Abstract
Increases in photosynthetic capacity (A1500) after defoliation have been attributed to changes in leaf-level biochemistry, water, and/or nutrient status. The hypothesis that transient photosynthetic responses to partial defoliation are regulated by whole-plant (e.g. source–sink relationships or changes in hydraulic conductance) rather than leaf-level mechanisms is tested here. Temporal variation in leaf-level gas exchange, chemistry, whole-plant soil-to-leaf hydraulic conductance (KP), and aboveground biomass partitioning were determined to evaluate mechanisms responsible for increases in A1500 of Eucalyptus globulus L. potted saplings. A1500 increased in response to debudding (B), partial defoliation (D), and combined B&D treatments by up to 36% at 5 weeks after treatment. Changes in leaf-level factors partly explained increases in A1500 of B and B&D treatments but not for D treatment. By week 5, saplings in B, B&D, and D treatments had similar leaf-specific KP to control trees by maintaining lower midday water potentials and higher transpiration rate per leaf area. Whole-plant source:sink ratios correlated strongly with A1500. Further, unlike KP, temporal changes in source:sink ratios tracked well with those observed for A1500. The results indicate that increases in A1500 after partial defoliation treatments were largely driven by an increased demand for assimilate by developing sinks rather than improvements in whole-plant water relations and changes in leaf-level factors. Three carbohydrates, galactional, stachyose, and, to a lesser extent, raffinose, correlated strongly with photosynthetic capacity, indicating that these sugars may function as signalling molecules in the regulation of longer term defoliation-induced gas exchange responses.
Key words: Carbohydrates, carbon limitation, defoliation, leaf water potential, photosynthesis, plant hydraulic conductance.
Introduction
After partial defoliation, a continuum of photosynthetic responses has been reported in the remaining foliage, with the direction, magnitude, and duration influenced by the type of damage, plant species, and environment (Sweet and Wareing, 1966; von Caemmerer and Farquhar, 1984; Lovett and Tobiessen, 1993; Reich et al., 1993; Pinkard et al., 2004; Turnbull et al., 2007; Eyles et al., 2009b, 2011). Explanations for photosynthetic responses have been largely focused on leaf-level mechanisms such as improvements in leaf N availability (Lavigne et al., 2001) and increases in the rates of biochemical reactions of photosynthesis (von Caemmerer and Farquhar, 1984; Layne and Flore, 1995; Ozaki et al., 2004; Turnbull et al., 2007). An alternative hypothesis is that whole-plant mechanisms explain the gas exchange responses. The scale at which photosynthetic responses are regulated affects the sorts of management strategies that may be effective in promoting recovery from defoliation, and influences the ways in which defoliation responses might be represented in productivity models (Pinkard et al., 2011a).
There are two hypotheses that might explain photosynthetic responses to defoliation at a whole-plant level. The first, the source:sink (S:S) hypothesis, suggests that increases in photosynthetic rates after partial defoliation are regulated by changes in whole-plant carbon S:S relationships (Neales and Incoll, 1968; Layne and Flore, 1995; Pinkard et al., 2011b). The second implies that photosynthetic responses to defoliation are regulated by changes in whole-plant hydraulic conductance (Whitehead, 1998; Brodribb et al., 2007). Plant organs can be defined by their functional role as either net exporters (sources) or net consumers of carbohydrates (sinks) (Laporte and Delph, 1996; Neales and Incoll, 1968). The S:S hypothesis suggests that defoliation decreases source size while leaving sink demand relatively unchanged. Increased demand for carbohydrates from remaining leaves reduces end-product inhibition, which in turn increases the photosynthetic rates of the remaining leaves (Sweet and Wareing, 1966; Neales and Incoll, 1968; Layne and Flore, 1995). If whole-plant S:S relationships were driving photosynthetic responses to defoliation, increases in the rates of biochemical reactions of photosynthesis and changes in leaf-level carbohydrate concentrations might be expected. While increases in stomatal conductance (gs) might also occur, this would not be a key driver of photosynthetic responses. Evidence to support the S:S hypothesis at the whole-plant scale is not well documented. While increases in the rates of biochemical reactions of photosynthesis are commonly reported following defoliation (von Caemmerer and Farquhar, 1984; Layne and Flore, 1995; Ozaki et al., 2004), these have not been linked explicitly to whole-plant S:S processes. Reductions in total non-structural carbohydrate (NSC) concentrations in the remaining leaves following defoliation have been observed (e.g. Layne and Flore, 1995; Myers et al., 1999; Zhou and Quebedeaux, 2003) and are consistent with an increased demand and export of NSC from source leaves to developing sinks. However, this response is not universal (see Lavigne et al., 2001; Turnbull et al., 2007; Eyles et al., 2009b). End-product inhibition may be related to a specific carbohydrate rather than total NSC concentration (Turnbull et al., 2007). In this study, the aim is to quantify not only total soluble sugar and starch concentrations but also up to eight specific carbohydrates in order to capture fully the effect of defoliation on carbohydrate metabolism.
Maximum rates of gas exchange and growth of plants are also regulated by plant hydraulic architecture (Whitehead, 1998; Brodribb et al., 2007). Leaves represent one of the largest sites of resistances to water flow in plants (Sack and Holbrook, 2006); thus, reductions in leaf area may result in a greater root-to-leaf hydraulic conductivity. Reductions in leaf area also represent reduced transpiring surface area and increased root:leaf ratio, which in turn improves the water and nutrient status of the remaining foliage per unit area (Welker and Menke, 1990). If hydraulic architecture was the key driver of photosynthetic responses to defoliation, a strong relationship between photosynthesis and gs, irrespective of defoliation treatment, would be expected, along with increases in root-to-leaf hydraulic conductivity. The few studies that have examined the impact of defoliation on whole-plant water relations reported less negative midday leaf water potential (Ψmd) (Syvertsen, 1994; Singh and Thompson, 1995; Vanderklein and Reich, 2000; Quentin et al., 2011) and higher transpiration (EL) per unit leaf area in response to defoliation (Meinzer and Grantz, 1990; Quentin et al., 2011; but see Oren et al., 1999). Meinzer and Grantz (1990) observed increased whole-plant soil-to-leaf hydraulic conductance (KP) within 24h after defoliation in sugar cane and they attributed this response to increased EL and not to any changes in Ψ. A recent study noted higher KP 2 months after defoliation, and this was due to an increase in EL and a less negative Ψmd in 4-year-old Eucalyptus globulus Labill. trees (Quentin et al., 2011).
The broad-leaved evergreen woody tree species, E. globulus was selected to examine the underlying mechanisms of photosynthetic up-regulation observed post-defoliation. This well-studied species exhibits an indeterminate growth habit and has a documented propensity for photosynthetic up-regulation (Pinkard et al., 2007; Eyles et al., 2009b). Temporal responses in leaf-level gas exchange, biochemistry, N, and carbohydrates as well as whole-plant changes in KP and S:S ratios of well-watered and fertilized saplings were examined to test specific whole-plant- and leaf-level mechanisms that may explain the increased photosynthetic performance after partial defoliation and/or budding. In seeking to determine the contribution of whole-plant mechanisms, the focus is not only on the S:S ratio and hydraulic conductance, but leaf-level responses are also investigated to examine fully the effects of defoliation on carbon use and storage, and water relations during the refoliation process. It was hypothesized that photosynthetic responses to partial defoliation were determined by whole-plant processes related to changes in either the S:S ratio or hydraulic conductance. It was predicted that (i) if the demand for assimilates by sinks regulates photosynthesis of source leaves, then there will be an inverse relationship between photosynthesis and the S:S ratio, followed by a decline in photosynthesis as the S:S ratio is restored during crown recovery, with concomitant leaf-level increases in rates of biochemical reactions of photosynthesis and changes in NSCs; and (ii) if whole-plant water relations are responsible for photosynthetic up-regulation, then there will be a positive relationship between A and KP.
Materials and methods
Plant material
Fifty-three open-pollinated saplings of E. globulus were planted in 300mm diameter pots (volume: 21 litres). In order to mimic natural growing conditions, saplings were supplied a low-phosphorus potting mix. They were grown outside for 5 months, watered until saturated daily, and fertilized with a slow-release pelletized fertilizer (Osmocote Native Gardens, N:P:K of 17.9:0.8:7.3, Scotts, Australia). The saplings were 68±2cm (mean ±SE) in height and had a diameter of 0.7±0.3cm at the start of the experiment (December 2010; aged 6 months). The saplings were exposed to ambient weather conditions for the full duration of the experiment.
Design
The experiment was established as a completely randomized design. Four foliage treatments were randomly applied to 12 saplings per treatment on 14 December, 2010. (i) Control (no defoliation) (C). (ii) Defoliated (remove leaves from 50% of crown length in the upper crown on December 2010 when saplings were 6 months old). The defoliation treatment removed leaves from the crown apex downwards (i.e. upper crown), excluding apical buds. Leaves were removed using long-nosed secateurs (D). (iii) A 100% removal of buds (remove all buds throughout the entire crown) (B). (iv) A combination of B and D (B&D).
These treatments were designed to manipulate S:S ratios (i.e. defoliation examined the effect of source leaf removal while budding examined the effect of sink removal). Previous studies have shown that 50% defoliation of the upper but not the lower crown elicits photosynthetic up-regulation (Pinkard et al., 2007). All foliage material was collected and total foliage area removed was determined as follows. A subsample of 10 leaves per sapling was taken and fresh leaf area was measured using the scanning software WinRhizo (Regent Instruments, Quebec). Leaves were then dried at 65 °C for 3 d and weighed. The remaining leaf material per tree was also dried at 65 °C for 7 d. The area:dry mass ratio (specific leaf area, SLA) was used to calculate leaf area removed per tree.
Gas exchange measurements
Photosynthetic measurements were made immediately before treatment (week 0) and 2 (week 2), 5 (week 5), 6 (week 6), 8 (week 8), and 12 (week 12) weeks after imposition of treatments on four saplings per treatment per measurement week. The A1500 and gs were measured with a portable open-path gas exchange system with CO2 control (Li-Cor LI-6400 portable IRGA, Li-Cor, Lincoln, NE, USA). Measurements were undertaken with a standard 20×30mm leaf chamber equipped with blue–red light-emitting diodes mounted on the top of the chamber (Model 6400-02B). Leaves of potted E. globulus saplings have been shown to be light saturated at a photosynthetic photon flux density (PPFD) of 1000 mmol m–2 s–1 (Eyles et al., 2009b); therefore, PPFD was set at 1500 μmol m–2 s–1. The ambient [CO2] was maintained at 39 Pa. Three of the youngest fully expanded leaves located within the lower crown of each sapling were recorded after A1500 and gs had stabilized (between 0.5min and 2min). In weeks 2, 5, and 6, it was not possible to follow this sampling protocol for the bud treatments (B&D and B treatments). In these two treatments, the leaf pair closest to the developing bud was selected for gas exchange measurements. Up to week 5, all leaves selected for measurement had been fully expanded before imposition of the defoliation/debudding treatments. To minimize the effects of time of measurement, a randomly chosen replicate from each treatment was measured.
The responses of A to varying [CO2] were measured at week 5, 8, and 12. For the photosynthetic rate–intercellular CO2 concentration (A/Ci) curves, PPFD was maintained at 2000 μmol m–2 s–1 and leaf temperature at 20 °C. Leaves were first equilibrated at a [CO2] of 39 Pa, after which [CO2] was reduced to 0 and then increased to 200 Pa in a total of 12 steps. The A/Ci curves were measured between 0900h and 1400h Eastern Standard Time (EST) over two consecutive days. Across all gas exchange measurements, leaf temperatures varied between 18 °C and 26 °C, vapour pressure deficit approximated ambient conditions, varying between 0.9 kPa and 1.3 kPa, while airflow through the chamber was 400 μmol–1 s–1. Each leaf used for photosynthetic measurements was sampled mid afternoon because maximum accumulation of sugars and starch occurs mid afternoon (1530–1630h EST) (Zhou et al., 2001). Leaves were immediately frozen and stored at –20 °C pending chemical analyses.
Biomass harvests
At week 0, a biomass harvest of five saplings was conducted to develop an allometric relationship between basal diameter and leaf area (leaf area=0.0028×basal diameter2.104, r2=0.89), which were used to estimate percentage leaf area removed for each treatment in week 1. Further biomass harvests were conducted at week 5, 8, and 12. Four saplings per treatment (a total of 16 saplings per harvest date) were harvested for measurements of aboveground biomass. At each harvest, saplings were enclosed immediately in plastic bags and stored at –20 °C until processed. Each tree was divided into its three main biomass components: leaves, buds, and woody tissue, and oven-dried to constant mass at 65 °C. Prior to drying, the leaf areas of 10 leaves, representing a range of sizes, were measured using the scanning software WinRhizo for determination of SLA. Biomass was coarsely ground with a Thomas-Wily mill model 4 (Thomas Scientific, Swedesboro, NJ, USA) and then subsampled for further grinding to a fine powder with a mixer mill MM200 grinder (Retsch, Haan, Germany) for N and carbohydrate analyses.
Plant hydraulic conductance
KP was measured 1, 5, 8, and 12 weeks after defoliation using the following equation:
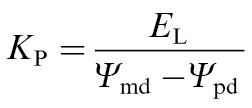 |
where EL is transpiration (mmol m–2 s–1) and Ψpd and Ψmd (MPa) are pre-dawn and mid-day leaf water potentials, respectively. On the day prior to Kp measurements, each pot was watered thoroughly and enclosed in a polypropylene bag sealed tightly around the base of the stem to prevent evaporation of soil water. EL was measured gravimetrically; that is, change in weight of the pot as measured over a 40–60min period between 1130h and 1230h EST during maximal rates of transpiration on sunny days. EL was normalized by dividing all values by the total leaf area of the sapling (as determined from the biomass harvests, described above). Ψ md and Ψpd were measured by collecting one leaf at 1300h and 2400h, respectively. They were placed immediately into plastic bags and kept in the dark until measurements were made using a 4.0MPa pressure chamber (PMS Instruments Co., Corvallis, OR, USA).
Chemical analyses
Soluble carbohydrates were extracted from ~50mg of dried plant tissue in 10ml of 80% (v/v) ethanol in a 60 ºC water bath for 30min. The extraction solvent included an internal standard of 0.01% trehalose, a sugar which had not been previously detected in initial qualitative studies. After centrifugation (10min, 2500rpm), the soluble sugars were separated and quantified using ultra-performance liquid chromatography–mass spectrometry (UPLC-MS). Full details of the method used are detailed in the Supplementary Method S1 available at JXB online. Starch and complex sugars remaining in the undissolved pellet of plant material after ethanol extractions were enzymatically (amyloglucosidase; Fluka-10115, Sigma-Aldrich, St. Louis, MO, USA) reduced to glucose using the method detailed in Eyles et al. (2009a). It would have been preferable to have used freeze- rather than oven-dried samples but, given that all samples were processed in the same manner, and relative rather than absolute values were used to compare treatment effects, it is considered that any treatment effects, if present, would have continued to be evident nonetheless. Although the sucrose concentration in the samples was lower than those found in other E. globulus studies (e.g. Merchant et al., 2011), it was still possible to observe significant differences across treatments (Table 1).
Table 1.
