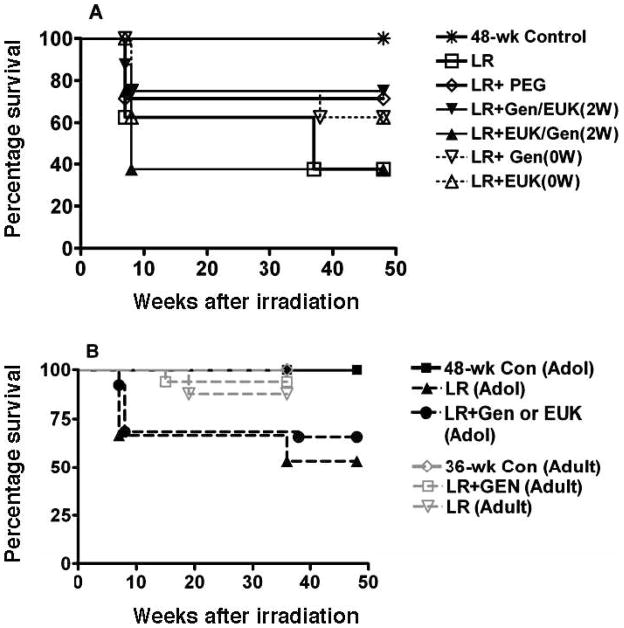
We observed significant morbidity (requiring euthanasia) in the irradiated rats occurring at 6–8 weeks consistent with the earlier onset of the pneumonitis phase in adolescent rats (Fig. 3A). Taken as a group, the rats receiving LR were significantly different (P < 0.05) from the control but the differences were not significant for the individual irradiated groups. Although a number of animals had to be euthanized in this time window, the rapid drop seen in the mean breathing rate at 7 weeks (Fig. 2) was not due to these early deaths. The rats that did not develop severe morbidity at this early time survived until the end the study period at 48 weeks, except for 3 rats that died at 37–38 weeks post irradiation. There was an initial decrease of body weight in irradiated rats during the pneumonitis stage. However, after the pneumonitis stage, the irradiated rats gained weight similarly to the other rats (data not shown). A combined analysis of these results with those from similarly treated young adult rats in our previous study (18) showed that the adolescent rats were significantly (P < 0.05) more sensitive (Fig. 3B) to developing severe morbidity requiring euthanasia (~37% in the adolescent rats vs. ~10% for the adult animals).
FIG. 3.
Panel A: Survival of the rats as a function of time after irradiation. Labeling of the treatment groups is as indicated in the legend to Fig. 2. Panel B: Comparison of survival of the adolescent (Adol) rats in the current experiment with adult rats given similar treatment in our previous study (18). An analysis of these results showed that the adolescent rats were significantly (P < 0.05) more sensitive to developing severe morbidity that required euthanasia. In our previous study, the rats were euthanized at 36 weeks vs. 48 weeks in the current study
Lung Fibrosis
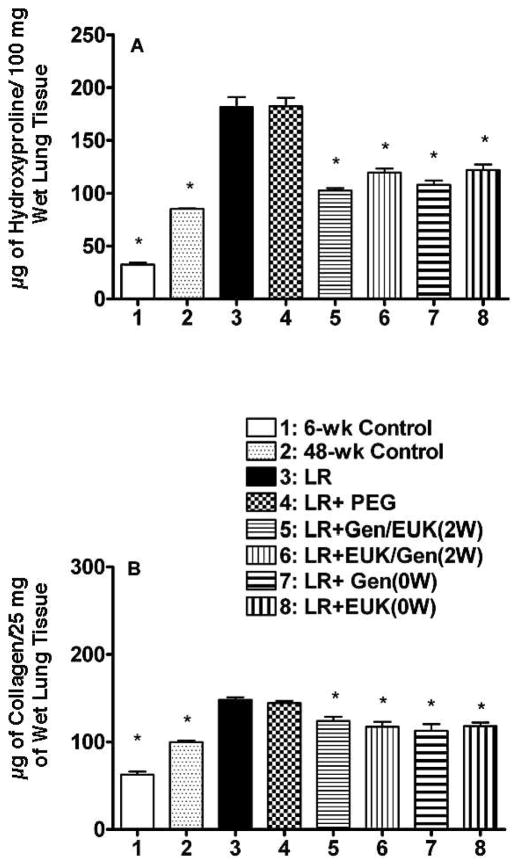
We measured hydroxyproline levels at 48 weeks as a quantitative measure of total lung collagen (Fig. 4A). Both LR and LR + PEG caused a large increase in hydroxyproline levels relative to the two untreated controls (P < 0.05). All of the drug treatment groups had significantly (P < 0.05) lower hydroxyproline content (61–81% reduction of the difference between the LR group and the 48-week control group), with only small differences among the groups (see supplementary Table S1; http://dx.doi.org/10.1667/RR2954.1.S1). A second measure of collagen in the lungs was obtained using the Sircol Assay that assesses the amount of recently synthesized (RS) collagen in the tissue. The RS collagen levels were increased to a lesser extent (than for total collagen as assessed by hydroxyproline) in the LR and LR + PEG groups relative to the 48-week control, but the decreases in these RS collagen levels expressed as a percentage of the difference between the LR group and the 48-week control group (50–78%) were similar to those for the hydroxyproline (see supplementary Table S1; http://dx.doi.org/10.1667/RR2954.1.S1). All of the drug treatments resulted in a significant decrease (P < 0.05) relative to the LR group (Fig. 4B) but the results for the different treatment groups were not significantly different from one another.
