Abstract
In this study, target compounds 5–12 were synthesized via acid amine coupling of ibuprofen and naproxen with methyl ester derivatives of amino acids, namely, l-proline, sarcosine, l-tyrosine, and l-glutamic acid. When tested for anti-inflammatory activity using the acute carrageenan-induced hind paw method in rats, compounds 5–12 showed significantly greater anti-inflammatory activity, in the range of 40.64%–87.82%, compared with a placebo control group (P < 0.001). Among the newly synthesized compounds 5–12, naproxen derivatives 9–12 with anti-inflammatory activity ranging between 66.99% and 87.82% showed significantly higher (P < 0.05) potency than ibuprofen derivatives 5–8 with inhibition in the range of 22.03%–52.91% and control groups of ibuprofen (76.34%) or naproxen (75.59%, P < 0.05). Moreover, derivatives 9–12 derived from naproxen, in particular compounds 9 and 10 which achieved 83.91% and 87.82% inhibition of inflammation, respectively, showed significantly (P < 0.05) higher potency than naproxen derivatives 11 and 12. Notably, among naproxen derivatives 9–12, the gastric ulcerogenicity for 9 (ulcer index 11.73) and 10 (ulcer index 12.30) was found to be significantly lower (P < 0.05) than that of the active ibuprofen and naproxen control groups with ulcer indices of 22.87 and 24.13, respectively. On the other hand, naproxen derivatives 9–11 showed significant inhibition (P < 0.05) of prostaglandin E2 synthesis when compared with the active control group receiving indomethacin, suggesting a correlation between the observed low ulcerogenicity and effect on prostaglandin E2 synthesis for compounds 9 and 10. However, significant inhibition of prostaglandin E2 observed for naproxen derivative 11 (107.51) did not correlate with its observed ulcer index (16.84). Our overall findings for carbamoylmethyl ester derivatives named 5–12 clearly suggest that the compounds showing potent antiinflammatory effect.
Keywords: carbamoylmethyl ester, anti-inflammatory, prostaglandin E2, inhibitory properties
Introduction
Ibuprofen and naproxen are well known nonsteroidal anti-inflammatory drugs (NSAIDs) belonging to the family of propionic acid derivatives. They often cause upper gastrointestinal damage, including lesions, peptic ulcers, bleeding, and perforation which are attributed to the presence of a free carboxylic acid group responsible for local irritation and ulceration of the gastrointestinal mucosa and local blockade of prostaglandin biosynthesis.1 NSAIDs belonging to the propionic acid class have greater selectivity for inhibition of constitutively expressed cytoprotective cyclo-oxygenase 1 than inducible cyclo-oxygenase 2, so long-term therapy with nonselective NSAIDs may cause gastrointestinal complications, ranging from stomach irritation to life-threatening gastrointestinal ulceration and bleeding.2–5 Mounting evidence has suggested that structural modification, especially esterification, of the free carboxylic moiety of representative NSAIDs maintains anti-inflammatory activity and a reduced potential for ulcerogenicity.6–11 Prompted by these observations and applying previously described efficient chemical procedures, we report herein the synthesis of novel carbamoyl derivatives 5–12 containing various amide-linked amino acid methyl esters.12 To achieve the desired target compounds 5–12, the carboxylic acid moieties of ibuprofen and naproxen were reacted with methyl ester derivatives of amino acids, including l-proline, sarcosine, l-tyrosine, and l-glutamic acid, in the presence of thionyl chloride and benzotriazole. Because of their nontoxicity, varying degrees of lipophilicity, and lower incidence of side effects, the amino acid group was chosen especially for amidation to mask the free carboxylic acid group of ibuprofen and naproxen.13–17 The newly synthesized compounds 5–12 were screened for anti-inflammatory and ulcerogenic properties, and their ability to inhibit prostaglandin E2 synthesis.
Materials and methods
General
The melting points are uncorrected and were determined in open capillaries using Buechi 512 Dr Tottoli apparatus. 1H-NMR spectra were recorded on a WC 300 spectrometer (Bruker, Billerica, MA, USA) with tetramethylsilane as the internal standard. Chemical shifts are reported in ppm downfield from internal tetramethylsilane used as the reference. 1H-NMR signals are reported in order: multiplicity (s, single; d, doublet; t, triplet; dt, doublet of triplet; q, quantet; m, multiplet), number of protons, and approximate coupling constants in Hz. 13C-NMR spectra were recorded on a DPX 400 Avance (75 mHz) instrument (Bruker). Chemical shifts are reported in ppm downfield from internal tetramethylsilane used as the reference. Elemental analyses were performed on 240B and 240C instruments (Perkin-Elmer, Boston, MA, USA). Analyses (C, H, N) indicated by the symbols of elements were within ±0.4% of the theoretical values. Chromatographic separations were done using a Chromatotron model 7924 (Harrison Research, Union, NJ, USA) with 4 mm layers of silica gel 60 PF containing gypsum (Merck, Whitehouse Station, NJ, USA). Electron ionization mass spectra were recorded using a Finnigan MAT CH7A (70 eV), a Finnigan MAT 711 (80 eV), or a Kratos MS 25 RF (70 eV) Shimadzu, Japan. +FAB-MS spectra were recorded on a Finnigan MAT CH5DF instrument (Thermo Fischer Scientific, MA, USA) (xenon, dimethyl sulfoxide/glycerol).
Preparation of benzotriazole-activated ibuprofen and naproxen 3 and 4
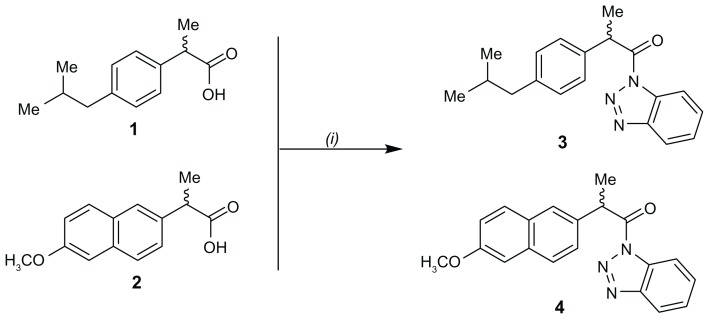
Thionyl chloride (2.64 mmol) was added to a solution of 1H-benzotriazole (8.7 mmol) in dry CH2Cl2 (60 mL) at room temperature and the reaction was stirred for 20 minutes. Ibuprofen (1) (2.2 mmol) or naproxen (2) (2.2 mmol) were added and the mixtures were stirred for 4 hours at room temperature. The white precipitate formed was filtered off and the filtrate was concentrated under reduced pressure. Each residue was diluted with 50 mL of ethyl acetate and each solution was washed with 4 N HCl (3 × 15 mL) and dried over MgSO4. Removal of the solvent under reduced pressure gave products 3 and 4, which were recrystallized from hexanes to give 3 and 4 as pure products.12
1-(1H-benzo[d][1,2,3]triazol-1-yl)-2-(4-isobutylphenyl)propan-1-one (3)
White microcrystals (95%) melting point 76.0°C–77.0°C [12]; 1H-NMR (CDCl3): 8.29 (d, J = 8.2 Hz, 1H) 8.07 (d, J = 8.1 Hz, 1H), 7.61 (t, J = 8.1 Hz, 1H), 7.4–7.5 (m, 3H), 7.11 (d, J = 7.6 Hz, 2H), 5.41 (q, J = 7.0 Hz, 1H), 2.41 (d, J = 7.2 Hz, 2H), 1.78–1.90 (m, 1H), 1.75 (d, J = 6.9 Hz, 3H), 0.86 (d, J = 6.4 Hz, 6H); 13C-NMR (CDCl3): 173.8, 146.4, 141.3, 136.6, 131.5, 130.5, 129.8, 128.0, 126.3, 120.3, 114.7, 45.2, 44.6, 30.3, 22.6,18.8; analysis calculated for C19H21 N3O: C, 74.24; H, 6.89; N, 13.67. Found: C, 74.29; H, 6.94; N, 13.83.
1-(1H-benzo[d][1,2,3]triazol-1-yl)-2-(6-methoxynaphthalen-2-yl)propan-1-one (4)
Microcrystals (92%) melting point 181.0°C–182.0°C [12]; 1H-NMR (CDCl3): 8.29 (d, J = 8.3 Hz, 1H), 8.07 (d, J = 10.1 Hz, 1H), 7.89 (d, J = 1.1 Hz, 1H), 7.70 (d, J = 8.6 Hz, 2H), 7.59–7.66 (m, 2H), 7.43–7.50 (m, 1H), 7.11 (dd, J = 8.9; 2.5 Hz, 1H), 7.07 (d, J = 2.3 Hz, 1H), 5.55 (q, J = 7.0 Hz, 1H), 3.89 (s, 3H), 1.83 (d, J = 7.0 Hz, 3H),; 13C-NMR (CDCl3): 173.5, 157.8, 146.2, 134.3, 133.8, 131.3, 130.3, 129.3, 128.9, 127.4, 126.9, 126.4, 126.1, 120.1, 119.1, 114.5, 105.5, 55.3, 44.7, 18.6; analysis calculated for C20H17 N3O2: C, 72.49; H, 5.17; N, 12.68. Found: C, 72.14; H, 5.28; N, 12.69.
Preparation of ibuprofen and naproxen containing derivatives 5–12
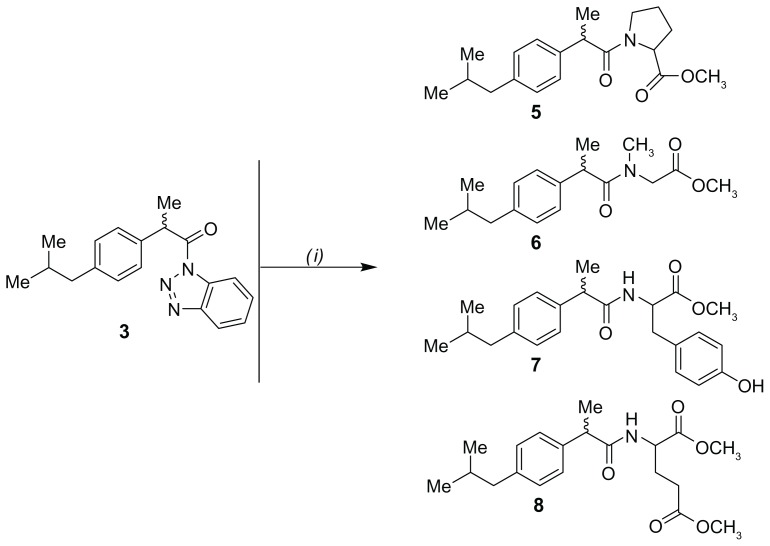
The appropriate benzotriazole 3 and 4 (0.65 mmol) was added to a 0.65 mmol solution of amino acid methyl ester in acetonitrile in the presence of triethylamine (1.8 mmol). Each reaction mixture was stirred at room temperature for 6–24 hours, and completion of the reaction was checked by thin layer chromatography using ethyl acetate-hexane (1:1) as an eluting solvent. Finally, the reaction was quenched by addition of water (10 mL), and the organic compound was extracted with ethyl acetate (3 × 20 mL). The organic layer was washed with brine (3 × 20 mL), dried over anhydrous Na2SO4, and concentrated in a vacuum. Purification was done by rotary chromatography using silica gel 60 PF containing gypsum and ethyl acetate-hexane as an eluting solvent to yield racemic products 5–12.
Methyl-1-(2-[4-isobutylphenyl]propanoyl) pyrrolidine-2-carboxylate (5)
Yield 82%, melting point 101°C–102°C; IR (KBr): ν(cm-1) 1743 (ester), 1668 (amide); 1H-NMR (CDCl3): δ ppm = 7.19 (d, J = 7.9 Hz, 2H, Ibu-2,6H), 7.11 (d, J = 7.9 Hz, 2H, Ibu-3,5H), 4.39 (q, J = 6.7 Hz, 1H, ArCH), 3.75 (s, 3H, COOCH3), 3.68–3.61 (m, 1H, pro-CH), 2.41 [d, J = 7.4 Hz, 2H, (CH3)2CHCH2], 2.35 (m, 2H, pro-CH2), 2.03 (m, 2H, pro-CH2), 1.98 (m, 2H, pro-CH2), 1.61 (heptet, J = 6.7 Hz, 1H, (CH3)2CHCH2), 1.50 (d, J = 6.7 Hz, 3H, CHCH3); 0.80 (d, J = 6.7 Hz, 6H, (CH3)2CHCH2); 13C-NMR (CDCl3) δ ppm = 173.10, 170.51, 129.69, 127.41, 59.71, 53.90, 47.86, 46.40, 45.05, 28.41, 26.44, 23.40, 22.41, 18.42; MS: m/z (%) 318 (M+, 90); analysis calculated for C19H27NO3: C, 71.89; H, 8.57; N, 4.41. Found: C, 71.68; H, 8.43; N, 4.51.
Methyl-2-(2-[4-isobutylphenyl]-N-methylpropanamido)acetate (6)
Yield 86%, melting point 89°C–90°C; IR (KBr): ν(cm-1) 1733 (ester), 1667 (amide); 1H-NMR (CDCl3): δ ppm = 7.19 (d, J = 7.9 Hz, 2H, Ibu-2,6H), 7.11 (d, J = 7.9 Hz, 2H, Ibu-3,5H), 4.04 (s, 2H, CH2COOCH3), 3.74 (s, 3H, COOCH3), 3.68–3.61 (m, 1H), 3.26 (s, 3H, NCH3), 2.41 (d, J = 7.4 Hz, 2H, (CH3)2CHCH2), 1.61 [heptet, J = 6.7 Hz, 1H, (CH3)2CHCH2], 1.50 (d, J = 6.7 Hz, 3H, CHCH3), 0.80 [d, J = 6.7 Hz, 6H, (CH3)2CHCH2]; 13C-NMR (CDCl3) δ ppm = 172.61, 168.81, 129.69, 127.41, 53.50, 47.86, 45.06, 40.21, 32.41, 26.44, 22.41, 18.41; MS: m/z (%) 292 (M+, 90); analysis calculated for C17H25 NO3: C, 70.07; H, 8.65; N, 4.81. Found: C, 70.17; H, 8.84; N, 4.64.
Methyl-3-(4-hydroxyphenyl)-2-(2-[4-isobutylphenyl]propanamido)propanoate (7)
Yield 54%, melting point 133°C–134°C (56–58°C for S(+)-Dexibuprofen derivatie)23; IR (KBr): ν(cm-1) 1734 (ester), 1668 (amide); 1H-NMR (CDCl3): δ ppm = 7.26 (d, J = 7.9 Hz, 2H, Ibu-2,6H), 7.22–7.09 (m, 4H, Ibu-H, Ph-H), 6.69 (d, J = 7.25 Hz, 2H, Ph-H), 6.67 (bs, 1H, CONH), 5.86 (s, 1H, Ph-OH), 4.87 (dd, J = 2.1 Hz, 5.9 Hz, 1H, COOCH), 3.72 (s, 3H, COOCH3), 3.69–3.61 (m, 1H, CHCH3), 3.29–3.13 (m, 2H, ArCH2), 2.41 (d, J = 7.4 Hz, 2H, (CH3)2CHCH2), 1.61 (heptet, J = 6.7 Hz, 1H, [CH3)2CHCH2], 1.50 (d, J = 6.7 Hz, 3H, CHCH3), 0.80 (d, J = 6.7 Hz, 6H, [CH3)2CHCH2]; 13C-NMR (CDCl3) δ ppm = 173.10, 170.11, 133.81, 129.67, 129.51, 128.21, 54.21, 53.70, 47.86, 45.05, 35.71, 26.44, 22.41, 18.42; MS: m/z (%) 383 (M+, 90); analysis calculated for C23H29 NO4: C, 72.04; H, 7.62; N, 3.65. Found: C, 72.28; H, 7.93; N, 4.01.
Dimethyl-2-(2-[4-isobutylphenyl]propanamido)pentanedioate (8)
Yield 87%, melting point 103°C–104°C; IR (KBr): ν(cm-1) 1738 (ester), 1662 (amide); 1H-NMR (CDCl3): δ ppm = 7.19 (d, J = 7.9 Hz, 2H, Ph-2H,6H), 7.11 (d, J = 7.9 Hz, 2H, Ph-3, 5H), 6.67 (bs, 1H, CONH), 4.58 (dd, J = 2.1 Hz, 5.9 Hz, 1H, COOCH), 2.41 [d, J = 7.4 Hz, 2H, (CH3)2CHCH2], 3.68– 3.60 (m, 1H, CHCH3), 3.65 (s, 3H, COOCH3), 3.60 (s, 3H, COOCH3), 2.17 (dt, J = 7.5 Hz, 14.1 Hz, 2H, COOCH2CH2), 1.92 (dt, J = 7.9 Hz, 14.2 Hz, 2H, COOCH2CH2), 1.61 [heptet, J = 6.7 Hz, 1H, (CH3)2CHCH2], 1.50 (d, J = 6.7 Hz, 3H, CHCH3), 0.80 [d, J = 6.7 Hz, 6H, (CH3)2CHCH2]; 13C-NMR (CDCl3) δ ppm = 173.10, 170.41, 129.69, 127.41, 53.70, 52.90, 47.86, 45.05, 39.20, 29.40, 26.44, 26.40, 22.41, 18.42; MS: m/z (%) 364 (M+, 100); analysis calculated for C20H29 NO5: C, 66.09; H, 8.04; N, 3.85. Found: C, 65.98; H, 8.24; N, 4.03.