Effect of treatments (B=bud damage; B&D=bud damage and defoliation; C=control; D=50% defoliation) on leaf-level factors of Eucalyptus globulus in week 5 only.
| Parameter | B | B&D | C | D | P-value |
|---|---|---|---|---|---|
| VCmax (μmol m–2 s–1) | 90.7 (0.4)a | 113.2 (5.9) b | 82.1 (1.4) a | 94.9 (5.7) a | *** |
| Jmax (μmol m–2 s–1) | 163.8 (5.4) a,b | 184.8 (5.4) b | 137.2 (9.6) a | 137.5 (9.3) a | ** |
| Leaf Narea (g m–2) | 1.24 (0.08) a | 1.70 (0.2) b | 0.939 (0.07) a | 1.163 (0.1) a | *** |
| Leaf Nmass (%) | 0.98 (0.07) | 1.34 (0.1) | 0.99 (0.08) | 1.24 (0.09) | P = 0.06 |
| SLA (cm2 g–1) | 78.3 (5.8) a | 78.6 (5.8) a | 106.6 (5.8) b | 106.9 (5.8) b | *** |
| Soluble sugars (%) | 5.9 (0.7) | 6.0 (0.4) | 5.4 (0.4) | 6.5 (0.3) | NS |
| Starch (%) | 10.7 (1.8) | 11.4 (1.1) | 9.2 (0.7) | 10.8 (1.2) | NS |
| Specific soluble sugars (mg g–1) | |||||
| Fructose | 22.7 (2.8) | 19.7 (2.2) | 21.9 (2.3) | 24.0 (1.8) | NS |
| Galactinol | 2.89 (0.2) a | 4.91 (0.2) b | 2.31 (0.2) a | 2.44 (0.4) a | *** |
| Gentiobiose | 4.54 (0.9)c | 1.38 (0.2) a,b | 3.77 (0.7) b,c | 1.18 (0.3)a | ** |
| Glucose | 19.5 (2.7) | 16.3 (1.9) | 17.8 (1.8) | 19.8 (1.2) | NS |
| Raffinose | 1.91 (0.6) a | 5.83 (0.6)b | 1.43 (0.4)a | 5.8 (0.2)b | *** |
| Stachyose | 0.31 (0.04) a | 0.62 (0.07) b | 0.24 (0.01) a | 0.38 (0.02) a | *** |
| Sucrose | 0.79 (0.3) a | 2.91 (0.5) b | 0.57 (0.2) a | 6.33 (0.6) c | *** |
Asterisks indicate significance of treatment (Trt), *P ≤ 0.05; **P ≤0.01, ***P ≤0.001; NS, non-significant. Different letters denote a significant treatment (α=0.05) effect within a measurement period. Error bars are 1 SE, n=4 for all treatments.
Leaf N concentration was determined on dried and ground material with an elemental analyser (Thermo Finnigan EA 1112 Series Flash Elemental Analyser, Thermo Scientific, MA, USA).
Data analyses
Photosynthetic responses to [CO2] were fitted to the biochemical model developed by Farquhar et al. (1980) and von Caemmerer and Farquhar (1981), and presented in full by von Caemmerer (2000). The values of the various parameters in the model are the standard values tabulated by von Caemmerer (2000), but VCmax and Jmax were estimated from A/Ci curves. The fitting protocol used here was based on an Excel workbook on the PhysEcol website (http://www.elsevierdirect.com/companion.jsp?ISBN=9780123744609) that accompanies Landsberg and Sands (2011). The standard errors of, and correlations between, the estimated parameters were obtained using the NonlinXL software also available from the PhysEcol website.
The whole-plant aboveground S:S ratio (g:g) was calculated in two ways. First, it was calculated as the ratio of biomass of photosynthetic tissues (leaves) to non-photosynthetic tissue (woody tissue+buds) [hereafter referred to as source:sink (biomass)). Biomass accumulation is a long-term integrated measure of physiological performance and this definition is similar to that used in previous manipulation studies (Evans, 1991; Laporte and Delph, 1996). Secondly, it was calculated as the ratio of the total NSC pool of photosynthetic tissues (leaves) to non-photosynthetic tissue (woody tissue) [hereafter referred to as source:sink (carbohydrates)]. This definition attempts to take into account stored NSC pools that may be utilized in recovery. The woody tissue was included with non-photosynthetic tissues because their CO2 exchange rates are negligibly small, especially compared with that of leaves (Eyles et al., 2009b). It is acknowledged that the calculations of both whole-plant S:S ratios would have been strengthened by taking into account the contribution of root biomass as a sink.
Effects of treatment and week and their interaction on A1500, gs, KP, EL, Ψpd, Ψmd, leaf area, and source:sink (biomass) were analysed by two-way analysis of variance (ANOVA), and the degrees of freedom were adjusted using the Satterthwaite method. Since destructive harvesting of saplings was used, there were no repeated measures. The largest difference between treatments in A1500 occurred in week 5; therefore, the effects of treatment on the following variables were explored: VCmax, Jmax, leaf N, soluble sugars, starch, and specific soluble sugars (fructose, galactinol, gentiobiose, glucose, raffinose, stachyose, and sucrose) in week 5 only using one-way ANOVA. The assumptions of ANOVA such as homogeneity of variance and the Gaussian distribution were checked by the use of qq plots and residual plots for all variables. Post-hoc comparisons of means were made using Tukey’s method. Initially, the relationships between A1500 and leaf- and whole-plant-level factors in week 5 were examined by linear regression. Associations between A1500 and the explanatory variables were then explored using stepwise regression methods to examine whether increases in A1500 could be best explained by (i) leaf-level factors only; (ii) whole-plant factors only; or (iii) a combination of leaf- and whole-plant-level factors. A1500, gs, and SLA were analysed by SAS version 9.2. The other factors were analysed by Genstat Version 10.1 (VSN International).
Results
Effect of defoliation treatments on leaf area
Leaf area at the start of the experiment in December averaged 0.586±0.008 m2. From the allometric relationships developed between basal diameter and leaf area, it was estimated that the percentage of leaf area removed in increasing order of severity was as follows: C, 0%; B, 3.96±0.2%; D, 32.8±1.3%, and B&D, 35.2±1.3%. By week 5, leaf area of the B&D treatment continued to be significantly reduced by 50% compared with the C treatment (C, 1.32±0.1; B, 0.97±0.1; B&D, 0.66±0.09, and D, 1.05±0.1 m2; P < 0.05).
Defoliation treatments induce photosynthetic up-regulation
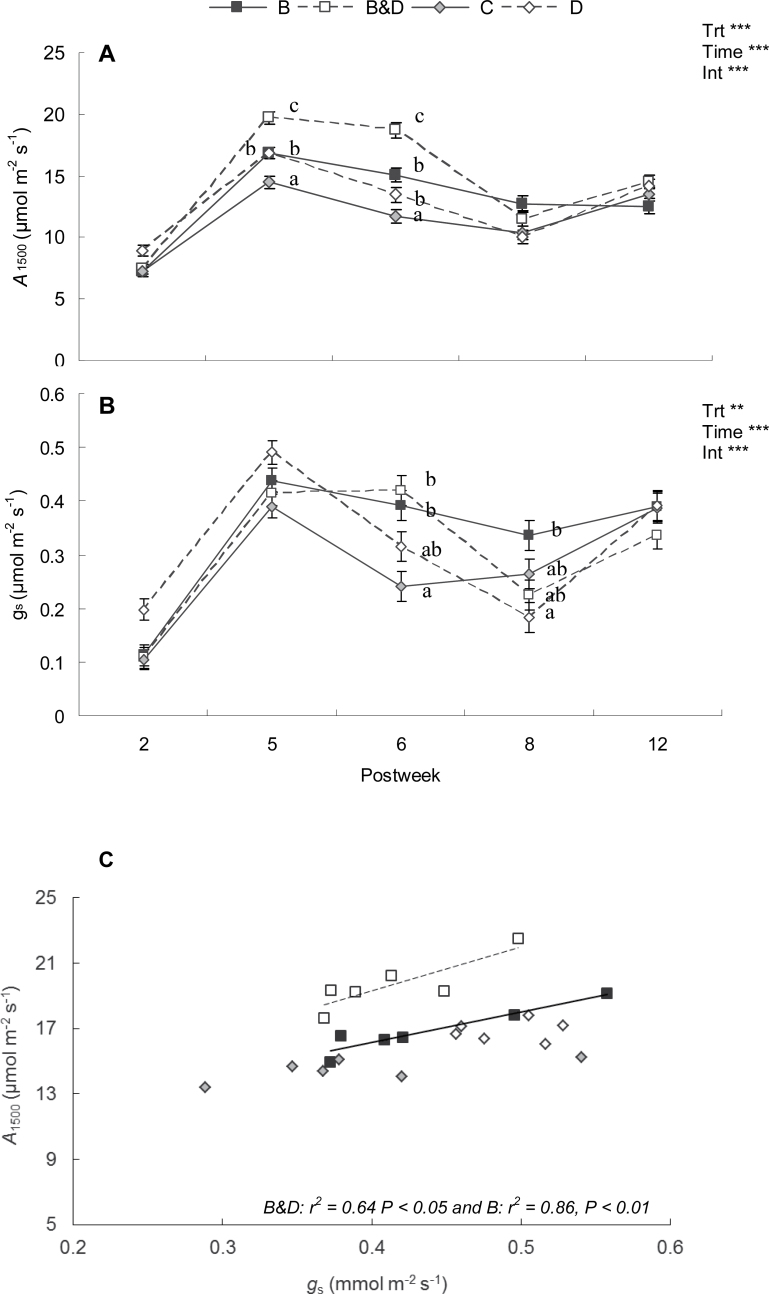
The A1500 and gs measured at week 0 were not significantly different across treatments (i.e. mean values of A1500=13.6±0.2 μmol m–2 s–1 and gs=0.343±0.01 mmol m–2 s–1; P > 0.05). Defoliation treatments significantly increased A1500 (Fig. 1A). By week 5, A1500 was 36, 18, and 17% higher in B&D, B, and D treatments, respectively, than in control saplings. These treatment differences were maintained through to week 6 but, by week 8, treatment effects were negligible. The changes in gs followed a similar pattern to A1500, with the largest difference occurring in week 6 (Fig. 1B). At this sampling point, the gs of both bud treatments was nearly double that measured in the C treatment. In week 8, it was only the gs of B and not the B&D treatment that remained significantly higher than that of the control value. In week 5, the relationship between A1500 and gs was significantly affected by treatment interactions (P < 0.05, Fig. 1C). In particular, significant regressions were found for the B and B&D treatments, and their respective slopes were different from those of the control saplings. This meant a higher A1500 per unit gs was exhibited by saplings in the B&D and B treatments than in the C treatment.
Fig. 1.
Effect of treatments (B=bud damage; B&D=bud damage and defoliation; C=control; D=50% defoliation) on A1500 (A) and (B) gs on leaves of Eucalyptus globulus. Asterisks indicate significance of treatment (Trt), time, or their interaction (Int): **P ≤ 0.01 and ***P ≤ 0.001. Different letters denote a significant treatment (α=0.05) effect within a measurement period. Error bars are 1 SE. (C) Relationship between A1500 and gs of E. globulus in weeks 5 and 6. The significant regressions shown are described by the following equations: A1500 (B)=18.63 gs+8.70 and A1500 (B&D)=27.05 gs+8.48.
Impact of defoliation treatment on whole-tree hydraulic conductivity per leaf area varies during refoliation
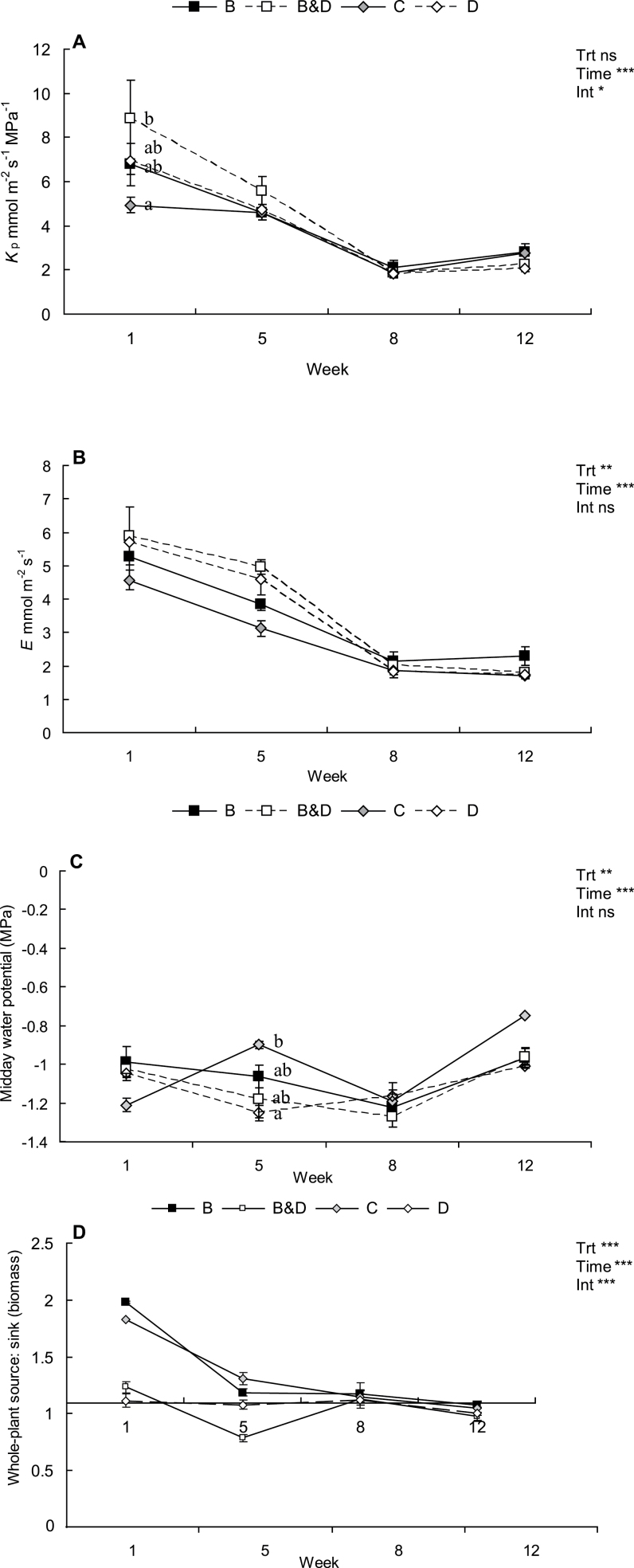
Ψpd was unaffected by defoliation treatment throughout the experiment (P > 0.05; data not shown). KP was significantly affected by defoliation treatments (Fig. 2A). In week 1, the KP in the B&D treatment was 78% higher than control values. The differences between the treatments and control saplings, however, decreased with time and, by week 5, the differences were <21%. In week 5, the reduction in total transpiring area brought about by the defoliation treatments was offset by a concomitant non-significant increase in EL (Fig. 2B) and a decrease inΨmd (Fig. 2C). That is, EL was 60% higher while Ψmd was 31% lower for saplings in the B&D treatment compared with control values.
Fig. 2.
Effect of treatments (B=bud damage; B&D=bud damage and defoliation; C=control; D=50% defoliation) on whole-plant leaf-specific conductance (Kp) per unit leaf area (A), whole-plant transpiration (E) (B), midday water potential (C), and whole-plant source:sink (biomass) (D) of Eucalyptus globulus. Asterisks indicate significance of treatment (Trt), time, or their interaction (Int): *P ≤ 0.05; **P ≤0.01, ***P ≤0.001; ns, non-significant. Different letters denote a significant treatment (α=0.05) effect within a measurement period. Error bars are 1 SE, n=4 for all treatments.
Whole-tree S:S ratios reflect defoliation treatments over time
The source: sink (biomass) of control trees was 1.8 (Fig. 2D). This declined with time to 1.05 by week 12, reflecting increased allocation of resources to woody tissue [i.e. woody tissue/total aboveground biomass %=32.6±0.02 (week 0), 42.9±0.9 (week 5), 46.1±0.9 (week 8), and 48±1.6% (week 12), P < 0.05; Fig. 2D]. At week 1, defoliation treatments brought about a large reduction in the source:sink (biomass) of B&D and D treatments by 32% and 39%, respectively, compared with the C treatment. In contrast, there was an immediate reduction in sinks and therefore an increase in source:sink (biomass) in the B treatment. However, by week 5, the release of auxiliary buds, which replaced the single debudded apical bud with a new pair of buds (one arising from each leaf axil), created new sinks and this was reflected as a reduced source:sink (biomass) that was similar to that of the control saplings. The source:sink (biomass) of saplings in the B&D treatment remained significantly lower than the control value in week 5. Thereafter, the differences between treatments and control saplings decreased over time.
Leaf-level responses in week 5 vary with defoliation treatment
The largest contrast between treatments in A1500 occurred in week 5 (Fig. 1). Therefore, for clarity, the effects of defoliation treatments on leaf-level parameters in week 5 only are presented (see summary in Table 1). VCmax and Jmax were significantly higher (~40%) than control values in the B&D treatment. SLA in both bud treatments was significantly lower (26%) than in the other two treatments. The leaf Nmass of saplings in the B&D treatment was almost twice as high as that of control saplings in week 5. Total soluble sugar concentrations showed a similar pattern to starch concentrations in week 5. The concentrations of five carbohydrates were significantly influenced by defoliation treatments in week 5 (Table 1). In contrast, no significant changes were observed for the inositol group, the unknown monosaccharide, and the two most dominant soluble sugars, glucose and fructose (data presented for fructose and glucose only, Table 1). Specifically, both defoliation treatments (D and B&D) resulted in significant increases in sucrose and raffinose but decreases in gentiobiose. For saplings in the B&D treatment, concentrations of stachyose and galactinol were 3- and 2-fold higher, respectively, than those in the C treatment
Photosynthetic up-regulation primarily related to whole-plant responses
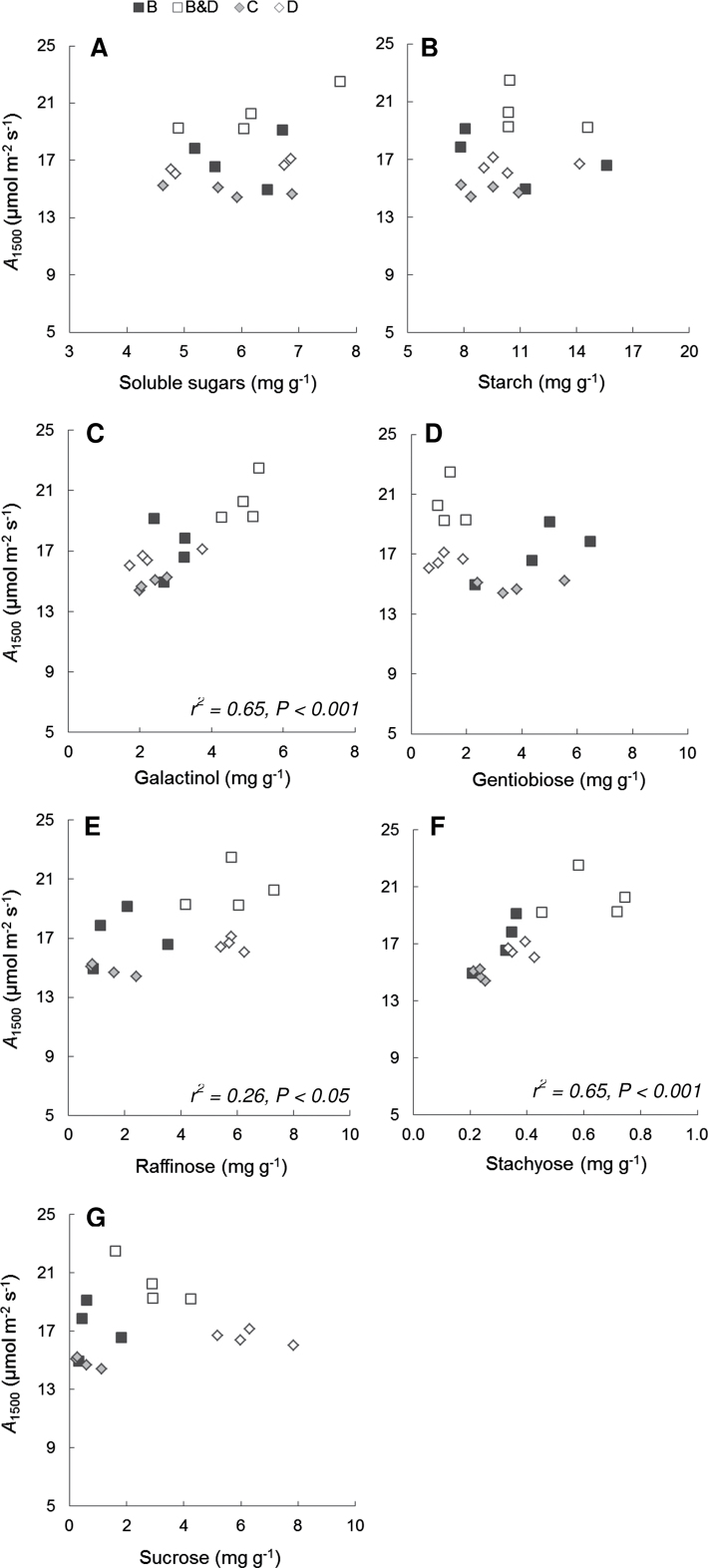
The relationships between A1500 and leaf- and whole-plant-level responses to defoliation treatments were explored in week 5 only. For leaf-level factors, A1500 was positively correlated with leaf Nmass, VCmax, and Jmax, and negatively correlated with SLA (Supplementary Fig. S1 at JXB online). A1500 was unrelated to starch and soluble sugar concentrations (Fig. 3A, B). These leaf-level relationships did not appear to be consistent across treatments (data not shown), suggesting that the changes in these values were not necessarily driving the photosynthetic up-regulation. Regression analyses between A1500 and specific soluble sugars showed that the direction and strength of these relationships varied with each soluble sugar (Fig. 3). Specifically, A1500 was positively correlated with stachyose, galactinol, and raffinose, but not sucrose and gentiobiose. Multiple regression analysis of all seven leaf-level factors that were found to be significantly related to A1500 (i.e. gs, VCmax, Jmax, leaf Nmass, stachyose, galactinol, and raffinose, Figs 1C, 3; Supplementary Fig. S1) showed that increases in A1500 were best explained by the predictor variables of galactinol and gs (A1500=7.06+1.58Galactinol+11.76gs, r2=0.76, P < 0.001). However, there was considerable between-treatment variation in the relationship between A1500 and gs (Fig. 1C), suggesting that galactinol had a large influence on the strength of this relationship.
Fig. 3.
Relationship between A1500 and soluble sugars (A), starch (B), galactinol (C), gentiobiose (D), raffinose (E), stachyose (F), and sucrose (G) from leaves of Eucalyptus globulus following treatments (B=bud damage; B&D=bud damage and defoliation; C=control; D=50% defoliation) in week 5. The significant regressions shown are described by the following equations: (C) A1500=1.59Galactinol+12.22, (E) A1500=0.55Raffinose+15.14, and (F) A1500=11.39Stachyose+12.81.
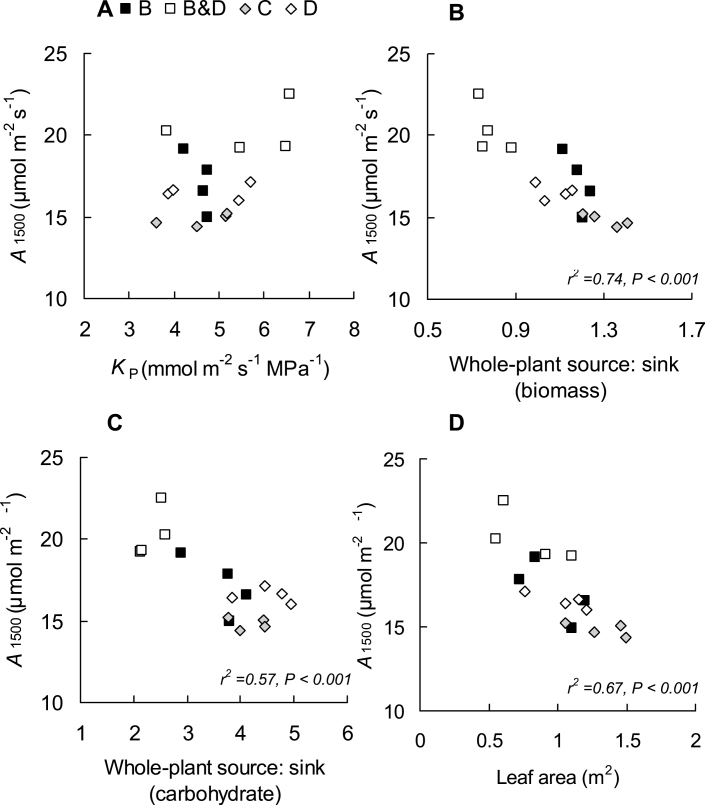
At a whole-plant level, there was no significant relationship between A1500 and KP (P > 0.05) (Fig. 4A). In contrast, there were significant negative relationships between A1500 and source:sink (biomass) (Fig. 4B), source:sink (carbohydrate) (Fig. 4C), and leaf area (Fig. 4D). Multiple regression analysis at the whole-plant scale showed that increases in A1500 were best explained by the predictor variable of source:sink (biomass) (r2=0.74, P < 0.001, Fig. 4B) alone. The addition of KP or leaf area did not improve the correlation. A final multiple regression analysis of all leaf- and whole-plant-level variables showed that the best whole-plant model was not significantly improved by the addition of any of the leaf-level variables (data not shown).
Fig. 4.
Relationship between A1500 and KP (A), whole-plant source:sink (biomass) (B), whole-plant source:sink (carbohydrate) (C), and leaf area (D) of Eucalyptus globulus following treatments (B=bud damage; B&D=bud damage and defoliation; C=control; D=50% defoliation) in week 5. The significant regressions shown are described by the following equations: (A) A1500= –9.58Source:sink (biomass)+27.64, (C) A1500= –1.92Source:sink (carbohydrate)+24.26, and (D) A1500= –6.37leaf area+23.56.
Discussion
It has been demonstrated that whole-plant S:S relationships explain photosynthetic responses to defoliation and debudding. Part of the whole-plant response revolved around leaf-level changes that facilitate increases in photosynthetic rates, although the role of some leaf-level responses alone in determining photosynthetic responses (e.g. the relationship between A1500 and galactinol+gs) cannot be discounted conclusively. There was only weak evidence for hydraulically mediated photosynthetic responses. Following an initial discussion of the variable evidence that links increases in A1500 with leaf-level responses, it is then proposed that the observed whole-plant responses improved understanding of photosynthetic responses to defoliation. In particular, it is argued that the temporal patterns of A1500 are closely related to changes in S:S ratios during crown recovery.
Leaf-level factors and photosynthetic up-regulation
Leaf-level changes similar to those reported in other studies (Lavigne et al., 2001; Layne and Flore, 1995; Ozaki et al., 2004; Pinkard et al., 2011b), and consistent with the whole-plant regulation of photosynthesis via S:S interactions, were observed. All treatments, and, in particular, the B&D and B treatments, increased A1500 per unit gs (Fig. 1C), suggesting that these saplings became more efficient at fixing CO2 per unit water lost, and that changes in gs were not driving photosynthetic responses to defoliation as would have been expected if photosynthetic changes were hydraulically mediated. Further, increases in A1500 were accompanied by increases in VCmax and Jmax (Table 1), suggestive of changes in the rates of biochemical reactions of photosynthesis and more rapid translocation of end-products. These responses, however, were only observed in the debudding and not in the D treatment. Decreases in SLA were also noted, but, once again, only in the debudding treatments (Table 1), providing some evidence that these treatments had affected the leaf thickness and density of apparently ‘fully expanded’ leaves, though it remains unclear exactly how. In response to pathogen infection, the adaxial palisade layer of fully expanded leaves of E. globulus is capable of undergoing cell division to produce, for example, a necrophylactic periderm (Smith et al., 2007). Debudding treatments may similarly induce modifications in leaf anatomy that might affect leaf-level hydraulics (Brodribb et al., 2007).
In previous studies examining photosynthetic responses in E. globulus after defoliation, leaf Narea has been reported either to increase (Turnbull et al., 2007; Pinkard et al., 2011b) or to not differ from the control value (Eyles et al., 2009b; Quentin et al., 2010). Turnbull et al. (2007) showed that increases in leaf Narea following defoliation did not translate into more Rubisco or chlorophyll. Turnbull et al. (2007) concluded that the immediate increases in A1500 following defoliation were largely attributed to increases in VCmax and Jmax. In this study, a positive relationship between A1500 and leaf Narea was observed, but regression analysis suggested that Narea did not explain the photosynthetic response to defoliation.
As reported in other studies, it was found that responses between A1500 and various leaf-level variables were inconsistent across treatments, suggesting that the direction and magnitude of the leaf-level responses were dependent on the nature of the treatment imposed, and that the observed photosynthetic responses were not sufficiently explained by leaf-level mechanisms. The strong relationship between A1500 and the combination of gs and galactinol does not reflect the observed strong among-treatment differences in the relationship between A1500 and gs, and may highlight the role of galactinol as a signalling molecule (see below).
Whole-plant water relations and photosynthetic up-regulation
Various measures of whole-plant responses to defoliation have arguably provided some support for hydraulically mediated control of gas exchange. In the current study, EL was significantly higher in both defoliation treatments (B&D and D) than control values in week 5 (P < 0.01). This result is consistent with previous studies (Meinzer and Grantz, 1990; Pataki et al., 1998; Oren et al., 1999; Quentin et al., 2011, 2012) and provides some evidence of improved water status. However, it was also found that a significantly more negative Ψmd enabled trees in the B&D, D, and B treatments to maintain similar KP values to that observed in the C treatment in week 5. While there was an immediate increase in KP 1 week after defoliation (especially in the B&D treatment) (Fig. 2), it is suggested that this initial increase in KP was not directly responsible for the increase in A1500 observed in week 5 because the increase was not sustained whereas the increases in A1500 were. The lack of correlation between Kp and A1500 across treatments in week 5 (Fig. 4A) provides good evidence that increases in A1500 were not sufficiently explained by changes in Kp alone. In additional support for this argument, it was found that Kp was unrelated to gs in week 5 (data not shown), suggesting a transient disconnection between leaf-level water status and whole-plant hydraulic capacity.
The poor correlation between Kp and A1500 could be explained by an unchanging root:shoot ratio. While partial defoliation is assumed to increase root:shoot ratios (Reich et al., 1993; Lavigne et al., 2001; Ozaki et al., 2004), in the current study it is unclear how debudding would lead to substantial shifts in the root:shoot ratio where even the 100% removal of buds failed to cause any changes in leaf area compared with the C treatment. Studies that examined the effects of defoliation on biomass partitioning have consistently reported either a decrease (due to root death) or an unchanging root:shoot ratio (Vanderklein and Reich, 199; Ovaska et al., 1993; Reich et al., 1993; Syvertsen, 1994; Singh and Thompson, 1995). Although the root:shoot ratio was not explicitly examined in this study, a comparable defoliation study in young E. globulus field trees similarly observed a reduction, rather than an increase, in this ratio (Eyles et al., 2009a). This response most probably reflects the strategy of defoliated plants to increase aboveground allocation of biomass to new photosynthetic tissue, not necessarily at the expense of, but instead of, root biomass (Eyles et al., 2009a).
Source–sink interactions and photosynthetic up-regulation
In accordance with the S:S hypothesis, A1500 was greater in the B&D, D, and B treatments than C treatment in weeks 5 and 6 (Fig. 1), which was followed by a decline in A1500 as the source:sink (biomass) was restored by week 8 (Figs 1, 2D). As predicted, an inverse relationship between A1500 and source:sink (biomass) was observed across treatments in week 5 (Fig. 4D), providing further support for S:S regulation of photosynthesis. The photosynthetic responses of the B treatment appears puzzling at first because debudding presumably should have increased source:sink (biomass). Previous studies examining photosynthetic responses to debudding suggest that the direction of the response is species specific. In herbaceous species, sink manipulation can decrease (Plaut et al., 1987), increase (von Caemmerer and Farquhar, 1984), or effect no change in A (Plaut et al., 1987; Evans, 1991). In woody species, debudding has mostly been found to increase A (Syvertsen, 1994; Lavigne et al., 2001;Ozaki et al., 2004; but see Myers et al., 1999). Consistent with the latter studies, significant increases in A1500 were also found in the B treatment, but it is necessary to keep in mind that this measurement was made 5 weeks after defoliation (Fig. 1). At this measurement date, the source:sink (biomass) of the B treatment was similar to control values, most probably reflecting the rapid stimulation of auxiliary bud development, which created new sinks.
The refoliation process, which includes the production of new leaf and buds, is critical for the restoration of the S:S ratio and, for evergreen woody tree species with an indeterminate growth habit such as E. globulus, rapid refoliation after defoliation is possible. It is unclear how evergreen trees with a deterministic growth habit would restore S:S ratios, particularly after a post-flush defoliation event. Interestingly, for some of these tree species, the increase in photosynthesis has been shown to be much more long-lived, up to 16 weeks rather than only 5 weeks (Reich et al., 1993; Eyles et al., 2011). Longer durations of photosynthetic up-regulation have also been observed for older trees, which potentially may require a longer time to restore S:S balance, as their sinks are relatively much larger than those of young trees. Alternatively, the demand for new resources by alternative sinks such as stem and/or roots may increase during periods when shoot growth ceases (Reich et al., 1993).
Reductions in NSC in source leaves have been suggested as evidence for a carbohydrate-mediated feedback between carbon sinks and sources (Zhou and Quebedeaux, 2003). Therefore, a negative relationship between carbohydrate and A1500 would have been expected. While no such relationships were observed for either soluble sugar or starch (Fig. 3E, F), there was a negative relationship between A1500 and sucrose for the two defoliation treatments (A1500= –1.02Sucrose+23.12, r2 =0.85, P < 0.001, Fig. 3G), which supports the hypothesis. Sucrose is the major transport carbohydrate in plants (Koch, 2004).
Role of carbohydrates as signalling molecules in photosynthetic up-regulation
The present results suggest that increases in A1500 were not related to variation in leaf-level bulk NSC, as similarly reported in other studies (Lavigne et al., 2001; Turnbull et al., 2007; Quentin et al., 2010). Instead, the analysis of specific soluble sugars showed that increases in A1500 were not accompanied by the same changes in the carbohydrate profile across treatments (Table 1). In particular, strong positive correlations were found between galactinol, stachyose, and, to a lesser extent, raffinose and photosynthetic responses (Fig. 3). In addition to their critical roles as carbon and energy sources, carbohydrates are also recognized as signalling molecules in growth and development, and stress-related responses (Valluru and Van den Ende, 2011; Bolouri Moghaddam and Van den Ende, 2012). While sucrose is the major transport carbohydrate in plants (Koch, 2004), other small oligosaccharides such as raffinose and galactinol are also phloem mobile (Keller and Pharr, 1996). The present results provide some evidence that galactinol, stachyose, and raffinose may potentially function as signalling molecules in the regulation of gas exchange. Future experiments should focus on the putative signalling role of these sugars, monitoring their presence not only in leaves but also in phloem sap. It is interesting to note that the carbon content of phloem sap of E. globulus is dominated by sucrose and raffinose (Merchant et al., 2010). The latter has received little attention in the context of defoliation studies but, given that phloem is the main pathway for the movement of solutes and signalling among tissues of higher plants, examining the effects of defoliation on the composition of phloem sap will provide insights into the regulatory role of specific carbohydrates.
Conclusion
Although photosynthetic responses to defoliation treatments were influenced by both leaf- and whole-plant-level factors, by directly comparing these variables with multiple linear regression, strong evidence was provided that the direction and duration of these photosynthetic responses were regulated by changes in whole-plant S:S ratios in E. globulus saplings. There was little evidence that A1500 was related to Kp in week 5, suggesting that changes in A1500 were not hydraulically mediated. At a leaf level, only the interaction of gs and galactinol explained photosynthetic responses to defoliation, and, given the between-treatment variation in the relationship between A1500 and gs alone, there cannot be confidence in the capacity of this relationship to explain photosynthetic responses to defoliation without further examination of the role of galactinol. Strong positive correlations were observed between three specific carbohydrates: galactional, stachyose, and raffinose, and photosynthetic responses, providing some evidence that these sugars may function as longer term signalling molecules in the regulation of gas exchange. Future studies examining whole-plant responses to defoliation treatments should be able to use the S:S hypothesis to predict the interactive effects of defoliation treatments with other stresses such as water or nutrient stress on photosynthetic responses (Pinkard et al., 2011b).
Supplementary data
Supplementary data are available at JXB online.
Method S1. Full description of the ultra-performance liquid chromatography–mass spectrometry (UPLC-MS) method used to analyse leaf soluble sugars.
Figure S1. Relationship between A1500 (measured directly) and leaf-level Nmass (a), SLA (b), VCmax (c), and Jmax (d) of Eucalyptus globulus following treatments in week 5.
Acknowledgements
We thank Dr Tim Brodribb (Plant Science, University of Tasmania), Dr Don White (CSIRO Ecosystem Sciences), and two anonymous reviewers for valuable comments on earlier drafts of the manuscript. We thank Craig Baillie, Dale Worledge, Malcolm Hall, and Karen Barry for field and laboratory assistance. Thanks to Dr Thomas Rodemann (Central Science Laboratory, University of Tasmania) for analysis of leaf N. We acknowledge the Cooperative Research Centre for Forestry (sub-project 1.2) for financial support.
Glossary
Abbreviations:
- A1500
photosynthetic rate
- A/Ci
photosynthetic rate–intercellular CO2 concentration
- B
debudding treatment
- B&D
debudding and defoliation treatment C, control (no defoliation)
- D
defoliation treatment
- DM
dry matter
- E
transpiration
- EST
Eastern Standard Time
- gs
stomatal conductance
- KP
whole-plant soil-to-leaf hydraulic conductance NSC, non-structural carbohydrate
- PPFD
photosynthetic photon flux density
- SLA
specific leaf area
- S:S
source:sink
- UPLC-MS
ultra-performance liquid chromatography–mass spectrometry
- Ψmd
midday leaf water potential
- Ψpd
pre-dawn leaf water potential.
References
- Brodribb TJ, Feild TS, Jordan GJ. 2007. Leaf maximum photosynthetic rate and venation are linked by hydraulics. Plant Physiology 144, 1890–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolouri Moghaddam MR, Van den Ende W. 2012. Sugars and plant innate immunity. Journal of Experimental Botany 63, 3989–3998 [DOI] [PubMed] [Google Scholar]
- Eyles A, Pinkard EA, Mohammed C. 2009a. Shifts in biomass and resource allocation patterns following defoliation in Eucalyptus globulus growing with varying water and nutrients. Tree Physiology 29, 753–764 [DOI] [PubMed] [Google Scholar]
- Eyles A, Pinkard EA, O’Grady AP, Worledge D, Warren C. 2009b. Role of corticular photosynthesis following defoliation in Eucalyptus globulus . Plant, Cell and Environment 32, 1004–1014 [DOI] [PubMed] [Google Scholar]
- Eyles A, Robinson AP, Smith D, Carnegie A, Smith I, Stone C, Mohammed C. 2011. Quantifying the growth loss in Pinus radiata plantation trees to repeated aphid attack by Essigella californica . Forest Ecology and Management 261, 120–127 [Google Scholar]
- Evans AS. 1991. Whole-plant responses of Brassica campestris (cruciferae) to altered sink–source relations. American Journal of Botany 78, 394–400 [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. 1980. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149, 78–90 [DOI] [PubMed] [Google Scholar]
- Koch K. 2004. Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Current Opinion in Plant Biology 7, 235–246 [DOI] [PubMed] [Google Scholar]
- Landsberg JJ, Sands P. 2011. Physiological ecology of forest production: principles, processes and models. London:Academic Press; [Google Scholar]
- Laporte MM, Delph LF. 1996. Sex specific physiology and source–sink relations in the dioecious plant Silene latifolia . Oecologia 106, 63–72 [DOI] [PubMed] [Google Scholar]
- Lavigne MB, Little CHA, Major JE. 2001. Increasing the sink:source balance enhances photosynthetic rate of 1-year-old Balsam-fir foliage by increasing allocation of mineral nutrients. Tree Physiology 21, 417–426 [DOI] [PubMed] [Google Scholar]
- Layne D, Flore J. 1995. End-product inhibition of photosynthesis in Prunus cerasus L. in response to whole-plant source–sink manipulation. Journal of the American Society for Horticultural Science 120, 583–599 [Google Scholar]
- Lovett GM, Tobiessen P. 1993. Carbon and nitrogen assimilation in red oaks Quercus rubra L. subject to defoliation and nitrogen stress. Tree Physiology 12, 259–269 [DOI] [PubMed] [Google Scholar]
- Merchant A, Peuke AD, Keitel C, Macfarlane C, Warren CR, Adams MA. 2010. Phloem sap and leaf δ13C, carbohydrates, and amino acid concentrations in Eucalyptus globulus change systematically according to flooding and water deficit treatment. Journal of Experimental Botany 61, 1785–1793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant A, Wild B, Richter A, Bellot S, Adams MA, Dreyer E. 2011. Compound-specific differences in (13)C of soluble carbohydrates in leaves and phloem of 6-month-old Eucalyptus globulus (Labill). Plant, Cell and Environment 34, 1599–1608 [DOI] [PubMed] [Google Scholar]
- Meinzer FC, Grantz DA. 1990. Stomatal and hydraulic conductance in growing sugarcane: stomatal adjustment to water transport capacity. Plant, Cell and Environment 13, 383–388 [Google Scholar]
- Myers DA, Thomas RB, DeLucia EH. 1999. Photosynthetic responses of loblolly pine (Pinus taeda) needles to experimental reduction in sink demand. Tree Physiology 19, 235–242 [DOI] [PubMed] [Google Scholar]
- Neales TF, Incoll LD. 1968. The control of leaf photosynthesis rate by the level of assimilate concentration in the leaf: a review of hypotheses. Botanical Reviews 34, 107–125 [Google Scholar]
- Oren R, Phillips N, Ewers BE, Pataki DE, Megonigal JP. 1999. Sap-flux-scaled transpiration responses to light, vapor pressure deficit, and leaf area reduction in a flooded Taxodium distichum forest. Tree Physiology 19, 337–347 [DOI] [PubMed] [Google Scholar]
- Ovaska J, Ruuska S, Rintamaki E, Vapaavuori E. 1993. Combined effects of partial defoliation and nutrient availability on cloned Betula pendula saplings. II. Changes in net photosynthesis and related biochemical properties. Journal of Experimental Botany 44, 1395–1402 [Google Scholar]
- Ozaki K, Saito H, Yamamuro K. 2004. Compensatory photosynthesis as a response to partial debudding in Ezo Spruce, Picea jezoensis seedlings. Ecological Research 19, 225–231 [Google Scholar]
- Pataki DE, Oren R, Phillips N. 1998. Responses of sap flux and stomatal conductance of Pinus taeda L. to stepwise reductions in leaf area. Journal of Experimental Botany 49, 871–878 [Google Scholar]
- Pinkard EA, Battaglia M, Mohammed CL. 2007. Defoliation and nitrogen effects on photosynthesis and growth of Eucalyptus globulus . Tree Physiology 27, 1053–1063 [DOI] [PubMed] [Google Scholar]
- Pinkard EA, Battaglia M, Roxburgh S, O’Grady AP. 2011a. Estimating forest net primary production under changing climate: adding pests into the equation. Tree Physiology 31, 686–699 [DOI] [PubMed] [Google Scholar]
- Pinkard EA, Eyles A, O’Grady AP. 2011b. Are gas-exchange responses to resource limitation and defoliation linked to source:sink relationships? Plant, Cell and Environment 34, 1652–1665 [DOI] [PubMed] [Google Scholar]
- Pinkard EA, Mohammed C, Beadle CL, Hall MR, Worledge D, Mollon A. 2004. Growth responses, physiology and decay associated with pruning plantation grown Eucalyptus globulus Labill. and E. nitens Deane and Maiden Maiden. Forest Ecology and Management 200, 263–277 [Google Scholar]
- Plaut Z, Mayoral ML, Reinhold L. 1987. Effect of altered sink:source ratio on photosynthetic metabolism of source leaves. Plant Physiology 85, 786–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quentin AG. 2010. Growth and physiological responses of Eucalyptus globulus Labilladiere following defoliation. PhD Thesis, University of Tasmania:Hobart, Australia: [Google Scholar]
- Quentin AG, O’Grady AP, Beadle CL, Worledge D, Pinkard EA. 2011. Responses of transpiration and canopy conductance to partial defoliation of Eucalyptus globulus trees. Agricultural and Forest Meteorology 151, 356–364 [Google Scholar]
- Quentin AG, O’Grady AP, Beadle CL, Worledge D, Pinkard EA. 2012. Interactive effects of water supply and defoliation on photosynthesis, plant water status and growth of Eucalyptus globulus Labill. Tree Physiology 32, 958–967 [DOI] [PubMed] [Google Scholar]
- Quentin AG, Pinkard EA, Beadle CL, Wardlaw TJ, O’Grady AP, Paterson S, Mohammed CL. 2010. Do artificial and natural defoliation have similar effects on physiology of Eucalyptus globulus Labill. seedlings? Annals of Forest Science 67, 203–211 [Google Scholar]
- Reich PB, Walters MB, Krause SC, Vanderklein DW, Raffa KF, Tabone T. 1993. Growth, nutrition and gas exchange of Pinus resinosa following artificial defoliation. Trees: Structure and Function 7, 67–77 [Google Scholar]
- Sack L, Holbrook NM. 2006. Leaf hydraulics. Annual Review of Plant Biology 57, 361–381 [DOI] [PubMed] [Google Scholar]
- Sands PJ. 1995. Modelling canopy production II. From single leaf photosynthetic parameters to daily canopy photosynthesis. Australian Journal of Plant Physiology 22, 603–614 [Google Scholar]
- Singh KA, Thompson FB. 1995. Effect of lopping on water potential, transpiration, regrowth, 14C-photosynthate distribution and biomass production in Alnus glutinosa . Tree Physiology 15, 197–202 [DOI] [PubMed] [Google Scholar]
- Smith AH, Gill WM, Pinkard EA, Mohammed CL. 2007. Anatomical and histochemical defence responses induced in juvenile leaves of Eucalyptus globulus and Eucalyptus nitens by Mycosphaerella infection. Forest Pathology 37, 361–373 [Google Scholar]
- Syvertsen JP. 1994. Partial shoot removal increases net CO2 assimilation and alters water relations of Citrus seedlings. Tree Physiology 14, 497–508 [DOI] [PubMed] [Google Scholar]
- Sweet GB, Wareing PF. 1966. Role of plant growth in regulating photosynthesis. Nature 210, 77–79 [Google Scholar]
- Turnbull TL, Adams MA, Warren CR. 2007. Increased photosynthesis following partial defoliation of field-grown Eucalyptus globulus seedlings is not caused by increased leaf nitrogen. Tree Physiology 27, 1481–1492 [DOI] [PubMed] [Google Scholar]
- Valluru R, Van den Ende W. 2011. Myo-inositol and beyond—emerging networks under stress. Plant Science 181, 387–400 [DOI] [PubMed] [Google Scholar]
- Vanderklein DW, Reich PB. 2000. European Larch and Eastern White Pine respond similarly during three years of partial defoliation. Tree Physiology 20, 283–287 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S. 2000. Biochemical models of leaf photosynthesis. Collingwood, Victoria, Australia:CSIRO Publishing; [Google Scholar]
- von Caemmerer S, Farquhar GD. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153, 376–387 [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Farquhar GD. 1984. Effects of partial defoliation, changes of irradiance during growth, short-term water stress and growth at enhanced pCO2 on the photosynthetic capacity of leaves of Phaseolus vulgaris L. Planta 160, 320–329 [DOI] [PubMed] [Google Scholar]
- Welker JM, Menke JW. 1990. The influence of simulated browsing on tissue water relations, growth and survival of Quercus douglasii Hook and Arn. seedlings under slow and rapid rates of soil drought. Functional Ecology 4, 807–817 [Google Scholar]
- Whitehead D. 1998. Regulation of stomatal conductance and transpiration in forest canopies. Tree Physiology 18, 633–644 [DOI] [PubMed] [Google Scholar]
- Zhou R, Quebedeaux B. 2003. Changes in photosynthesis and carbohydrate metabolism in mature apple leaves in response to whole plant source–sink manipulation. Journal of the American Society for Horticultural Science 128, 113–119 [Google Scholar]
- Zhou R, Sicher R, Quebedeaux B. 2001. Diurnal changes in carbohydrate metabolism in mature apple leaves. Australian Journal of Plant Physiology 28, 1143–1150 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.