FIG. 4.
Measures of fibrosis in the lungs of the rats at time of euthanasia at 48 weeks after irradiation. Panel A: Hydroxyproline (μg of hydroxyproline/100 mg of wet lung tissue) content of the lung tissue. Panel B: recently synthesized collagen (μg of soluble collagen/ 25 mg of wet lung tissue). Each bar represents the mean (±SEM) for all rats available for analysis (Group 1 n = 4; Group 2 n = 8; Group 3 n = 3, Group 4 n = 5; Group 5 n = 5; Group 6 n = 3; Group 7 n = 4 and Group 8 n = 4). Labeling of the treatment groups is as indicated in the legend to Fig. 2. Six-week control animals were euthanized at the start of the experiment and 48-week controls were euthanized at 48 weeks post irradiation. The asterisks indicate groups that are significantly different from the LR group in each panel.
Oxidative Damage
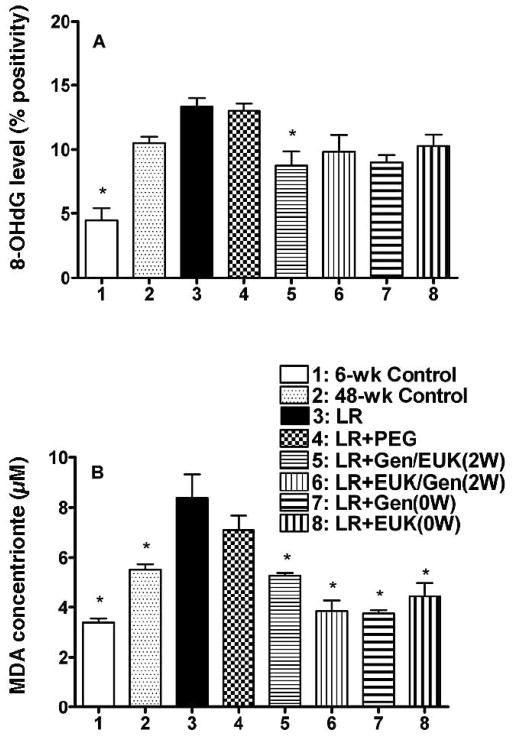
The presence of oxidative damage in the lung tissue was measured using both levels of 8-hydroxy-2-deoxyguanosine (8-OHdG), a marker of oxidative damage in the DNA and levels of malondialdehyde (MDA), a measure of lipid peroxidation. Analysis of staining of the lung sections for 8-OHdG (see supplementary Fig. S1; http://dx.doi.org/10.1667/RR2954.1.S2) showed that the LR only and the LR + PEG groups showed a significant increase relative to the 6-week control and only a trend for increased levels of 8-OHdG compared to the 48-week control rats after irradiation (Fig. 5A). All of the drug treatment groups showed a trend for a reduction in 8-OHdG levels to values similar to those seen in the 48-week controls, but only the Gen/EUK group that received treatment starting 2 weeks after irradiation showed a significant (P < 0.05) decrease. Results from the TBARS assay (Fig. 5B) to quantify the concentrations of MDA at 48 weeks showed a significant (P < 0.05) increase in the LR group relative to both controls (P < 0.05). All of the treatment groups showed significant (P < 0.05) lowering of MDA concentration at 48 weeks post irradiation to levels similar to or lower than the 48-week control. The reductions in these levels, expressed as a fraction of the difference between the LR group and the 48-week control group are shown in supplementary Table S1; http://dx.doi.org/10.1667/RR2954.1.S1.
FIG. 5.
Measures of oxidative damage in the lungs of the rats at time of euthanasia at 48 weeks after irradiation. Panel A: Analysis of 8-OHdG staining as percent positivity (the ratio of positive pixels/total number of positive and negative pixels in the tissue section with air spaces excluded). Panel B: Analysis of MDA levels (μM) in the lung tissue. Each bar represents the mean (±SEM) for all rats available for analysis (Group 1 n = 4; Group 2 n = 8; Group 3 n = 3; Group 4 n = 5; Group 5 n = 5; Group 6 n = 3; Group 7 n = 4 and Group 8 n = 4). Labeling of the treatment groups is as indicated in the legend to Fig. 2. Six-week control animals were euthanized at the start of the experiment and 48-week controls were euthanized at 48 weeks post irradiation. The asterisks indicate groups that are significantly different from the LR group in each panel.
Levels of Inflammatory Cytokines and Activated Macrophages
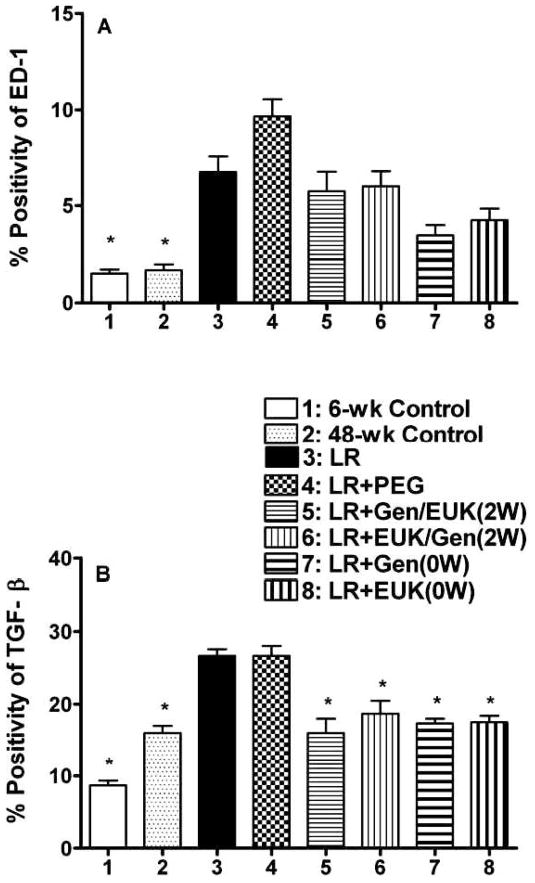
We assessed levels of inflammatory cytokines (IL-1α, IL-1β, IL-6 and TNF-α) using immunohistochemistry. All four of the cytokines were increased at 48 weeks post irradiation relative to the controls but the treatments had limited effects on these levels at 48-weeks post irradiation (see supplementary Fig. S2; http://dx.doi.org/10.1667/RR2954.1.S2). We also assessed levels of activated macrophages using ED-1 and levels of TGF-β1, since this cytokine is one of the key players in the formation of radiation-induced fibrosis in lung tissue. For ED-1 staining (see supplementary Fig. S3; http://dx.doi.org/10.1667/RR2954.1.S2) the analysis of staining levels showed that the LR and the LR + PEG groups were significantly (P < 0.05) elevated relative to both controls (Fig. 6A). All of the drug treatment groups showed a trend for a reduction relative to the reference LR group. For the TGF-β1 staining (see supplementary Fig. S4; http://dx.doi.org/10.1667/RR2954.1.S2), analysis of the staining levels is shown in Fig. 6B. In both the LR and the LR+ PEG groups, there was a significant (P < 0.05) increase in levels of TGF-β1 expression at 48 weeks post irradiation. All of the drug treatment groups showed a similar significant (P < 0.05) reduction in TGF-β1 to levels close to those observed in the 48-week control (Fig. 6B). These reductions, expressed as a fraction of the difference between the LR group and the 48-week control group, are shown in supplementary Table S1; http://dx.doi.org/10.1667/RR2954.1.S1.
FIG. 6.
Analysis of macrophage activation and TGF-β1 levels in the lungs of the rats at time of euthanasia at 48 weeks after irradiation. Panel A: Analysis of macrophage activation (ED-1 staining) as percent positivity (the ratio of positive pixels/total number of positive and negative pixels in the tissue section with air spaces excluded). Panel B: Analysis of TGF-β1 staining as percent positivity. Each bar represents the mean (±SEM) for all rats available for analysis (Group 1 n = 4; Group 2 n = 8; Group 3 n = 3; Group 4 n = 5; Group 5 n = 5; Group 6 n = 3; Group 7 n = 4 and Group 8 n = 4). Labeling of the treatment groups is as indicated in the legend to Fig. 2. Six-week control animals were euthanized at the start of the experiment and 48-week controls were euthanized at 48 weeks post irradiation. The asterisks indicate groups that are significantly different from the LR group in each panel.
DISCUSSION
Lung is one of the most susceptible late-responding organs to potentially debilitating radiation toxicity. It has been proposed that radiation-induced late effects are caused, in part, by chronic oxidative stress and inflammation. Increased production of reactive oxygen species, which leads to lipid peroxidation, oxidation of DNA and proteins, as well as activation of proinflammatory factors has been observed in vitro and in vivo following irradiation (12, 36, 37). The work presented here builds on our previous studies with genistein and EUK-207 as mitigators of radiation-induced lung damage. These agents are both antioxidants and can block activation of NFkB and they also have low toxicity (19–29). We previously found that treatment with both agents starting 1 week after irradiation could largely prevent breathing rate increases (radiation-induced pneumonitis) in Sprague-Dawley rats after irradiation, even when treatment was ceased at 14 weeks. There was also a substantial decrease in fibrosis (17). In another study (18), we found in young adult Fischer rats that genistein could delay the onset of a breathing rate increase, but caused only limited suppression suggesting that radiation damage in Fischer rats may be less responsive to these compounds. In the current study, we observed a similar effect in adolescent Fischer rats with a delay and limited suppression of the breathing rate increase, although the effect was observed earlier after irradiation in the young adolescent rats. It is unlikely that the lesser effect in the Fischer rats is due to the different dosing schedule for genistein, since plasma levels were higher than in those rats given genistein in the diet we used previously (17).
Interestingly, there was also no secondary elevation of breathing rate at 20–40 weeks in the Fischer rats after irradiation as we have observed in Sprague-Dawley rats in our previous studies (16, 17). Similar differences in breathing rate changes in Sprague-Dawley and Fischer rats have been reported previously (38). This might suggest that Fischer rats are more resistant to radiation-induced fibrosis. However, our analyses of collagen content in the lungs of the rats demonstrated significant increases in collagen content (consistent with fibrosis) at 48 weeks post irradiation (Fig. 4). Treatment with both genistein and EUK-207 resulted in a significant reduction in hydroxyproline levels in the rat lungs at 48 weeks, indicating that even though the treatment was stopped at 28 weeks it had a prolonged effect. These results are similar to those we reported previously for adult Fischer rats (18), but the reduction is less than that which we observed in Sprague-Dawley rats (17), again suggesting that radiation damage in the Fischer rats may be less responsive to these compounds. Interestingly, levels of recently synthesized collagen were still slightly elevated at these late times relative to the 48-week control (Fig. 4), implying an ongoing stimulation of collagen production. This is not entirely consistent with the measured TGF-β1 levels (Fig. 6) where all of the drug treatments caused a 75–100% reduction of the increase in the TGF-β1 levels.
Because of the importance of a chronic inflammatory response and the expected resulting exposure to ongoing oxidative stress, we also assessed both DNA oxidation (8-OHdG) and lipid peroxidation (MDA). Both drug treatments significantly reduced levels of radiation-induced lipid peroxidation, as assessed by MDA in the lungs of the rats (Fig. 5) to levels similar to, or below, the 48-week control level, consistent with a reduction in the chronic inflammatory response. The 8-OHdG levels were also reduced to levels similar or below the 48-week control, but assessing this as a mitigating effect is uncertain because, unlike with MDA, radiation alone did not increase 8-OHdG significantly above the levels of the age-matched (48-week) controls. Only one treatment group showed a significantly lowered 8-OHdG versus radiation alone, and this result was modest (Fig. 5). These smaller changes in DNA oxidative damage may reflect the fact that ROS produced by a chronic inflammatory response would likely be mostly extracellular and hence have better access to lipid membranes than to cellular DNA. A long-term reduction in oxidative stress is consistent with the lower levels of activated macrophages and TGF-β1 levels (Fig. 6) at 48 weeks post irradiation, which is 20 weeks after the end of the drug treatment. In this context, it is also of interest that crossing-over the drugs at 14 weeks had no significant impact on the results obtained suggesting that there is no development of specific resistance to the actions of the drugs, although the similarity in the activities might mask such an effect if it is downstream from the site of action.
Overall, these results might suggest that the cessation of the treatment at 28 weeks does not result in a resurgence of an ongoing chronic inflammatory response. However, increased levels of several inflammatory cytokines were observed at this late time point and were, except for TGF-β1 levels (Fig. 6), little affected by the drug treatments (supplementary Fig. S2; http://dx.doi.org/10.1667/RR2954.1.S2). The results on oxidative damage are of particular interest because, depending on repair and turnover rates, the lesions measured should reflect ongoing levels of oxidative stress rather than that induced at the time of irradiation. The higher levels of MDA in the irradiated groups at 48 weeks thus suggest that chronic oxidative stress is prolonged in the irradiated animals. However, the control data with both MDA and 8-OHdG show that there is also an age-associated increase in oxidative injury that is unrelated to irradiation.
Similar to our findings, Rabbani et al. (39) have demonstrated that chronic administration of small molecular weight catalytic metalloporphyrin antioxidants over a 10 week period post irradiation to female Fisher rats mitigated the effects of a single dose of 28 Gy given to the hemithorax. These studies were performed with rats irradiated at 160–180 g weight suggesting that they were equivalent to the adolescent rats used in our study. A more recent study (40) using the same model also reported mitigation with treatments of small molecular weight catalytic metalloporphyrin antioxidants that were started at various times after irradiation. These authors measured effects at 10 weeks post irradiation and found maximal mitigation occurred when the treatment was initiated within the first 12 h after irradiation. Both of these studies support our findings of mitigation with antioxidants, although they are not entirely in agreement with our findings that starting treatment at 1–2 weeks post irradiation is equally effective.
An interesting novel aspect of these studies is the finding of increased sensitivity and earlier onset of radiation-induced pneumonitis in the adolescent rats (4–8 weeks post irradiation) relative to older rats (7–13 weeks post irradiation) (see Fig. 2A). The observation that these adolescent rats also had a greater level of severe morbidity leading to euthanasia than we saw previously with older animals given identical radiation treatment (18), and that this occurred in the same (early) time window as the increased breathing rate (Fig. 3), further emphasizes the increased sensitivity of the animals. No obvious differences were observed for hydroxyproline levels at late times [comparison data not shown, but can be accessed in ref. (18)]. Further studies are required to confirm this possible effect of age but the results are consistent with our earlier report in mice that time of onset of severe morbidity after lung irradiation is prolonged in older animals, although in this study the age of the animals at time of irradiation was 3–12 months (41). Travis et al. (42) reported no difference in LD50 values (at 180 days post irradiation) for mice at 3, 5 and 8 months of age at the time of whole-lung irradiation and van den Aardweg et al. (43) reported no difference to late rectal toxicity in rats at 12 weeks vs. 77–80 weeks of age at the time of irradiation. It is possible that our results with the younger rats may reflect more rapid proliferation of alveolar cells in these rats as they grow. Damage to alveolar Type II cells is one of the mechanisms that has been implicated in radiation-induced pneumonitis (44–47). A recent review of radiation-induced lung damage in clinical studies did not clearly show an increased level of pneumonitis in children but the authors emphasized the difficulty in making comparisons across age groups because of different indications for treatment (and often different volumes irradiated) (48).
SUMMARY
In this study, we demonstrated that the antioxidant and anti-inflammatory agents genistein and EUK-207 can provide mitigation of radiation damage to the lung even when treatment is initiated 2 weeks after irradiation. Furthermore ceasing treatment at 28 weeks did not result in a re-emergence of lung damage at late times (48 weeks) post irradiation. We observed that the drugs appear to be somewhat less effective in the Fischer rats relative to Sprague-Dawley rats. We also found evidence of increased lung sensitivity to irradiation in the adolescent rats used in this study relative to that observed in our previous study with young adult rats. Further studies are needed to confirm this observation. Although the initial impetus for these studies related to the treatment of accidentally exposed individuals, genistein is also of interest therapeutically for cancer patients. It has been reported to inhibit invasion, metastasis and angiogenesis in vitro and in vivo in a number of cancers, including breast cancer, although recent data have questioned its effect in prostate cancers (49–57). A recent pilot study has reported that treatment with an isoflavone cocktail including genistein can reduce side effects after radiation treatment of prostate cancer (58). Our results suggest that this agent should be tested in clinical studies involving lung irradiation and the possibility of radiation-induced lung injury.
Supplementary Material
Acknowledgments
This work was supported by funds from an NIAID/NIH U19 program (U19 AI-067734) and by funds from the Canadian Institutes of Health Research (no. 144089). Partial support was also provided by the Ontario Ministry of Health and Long Term Care. The views expressed do not necessarily reflect those of OMHLTC.
Footnotes
Editor’s note. The online version of this article (DOI: 10.1667/ RR2954.1) contains supplementary information that is available to all authorized users.
References
- 1.Hamada N, Ogino H. Food safety regulations: what we learned from the Fukushima nuclear accident. J Environ Radioact. 2012;111:83–99. doi: 10.1016/j.jenvrad.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 2.Drouet M, Herodin F. Radiation victim management and the haematologist in the future: time to revisit therapeutic guidelines? Int J Radiat Biol. 2010;86(8):636–48. doi: 10.3109/09553001003789604. [DOI] [PubMed] [Google Scholar]
- 3.Vlasov PA, Kvacheva Iu E. The pathomorphology of the pulmonary infectious complications in acute radiation sickness based on the autopsy data from persons who died as a result of the accident at the Chernobyl Atomic Electric Power Station. Ter Arkh. 1996;68(3):23–6. [PubMed] [Google Scholar]
- 4.Goans RE, Wald N. Radiation accidents with multi-organ failure in the United States. BJR Suppl. 2005;27:41–6. [Google Scholar]
- 5.Hirama T, Akashi M. Multi-organ involvement in the patient who survived the Tokai-mura criticality accident. BJR Suppl. 2005;27:17–20. [Google Scholar]
- 6.Svendsen ER, Kolpakov IE, Stepanova YI, Vdovenko VY, Naboka MV, Mousseau TA, et al. 137Cesium exposure and spirometry measures in Ukrainian children affected by the Chernobyl nuclear incident. Environ Health Perspect. 2010;118(5):720–5. doi: 10.1289/ehp.0901412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uozaki H, Fukayama M, Nakagawa K, Ishikawa T, Misawa S, Doi M, et al. The pathology of multi-organ involvement: two autopsy cases from the Tokai-mura criticality accident. BJR. 2005;27:13–6. [Google Scholar]
- 8.Kim K, Pollard JM, Norris AJ, McDonald JT, Sun Y, Micewicz E, et al. High-throughput screening identifies two classes of antibiotics as radioprotectors: tetracyclines and fluoroquinolones. Clin Cancer Res. 2009;15(23):7238–45. doi: 10.1158/1078-0432.CCR-09-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17(2):141–8. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Williams JP, Johnston CJ, Finkelstein JN. Treatment for radiation-induced pulmonary late effects: spoiled for choice or looking in the wrong direction? Curr Drug Targets. 2010;11(11):1386–94. doi: 10.2174/1389450111009011386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao M, Whitnall MH. Pharmacological countermeasures for the acute radiation syndrome. Curr Mol Pharmacol. 2009;2(1):122–33. doi: 10.2174/1874467210902010122. [DOI] [PubMed] [Google Scholar]
- 12.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–43. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 13.Hensley ML, Schuchter LM, Lindley C, Meropol NJ, Cohen GI, Broder G, et al. American Society of Clinical Oncology clinical practice guidelines for the use of chemotherapy and radiotherapy protectants. J Clin Oncol. 1999;17(10):3333–55. doi: 10.1200/JCO.1999.17.10.3333. [DOI] [PubMed] [Google Scholar]
- 14.Stone HB, Moulder JE, Coleman CN, Ang KK, Anscher MS, Barcellos-Hoff MH, et al. Models for evaluating agents intended for the prophylaxis, mitigation and treatment of radiation injuries. Report of an NCI Workshop, December 3–4, 2003. Radiat Res. 2004;162(6):711–28. doi: 10.1667/rr3276. [DOI] [PubMed] [Google Scholar]
- 15.Day RM, Barshishat-Kupper M, Mog SR, McCart EA, Prasanna PG, Davis TA, et al. Genistein protects against biomarkers of delayed lung sequelae in mice surviving high-dose total body irradiation. J Radiat Res (Tokyo) 2008;49(4):361–72. doi: 10.1269/jrr.07121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173(5):602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of radiation-induced lung injury by Genistein and EUK-207. Int J Radiat Biol. 2011;87(8):889–901. doi: 10.3109/09553002.2011.583315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahmood J, Jelveh S, Calveley V, Zaidi A, Doctrow SR, Hill RP. Mitigation of lung injury after accidental exposure to radiation. Radiat Res. 2011;176(6):770–80. doi: 10.1667/rr2562.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang HJ, Doctrow SR, Xu L, Oberley LW, Beecher B, Morrison J, et al. Redox modulation of the liver with chronic antioxidant enzyme mimetic treatment prevents age-related oxidative damage associated with environmental stress. Faseb J. 2004;18(13):1547–9. doi: 10.1096/fj.04-1629fje. [DOI] [PubMed] [Google Scholar]
- 20.Clausen A, Doctrow S, Baudry M. Prevention of cognitive deficits and brain oxidative stress with superoxide dismutase/catalase mimetics in aged mice. Neurobiol Aging. 2010;31(3):425–33. doi: 10.1016/j.neurobiolaging.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, et al. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987;262(12):5592–5. [PubMed] [Google Scholar]
- 22.Baxa DM, Yoshimura FK. Genistein reduces NF-kappa B in T lymphoma cells via a caspase-mediated cleavage of I kappa B alpha. Biochem Pharmacol. 2003;66(6):1009–18. doi: 10.1016/s0006-2952(03)00415-5. [DOI] [PubMed] [Google Scholar]
- 23.Choi C, Cho H, Park J, Cho C, Song Y. Suppressive effects of genistein on oxidative stress and NFkappaB activation in RAW 264. 7 macrophages. Biosci Biotechnol Biochem. 2003;67(9):1916–22. doi: 10.1271/bbb.67.1916. [DOI] [PubMed] [Google Scholar]
- 24.Michael McClain R, Wolz E, Davidovich A, Pfannkuch F, Edwards JA, Bausch J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem Toxicol. 2006;44(1):56–80. doi: 10.1016/j.fct.2005.05.021. [DOI] [PubMed] [Google Scholar]
- 25.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79(2):231–8. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Liu R, Liu IY, Bi X, Thompson RF, Doctrow SR, Malfroy B, et al. Reversal of age-related learning deficits and brain oxidative stress in mice with superoxide dismutase/catalase mimetics. Proc Natl Acad Sci U S A. 2003;100(14):8526–31. doi: 10.1073/pnas.1332809100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng J, Stevenson FF, Doctrow SR, Andersen JK. Superoxide dismutase/catalase mimetics are neuroprotective against selective paraquat-mediated dopaminergic neuron death in the substantial nigra: implications for Parkinson disease. J Biol Chem. 2005;280(32):29194–8. doi: 10.1074/jbc.M500984200. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal RA, Huffman KD, Fisette LW, Damphousse CA, Callaway WB, Malfroy B, et al. Orally available Mn porphyrins with superoxide dismutase and catalase activities. J Biol Inorg Chem. 2009;14(6):979–91. doi: 10.1007/s00775-009-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vorotnikova E, Rosenthal RA, Tries M, Doctrow SR, Braunhut SJ. Novel synthetic SOD/catalase mimetics can mitigate capillary endothelial cell apoptosis caused by ionizing radiation. Radiat Res. 2010;173(6):748–59. doi: 10.1667/RR1948.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams JP, Jackson IL, Shah JR, Czarniecki CW, Maidment BW, Dicarlo AL. Animal models and medical countermeasures development for radiation-induced lung damage: report from an NIAID workshop. Radiat Res. 2012;177(5):e0025–39. doi: 10.1667/rrol04.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Dev Cogn Neurosci. 2011;1(4):444–58. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaneko T, Tahara S, Tanno M, Taguchi T. Effect of age on the induction of 8-oxo-2′-deoxyguanosine-releasing enzyme in rat liver by gamma-ray irradiation. Arch Gerontol Geriatr. 2003;36(1):23–35. doi: 10.1016/s0167-4943(02)00056-0. [DOI] [PubMed] [Google Scholar]
- 33.Malfroy-Camine B, Doctrow S. U.S. Patent Number 7, 123, 527. Cyclic salen Mn compounds as scavengers for oxygen radicals and useful as antioxidants in the treatment and prevention of disease. 2006
- 34.Rosenthal RA, Fish B, Hill RP, Huffman KD, Lazarova Z, Mahmood J, et al. Salen Mn complexes mitigate radiation injury in normal tissues. Anticancer Agents Med Chem. 2011;11(4):359–72. doi: 10.2174/187152011795677490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clarkson R, Lindsay PE, Ansell S, Wilson G, Jelveh S, Hill RP, et al. Characterization of image quality and image-guidance performance of a preclinical microirradiator. Med Phys. 2011;38(2):845–56. doi: 10.1118/1.3533947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17(2):89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 37.Robbins ME, Zhao W. Chronic oxidative stress and radiation-induced late normal tissue injury: a review. Int J Radiat Biol. 2004;80(4):251–9. doi: 10.1080/09553000410001692726. [DOI] [PubMed] [Google Scholar]
- 38.van Eerde MR, Kampinga HH, Szabo BG, Vujaskovic Z. Comparison of three rat strains for development of radiation-induced lung injury after hemithoracic irradiation. Radiother Oncol. 2001;58(3):313–6. doi: 10.1016/s0167-8140(00)00301-7. [DOI] [PubMed] [Google Scholar]
- 39.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, et al. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67(2):573–80. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gauter-Fleckenstein B, Fleckenstein K, Owzar K, Jiang C, Reboucas JS, Batinic-Haberle I, et al. Early and late administration of MnTE-2-PyP5+ in mitigation and treatment of radiation-induced lung damage. Free Radic Biol Med. 2010;48(8):1034–43. doi: 10.1016/j.freeradbiomed.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siemann DW, Sutherland RM. A comparison of the pharmacokinetics of multiple and single dose administrations of adriamycin. Int J Radiat Oncol Biol Phys. 1979;5(8):1271–4. doi: 10.1016/0360-3016(79)90652-7. [DOI] [PubMed] [Google Scholar]
- 42.Travis EL, Bucci L, Fang MZ. Residual damage in mouse lungs at long intervals after cyclophosphamide treatment. Cancer Res. 1990;50(7):2139–45. [PubMed] [Google Scholar]
- 43.van den Aardweg GJ, Olofsen-van Acht MJ, van Hooije CM, Levendag PC. Radiation-induced rectal complications are not influenced by age: a dose fractionation study in the rat. Radiat Res. 2003;159(5):642–50. doi: 10.1667/0033-7587(2003)159[0642:rrcani]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 44.Diao RY, Song LW, Wang SX, Li M. Biological effect of (60)Cogamma ray on alveolar type II cells and interstitial cells of alveoliar septum in radiation pulmonary injury. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi (Chin J Cell Mol Immun) 2008;24(2):119–21. [PubMed] [Google Scholar]
- 45.Osterreicher J, Pejchal J, Skopek J, Mokry J, Vilasova Z, Psutka J, et al. Role of type II pneumocytes in pathogenesis of radiation pneumonitis: dose response of radiation-induced lung changes in the transient high vascular permeability period. Exp Toxicol Pathol. 2004;6(3):181–7. doi: 10.1016/j.etp.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 46.Ryu SY, Do SH, Chung JY, Kim TH, Kim SH, Choi CY, et al. Activation of MAP kinases during progression of radiation-induced pneumonitis in rats. Hum Exp Toxicol. 2010;30(8):876–83. doi: 10.1177/0960327110382562. [DOI] [PubMed] [Google Scholar]
- 47.Travis EL. Organizational response of normal tissues to irradiation. Semin Radiat Oncol. 2001;11(3):184–96. doi: 10.1053/srao.2001.25243. [DOI] [PubMed] [Google Scholar]
- 48.Krasin MJ, Constine LS, Friedman DL, Marks LB. Radiation-related treatment effects across the age spectrum: differences and similarities or what the old and young can learn from each other. Semin Radiat Oncol. 2010;20(1):21–9. doi: 10.1016/j.semradonc.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Magee PJ, McGlynn H, Rowland IR. Differential effects of isoflavones and lignans on invasiveness of MDA-MB-231 breast cancer cells in vitro. Cancer Lett. 2004;208(1):35–41. doi: 10.1016/j.canlet.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Vantyghem SA, Wilson SM, Postenka CO, Al-Katib W, Tuck AB, Chambers AF. Dietary genistein reduces metastasis in a postsurgical orthotopic breast cancer model. Cancer Res. 2005;65(8):3396–403. doi: 10.1158/0008-5472.CAN-04-4109. [DOI] [PubMed] [Google Scholar]
- 51.Farina HG, Pomies M, Alonso DF, Gomez DE. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol Rep. 2006;16(4):885–91. [PubMed] [Google Scholar]
- 52.Singh AV, Franke AA, Blackburn GL, Zhou JR. Soy phytochemicals prevent orthotopic growth and metastasis of bladder cancer in mice by alterations of cancer cell proliferation and apoptosis and tumor angiogenesis. Cancer Res. 2006;66(3):1851–8. doi: 10.1158/0008-5472.CAN-05-1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lakshman M, Xu L, Ananthanarayanan V, Cooper J, Takimoto CH, Helenowski I, et al. Dietary genistein inhibits metastasis of human prostate cancer in mice. Cancer Res. 2008;68(6):2024–32. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 54.Chambers AF. Influence of diet on metastasis and tumor dormancy. Clin Exp Metastasis. 2009;26(1):61–6. doi: 10.1007/s10585-008-9164-4. [DOI] [PubMed] [Google Scholar]
- 55.El Touny LH, Banerjee PP. Identification of a biphasic role for genistein in the regulation of prostate cancer growth and metastasis. Cancer Res. 2009;69(8):3695–703. doi: 10.1158/0008-5472.CAN-08-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Martinez-Montemayor MM, Otero-Franqui E, Martinez J, De La Mota-Peynado A, Cubano LA, Dharmawardhane S. Individual and combined soy isoflavones exert differential effects on metastatic cancer progression. Clin Exp Metastasis. 2010;27(7):465–80. doi: 10.1007/s10585-010-9336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nakamura H, Wang Y, Kurita T, Adomat H, Cunha GR, Wang Y. Genistein increases epidermal growth factor receptor signaling and promotes tumor progression in advanced human prostate cancer. PLoS One. 2011;6(5):e20034. doi: 10.1371/journal.pone.0020034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, et al. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62(7):996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.