Abstract
Many plant-growth-promoting rhizobacteria (PGPR) associated with plant roots contain the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase and can metabolize ACC, the immediate precursor of the plant hormone ethylene, thereby decreasing plant ethylene production and increasing plant growth. However, relatively few studies have explicitly linked ethylene emission and/or action to growth promotion in these plant–microbe interactions. This study examined effects of the PGPR Variovorax paradoxus 5C-2 containing ACC deaminase on the growth and development of Arabidopsis thaliana using wild-type (WT) plants and several ethylene-related mutants (etr1-1, ein2-1, and eto1-1). Soil inoculation with V. paradoxus 5C-2 promoted growth (leaf area and shoot biomass) of WT plants and the ethylene-overproducing mutant eto1-1, and also enhanced floral initiation of WT plants by 2.5 days. However, these effects were not seen in ethylene-insensitive mutants (etr1-1 and ein2-1) even though bacterial colonization of the root system was similar. Furthermore, V. paradoxus 5C-2 decreased ACC concentrations of rosette leaves of WT plants by 59% and foliar ethylene emission of both WT plants and eto1-1 mutants by 42 and 37%, respectively. Taken together, these results demonstrate that a fully functional ethylene signal transduction pathway is required for V. paradoxus 5C-2 to stimulate leaf growth and flowering of A. thaliana.
Key words: ACC deaminase, Arabidopsis, ethylene, growth, floral transition, rhizobacteria
Introduction
The regulation of growth and functioning of plant root systems has attracted increased scientific attention in studies which aim to increase crop production but decrease environmental impacts of agriculture by decreasing water and nutrient inputs (Lynch, 2007; Ghanem et al., 2011). Manipulating rhizosphere microbial populations potentially offers a low-cost and flexible method to increase plant growth by regulating the growth and functioning of the root system. Plant-growth-promoting rhizobacteria (PGPR) can stimulate plant growth directly by producing or metabolizing plant hormones or enhancing plant nutrient uptake (Arshad and Frankenberger, 1991; Vessey, 2003; Dodd et al., 2010; Dodd and Ruiz-Lozano, 2012) or indirectly by mechanisms such as biocontrol of phytopathogens (Kloepper et al., 1992). A single PGPR strain can provide multiple beneficial effects to plants (Lugtenberg and Kamilova, 2009; Jiang et al., 2012). Many PGPRs contain the enzyme 1-aminocyclopropane-1-carboxylate (ACC) deaminase (ACCd), which can hydrolyse ACC (the immediate precursor of the plant hormone ethylene) to ammonia and α-ketobutyrate. These bacteria take up ACC from plant root exudates and use it as a nitrogen (Jacobson et al., 1994; Glick et al., 1995) and carbon (Belimov et al., 2005) source. Growth promotion of different plant species was repeatedly observed following substrate inoculation with various strains containing ACCd, particularly in plants subjected to environmental stresses that stimulate ethylene production (Glick et al., 1998, 2007; Belimov et al., 2001).
Ethylene can inhibit plant growth in several ways (Pierik et al., 2006). The triple response of dark-grown seedlings is a classic example illustrating that ethylene inhibits plant growth by reducing hypocotyl elongation and root growth (Guzman and Ecker, 1990). Application of ethylene or its precursor ACC or the ethylene-releasing chemical ethephon (which both can be converted to ethylene by plants) reduced leaf expansion and shoot growth (Lee and Reid, 1997; Pierik et al., 2006). In addition to plant growth regulation, floral transition time in Arabidopsis was delayed in WT plants which were supplied with 10 μM ACC to the root-zone, and in ctr1-1 mutants in which the ethylene signal transduction pathway is constantly stimulated (Achard et al., 2007).
Ethylene is perceived by a family of receptors including ETR1, ETR2, ERS1, ERS2, and EIN4 in Arabidopsis (Chang et al., 1993; Hua et al., 1995, 1998; Hua and Meyerowitz, 1998). In the presence of ethylene, ETR1 inactivates the negative regulator CTR1 (a Ser/Thr kinase) which is activated by air (Kieber et al., 1993). The positive regulator EIN2, which is downstream of CTR1 (Guzman and Ecker, 1990), can activate EIN3 and EIN3-like (EIL) transcription factors (Roman et al., 1995), thus inducing the expression of ethylene-responsive genes (Solano et al., 1998). Both etr1-1 and ein2-1 (the mutants used in this study) showed an ethylene-insensitive phenotype in the triple response assay (Guzman and Ecker, 1990; Chang et al., 1993).
PGPR containing the enzyme ACCd decreased root ACC concentrations (Penrose et al., 2001) and mitigated ethylene’s inhibitory effects on shoot growth (Glick et al., 1998; Belimov et al., 2001, 2009a; Glick, 2005). Inoculation of plants with rhizobacteria containing ACCd attenuated an increase in xylem sap ACC levels induced by soil drying (Belimov et al., 2009b) and decreased whole-seedling ethylene accumulation (Penrose et al., 2001; Mayak et al., 2004a, b; Madhaiyan et al., 2006). Inhibitors of ethylene biosynthesis (aminoethoxyvinylglycine) or action (silver ions) had similar physiological effects to those of bacterial inoculation (Hall et al., 1996; Belimov et al., 2002, 2007), suggesting that bacterial growth stimulation was mediated by ethylene. Furthermore, transposon mutagenesis of microorganisms to downregulate ACCd activity reduced or eliminated their growth-promoting effect, in plant–microbe interactions such as canola–Enterobacter cloacae (Li et al., 2000), tomato–Pseudomonas brassicacearum (Belimov et al., 2007), pea–Variovorax paradoxus (Belimov et al., 2009b) and canola–Trichoderma asperellum (Viterbo et al., 2010). These findings suggested that ACCd plays a key role in promoting plant growth. However, relatively few studies have examined the effects of bacterial inoculation on ethylene production of adult plants, the ethylene-signalling pathway involved, or the possible regulation of flowering by this group of rhizobacteria. Previously, preliminary studies in potato demonstrated that V. paradoxus 5C-2 accelerated tuber sprouting in potato, thereby decreasing time to flowering (Belimov et al., 2009a), although the possible mechanism causing this developmental regulation was not fully explored.
V. paradoxus 5C-2 also produced indoles in vitro (Belimov et al., 2005; Jiang et al., 2012). Canola (Brassica campestris) root inoculation with the rhizobacterium Methylobacterium fujisawaense that contains ACCd not only decreased ethylene accumulation, but also increased indole-3-acetic acid concentrations in young seedlings (Madhaiyan et al., 2006). However, it is not clear whether there is a correlation between increased indole-3-acetic acid concentrations and growth promotion effects from M. fujisawaense. Although V. paradoxus 5C-2 increased xylem abscisic acid (ABA) concentrations of pea plants exposed to drying soil (Belimov et al., 2009b), it decreased root ABA concentration (and xylem ABA flows) of well-watered and well-fertilized pea plants (Jiang et al., 2012). A minimum endogenous ABA concentration seems necessary to maintain optimal shoot and root development (Sharp et al., 2000; Spollen et al., 2000), even under well-watered conditions. Thus growth promotion by V. paradoxus 5C-2 may involve other phytohormonal mechanisms.
Several phytohormone-insensitive mutants of Arabidopsis thaliana were used to investigate effects of PGPR such as Bacillus megaterium (Lopez-Bucio et al., 2007) and Phyllobacterium brassicacearum (Galland et al., 2012) on root architecture. Results suggested that the effects of B. megaterium were ethylene and auxin independent, while ethylene was involved in mediating effects of P.brassicacearum. To determine whether the ethylene-signalling pathway regulated effects of V. paradoxus 5C-2 on growth and development, several ethylene-related A. thaliana mutants were used in this study. Furthermore, to determine whether rhizobacterial inoculation of the growing substrate affected long-distance ethylene signalling, ACC concentrations and ethylene accumulation of rosette leaves were measured.
Materials and methods
Seed lines
The A. thaliana lines used in this study were Columbia (Col) wild-type (WT), the ethylene-insensitive mutants etr1-1 (Bleecker et al., 1988) and ein2-1 and the ethylene over-producing mutant eto1-1 (Guzman and Ecker, 1990). Col-O, etr1-1, and ein2-1 were kindly given by Mike Roberts (Lancaster Environment Centre, Lancaster University, UK). eto1-1 was obtained from the European Arabidopsis Stock Centre (NASC, University of Nottingham). All mutant lines were derived from parental A. thaliana Columbia. In an attempt to avoid any surface contamination from microbes, all seeds were surface sterilized before sowing in the substrate by rinsing with 70% ethanol, followed by 95% ethanol for 1min. Surface-sterilized seeds were kept at 4 °C for 2 days and then sown on top of the growth medium (All Purpose Growth medium, Sinclair Hort Products, Lincoln, UK, mixed with sand and vermiculite at a ratio of 3:1:0.5, v/v/v) into individual 130-ml pots (63mm high and 65mm diameter). The compost was sieved and then sterilized by autoclaving (121 °C for 15 minutes) before mixing with sand and vermiculite, which were also autoclaved individually under the same conditions. Pots were placed in propagator trays (56×37.5cm) and covered with a propagator lid (comprising six ventilation holes 1×5cm) to minimize the ingress of airborne microbes. Plants were well watered throughout and grown on a bench in a walk-in controlled environment room with an average temperature of 23 °C ± 2 °C, and at 230±20 μmol m–2 s–1 photosynthetically active radiation (PAR) with a 16/8h light/dark cycle.
Bacterial cultures and inoculation
V. paradoxus 5C-2 was obtained from the All-Russia Research Institute for Agricultural Microbiology (St Petersburg). Bacteria were cultured on Bacto Pseudomonas F (BPF) medium and used for liquid suspension preparation as previously described (Belimov et al., 2001). Liquid suspension was applied to the plant growth medium by thorough mixing prior to filling the pots. The final bacterial concentration in the growth medium was 106 cells g–1substrate. Arabidopsis seeds were planted (four seeds per pot) on the surface of the soil. Ten days after planting (DAP), germinated seedlings were thinned to one per pot.
Estimation of bacteria on roots
Colonization of roots by V. paradoxus 5C-2 was determined as previously described (Belimov et al., 2009b). Briefly, plant roots were removed from the growth substrates by gently shaking to remove any adhering substrate particles. Both primary and lateral roots were used for analysis immediately after sampling. Root samples were homogenized in sterile tap water with a sterile mortar and pestle, the homogenates serially diluted in 10-fold steps, and 50 μl aliquots plated in duplicate on BPF medium supplemented with (l–1) 20mg rifampicin and 30mg kanamycin (to which V. paradoxus 5C-2 shows resistance) and 40mg nystatin (to prevent fungal growth). Colony-forming units were counted by comparing with the morphology of the original strain grown on BPF agar after incubation of plates for 4 days at 28 °C. Roots from uninoculated plants were the control treatment. Plates from these roots had few (if any) bacterial colonies, none of which morphologically resembled the inoculated strain (Supplementary Fig. S1, available at JXB online).
Physiological and biochemical measurements
Flowering time of Arabidopsis was determined by recording the total number of rosette leaves (excluding cotyledons) when the floral stem was 1cm long as described (Achard et al., 2007); and recording days at the same time. To determine rosette fresh weight, leaf number, and leaf area, WT plants were harvested at three points during the growth period: 17, 22, and 33 DAP. To ensure similarities of developmental stages between WT and mutants at harvesting, the mutants were harvested at 33 DAP for etr1-1, 35 DAP for ein2-1, and 28 DAP for eto1-1.
To determine rates of ethylene production, rosette leaves (0.5g fresh weight) from WT plants and the eto1-1 mutant were taken when the floral stem emerged and placed in 7.8-ml glass vials to incubate for 1h before 1ml of headspace was withdrawn and injected into a gas chromatograph for the quantification of ethylene as described previously (Dodd et al., 2009). Leaf samples for ACC measurement were collected when the floral stem emerged, frozen in liquid nitrogen and stored at –20 °C. ACC content was determined by GC-MS as previously described (Dodd et al., 2009).
Statistics
Two-way analysis of variance (ANOVA) was performed to determine effects of rhizobacteria, genotype, and their interactions, using SPSS version 19 (SPSS, Chicago, USA). One-way ANOVA with Tukey’s HSD (P < 0.05) was used to discriminate means. Pairwise comparisons used Student’s t-test and standard error (SE) in SigmaPlot for Windows version 7.0 (Jandel Scientific, Erkrath, Germany).
Results
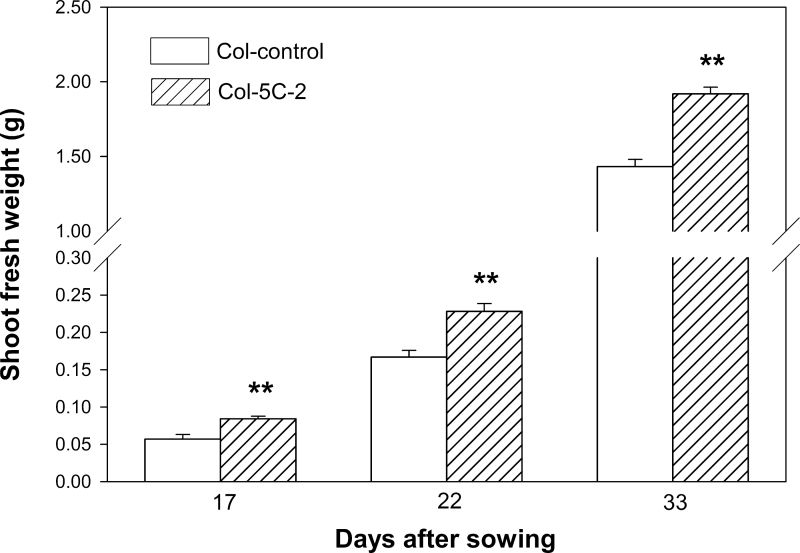
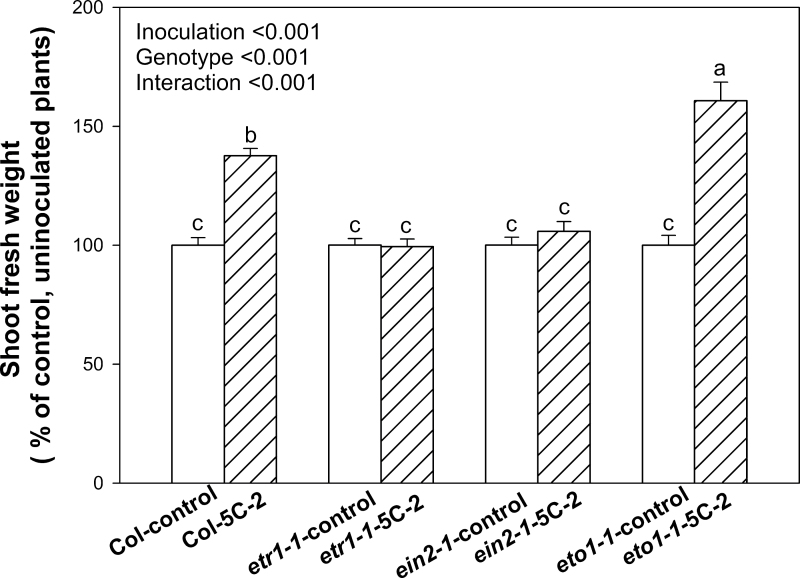
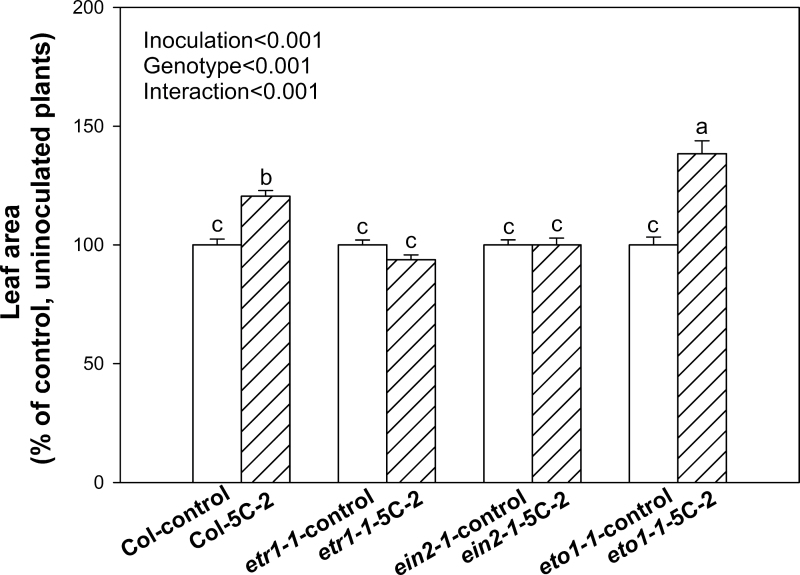
Inoculation of V. paradoxus 5C-2 significantly (P < 0.01) increased fresh biomass of WT plants by 34–47% throughout development (Fig. 1). Leaf areas (cm2) of different genotypes from uninoculated controls were 41±1 for WT, 6.9±0.2 for eto1-1, 64±2 for etr1-1, and 62±2 for ein2-1; while fresh weights (g) for control plants were 1.4±0.04 for WT, 0.2±0.01 for eto1-1, 2.0±0.1 for etr1-1, and 2.6±0.1 for ein2-1. Effects of genotype and rhizobacterial inoculation on shoot biomass (% of control) were analysed using two-way ANOVA. Both factors were highly significant (P < 0.001), as was the interaction (P < 0.001; Fig. 2), indicating that bacterial inoculation significantly (P < 0.01) stimulated growth of the WT and eto1-1 plants but not the etr1-1 and ein2-1 mutants (Fig. 2). Similarly, bacterial inoculation and genotype significantly (P<0.001) affected leaf area and again, there was a significant genotype × inoculation interaction (P < 0.001; Fig. 3). V. paradoxus 5C-2 inoculation significantly (P < 0.01) increased whole-plant leaf area of the WT and eto1-1 plants, but there was no promotion effect on the etr1-1 and ein2-1 mutants (Fig. 3).
Fig. 1.
Shoot fresh weight (g) of WT plants harvested at 17, 22, and 33 days after planting in response to V. paradoxus 5C-2 inoculation. Bars indicate mean ± SE (n = 25–30). Asterisks indicate significant differences (P < 0.01).
Fig. 2.
Shoot fresh weight (% of control plants of each genotype) of WT, eto1-1, etr1-1, and ein2-1 plants in response to V. paradoxus 5C-2 inoculation. WT plants were harvested at 29 DAP, while eto1-1, etr1-1 and ein2-1 were harvested at the corresponding development stage. Values are mean ± SE. Data for control, etr1-1 and ein2-1 were from two or three independent experiments and data for eto1-1 from one representative experiment; 25–30 replicates were used for each experiment. Different letters above bars indicate significant differences (P < 0.05; Tukey test). P-values are shown for two-way ANOVA for bacterial inoculation, genotype, and their interaction.
Fig. 3.
Leaf area (% of control plants of each genotype) of WT, eto1-1, etr1-1, and ein2-1 plants in response to V. paradoxus 5C-2 inoculation. WT plants were harvested at 29 DAP, while eto1-1, etr1-1 and ein2-1 were harvested at the corresponding development stage. Values are mean ± SE. Data of control, etr1-1 and ein2-1 were from two or three independent experiments and data for eto1-1 from one representative experiment; 25–30 replicates were used for each experiment. Different letters above bars indicate significant differences (P < 0.05; Tukey test). P-values are shown for two-way ANOVA for bacterial treatment inoculation, genotype, and their interaction.
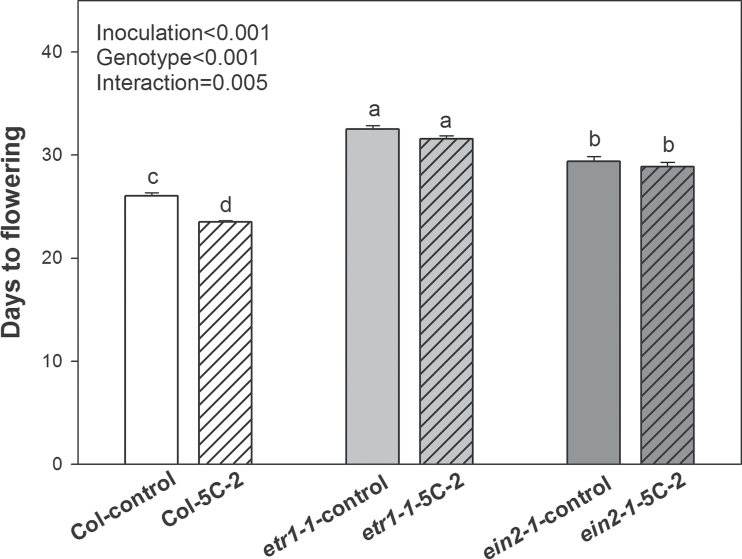
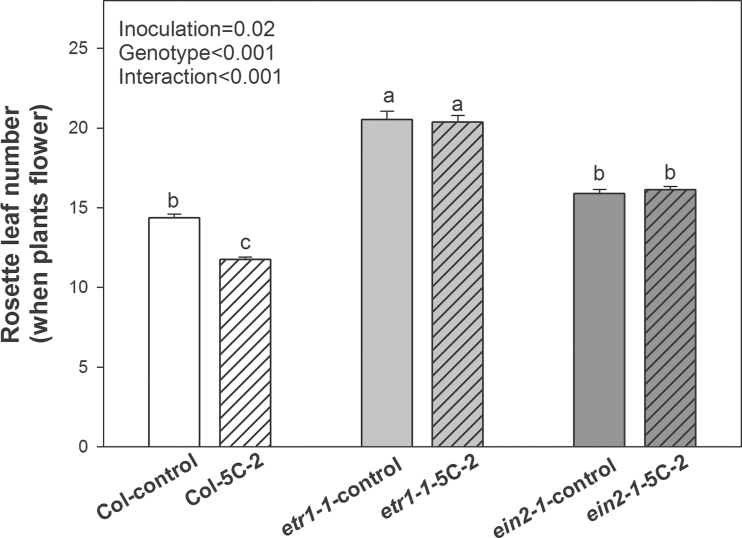
Both genotype and rhizobacterial inoculation and also their interaction had significant effects on both days to flowering and rosette leaf number at flowering. WT plants inoculated with V. paradoxus 5C-2 flowered significantly earlier than control plants (P < 0.01; Fig. 4). However, inoculation of the ethylene-insensitive mutants (etr1-1, and ein2-1) caused no statistically significant (P > 0.1; Fig. 4) decreases in days to flowering. Inoculation with V. paradoxus 5C-2 significantly (P < 0.01) decreased the number of WT rosette leaves at flowering (Fig. 5) but this did not occur in the ethylene-insensitive mutants etr1-1 and ein2-1 (P > 0.1; Fig. 5). Thus developmental effects of V. paradoxus 5C-2 were dependent on plant genotype.
Fig. 4.
Days to flowering of V. paradoxus 5C-2-treated WT, etr1-1, and ein2-1 plants when the floral stem extended to 1cm. Values are mean ± SE (n = 30). Different letters above bars indicate significant differences (P < 0.05; Tukey test). P-values are shown for two-way ANOVA for bacterial treatment inoculation, genotype, and their interaction.
Fig. 5.
Rosette leaf numbers of V. paradoxus 5C-2-treated WT, etr1-1, and ein2-1 plants when the floral stem was extended to 1cm. Values are mean ± SE (n = 30). Different letters above bars indicate significant differences (P < 0.05; Tukey test). P-values are shown for two-way ANOVA for bacterial treatment inoculation, genotype, and their interaction.
At the end of experiments, V. paradoxus 5C-2 was detected on roots of inoculated WT plants and ethylene-insensitive mutants (Table 1), but there was no significant genotypic effect. Bacterial root colonization was not determined in the eto1-1 mutant, as it was difficult to extract root tissues from the small root system of this mutant.
Table 1.
Number of bacteria isolated from roots of inoculated plants. Data are mean ± SE (n = 4). There were no significant differences (P > 0.05). DAP, days after planting.
| Genotypes | |||
|---|---|---|---|
| Col (29 DAP) | etr1-1 (33 DAP) | ein2-1 (35 DAP) | |
| Number of bacteria (106 CFU (g FW)–1) | 1.85±0.70 | 2.44±0.07 | 2.46±0.19 |
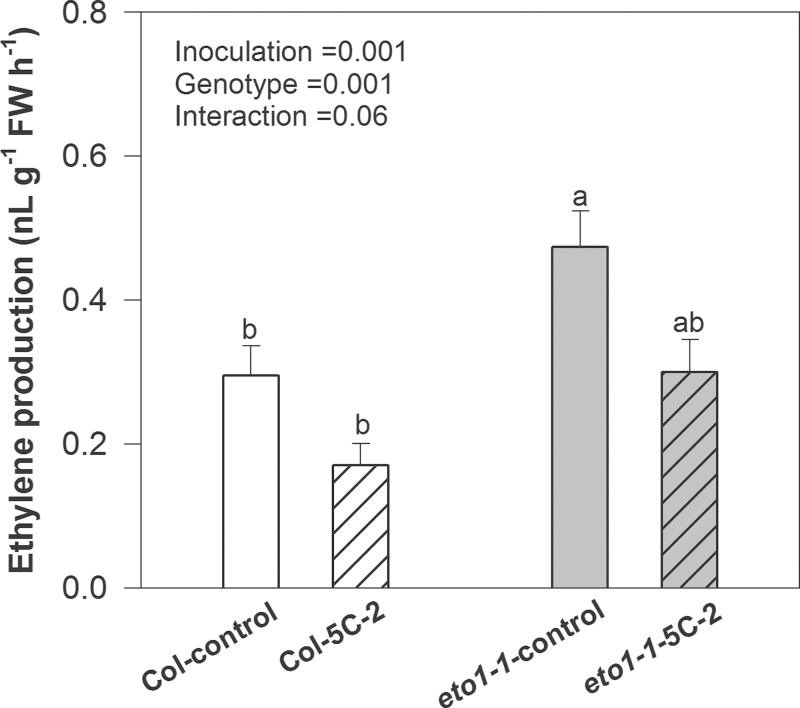
V. paradoxus 5C-2 inoculation significantly (P < 0.05) decreased ACC concentrations of rosette leaves of mature WT plants (Table 2). Furthermore, bacterial inoculation significantly decreased (P < 0.01) ethylene emission from rosette leaves of mature WT and eto1-1 mutants (Fig. 6). Ethylene emission of both genotypes responded similarly to inoculation, as indicated by the lack of a significant genotype × inoculation interaction (P > 0.05; Fig. 6).
Table 2.
ACC concentration of rosette leaves from control and inoculated WT plants. Plant leaves were harvested when the floral stem emerged. Data are mean ± SE (n = 7–8).
| Treatment | |||
|---|---|---|---|
| Control | Bacterial inoculation | P-value | |
| ACC (pmol (g FW)–1) | 817±174 | 336±49 | 0.014 |
Fig. 6.
Ethylene emission from mature leaves of control and uninoculated plants (WT and eto1-1). Plant leaves were harvested when the floral stem emerged from rosette leaves. Values are mean ± SE (Col n = 12, eto1-1 n = 6). Different letters above bars indicate significant differences (P < 0.05; Tukey test). P-values are shown for two-way ANOVA for bacterial treatment inoculation, genotype, and their interaction.
Discussion
Since V. paradoxus 5C-2 containing ACCd had multiple impacts on plant growth by stimulating leaf expansion, biomass accumulation, and flowering (Figs. 1–5), further evidence of the importance of ethylene in its interaction with A. thaliana was sought here by comparing growth responses of WT plants and ethylene-insensitive mutants (etr1-1 and ein2-1) to soil inoculation with this PGPR. Previously, both etr1-1 (Ruzicka et al., 2007) and ein2-1 (Stepanova et al., 2007) showed a response of root growth to exogenous auxin similar to WT plants. Although V. paradoxus 5C-2 can produce auxins in vitro (Belimov et al., 2005; Jiang et al., 2012), differences in growth and flowering between ethylene-insensitive mutants and WT plants (Figs. 2–5) suggests that ethylene, rather than auxin, was responsible for the growth promotion observed.
An alternative possible explanation for the lack of growth promotion in the ethylene-insensitive mutants may be that there was insufficient bacterial colonization of the root system. However, all studied genotypes showed similar levels of bacterial colonization of roots (Table 1). Comparatively little is known about putative plant regulation of rhizobacterial populations, and it is not clear whether root ACC exudation could stimulate colonization by bacteria containing ACCd. Circumstantial evidence showed that rhizobacterial populations of Hordeum spontaneum plants were enriched in ACCd-containing organisms in sun-exposed plants (which may have been more stressed) compared to plants grown on the opposite (shaded) sides of the valley (Timmusk et al., 2011). Although the impaired ability of the etr1-1 and ein2-1 plants to perceive ethylene was no impediment to colonization by rhizobacteria containing ACCd (Table 1), it is unknown whether root ACC efflux is independent of the ethylene-signalling pathway or rhizobacterial colonization depends on ACC efflux.
As shown previously in potato (Belimov et al. 2009a), promotion of floral initiation was noted in WT Arabidopsis plants inoculated with V. paradoxus 5C-2 (Figs. 4, 5). Together with promotion of shoot growth (Figs. 1–3) following inoculation of V. paradoxus 5C-2, these data are consistent with an inhibitory effect of ethylene on flowering (Achard et al., 2007) and growth (Lee and Reid, 1997). Although a systemic effect of V. paradoxus 5C-2 inoculation was suggested by the decreased xylem ACC concentrations of peas grown in drying soil (Belimov et al., 2009b), this conclusion is based on the assumption that root ACC export to the xylem quantitatively correlates with shoot ethylene evolution (Else and Jackson, 1998). However, ACC synthase, the rate-limiting enzyme in ethylene production (Wang et al., 2002), is encoded by multigene families which can be expressed in roots and shoots (Liang et al., 1992; Johnson and Ecker, 1998), indicating that plants can regulate their ACC pool (and hence ethylene production) in many ways.
Even though eto1-1 overproduces ethylene as a result of increased stability of the ACC synthesis protein 5 (Chae et al., 2003), here the increased shoot biomass and leaf area (Figs. 2, 3) but decreased ethylene accumulation (Fig. 6) of eto1-1 following root inoculation with V. paradoxus 5C-2 suggest that long-distance ACC signalling regulated by the bacteria can still be influential. Similar decreases in foliar ACC levels and ethylene evolution of mature WT plants (Table 2, Fig. 6), together with the results from eto1-1, further indicate that bacterial inoculation not only causes local effects on roots (Glick et al., 1997; Madhaiyan et al., 2006) but also systemic effects via long-distance ACC signalling.
Interestingly, ACCd enzyme activity is not only reported in bacteria or fungi, but also detected in Arabidopsis, tomato, and poplar (McDonnell et al., 2009; Plett et al., 2009). In particular, two putative ACCd genes were identified in the genome of Arabidopsis, and downregulating one of these two genes increased ethylene production of 3-day-old Arabidopsis seedlings (McDonnell et al., 2009). However, data presented here suggest that root inoculation of rhizobacteria containing ACCd can stimulate leaf growth of Arabidopsis, even during early growth (Fig. 1), despite any impact of Arabidopsis ACCd genes. These genes of Arabidopsis ACCd may be tightly regulated at transcriptional and translational levels to regulate its ethylene production, but rhizobacterial ACCd seems relatively independent of plant regulation and thus could affect plant ethylene evolution further.
Taken together, the work presented here elucidated that root inoculation of rhizobacteria containing ACCd promoted plant growth and floral initiation via an ethylene-dependent signalling pathway by regulating shoot ethylene production. Ethylene is generally regarded as a ‘stress hormone’ as it can be stimulated by various stresses such as drought (Morgan and Drew, 1997; Sobeih et al., 2004). Chemical inhibitors of ethylene synthesis or action have been used in agriculture to alleviate ethylene-induced growth inhibition, but suffer from practical disadvantages such as expense (aminoethoxyvinylglycine), toxicity (silver ions), or localized action (1-methylcyclopropene is only effective in disrupting shoot ethylene biology as it cannot penetrate the soil). Compared to these inhibitors, rhizobacteria containing ACCd offer relatively non-toxic, environmentally friendly, and low-cost benefits to mitigate effects of ethylene on both roots and shoots. Rhizobacteria containing ACCd may be used in agriculture to increase crop water use efficiency, especially by maintaining leaf expansion of plants in drying soil (Belimov et al., 2009b), allowing greater coverage of the soil surface to lessen soil evaporation and greater light capture to maximize whole-plant assimilation. Furthermore, stimulating crop development (as suggested by the promotion of flowering observed here) may accelerate the cropping cycle, of advantage both in avoiding late-season water deficits or other stresses, but also under well-watered conditions.
Supplementary material
Supplementary data are available at JXB online.
Supplementary Fig. S1. Plates from inoculated and control roots at different dilution levels.
Acknowledgements
The authors are very grateful to the BBSRC (Grant ref: BB/DO12821/1), and the UK-China Science Bridge project funded by the Research Councils UK (EP/G042683/1) for supporting this work, and the Royal Society for sponsoring Andrey A. Belimov to visit Lancaster University. The authors also would like to thank Maureen Harrison for valuable technical support.
References
- Achard P, Baghour M, Chapple A, Hedden P, Van der Straeten D, Genschik P, Moritz T, Harberd NP. 2007. The plant stress hormone ethylene controls floral transition via DELLA-dependent regulation of floral meristem-identity genes. Proceedings of the National Academy of Sciences, USA 104, 6484–6489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arshad M, Frankenberger WT. 1991. Microbial production of plant hormones. Plant and Soil 133, 1–8 [Google Scholar]
- Belimov A, Dodd IC, Hontzeas N, Theobald JC, Safronova VI, Davies WJ. 2009b. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase yield of plants grown in drying soil via both local and systemic hormone signalling. New Phytologist 181, 413–423 [DOI] [PubMed] [Google Scholar]
- Belimov A, Dodd IC, Safronova VI, Davies WJ. 2009a. ACC deaminase-containing rhizobacteria improve vegetative development and yield of potato plants grown under water-limited conditions. Aspects of Applied Biology 98, 163–169 [Google Scholar]
- Belimov A, Dodd IC, Safronova VI, Hontzeas N, Davies WJ. 2007. Pseudomonas brassicacearum strain Am3 containing 1-aminocyclopropane-1-carboxylate deaminase can show both pathogenic and growth-promoting properties in its interaction with tomato. Journal of Experimental Botany 58, 1485–1495 [DOI] [PubMed] [Google Scholar]
- Belimov A, Hontzeas N, Safronova VI, Demchinskaya SV, Piluzza G, Bullitta S, Glick BR. 2005. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.). Soil Biology and Biochemistry 37, 241–250 [Google Scholar]
- Belimov A, Safronova VI, Mimura T. 2002. Response of spring rape (Brassica napus var. oleifera L.) to inoculation with plant growth promoting rhizobacteria containing 1-aminocyclopropane-1-carboxylate deaminase depends on nutrient status of the plant. Canadian Journal of Microbiology 48, 189–199 [DOI] [PubMed] [Google Scholar]
- Belimov A, Safronova VI, Sergeyeva TA, et al. 2001. Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Canadian Journal of Microbiology 47, 642–652 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H. 1988. Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana . Science 241, 1086–1089 [DOI] [PubMed] [Google Scholar]
- Chae HS, Faure F, Kieber JJ. 2003. The eto1, eto2, and eto3 mutations and cytokinin treatment increase ethylene biosynthesis in Arabidopsis by increasing the stability of ACS protein. The Plant Cell 15, 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Kwok SF, Bleecker AB, Meyerowitz EM. 1993. Arabidopsis ethylene-response gene Etr1– similarity of product to 2-component regulators. Science 262, 539–544 [DOI] [PubMed] [Google Scholar]
- Dodd IC, Ruiz-Lozano JM. 2012. Microbial enhancement of crop resource use efficiency. Current Opinion in Biotechnology 23, 236–242 [DOI] [PubMed] [Google Scholar]
- Dodd IC, Theobald JC, Richer SK, Davies WJ. 2009. Partial phenotypic reversion of ABA-deficient flacca tomato (Solanum lycopersicum) scions by a wild-type rootstock: normalizing shoot ethylene relations promotes leaf area but does not diminish whole plant transpiration rate. Journal of Experimental Botany 60, 4029–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd IC, Zinovkina NY, Safronova VI, Belimov A. 2010. Rhizobacterial mediation of plant hormone status. Annals of Applied Biology 157, 361–379 [Google Scholar]
- Else MA, Jackson MB. 1998. Transport of 1-aminocyclopropane-1-carboxylic acid (ACC) in the transpiration stream of tomato (Lycopersicon esculentum) in relation to foliar ethylene production and petiole epinasty. Australian Journal of Plant Physiology 25, 453–458 [Google Scholar]
- Galland M, Gamet L, Varoquaux F, Touraine B, Touraine B, Desbrosses G. 2012. The ethylene pathway contributes to root hair elongation induced by the beneficial bacteria Phyllobacterium brassicacearum STM196. Plant Science 190, 74–81 [DOI] [PubMed] [Google Scholar]
- Ghanem ME, Hichri I, Smigocki AC, Albacete A, Fauconnier ML, Diatloff E, Martinez-Andujar C, Lutts S, Dodd IC, Perez-Alfocea F. 2011. Root-targeted biotechnology to mediate hormonal signalling and improve crop stress tolerance. Plant Cell Reports 30, 807–823 [DOI] [PubMed] [Google Scholar]
- Glick BR. 2005. Modulation of plant ethylene levels by the bacterial enzyme ACC deaminase. FEMS Microbiology Letters 251, 1–7 [DOI] [PubMed] [Google Scholar]
- Glick BR, Cheng Z, Czarny J, Duan J. 2007. Promotion of plant growth by ACC deaminase-producing soil bacteria. European Journal of Plant Pathology 119, 329–339 [Google Scholar]
- Glick BR, Karaturovic DM, Newell PC. 1995. A novel procedure for rapid isolation of plant-growth promoting pseudomonads. Canadian Journal of Microbiology 41, 533–536 [Google Scholar]
- Glick BR, Liu CP, Ghosh S, Dumbroff EB. 1997. Early development of canola seedlings in the presence of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2. Soil Biology and Biochemistry 29, 1233–1239 [Google Scholar]
- Glick BR, Penrose DM, Li JP. 1998. A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. Journal of Theoretical Biology 190, 63–68 [DOI] [PubMed] [Google Scholar]
- Guzman P, Ecker JR. 1990. Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. The Plant Cell 2, 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall JA, Peirson D, Ghosh S, Glick BR. 1996. Root elongation in various agronomic crops by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Israel Journal of Plant Sciences 44, 37–42 [Google Scholar]
- Hua J, Chang C, Sun Q, Meyerowitz EM. 1995. Ethylene insensitivity conferred by Arabidopsis ERS gene. Science 269, 1712–1714 [DOI] [PubMed] [Google Scholar]
- Hua J, Meyerowitz EM. 1998. Ethylene responses are negatively regulated by a receptor gene family in Arabidopsis thaliana . Cell 94, 261–271 [DOI] [PubMed] [Google Scholar]
- Hua J, Sakai H, Nourizadeh S, Chen QHG, Bleecker AB, Ecker JR, Meyerowitz EM. 1998. EIN4 and ERS2 are members of the putative ethylene receptor gene family in Arabidopsis . The Plant Cell 10, 1321–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson CB, Pasternak JJ, Glick BR. 1994. Partial-purification and characterization of 1-aminocyclopropane-1-carboxylate deaminase from the plant-growth promoting rhizobacterium Pseudomonas putida Gr12-2. Canadian Journal of Microbiology 40, 1019–1025 [Google Scholar]
- Jiang F, Chen L, Belimov AA, Shaposhnikov AI, Gong F, Meng X, Hartung W, Jeschke DW, Davies WJ, Dodd IC. 2012. Multiple impacts of the plant growth-promoting rhizobacterium Variovorax paradoxus 5C-2 on nutrient and ABA relations of Pisum sativum . Journal of Experimental Botany 63, 6421–6430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PR, Ecker JR. 1998. The ethylene gas signal transduction pathway: a molecular perspective. Annual Review of Genetics 32, 227–254 [DOI] [PubMed] [Google Scholar]
- Kieber JJ, Rothenberg M, Roman G, Feldmann KA, Ecker JR. 1993. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinases. Cell 72, 427–441 [DOI] [PubMed] [Google Scholar]
- Kloepper JW, Tuzun S, