Abstract
In contrast to the well-documented roles of its mono- and bisphosphate esters, the occurrence of free sedoheptulose in plant tissues remains a matter of conjecture. The present work sought to determine the origin of sedoheptulose formation in planta, as well as its physiological importance. Elevated CO2 and sucrose induction experiments were used to study sedoheptulose metabolism in the Crassulacean acid metabolism (CAM) plants Kalanchoë pinnata and Sedum spectabile. Experimental evidence suggested that sedoheptulose is produced from the oxidative pentose phosphate pathway intermediate sedoheptulose-7-phosphate, by a sedoheptulose-7-phosphate phosphatase. Carbon flux through this pathway was stimulated by increased triose-phosphate levels (elevated CO2, compromised sink availability, and sucrose incubation of source leaves) and attenuated by ADP and inorganic phosphate (Pi). The accumulation of free sedoheptulose is proposed to act as a mechanism contributing to both C and P homeostasis by serving as an alternative carbon store under elevated CO2 or a compromised sink capacity to avoid sucrose accumulation, depletion of inorganic phosphate, and suppression of photosynthesis. It remains to be established whether this acclimation-avoiding mechanism is confined to CAM plants, which might be especially vulnerable to Pi imbalances, or whether some C3 and C4 plants also dispose of the genetic capacity to induce and accelerate sedoheptulose synthesis upon CO2 elevation.
Key words: C homeostasis, Crassulacean acid metabolism, elevated CO2, P homeostasis, photosynthetic acclimation, sedoheptulose, sucrose.
Introduction
During the last 100 years, human activities have strongly contributed to the rising CO2 levels in the atmosphere. The impact of this change and its putative correlation with global warming has been extensively debated (Rogelj et al., 2010). It is clear that the increasing CO2 concentrations will also affect the production of future crops and algae, but the effect might differ greatly depending on the species, nutritional status, and other environmental factors (Xu et al., 2010). In general, elevation of atmospheric CO2 concentration enhances photosynthesis and stimulates growth and productivity in most plant species covering different photosynthetic pathways, i.e. C3, C4, and Crassulacean acid metabolism (CAM) (Poorter and Navas, 2002; Ainsworth and Long, 2005; Ceusters and Borland, 2011). However, the initial enhancement is often found to diminish during long-term exposure to elevated CO2 by excessive starch and sugar accumulation causing feedback inhibition of photosynthesis (Sage et al., 1989; Stitt, 1991). As this acclimation process is especially noted in annual C3 plants whilst some C3 trees and perennial CAM plants do not suffer from acclimation, the availability of sink capacity has been attributed as an important determinant for the long-term responses of plants to elevated CO2 (Nobel and Israel, 1994; Ceulemans et al., 1995).
In contrast to the universal presence of its mono- and bisphosphate esters in the plant kingdom, free sedoheptulose only accumulates to a high extent in some members of the Crassulaceae family such as Sedum spectabile (La Forge and Hudson, 1917; Hegnauer, 1964). However, its presence, albeit at lower levels, in a range of other families including Apiaceae, Aquifoliaceae, Euphorbiaceae, Lamiaceae, Primulaceae, and Saxifragaceae suggests a broader range of occurrence (Tolbert et al., 1957: Okuda and Mori, 1974; Häflinger et al., 1999; Soria et al., 2009). The only other free C7 sugar described in plants is mannoheptulose (Tesfay et al., 2012). Nordall et al. (1956) postulated that the presence of free sedoheptulose in amounts exceeding the steady-state concentration of its phosphate might be attributed to weak activity of sedoheptulokinase (EC 2.7.1.14), specifically producing sedoheptulose-7-phosphate from sedoheptulose. However, except for some studies involving sedoheptulokinase deficiency in humans, this enzymatic activity has been poorly studied (Kardon et al., 2008), and sedoheptulokinase activity has never been reported in plants. Alternatively, starting from sedoheptulose-7-phosphate, two routes might be considered in plant tissues that potentially yield the free heptose sugar, as both the Calvin–Benson cycle and the oxidative pentose phosphate pathway (oxPPP) contain sedoheptulose-7-phosphate as an intermediary reagent. Although the basic features of the oxPPP are well established (Kruger and von Schaewen, 2003), details on how exactly the pathway influences other processes are subject to further investigation. In addition to its putative origin, the role of free sedoheptulose in plants remains a matter of debate, except for serving as a precursor for the polyol volemitol in the horticultural hybrid polyanthus (Häflinger et al., 1999). A possible function as a carbohydrate reserve in CAM (such as starch or soluble sugars), essential to catalyse nocturnal CO2 uptake, was ruled out earlier by Kull (1965, 1967) based on the absence of clear changing diel patterns of sedoheptulose. Therefore, only speculation exists about its physiological roles in plant tissues (Kardon et al., 2008).
To gain more insight into the occurrence of free sedoheptulose and its physiological function in plant tissues, elevated CO2 and sucrose induction experiments were used to study sedoheptulose metabolism in K. pinnata and S. spectabile, two closely related members of the Crassulaceae family performing CAM. The results indicated that sedoheptulose formation in planta might occur by a sedoheptulose-7-phosphate phosphatase acting on the oxPPP intermediate sedoheptulose-7-phosphate. Furthermore, experimental evidence suggested that sedoheptulose accumulation might confer a novel mechanism to contribute to C and P homeostasis in CAM plants. Other possible complimentary functions are also discussed.
Materials and methods
Plant material and sampling
The closely related Kalanchoë pinnata and Sedum spectabile species belong both to the Crassulaceae and perform CAM (Brulfert et al., 1988; Lüttge et al., 1991). Plants were originated from leaf cuttings and grown under controlled greenhouse conditions (Leuven, Belgium). The elevated CO2 experiment was performed with K. pinnata plants that were divided equally between two greenhouse compartments (Leuven, Belgium). Control plants were grown under ambient atmospheric CO2 concentrations (~380 μmol mol–1). The CO2-treated plants were exposed to ~700 μmol mol–1, as described previously (Ceusters et al., 2008). All other environmental conditions in both compartments were identical. During the day, a minimum temperature of 21 °C was maintained, whilst at night a minimum of 19.5 °C was achieved. Between 6:00 and 22:00h, additional artificial lighting was provided [photosynthetic photon flux density (PPFD)=30 μmol m–2 s–1] and the average integrated daily PPFD was ~7mol photons m–2 d–1. Plants were watered twice weekly with a conventional nutrient solution, as described by Londers et al. (2009).
After 12 weeks, young fully developed leaves were cut in the afternoon (15:00h) from plants (n=5) under both CO2 regimens and immediately frozen in liquid nitrogen to arrest any enzyme activity.
Purification of sedoheptulose and confirmation by nuclear magnetic resonance (NMR)
About 50g of leaf material (cut into pieces of 1cm2) was combined with 100ml of Milli-Q water, boiled for 15min, and homogenized with a Waring blender. The homogenate was centrifuged at 20 000g for 20min. The supernatant was put on a mixed-bed Dowex column [50ml resin of acetate– (Ac–) and 50ml of H+ Dowex; Acros Organics, Morris Plains, NJ, USA] and further concentrated with a Rotavap to 15ml, which was loaded in three 5ml aliquots onto a Ca-Dowex column (50×300mm) and fractionated. Sedoheptulose-containing fractions were checked with analytical high-performance anion-exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and combined. Excess glucose was removed by the addition of glucose oxidase (500U). The resulting glucuronic acid was removed by loading the mixture onto 50ml Ac– Dowex. The column flowthrough was concentrated to 4ml with a Rotovap and subjected in ten 400 μl aliquots to preparative HPAEC-PAD as described previously (Vanhaecke et al., 2006). Manually collected fractions were neutralized, combined, and loaded onto an active charcoal column (25×150mm, 12-20 mesh; Sigma). The column was subsequently washed with 500ml Milli-Q water. Sedoheptulose was eluted with 20 % ethanol, which was subsequently removed with a Rotovap. Finally, the remaining volume was lyophilized (LSL Secfroid) and pure sedoheptulose (10mg) was dissolved in 0.6ml D2O for NMR analysis. Spectra were recorded at 22 °C on a Bruker Avance II 600 equipped with a 5mm TCI HCN Z gradient cryoprobe. Bruker Topspin 2.1 software was used to process all spectra. The two-dimensional pulse programs of 1H-1H correlation spectroscopy (COSY), heteronuclear single quantum correlation (HSQC), heteronuclear multiple bond correlation (HMBC), and nuclear Overhauser effect spectroscopy experiments were carried out as described previously (Vanhaecke et al., 2008).
Induction with sucrose
K. pinnata leaves were cut in pieces of 0.5cm3 and for the (3,4-dichlorophenyl)-1,1-dimethylurea (DCMU) experiment they were first incubated overnight in 100ml of 20 μM DCMU (or water as a control) in Petri dishes (17cm diameter), before transferring them for 2 d in 100ml of 200mM sucrose (or water) with or without DCMU in the dark or in continuous light (PPFD=100 μmol m–2 s–1) at 22 °C. Sucrose induction was also performed by placing flower-decapitated S. spectabile stem parts (with leaves included) in a beaker with 200mM sucrose for 2 d under continuous light. Subsequently, sugar concentrations and sedoheptulose-7-phosphate phosphatase activities were determined in the leaves.
Sugar extraction and quantification
Leaves were boiled in 2 volumes of water for 15min and further homogenized with a mortar and pestle. The extract was centrifuged at 13 200rpm for 5min. Subsequently, 200 μl of the supernatant was added to a mixed-bed Dowex column (300 μl Dowex H+ and 300 μl Dowex Ac–; both 100-200 mesh; Acros Organics). The column was eluted six times with 200 μl ddH2O (Vergauwen et al., 2000). From the supernatant, an aliquot was analysed using HPAEC-PAD, as described previously (Vergauwen et al., 2000). Peak quantification and identification was performed using the external standards method (Vergauwen et al., 2000). No losses in sedoheptulose occurred during the applied extraction procedures, as verified by adding a known amount of sedoheptulose to an extract from a non-sedoheptulose producing species followed by quantification of the amount found after the purification procedure (not shown).
Enzyme extraction and enzyme activity measurements
Standardized procedures were followed (Eisenthal and Danson, 1992). Leaves were extracted in 2 volumes of extraction buffer (20mM KMES, pH 6.0, containing 2mM mercaptoethanol, 2mM MgCl2, 10mM NaHSO3, 0.1 % Polyclar and 1mM PMSF). The extract was dialysed overnight against 500 volumes of 20mM KMES buffer. After dialysis, the enzyme was centrifuged for 5min at 16 000g. For the sedoheptulose-7-phosphate phosphatase assay, an enzyme aliquot was combined with 2mM sedoheptulose-7- phosphate, 4mM MgCl2, 50mM KMES (pH 6.0) (final concentrations), with (or without) the extra addition of 4mM ADP or 4mM potassium phosphate (pH 6.0) and incubated at 30 °C. After 0, 3, and 20h of incubation, an aliquot of 10 μl was removed and added to 50 μl of 20 μM mannitol (internal standard) with 0.04% sodium azide and heated to 90 °C for 5min. Identical concentrations and treatments were used in the sedoheptulose kinase assay, except that sedoheptulose and ATP (4mM) were used as substrates. All reaction products were analysed by HPAEC-PAD, as described above.
Chloroplast isolation
S. spectabile chloroplasts were isolated using the method of Kley et al. (2010) with minor modifications. A layer of 3ml of 50 % Percoll (GE Healthcare) was covered with 7ml of chloroplasts and centrifuged in a Sigma 203 centrifuge (13020 swing-out rotor) at 2800rpm for 10min. After washing in 10ml Clp buffer (Kley et al., 2010), the chloroplasts were spun down at 2500rpm for 5min.
Data analysis
Data were analysed using the statistical software package SAS Enterprise Guide 4.0. Before carrying out statistical tests, the normality of the datasets was checked by means of the Kolmogorov–Smirnoff statistic (P>0.05). Means were compared using a two-sample t-test (α=0.05).
Results
Detection of sedoheptulose in K. pinnata by NMR
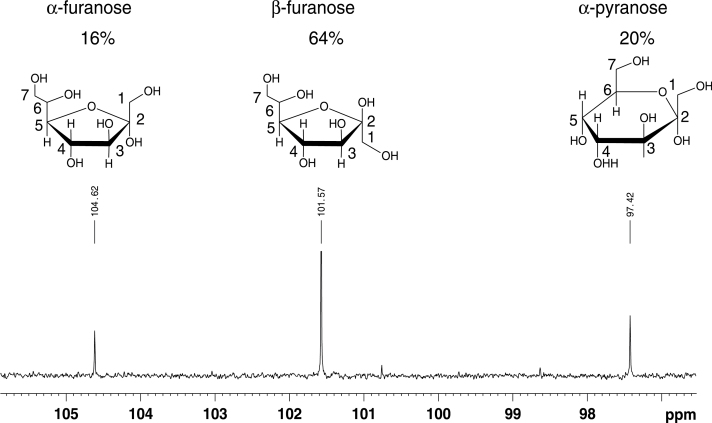
The studied sugar adopted three conformations in a D2O solution. The 13C signals of the three anomeric carbon atoms could easily be recognized by their typical chemical shift close to 100 ppm (Fig. 1). Starting from these quaternary carbons, assignment in the individual spin systems was possible using a combination of two-dimensional HMBC, HSQC–total correlation spectroscopy, HSQC, and COSY spectra. The observed ratio of three conformations and the obtained 13C data corresponded to those published for d-sedoheptulose-7-phosphate (Charmantray et al., 2009), despite minor differences that are expected from its phosphorylation state. Chemical shift assignments and 3JHH couplings within each ring are listed in Table 1. The obtained NMR spectra could be differentiated clearly from those obtained for mannoheptulose (Waschke et al., 2011).
Fig. 1.
Region of the 13C-spectrum in D2O with signals of anomeric C2 carbons. Chemical structures of the three observed conformations are depicted above their anomeric signal. Relative amounts of the different conformations were calculated from resolved signals in a one-dimensional proton spectrum.
Table 1.
Chemical shift assignments for the three forms of sedoheptulose that appear in D2O and 3JHH couplings within each ring.
| α-Furanose | β-Furanose | α-Pyranose | |
|---|---|---|---|
| C1 | 62.7 | 62.4 | 64.0 |
| C2 | 104.6 | 101.6 | 97.4 |
| C3 | 81.7 | 75.6 | 68.2 |
| C4 | 76.4 | 75.4 | 70.1 |
| C5 | 81.6 | 80.1 | 63.4 |
| C6 | 71.6 | 72.6 | 68.7 |
| C7 | 62.2 | 62.2 | 61.2 |
| H11/H12 | 3.51 | 3.41 | 3.54 |
| H3 | 3.94 | 3.94 | 3.80 |
| H4 | 4.03 | 4.15 | 3.92 |
| H5 | 3.85 | 3.61 | 3.69 |
| H6 | 3.73 | 3.69 | 3.88 |
| H71/H72 | 3.59 / 3.47 | 3.61 / 3.46 | 3.74 / 3.62 |
| 3J34 | 4.6 | 7.4 | 3.6 |
| 3J45 | 5.8 | 7.4 | 4.4 |
| 3J56 | ND | ND | 11.0 |
Sedoheptulose accumulation under elevated CO2 in K. pinnata in vivo
In the leaves of K. pinnata, CO2 elevation caused the levels of glucose and fructose to increase (P<0.05), whilst sucrose was maintained at a similar level compared with control plants (P>0.05; Table 2). Initially, free sedoheptulose was present only at values representing 20-30 % in comparison with hexoses and sucrose, but after 12 weeks of CO2 enhancement, sedoheptulose had increased by 500 % (P<0.05) and became the dominant sugar in the leaves of K. pinnata. No other major carbohydrate accumulations were detected by HPAEC-PAD.
Table 2.
Concentrations of glucose, fructose, sucrose, and sedoheptulose (mM) in young, fully expanded leaves of K. pinnata after 12 weeks at ambient (380 μmol mol–1) or elevated (700 μmol mol–1) CO2. The data are means ± standard error for five leaves, each from a separate plant and were compared by a two-sample t-test (α=0.05).
| CO2 (μmol mol–1) | Glucose | Fructose | Sucrose | Sedoheptulose |
|---|---|---|---|---|
| 380 | 1.6±0.2 | 2.1±0.4 | 1.7±0.2 | 0.5±0.1 |
| 700 | 2.2±0.2 | 3.0±0.4 | 1.7±0.1 | 2.5±0.5 |
| P value | <0.05 | <0.05 | >0.05 | <0.05 |
Artificial induction of sedoheptulose accumulation in isolated leaves of K. pinnata
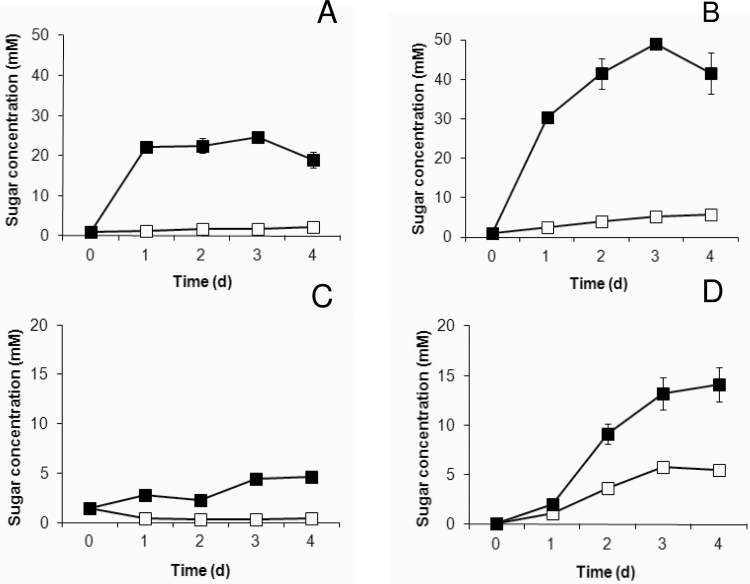
To gain more insight into the strong accumulation of sedoheptulose noted under CO2 enrichment, additional experiments were set up under ambient CO2 involving the elimination of sinks by isolating source leaf pieces and incubating them in water or 200mM sucrose under continuous illumination for 4 d.
Incubation of source leaves in a water solution showed a steady small but significant increase (P<0.05) in hexose sugars over a time course of 4 d (Fig. 2A, B). After 1 d of incubation, sucrose levels decreased threefold (P<0.05) and remained invariable thereafter for the considered time period (Fig. 2C). Sedoheptulose showed a tenfold increase (P<0.05) after 3 d of incubation (Fig. 2D). Feeding the leaves with 200mM sucrose solution resulted in a massive accumulation of 20 and 40 times the original concentration for glucose and fructose, respectively (Fig. 2A, B). Glucose build-up only occurred for 1 d, whilst fructose enhancement took about 2 d before reaching a maximum steady-state level (P>0.05). The accumulation of sucrose was much more limited compared with the hexoses, and showed only a threefold increase over 3 d (Fig. 2C), suggesting that the sucrose concentration is strictly controlled in K. pinnata. After the first day of incubation in a 200mM sucrose solution, sedoheptulose showed a small increase (P<0.05), similar to that in leaves incubated in a water solution (P>0.05) (Fig. 2D). However, over the next 2 d, sedoheptulose increased at a much higher rate, resulting in a 30-fold accumulation after 3 d.
Fig. 2.
Changes in glucose (A), fructose (B), sucrose (C), and sedoheptulose (D) concentrations (mM) in young, fully expanded, isolated source leaves of K. pinnata incubated in water (open squares) or in a sucrose solution (filled squares, 200mM).
Inhibition of photosynthesis reduces sedoheptulose formation
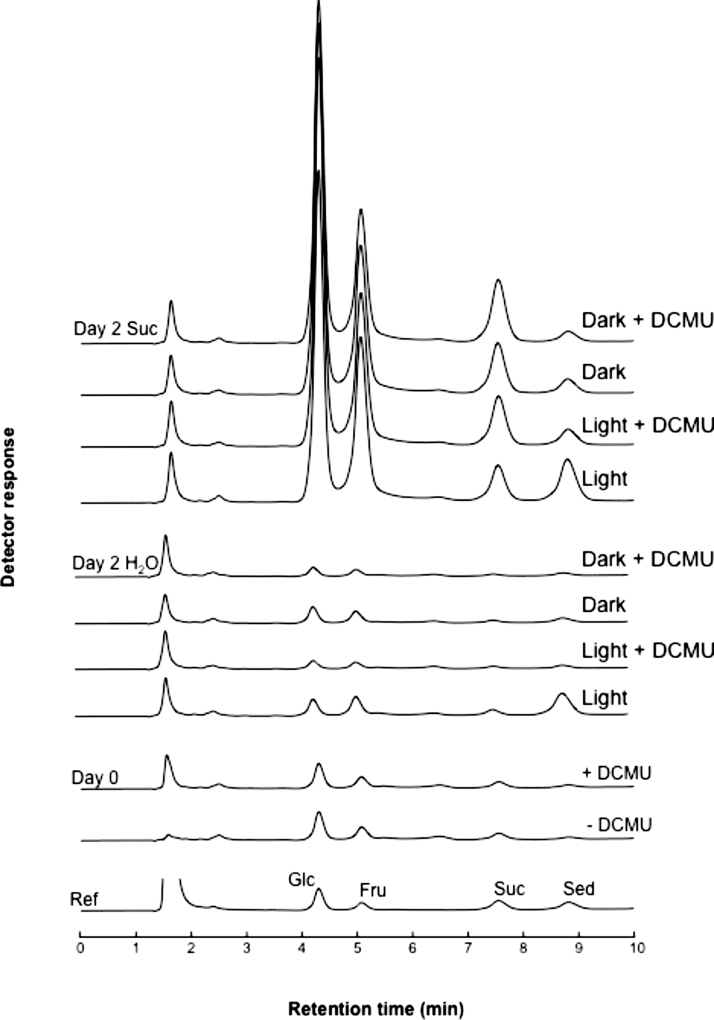
Leaf pieces of K. pinnata were incubated overnight in the presence and absence of DCMU, an inhibitor of photosynthesis. Thereafter, they were kept under continuous light and in the dark for 2 d, respectively (±DCMU), both in the presence or absence of 200mM sucrose (Fig. 3). Without additional sucrose, only the treatment with light and without DCMU yielded a significant amount of sedoheptulose, whilst only negligible amounts were detected in the light plus DCMU condition and in the dark. All sucrose-treated leaf pieces accumulated high levels of glucose and fructose, and showed an increased level of sucrose and sedoheptulose. However, in the light without DCMU, the sedoheptulose:sucrose ratio was substantially higher than in the three other conditions (Fig. 3). These results suggested that photosynthesis stimulates sedoheptulose formation. Nevertheless, synthesis also appeared to be possible in the dark, although to a lower extent. This was further corroborated using etiolated K. pinnata material (not shown). When green stem parts (with leaves) of S. spectabile were induced with 200mM sucrose, sedoheptulose concentrations in the leaves increased from 6.7mM (control) to 15.5mM.
Fig. 3.
Typical chromatograms showing glucose (Glc), fructose (Fru), sucrose (Suc), and sedoheptulose (Sed) in K. pinnata leaf pieces incubated with or without the photosynthesis inhibitor DCMU (day 0 + and – DCMU), before transferring them to 100ml water or 200mM sucrose solution for 2 d in the dark or in continuous light (PPFD 100 μmol m–2 s–1) at 22 °C. The reference chromatogram (10 μM of Glc, Fru, Suc, and Sed) is displayed at the bottom.
Sedoheptulose is produced from sedoheptulose-7-phosphate in vitro
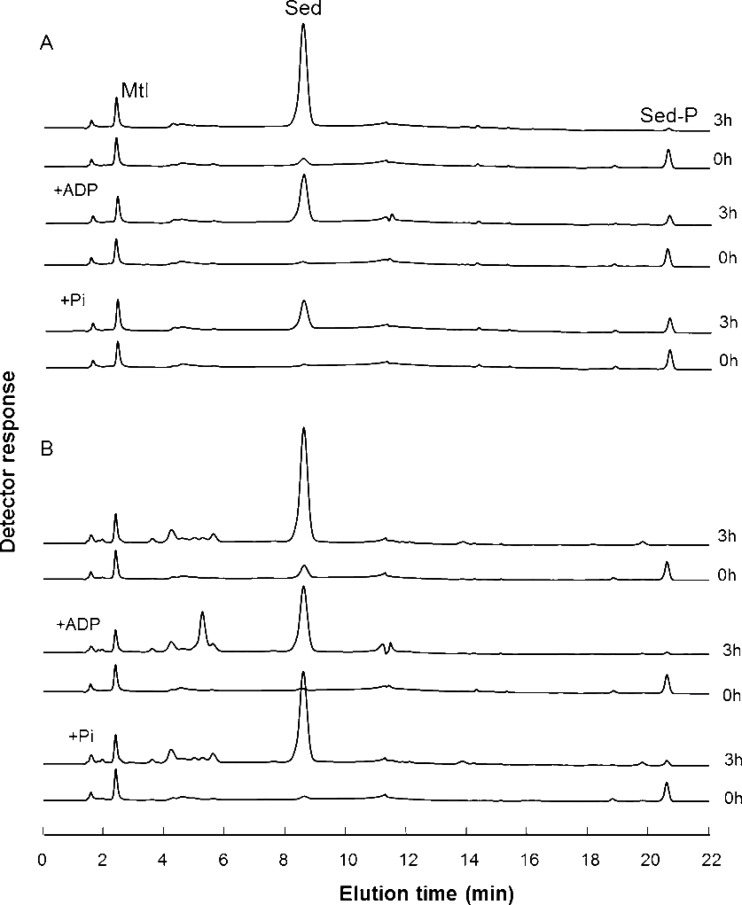
Because it proved practically impossible to obtain substantial amounts of proteins from K. pinnata, the close relative species S. spectabile, known to accumulate high levels of sedoheptulose under standard conditions in its organs (La Forge and Hudson, 1917; Fig. S1 at JXB online), was used for protein extraction. Both species belong to the Crassulaceae and are situated in the same subfamily, Sedoideae (Mort et al., 2001). Desalted protein extracts of sucrose-induced leaves were incubated with 2mM sedoheptulose-7-phosphate. Fig. 4A shows the time-dependent formation of sedoheptulose from sedoheptulose-7-phosphate. No sedoheptulose kinase activity (the reverse reaction) could be detected by combining sedoheptulose and ATP (not shown), strongly suggesting that the sedoheptulose concentration in planta might be controlled by the sedoheptulose-7-phosphate supply and the sedoheptulose-7-phosphate phosphatase activity. Strikingly, the addition of both ADP and inorganic phosphate (Pi) at a concentration of 4mM attenuated the sedoheptulose-7-phosphate phosphatase activity (Fig. 4A). This was not observed for the non-specific phosphatases present in enzyme extracts of the C3 plant wheat (Fig. 4B), suggesting the presence of a rather specific sedoheptulose-7-phosphate phosphatase in S. spectabile that can be regulated by ADP and Pi. Moreover, sedoheptulose-7-phosphate phosphatase activities increased to 490 nmol mg–1 of protein min–1 in the leaves of sucrose-induced stems, compared with 25 nmol mg–1 of protein min–1 in control leaves (Table S1 at JXB online). Unfortunately, our continued efforts to purify this enzyme remained unsuccessful due to enzyme instability or removal of an essential co-factor.
Fig. 4.
Typical chromatograms showing the time-dependent (0 and 3h) formation of sedoheptulose from 2mM sedoheptulose-7-phosphate. Desalted protein extracts of sugar-induced S. spectabile (A) and wheat (B) leaves were compared. The attenuation of sedoheptulose formation by ADP and Pi is much more pronounced in (A). Mannitol (Mtl) served as an internal standard.
Sedoheptulose formation probably originates in the cytosol via the oxPPP and is phloem transportable
To determine the putative subcellular location of sedoheptulose synthesis in S. spectabile, chloroplasts were isolated and lysed and the supernatant was analysed. The amount of sedoheptulose was negligible (Fig. S1), arguing for the actual synthesis of sedoheptulose in the cytosol via the oxPPP, as the Calvin-Benson cycle solely acts inside the chloroplasts. However, the absence of sedoheptulose in freshly prepared chloroplasts should be confirmed by non-aqueous fractionation methods.
Compared with leaves under full light (maximal activities up to 1100 nmol mg–1 of protein min–1 were recorded), much lower sedoheptulose-7-phosphate phosphatase activities (up to 33 nmol mg–1 of protein min–1) could be detected in S. spectabile roots, despite the fact that roots also accumulate sedoheptulose, although to a lower extent than leaves (Nordall et al., 1956; Fig. S1). This implied that sedoheptulose is produced mainly in the leaves and transported to the roots through the phloem, as suggested previously by Liu et al. (2002). Typically, the honeydew of aphids still partly contains phloem-derived sugars, although substantial sugar pattern modulation occurs by the action of glycosyl transferases in the aphid gut (e.g. producing melezitose from sucrose; Vantaux et al., 2011). Intriguingly, next to melezitose as a major sugar, honeydew from S. spectabile-resident aphids contained substantial amounts of sedoheptulose (Fig. S2 at JXB online), providing further evidence for the phloem transport of sedoheptulose in S. spectabile.
Discussion
In contrast to the well-documented roles of the phosphate esters of sedoheptulose, either in the regenerative part of the Calvin cycle or in the oxPPP, the occurrence of substantial amounts and the physiological role of free sedoheptulose in a range of CAM plants has remained enigmatic, despite the fact that sedoheptulose was discovered almost a century ago in S. spectabile (La Forge and Hudson, 1917). Here, we have shed light on the putative importance of sedoheptulose ana bolism in the CAM plants K. pinnata and S. spectabile.
We showed that sedoheptulose, whose identity was confirmed by NMR (Fig. 1, Table 1), was produced from sedoheptulose-7-phosphate in vitro, probably by the activity of a specific sedoheptulose-7-phosphate phosphatase (Fig. 4) operating in the leaves. Increased sedoheptulose-7-phosphate phosphatase activities were noticed concomitantly with elevated sedoheptulose concentrations in the leaves of sucrose-induced S. spectabile stems, suggesting that this enzyme is involved in the biosynthesis of sedoheptulose in planta. Sedoheptulose-7-phosphate constitutes an intermediary product in two pathways in plants: the Calvin-Benson cycle and the oxPPP. In contrast to the first, which is restricted to a plastid origin, the oxPPP can be completed in the cytosol alone or involve compartmentalization between cytosol and chloroplast, depending on species, tissue, developmental stage, and environmental conditions (Kruger and von Schaewen, 2003). The absence of sedoheptulose in chloroplasts suggested an oxPPP-mediated synthesis of sedoheptulose in the cytosol. However, by converting sedoheptulose-7-phosphate to sedoheptulose, significant amounts of carbohydrate may be withdrawn from the actual oxPPP. This observation prompted further investigation into its physiological significance.
Under control conditions, free sedoheptulose concentrations were only of minor importance in K. pinnata, but doubling of the atmospheric CO2 concentration caused sedoheptulose to increase by 500 % whilst sucrose was not influenced in source leaves (Table 2). Strict metabolic control of the amount of sucrose under CO2 elevation has been noted previously in other CAM plants, which show no downward acclimation of photosynthesis in response to elevated CO2, but the precise underlying mechanisms have not been elucidated (Wang and Nobel, 1996; Ceusters and Borland, 2011). It is well known that, as well as reducing expression of genes implicated in photosynthesis, accumulated sucrose might reduce the rate of its own synthesis. This might cause sugar phosphates to accumulate and inorganic phosphate (Pi) pools to be depleted in the cytosol and in chloroplasts, ultimately inhibiting photophosphorylation and leading to long-term acclimation of photosynthesis under elevated CO2 in a range of plants (Stitt, 1991; Sheen, 1994). In different CAM plants, a lack of long-term acclimation has been attributed mainly to increased rates of phloem sucrose transport to supply growing sinks and avoiding sucrose accumulation in the source leaves (Wang and Nobel, 1996). In addition to this mechanism, we propose in our model (Fig. 5) that oxPPP-mediated sedoheptulose formation in the cytosol in K. pinnata might function as an excess carbon escape valve to deal with a surplus of triose phosphates originating from the chloroplasts (Calvin-Benson cycle), and as such avoiding sucrose accumulation under CO2 enrichment when sink capacities might become saturated. To test this hypothesis, source-sink transport was compromised by detaching K. pinnata leaves and incubating them in solutions containing extra sucrose (200mM) under full light (Fig. 2). The sucrose concentration inside the leaves rose slightly from 1.5 to about 5mM, representing only a threefold increase, indicating that there is a strict metabolic control over the sucrose concentration inside the leaves but without the possibility of diverting excess sucrose to sinks. Initially, significant amounts were converted to glucose and fructose (Fig. 2), but after 2 d, the levels of these sugars were also saturated and a massive 30-fold increase in sedoheptulose level occurred (Fig. 2). These results demonstrated that sedoheptulose synthesis can act as an alternative or additional mechanism to deal with excess carbohydrate in the leaf or even at the cell level, whilst elevated increased phloem transport of sucrose requires coordination between different plant organs. In this context, sedoheptulose formation offers a higher degree of flexibility in comparison with sucrose. Both sugars are phloem transportable, but when this possibility is restricted, significant accumulation of sedoheptulose can take place inside source leaves without suppressing photosynthesis. Moreover, CAM plants are particularly well established to store large amounts of sugars in the typically large central vacuoles, which dominate about 95% of the cell’s volume (Steudle et al., 1980; Kenyon et al., 1985). Of course, the drawback of carbon through sedoheptulose formation in vivo should only be active when triose phosphates are accumulating excessively, i.e. when sink capacity is limited and triose phosphates are synthesized at high rates under elevated CO2. Our findings that incubating leaf pieces in a water solution without extra sucrose in the dark or under photosynthesis-inhibiting conditions (DCMU) resulted in only marginal sedoheptulose formation (Fig. 3) confirm this view, and significant sedoheptulose accumulation was restored when leaf pieces were incubated in a water solution in the light.
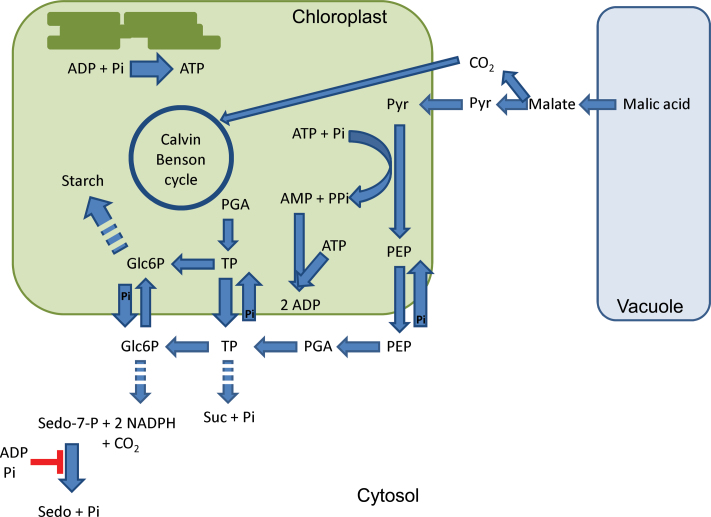
Fig. 5.
Hypothetical model of daytime metabolite fluxes in starch-storing CAM species such as Mesembryanthemum crystallinum, K. pinnata and S. spectabile (based on Silvera et al., 2010; Holtum et al., 2005). Dotted arrows represent multiple steps. Free sedoheptulose formation under elevated CO2 in K. pinnata is indicated and originates from the action of a sedoheptulose-7-phosphate phosphatase acting on the oxPPP intermediate sedoheptulose-7-phosphate. A negative feedback control by ADP and Pi is proposed, contributing to both C and P homeostasis. Glc6P, glucose-6-phosphate; PGA, 3-phosphoglycerate; PEP, phosphoenolpyruvate; Pyr, pyruvate; Sedo, sedoheptulose; Sedo-7-P, sedoheptulose-7-phosphate; Suc, sucrose; TP, triose phosphate. (This figure is available in colour at JXB online.)
Diverting sedoheptulose from triose phosphates via glucose-6-phosphate in the oxPPP also involves the liberation of Pi and reducing power (NADPH) (Fig. 5). NADPH might be utilized in biosynthetic processes, for the production of antioxidants (e.g. ascorbate, glutathione), or, after conversion to NADH, oxidized in the mitochondrial electron transport chain. An elevated amount of reducing power could also favour CAM operation under elevated CO2 because it energizes the conversion of oxaloacetic acid to malate during nocturnal CO2 uptake. However, previous measurements from Winter et al. (1997) showed that K. pinnata only showed an elevated daytime uptake of CO2, whilst nocturnal sequestration and consequently malate formation remained unaffected upon CO2 treatment. As already mentioned above, the availability of Pi is crucial to drive photosynthesis, and in this respect, CAM plants can be particularly vulnerable to Pi imbalances as they generally utilize more ATP and as such more Pi to convert carbon, originating from CO2, into sugars during photosynthesis (Iglesias et al., 1993; Winter and Smith, 1996). Moreover, pyruvate phosphatase dikinase also requires both ATP and Pi to convert pyruvate to phosphoenolpyruvate (PEP) during the daytime (Fig. 5), and as such these reactions might compete with direct Rubisco assimilation for Pi during the transition phases II and IV, leading to Pi limitation in the chloroplast. It is clear that the production and transport of PEP are crucial for the functioning of CAM plants to provide consistent carbohydrate storage each day to ensure the continuation of nocturnal carboxylation via PEP carboxylase (Ceusters et al., 2010; Borland et al., 2011). As such, the formation of sedoheptulose is not only beneficial with regard to carbon allocation under elevated CO2 but also contributes to Pi homeostasis. Of course, this operation is only useful when chloroplast and cytosolic Pi and ADP are low, and this is completely in line with our finding that Pi and ADP exerted a negative feedback control on sedoheptulose-7-phosphate phosphatase activity (Figs 4 and 5). Besides the proposed functions of sedoheptulose in CO2-enriched K. pinnata, the free heptose sugar might also serve a role in cellular antioxidant mechanisms. CAM habitats are generally characterized by high solar radiations, e.g. exposed terrestrial or epiphytic habitats where photosynthetic active radiation is not limiting photosynthesis but overenergization of the photosynthetic apparatus in CAM plants might constitute a real threat (Lüttge 2002). Moreover, it has been proposed that sugar(like) compounds, when accumulating at higher concentrations, might act as reactive oxygen species scavengers (Van den Ende and Valluru, 2009; Van den Ende et al., 2011). Typically, resurrection species maintain higher soluble sugar levels compared with closely related non-resurrection species (Djilianov et al., 2011).
In conclusion, we propose that sedoheptulose formation in planta occurs via the oxPPP intermediary sedoheptulose-7-phosphate, probably by a dedicated cytosolic sedoheptulose-7-phosphate phosphatase, although the exact subcellular localization is a subject for further investigation. Furthermore, it is proposed that sedoheptulose formation contributes to both C and P homeostasis in K. pinnata by acting as an alternative carbon store under elevated CO2 to avoid sucrose accumulation and depletion of Pi. Our experimental evidence (elevated CO2, external sugar, and light) strongly suggest a stimulated flux of C through this pathway, mediated by increased triose-phosphate levels but attenuated by ADP and Pi. It remains to be established whether this mechanism is confined to CAM plants, which might be especially vulnerable to Pi imbalances, or whether some C3 and C4 plants also dispose of the genetic capacity to induce and accelerate sedoheptulose synthesis upon CO2 elevation.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Typical chromatograms showing the sugar composition of different organs and isolated chloroplasts of S. spectabile.
Fig. S2. Typical chromatogram showing the sugar composition of honeydew from S. spectabile-resident aphids.
Table S1. Sedoheptulose-7-phosphate (S7P) phosphatase activities and sedoheptulose concentrations in leaves derived from sucrose-induced (200mM, 2d) stem fragments of S. spectabile compared with an untreated control.
Acknowledgements
This research was supported by the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen). J.C. currently holds a Marie Curie Intra European Fellowship and W.V.d.E. is supported by grants from FWO-Vlaanderen. The authors wish to thank Dr Anne Borland (Newcastle University, UK) for constructive comments and discussions.
Glossary
Abbreviations:
- Ac
acetate
- CAM
Crassulacean acid metabolism
- COSY
correlation spectroscopy
- DCMU
(3,4-dichlorophenyl)-1,1-dimethylurea
- HMBC
heteronuclear multiple bond correlation
- HPAEC-PAD
high-performance anion-exchange chromatography with pulsed amperometric detection
- HSQC
heteronuclear single quantum correlation
- NMR
nuclear magnetic resonance
- oxPPP
oxidative pentose phosphate pathway
- PEP
phosphoenolpyruvate
- Pi
inorganic phosphate
- PPFD
photosynthetic photon flux density.
References
- Ainsworth EA, Long SP. 2005. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165, 351–371 [DOI] [PubMed] [Google Scholar]
- Borland AM, Barrera Zambrano VA, Ceusters J, Shorrock K. 2011. The photosynthetic plasticity of crassulacean acid metabolism: an evolutionary innovation for sustainable productivity in a changing world. New Phytologist 191, 619–633 [DOI] [PubMed] [Google Scholar]
- Brulfert J, Kluge M, Güclü S, Quieroz O. 1988. Combined effects of drought, daylength and photoperiod on rapid shifts in the photosynthetic pathway of Sedum spectabile, a CAM species. Plant Physiology and Biochemistry 26, 7–16 [Google Scholar]
- Ceulemans R, Jiang XN, Shao BY. 1995. Growth and physiology of one-year old poplar (Populus) under elevated atmospheric CO2 levels. Annals of Botany 75, 609–617 [Google Scholar]
- Ceusters J, Borland AM. 2011. Impacts of elevated CO2 on the growth and physiology of plants with crassulacean acid metabolism. Progress in Botany 72, 163–181 [Google Scholar]
- Ceusters J, Borland AM, Ceusters N, Verdoodt V, Godts C, De Proft MP. 2010. Seasonal influences on carbohydrate metabolism in the CAM bromeliad Aechmea ‘Maya’: consequences for carbohydrate partitioning and growth. Annals of Botany 105, 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceusters J, Borland AM, Londers E, Verdoodt V, Godts C, De Proft MP. 2008. Diel shifts in carboxylation pathway and metabolite dynamics in the CAM bromeliad Aechmea ‘Maya’ in response to elevated CO2. Annals of Botany 102, 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmantray F, Hélaine V, Legeret B, Hecquet L. 2009. Preparative scale enzymatic synthesis of d-sedoheptulose-7-phosphate from β-hydroxypyruvate and d-ribose-5-phosphate. Journal of Molecular Catalysis B: Enzymatic 57, 6–9 [Google Scholar]
- Djilianov D, Ivanov S, Moyankova D, Miteva L, Kirova E, Alexieva V, Joudi M, Peshev D, Van den Ende W. 2011. Sugar ratios, glutathione redox status and phenols in the resurrection species Haberlea rhodopensis and the closely related non-resurrection species Chirita eberhardtii. Plant Biology 13, 767–776 [DOI] [PubMed] [Google Scholar]
- Eisenthal R, Danson MJ. 1992. Enzyme assays: a practical approach. Oxford: IRL Press; [Google Scholar]
- Häflinger B, Kindhauser E, Keller F. 1999. Metabolism of d-glycero-d-manno-heptitol, Volemitol, in polyanthus. Discovery of a novel ketose reductase. Plant Physiology 119, 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegnauer R. 1964. Chemotaxonomie der Pflanzen, Vol 3 Basel, Switzerland: Birkhäuserverlag; [Google Scholar]
- Holtum JAM, Smith JAC, Neuhaus H. 2005. Intracellular transport and pathways of carbon flow in plants with crassulacean acid metabolism. Functional Plant Biology 32, 429–449 [DOI] [PubMed] [Google Scholar]
- Iglesias AA, Plaxton WC, Podesta FE. 1993. The role of inorganic phosphate in the regulation of C4 photosynthesis. Photosynthesis Research 35, 205–211 [DOI] [PubMed] [Google Scholar]
- Kardon T, Stroobant V, Veiga-da-Cuncha M, Van Schaftingen E. 2008. Characterization of mammalian sedoheptulokinase and mechanism of formation of erythrol in sedoheptulokinase deficiency. FEBS Letters 582, 3330–3334 [DOI] [PubMed] [Google Scholar]
- Kenyon WH, Severson RF, Black CC. 1985. Maintenance carbon cycle in crassulacean acid metabolism leaves. Plant Physiology 77, 183–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kley J, Heil M, Muck A, Svatos A, Boland W. 2010. Isolating intact chloroplasts from small Arabidopsis samples for proteomic studies. Analytical Biochemistry 398, 198–202 [DOI] [PubMed] [Google Scholar]
- Kruger NJ, von Schaewen A. 2003. The oxidative pentose phosphate pathway: structure and organization. Current Opinion in Plant Biology 6, 236–246 [DOI] [PubMed] [Google Scholar]
- Kull U. 1965. Über das vorkommen und das physiologischen Verhalten der Sedoheptulose im Rahmen das Kohlenhydrathaushaltes vegetativer Pflanzenteile. Beiträge zur Biologie der Pflanzen 41, 231–300 [Google Scholar]
- Kull U. 1967. Zum physiologischen Verhalten der Sedoheptulose im Rahmen des Kohlenhydrathaushaltes einiger Crassulaceen. Berichte der Deutschen Botanischen Gesellschaft 80, 187–198 [Google Scholar]
- La Forge FB, Hudson CS. 1917. Sedoheptulose, a new sugar from Sedum spectabile. Journal of Biological Chemistry 30, 61–77 [Google Scholar]
- Liu X, Sievert J, Arpaia ML, Madore MA. 2002. Postulated physiological roles of the seven-carbon sugars, mannoheptulose and perseitol in avocado. Journal of the American Society for Horticultural Science 127, 108–114 [Google Scholar]
- Londers E, Ceusters J, Godts C, De Proft MP. 2009. Impact of fertiliser level on plant growth, shape and physiological leaf damage risk of two Aechmea cultivars characterized by the crassulacean acid metabolism. Journal of Horticultural Science and Biotechnology 84, 531–535 [Google Scholar]
- Lüttge U, Ball E, Fetene M, Medina E. 1991. Flexibility of crassulacean acid metabolism in Kalanchoë pinnata (Lam.) Pers. I. Response to irradiance and supply of nitrogen and water. Journal of Plant Physiology 137, 259–267 [Google Scholar]
- Lüttge U. 2002. CO2-concentrating: consequences in crassulacean acid metabolism. Journal of Experimental Botany 53, 2131–2142 [DOI] [PubMed] [Google Scholar]
- Mort ME, Soltis DE, Soltis PS, Francisco-Ortega J, Santos-Guerra A. 2001. Phylogenetic relationships and evolution of Crassualceae inferred from matK sequence data. American Journal of Botany 88, 76–91 [PubMed] [Google Scholar]
- Nobel PS, Israel AA. 1994. Cladode development, environmental responses of CO2 uptake, and productivity for Opuntia ficus-indica under elevated CO2. Journal of Experimental Botany 45, 295–303 [Google Scholar]
- Nordall A, Benson AA, Calvin M. 1956. Photosynthesis of sedoheptulose-C14. Archives of Biochemistry and Biophysics 62, 435–445 [DOI] [PubMed] [Google Scholar]
- Okuda T, Mori K. 1974. Coriose and related compoumds. 5. Distribution of manno-heptulose and sedoheptulose in plants. Phytochemistry 13, 961–964 [Google Scholar]
- Poorter H, Navas ML. 2002. Plant growth and competition at elevated CO2: on winners, losers and functional groups. New Phytologist 157, 175–198 [DOI] [PubMed] [Google Scholar]
- Rogelj J, Nabel J, Chen C, Hare W, Markmann K, Meinshausen M, Schaeffer M, Macey K, Höhne N. 2010. Copenhagen Accord pledges are paltry. Nature 464, 1126–1128 [DOI] [PubMed] [Google Scholar]
- Sage RF, Sharkey TD, Seemann JR. 1989. Acclimation of photosynthesis to elevated CO2 in five C3 species. Plant Physiology 89, 590–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J. 1994. Feedback control of gene expression. Photosynthesis Research 39, 427–438 [DOI] [PubMed] [Google Scholar]
- Silvera K, Neubig KM, Whitten WM, Williams MH, Winter K, Cushman JC. 2010. Evolution along the crassulacean acid metabolism continuum. Functional Plant Biology 37, 995–1010 [Google Scholar]
- Soria AC, Sanz ML, Villamiel M. 2009. Determination of minor carbohydrates in carrot (Daucus carota L.) by GC-MS. Food Chemistry 114, 758–762 [Google Scholar]
- Steudle E, Smith JAC, Lüttge U. 1980. Water-relation parameters of individual mesophyll cells of the crassulacean acid metabolism plant Kalanchoë daigremontiana. Plant Physiology 66, 1155–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. 1991. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant, Cell & Environment 14, 741–762 [Google Scholar]
- Tesfay SZ, Bertling I, Bower JP. 2012. d-Mannoheptulose and perseitol in ‘Hass’ avocado: metabolism in seed and mesocarp tissue. South African Journal of Botany79, 159–165 [Google Scholar]
- Tolbert NE, Nystrom CW, Kerr PC. 1957. Sedoheptulose in Coleus. Plant Physiology 32, 269–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Ende W, Peshev D, De Gara L. 2011. Disease prevention by natural antioxidants and prebiotics acting as ROS scavengers in the gastrointestinal tract. Trends in Food Science & Technology 22, 689–697 [Google Scholar]
- Van den Ende W, Valluru R. 2009. Sucrose, sucrosyl oligosaccharides and oxidative stress: scavenging and salvaging? Journal of Experimental Botany 60, 9–18 [DOI] [PubMed] [Google Scholar]
- Vanhaecke M, Van den Ende W, Lescrinier E, Dyubankova N. 2008. Isolation and characterization of a pentasaccharide from Stellaria media. Journal of Natural Products 71, 1833–1836 [DOI] [PubMed] [Google Scholar]
- Vanhaecke M, Van den Ende W, Van Laere A, Herdewijn P, Lescrinier E. 2006. Complete NMR characterization of lychnose from Stellaria media (L.) Vill. Carbohydrate Research 341, 2744–2750 [DOI] [PubMed] [Google Scholar]
- Vantaux A, Van den Ende W, Billen J, Wenseleers T. 2011. Large interclone differences in melezitose secretion in the facultatively ant-tended black bean aphid Aphis fabae. Journal of Insect Physiology 57, 1614–1624 [DOI] [PubMed] [Google Scholar]
- Vergauwen R, Van den Ende W, Van Laere A. 2000. The role of fructan in flowering of Campanula rapunculoides. Journal of Experimental Botany 51, 1261–1266 [PubMed] [Google Scholar]
- Wang N, Nobel PS. 1996. Doubling the CO2 concentration enhanced the activity of carbohydrate-metabolism enzymes, source carbohydrate production, photoassimilate transport and sink strength for Opuntia ficus-indica. Plant Physiology 110, 893–902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke D, Thimm J, Thiem J. 2011. Highly efficient synthesis of ketoheptoses. Organic Letters 13, 3628–3631 [DOI] [PubMed] [Google Scholar]
- Winter K, Richter A, Engelbrecht B, Posada J, Virgo A, Popp M. 1997. Effect of elevated CO2 on growth and crassulacean-acid-metabolism activity of Kalanchoë pinnata under tropical conditions. Planta 201, 389–396 [Google Scholar]
- Winter K, Smith JAC. 1996. Crassulacean acid metabolism: current status and perspectives. In: Winter K, Smith JAC, eds. Crassulacean acid metabolism: biochemistry, ecophysiology and evolution. Berlin, Germany: Springer-Verlag, 389–426 [Google Scholar]
- Xu ZG, Zou DH, Gao KS. 2010. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Botanica Marina 53, 123–129 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.