Methyl-1-(2-[6-methoxynaphthalen-2-yl]propanoyl)pyrrolidine-2-carboxylate (9)
Yield 80%, melting point 109°C–110°C; IR (KBr): ν(cm-1): 1742 (ester), 1668 (amide); 1H-NMR δ/ppm (CDCl3): 6.75–7.77 (m, 6 H, Naphth-H), 4.02 (q, J = 7.2 Hz, 1H, CHCH3), 3.96 (s, 3H, OCH3), 1.69 (d, J = 7.2 Hz, 3H, CHCH3), 2.35 (m, 2H, pro-CH2), 2.03 (m, 2H, pro-CH2), 1.98 (m, 2H, pro-CH2-CH2), 3.71 (s, 3H, COOCH3), 3.68–3.61 (m, 1H, pro-CH); 13C-NMR (CDCl3) δ ppm = 175.30, 171.50, 156.40, 129.30, 128.90, 127.40, 126.40, 126.10, 117.60, 105.50, 66.10, 55.30, 51.70, 50.20, 24.10, 23.90; MS: m/z (%) 341 (M+, 100); analysis calculated for C20H23NO4: C, 70.36; H, 6.79; N, 4.10. Found: C, 70.68; H, 6.83; N, 4.07.
Methyl-2-(2-[6-methoxynaphthalen-2-yl]-N-methylpropanamido)acetate (10)
Yield 89%, melting point 113°C–114°C; IR (KBr): ν(cm-1): 1733 (ester), 1667 (amide); 1H-NMR δ/ppm (CDCl3): 6.75–7.78 (m, 6 H, Naphth-H), 4.01–4.05 (m, 3H, CHCH3, CH2COOCH3), 3.96 (s, 3H, OCH3), 3.74 (s, 3H, COOCH3), 3.26 (s, 3H, NCH3), 1.69 (d, J = 6.2 Hz, 3H, CHCH3); 13C-NMR (CDCl3) δ ppm = 171.5, 158.4, 129.3, 128.9, 127.4, 126.4, 126.1, 117.6, 105.5, 66.1, 55.3, 51.7, 50.2, 24.1, 23.9; MS: m/z (%) 315 (M+, 90); analysis calculated for C18H21 NO4: C, 68.55; H, 6.71; N, 4.44. Found: C, 68.98; H, 6.75; N, 4.13.
Methyl-3-(4-hydroxyphenyl)-2-(2-[6-methoxynaphthalen-2-yl]propanamido) propanoate (11)
Yield 59%, melting point 138°C–139°C; IR (KBr): ν(cm-1): 1734 (ester), 1668 (amide); 1H-NMR δ/ppm (CDCl3): 6.75–7.77 (m, 8H, Naphth-H, Ph-H), 6.69 (d, J = 7.25 Hz, 2H, Ph-H), 6.67 (bs, 1H, CONH), 5.86 (s, 1H, Ph-OH), 4.87 (dd, J = 2.1 Hz, 5.9 Hz, 1H, COOCH), 4.02 (q, J = 7.2 Hz, 1H, CHCH3), 3.96 (s, 3H, OCH3), 1.69 (d, J = 7.2 Hz, 3H, CHCH3), 3.72 (s, 3H, COOCH3), 3.29–3.13 (m, 2H, ArCH2); 13C-NMR (CDCl3) δ ppm = 173.2, 172.8, 156.9, 137.6, 136.9, 133.0, 129.1, 129.0, 128.2, 128.0, 126.5, 126.3, 126.2, 125.3, 118.4, 105.5, 55.0, 53.2, 51.1, 44.4, 36.6, 18.5; MS: m/z (%) 407 (M+, 100); analysis calculated for C24H25NO5: C, 70.74; H, 6.18; N, 3.44. Found: C, 70.68; H, 6.73; N, 3.21.
Dimethyl-2-(2-[6-methoxynaphthalen-2-yl]propanamido)pentanedioate (12)
Yield 83%, melting point 107°C–108°C; IR (KBr): ν(cm-1): 1738 (ester), 1662 (amide); 1H-NMR δ/ppm (CDCl3): 6.75–7.77 (m, 6H, Naphth-H), 6.67 (bs, 1H, CONH), 4.58 (dd, J = 2.1 Hz, 5.9 Hz, 1H, COOCH), 4.02 (q, J = 7.2 Hz, 1H, CHCH3), 3.96 (s, 3H, OCH3), 3.65 (s, 3H, COOCH3), 3.60 (s, 3H, COOCH3), 1.69 (d, J = 7.2 Hz, 3H, CHCH3), 2.17 (dt, J = 7.5 Hz, 14.1 Hz, 2H, COOCH2CH2), 1.92 (dt, J = 7.9 Hz, 14.2 Hz, 2H, COOCH2CH2); 13C-NMR (CDCl3) δ ppm = 173.1, 170.3, 129.6, 127.4, 53.7, 51.1, 51.2, 47.8, 45.1, 39.2, 29.3, 26.4, 26.5, 22.4, 18.5; MS: m/z (%) 401 (M+, 90); analysis calculated for C22H25NO6: C, 65.82; H, 6.78; N, 3.49. Found: C, 65.98; H, 6.34; N, 3.05.
Pharmacology
Male Wistar rats weighing 170–200 g (five per group) were used for the in vivo anti-inflammatory and ulcerogenicity studies. Age-matched controls (n = 5) were used for each test group. The animals were housed in groups of five per cage under standard temperature, humidity, and laboratory conditions. All experimental procedures were carried out according to standard guidelines and were approved by the Institutional Animal Ethics Committee of the Faculty of Medicine and Health Sciences/United Arab Emirates University (A7–13).
Anti-inflammatory activity
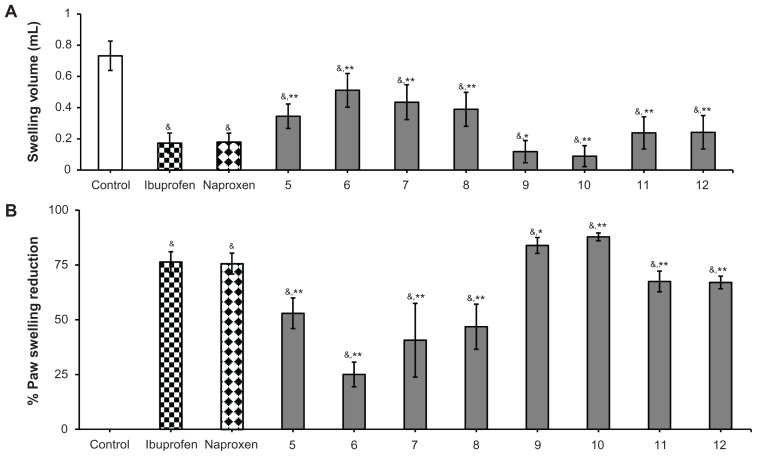
The anti-inflammatory activity of the study compounds was determined in vivo using the standard acute carrageenan-induced paw edema method in rats.18–20,23 Male Wistar rats weighing 170–200 g were randomly divided into 11 groups of five animals each. The reference compounds ibuprofen (50 mg/kg, 0.242 mmol/kg) and naproxen (50 mg/kg, 0.271 mmol/kg), as well as the study compounds (5–8 at 0.242 mmol/kg and 9–12 at 0.271 mmol/kg) dissolved in dimethyl sulfoxide (1.5%), were administered intraperitoneally, while the placebo control group received only dimethyl sulfoxide (1.5%) one hour before induction of inflammation. Carrageenan paw edema was induced by subcutaneous injection of a 1% solution of carrageenan in saline (0.1 mL per rat) into the right hind paw. Paw volumes were measured volumetrically after 4 hours using a plethysmometer (7140, Ugo Basile, Comerio VA, Italy) and compared with the initial hind paw volume of each rat for determining the edema volume. Anti-inflammatory activity was expressed as mean swelling volume ± standard error of the mean (SEM) and mean ± SEM percentage inhibition of edema in treated animals in comparison with the active control group (Figure 1A and B):
Figure 1.
Anti-inflammatory activity of ibuprofen derivatives 5–8 and naproxen derivatives 9–12 in rats. (A) Swelling volumes are expressed as the mean ± standard error of five replicates. (B) Percentage of inhibition of edema expressed as means of five replicates ± standard error of the mean.
Notes: Doses administered: ibuprofen 50 mg/kg, (0.242 mmol/kg); ibuprofen containing derivatives 5–8 (0.242 mmol/kg); naproxen 50 mg (0.217 mmol/kg); naproxen containing derivatives 9–12 (0.217 mmol). *Significantly different from control value of ibuprofen and naproxen (P < 0.05); **significantly different from control value of ibuprofen and naproxen (P < 0.01); &significantly different from placebo control value (P < 0.001). The significant difference between groups was tested by using one-way analysis of variance followed by Dunnett’s test at P < 0.05.
where Vc and Vt are the volumes of edema for the placebo control and drug-treated animal groups, respectively.
Ulcerogenicity
The ulcerogenic index was determined in male Wistar rats following a previously reported standard method.20,21 Rats weighing 170–200 g were divided into seven groups of five animals each. The animals were fasted for 18 hours before drug administration. The reference drug naproxen (50 mg/kg, 0.271 mmol/kg) and the test compounds (9–12 at 0.271 mmol/kg) were suspended in saline solution with the help of a few drops of Tween® 80 and administered orally for three successive days to the fasted rats. Animals in the placebo control group were given saline with a few drops of Tween 80. One hour after the final dose, the animals were sacrificed by cervical dislocation and their stomachs were excised, opened along the greater curvature, and rinsed with chilled saline. The gastric mucosa was examined for the presence of microscopic lesions and erosions. The ulcer index was determined by measuring the length (in millimeters) of the individual lesions in the mucosa using Vernier calipers, and the sum of lengths of all lesions in each stomach was regarded as the ulcer index.
Measurement of prostaglandin E2 levels
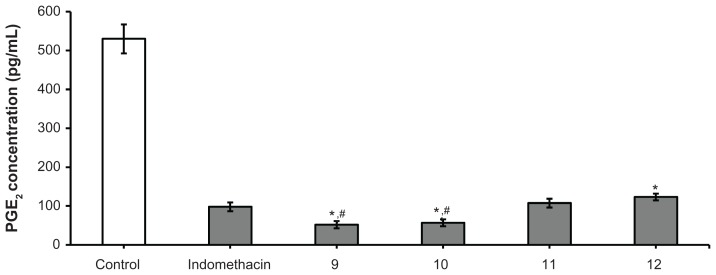
Measurement of prostaglandin E2 levels was done using the previously described standard 6-day air pouch method for rats.22,24 Male Wistar rats weighing 170–200 g were divided into six groups of five animals each. The air pouch was induced as follows: on the first day of the experiment, 20 mL of air was injected subcutaneously in the back of each rat. Two days later, another 10 mL of air was injected at the same site. On the fifth day after the first injection, a further 10 mL of air was injected into the pouch. One day later and one hour before injecting the pouch with carrageenan (2 mL of 1% solution in saline), four groups of animals were treated orally with the test compounds 9–12 at a dose of 0.271 mmol/kg body weight, one group with the reference drug indomethacin (active control group, 10 mg/kg body weight, 0.028 mmol/kg) suspended in saline solution with the aid of a few drops of Tween 80, and the last group with sterile saline (placebo control group). All injections were conducted under light ether anesthesia. Six hours after injection of carrageenan, the animals were lightly anesthetized with ether and the contents of the pouch were aspirated using a Pasteur pipette and transferred into graduated plastic tubes kept in ice. The bulk of the exudates was frozen and stored at −20°C until required for prostaglandin E2 assay. Prostaglandin E2 was measured using an enzyme-linked autoimmune assay (Beckman Biomek™ 1000 Automated Laboratory Workstation apparatus, Fullerton, CA, USA) technique using a prostaglandin E2 assay kit and the reference drug (indomethacin) according to the manufacturer’s specifications (Figure 2).
Figure 2.
Prostaglandin E2 inhibition of test compounds 9–12.
Notes: Doses administered: indomethacin 10 mg/kg; naproxen-containing derivatives 9–12 (0.217 mmol). Results are expressed as the mean ± standard error of six replicates. *Significantly different from control value of indomethacin (P < 0.05); #significantly different from 11 and 12 (P < 0.05). The significant difference between groups was tested by using one-way analysis of variance followed by Dunnett’s test at P < 0.05.
Acute toxicity
At the end of the experiments, all animals used in the anti-inflammatory study were observed closely for 48 hours. No morbidity or mortality was documented.
Data analysis
The results are expressed as the mean ± SEM per group. The statistical significance of differences between groups was determined by one-way analysis of variance followed by Dunnett’s test. SigmaStat software was used to determine statistical significance. A probability (P) value of less than 0.05 was considered to be statistically significant.
Results
Chemistry
The carboxylic acid moiety of the parent compounds ibuprofen (1) and naproxen (2) was activated by reaction with thionyl chloride and benzotriazole at room temperature to yield the intermediate benzotriazoles 3 and 4, respectively (Figure 3). The reaction of 3 and 4 with the corresponding amino acid methyl esters in acetonitrile in the presence of triethylamine successfully afforded good yields (79%–89%) of the ibuprofen derivatives 5–8 and naproxen derivatives 9–12, respectively (Figures 4 and 5). Chemical structures of all the newly developed compounds (5–12) were established through spectroscopic (infrared, 1H-NMR, 13C-NMR, mass spectrometry) as well as elemental analysis data.
Figure 3.
Synthesis of ibuprofen and naproxen benzotriazoles 3 and 4.
Note: (i) Benzotriazole, SOCl2, CH2Cl2, room temperature, 20 minutes.
Figure 4.
Synthetic pathway of ibuprofen containing derivatives 5–8.
Note: (i) Methyl ester of corresponding amino acid, acetonitrile, triethylamine, 20°C, 6–24 hours.
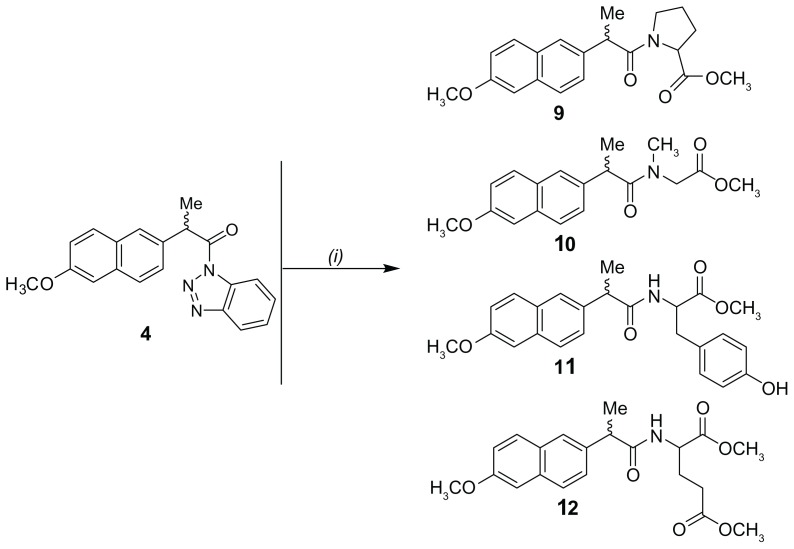
Figure 5.
Synthetic pathway of naproxen containing derivatives 9–12.
Note: (i) Methyl ester of corresponding amino acid, acetonitrile, triethylamine, 20°C, 6–24 hours.
Pharmacology
When subjected to anti-inflammatory activity, compounds 5–12 showed significantly higher anti-inflammatory activity in the range of 40.64%–87.82% when compared with the placebo control group. Among all the newly synthesized compounds 5–12, the naproxen derivatives 9–12 with anti-inflammatory activity ranging between 66.99% and 87.82% were found to be significantly more potent than their corresponding ibuprofen derivatives 5–8 with inhibition in the range of 22.03%–52.91%. Moreover, the anti-inflammatory activity measured for derivatives derived from naproxen (compounds 9–12) were significantly more potent than the control groups of ibuprofen (76.34%) or naproxen (75.59%). Noticeably, within the current series of derivatives derived from naproxen (9–12), especially 9 and 10, with respective 83.91% and 87.82% inhibition of inflammation, exhibited significantly higher potency than those of 11 and 12 (P < 0.05).
Furthermore, the gastric ulcerogenicity for naproxen derivatives 9 (ulcer index 11.73) and 10 (ulcer index 12.30) was found to be significantly lower than that of the ibuprofen and naproxen control groups which had ulcer indices of 22.87 and 24.13, respectively. Conversely, both naproxen derivatives (9 and 10) failed to show significant inhibition of prostaglandin E2 synthesis when compared with compounds 11 and 12 or the control group of indomethacin.
Discussion
Chemistry
Having free carboxylic acid moieties, ibuprofen and naproxen were modified into various carbamoylmethyl esters (5–12) using different aliphatic as well as aromatic amino acid methyl esters, which resulted in masking of the carboxylic acid moiety. The infrared spectra of compounds 5–12 revealed the presence of a strong band at ν = 1668 assignable to their carbonyl amide function, in addition to an amidic NH stretching vibration band at ν = 3332–3340. The 1H-NMR spectra of compounds 7–8 and 11–12 revealed the presence of an amidic -NH group as a sharp singlet signal at δ = 6.67, whereas the 1H-NMR spectra of 5 and 9 revealed the presence of a methyl group on the amide nitrogen of sarcosine as a sharp singlet signal at δ = 3.26. The mass spectra of compounds 5–12 exhibiting the parent molecular ion peaks confirmed the assumed structures.
Pharmacology
From the results obtained, it appears that animals pretreated with ibuprofen derivatives 5–8 were significantly protected against inflammation (23.3%–52.9% inhibition of edema) as compared with the placebo control group, but the anti-inflammatory effects observed for compounds 5–8 were significantly lower in comparison with those of the control groups ibuprofen-treated (P < 0.05), naproxen-treated (P < 0.05) control group, as well as naproxen derivatives (P < 0.05) (Figure 4)
Among the derivatives (compounds 5–12) investigated in the current study, our findings clearly suggest that structural modification via amidation of the carboxylic acid moiety of the parent compound naproxen (9–12) showed more potent antiinflammatory effect than derivatives derived from ibuprofen. Moreover, our results show that chemical modification of naproxen, especially with small-sized amino acid esters, as achieved in derivatives 9 (83.91%) and 10 (87.82%) potently and significantly elevate their anti-inflammatory activity within the series of naproxen derivatives 9–12 as compared with the control of the naproxen group (76.34%, P < 0.05). In contrast, naproxen when modified through bulkier aromatic substitution, eg, the l-tyrosine methyl ester moiety present in compound 11, or even an aliphatic elongation of the amino acid ester side chain as found in compound 12, negatively affected the anti-inflammatory activity measured for compounds 11 and 12 (Figure 1).
Furthermore, the ulcerogenic indices for the naproxen derivatives 9–12 with more promising anti-inflammatory potencies were studied in rats (Table 1). From the data observed, it is concluded that rats pretreated with test compounds 9 and 10 had significantly less ulcerogenic potential (ulcer indices of 11.73 and 12.30, respectively) than that of the control groups pretreated with ibuprofen or naproxen (ulcer indices of 22.87 and 24.13, respectively, P < 0.05).
Table 1.
Effect of study compounds 9–12 on ulcer indices
| Compound | Animals with ulcers (n) | Incidence (%) | Ulcer index mean ± SEM (mm) |
|---|---|---|---|
| Control | 0/5 | 0 | 0 |
| Ibuprofen | 5/5 | 100 | 22.87 ± 6.73 |
| Naproxen | 5/5 | 100 | 24.13 ± 6.34 |
| 9 | 4/5 | 80 | 11.73 ± 3.96a,b |
| 10 | 4/5 | 80 | 12.30 ± 4.28a,b |
| 11 | 5/5 | 100 | 16.84 ± 8.14 |
| 12 | 5/5 | 100 | 15.90 ± 7.36 |
Notes:
Significantly different from control values of ibuprofen and naproxen, respectively (P < 0.05).
Abbreviation: SEM, standard error of the mean.
On the other hand, inhibition of prostaglandin E2 synthesis determined for naproxen derivatives 9–12 showed that compounds 9 and 10 had significantly reduced (P < 0.05) prostaglandin E2 levels compared with the control groups pretreated with the reference drug indomethacin (97.97 pg/mL, Figure 2). In contrast, naproxen derivatives 11 and 12 were found to show a significant decrease in prostaglandin E2 levels (P < 0.05) when compared with the placebo control group, but failed to exert significantly higher inhibition when compared with the control groups pretreated with the reference compound, indomethacin, or their structurally related naproxen derivatives 9 and 10 (Figure 2).
Conclusion
Our results show that naproxen derivatives 9–12 had better anti-inflammatory activity compared with their corresponding ibuprofen derivatives 5–8 when ibuprofen and naproxen were used as the reference drugs. The concept of a relationship between type of chemical substitution and anti-inflammatory activity has been clarified in the present study. It can be seen clearly that amidation of the carboxylic acid moiety of naproxen with small-sized amino acid methyl esters led to a significant increase in the anti-inflammatory activity observed in the newly developed compounds, 9 and 10. However, bulkier aromatic substitution, eg, l-tyrosine methyl ester moiety as in naproxen derivative 11, or even an aliphatic elongation of the amino acid ester side chain found in 12, negatively influenced the anti-inflammatory activity measured in the current study. On the other hand, the anti-inflammatory results revealed remarkable activity for naproxen derivatives 9 and 10, which correlate largely with the results observed for inhibition of prostaglandin E2 synthesis.
These distinct characteristics, especially for compounds 9 and 10, with high anti-inflammatory activity, low ulcerogenicity, and potent inhibitory action on prostaglandin E2 synthesis make the newly developed derivatives, especially those derived from naproxen, potentially interesting for future structural optimization and pharmacological investigation of their ability to inhibit cyclo-oxygenase enzymes 1 and 2, given that gastric toxicity of NSAIDs is linked closely to the ability of these drugs to inhibit prostaglandin synthesis, with both cyclo-oxygenase 1 and 2 contributing significantly to mucosal defense and repair.25,26 It would also be worth performing further assays in appropriate cell lines to confirm that these new compounds do not have intrinsic cytotoxicity.
Acknowledgment
The financial support of this work by the College of Medicine and Health Sciences/United Arab Emirates University is gratefully acknowledged.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 2.Marnett LJ, Kalgutkar AS. Cyclooxygenase 2 inhibitors: discovery, selectivity and the future. Trends Pharmacol Sci. 1999;20:465–469. doi: 10.1016/s0165-6147(99)01385-1. [DOI] [PubMed] [Google Scholar]
- 3.Dannhardt G, Kiefer W. Cyclooxygenase inhibitors – current status and future prospects. Eur J Med Chem. 2001;36:109–126. doi: 10.1016/s0223-5234(01)01197-7. [DOI] [PubMed] [Google Scholar]
- 4.Almansa C, Alfon J, De Arriba AF, et al. Synthesis and structure-activity relationship of a new series of COX-2 selective inhibitors: 1,5-diarylimidazoles. J Med Chem. 2003;46:3463–3475. doi: 10.1021/jm030765s. [DOI] [PubMed] [Google Scholar]
- 5.Jackson LM, Hawkey CJ. Gastrointestinal effects of COX-2 inhibitors. Expert Opin Investig Drugs. 1999;8:963–971. doi: 10.1517/13543784.8.7.963. [DOI] [PubMed] [Google Scholar]
- 6.Anikina LV, Levit GL, Demin AM, et al. Synthesis and anti-inflammatory and analgesic activity of amino acids acylated with ibuprofen. Pharm Chem J. 2002;36:237–239. [Google Scholar]
- 7.Mishra A, Ravichandran V, Jain PK, Dixit VK, Agrawal RK. Synthesis, characterization and pharmacological evaluation of amide prodrugs of flurbiprofen. J Braz Chem Soc. 2008;19:89–100. doi: 10.1016/j.ejmech.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Shanbhag VR, Crider AM, Gokhale R, Harpalani A, Dick RM. Ester and amide prodrugs of ibuprofen and naproxen: synthesis, antiinflammatory activity and gastrointestinal toxicity. J Pharm Sci. 1992;81:149–154. doi: 10.1002/jps.2600810210. [DOI] [PubMed] [Google Scholar]
- 9.Roy SD, Manoukian E. Permeability of ketorolac acid and its ester analogs (prodrug) through human cadaver skin. J Pharm Sci. 1994;83:1548–1553. doi: 10.1002/jps.2600831106. [DOI] [PubMed] [Google Scholar]
- 10.Levit GL, Anikina LV, Vikharev YB, et al. Synthesis and antiinflammatory and analgesic activity of naproxen amides with amino acid derivatives. Pharm Chem J. 2002;36:232–236. [Google Scholar]
- 11.Barsoum F, Georgey H, Abdel-Gawad N. Anti-inflammatory activity and PGE2 inhibitory properties of novel phenylcarbamoylmethyl ester-containing compounds. Molecules. 2009;14:667–681. doi: 10.3390/molecules14020667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katritzky AR, Jishkariani D, Narindoshvili T. Convenient synthesis of ibuprofen and naproxen aminoacyl, dipeptidoyl and ester derivatives. Chem Biol Drug Des. 2009;73:618–626. doi: 10.1111/j.1747-0285.2009.00811.x. [DOI] [PubMed] [Google Scholar]
- 13.Mishra A, Veerasamy R, Kumar P, Jain PK, Dixit VK, Agrawal RK. Synthesis, characterization and pharmacological evaluation of amide prodrugs of ketorolac. Eur J Med Chem. 2008;43:2464–2472. doi: 10.1016/j.ejmech.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 14.Rasheed A, Kumar CKA. Novel approaches on prodrug based drug design. Pharm Chem J. 2008;42:677–686. [Google Scholar]
- 15.Parmeshwari KH, Prashant RM, Rajani G, Mange RY. Prodrug designing of NSAIDs. Mini Rev Med Chem. 2009;9:124–139. doi: 10.2174/138955709787001695. [DOI] [PubMed] [Google Scholar]
- 16.Kumar R, Jain S, Jain N, Singh M. Synthesis and biological evaluation of some novel analogue of p-hydroxyaniline. Acta Pharm Sci. 2008;50:183–188. [Google Scholar]
- 17.Rasheed A, Kumar CKA. Design, synthesis, hydrolysis kinetics and phamacodynamic profiles of histidine and alanine conjugates of aceclofenac. Acta Pharm. 2010;60:99–109. doi: 10.2478/v10007-010-0003-1. [DOI] [PubMed] [Google Scholar]
- 18.Winter CA, Fisley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–547. doi: 10.3181/00379727-111-27849. [DOI] [PubMed] [Google Scholar]
- 19.Franklin PX, Pillai AD, Rathod PD, et al. 2-amino-5-thiazolyl motif: a novel scaffold for designing anti-inflammatory agents of diverse structures. Eur J Med Chem. 2008;43:129–134. doi: 10.1016/j.ejmech.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 20.Barsoum FF, Hosni HM, Girgis AS. Novel bis(1-acyl-2-pyrazolines) of potential antiinflammatory and molluscicidal properties. Bioorg Med Chem. 2006;14:3929–3937. doi: 10.1016/j.bmc.2006.01.042. [DOI] [PubMed] [Google Scholar]
- 21.Hamza YE, Sammour OA, Abdel-Latif HA. Enhancement of dissolution of indometacin and modulation of its pharmacodynamics and ulcerogenicity via solid dispersions. Pharm Ind. 1994;56:286–291. [Google Scholar]
- 22.Khayyal MT, El-Ghazaly MA, Abdallah DM, Okpanyi SN, Kelber O, Weiser D. Mechanisms involved in the anti-inflammatory effect of a standardized willow bark extract. Arzneimittelforschung. 2005;55:677–687. doi: 10.1055/s-0031-1296917. [DOI] [PubMed] [Google Scholar]
- 23.Rasheed A, Kumar CK, Mishra A. Synthesis, hydrolysis studies and phamacodynamic profiles of amide prodrugs of dexibuprofen with amino acids. J Enzyme Inhib Med Chem. 2011;26:688–695. doi: 10.3109/14756366.2010.548327. [DOI] [PubMed] [Google Scholar]
- 24.Edwards JCW, Sedgwick AD, Willoughby DA. The formation of a structure with the features of synovial lining by subcutaneous injection of air: an in vivo tissue culture system. J Pathol. 1981;134:147–156. doi: 10.1002/path.1711340205. [DOI] [PubMed] [Google Scholar]
- 25.Wallace JL, McKnight W, Reuter BK, Vergnolle N. NSAID-induced gastric damage in rats: requirement for inhibition of both cyclooxygenase 1 and 2. Gastroenterology. 2000;119:706–714. doi: 10.1053/gast.2000.16510. [DOI] [PubMed] [Google Scholar]
- 26.Wallace JL. Prostaglandins, NSAIDs, and gastric mucosal protection: why doesn’t the stomach digest itself? Physiol Rev. 2008;88:1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]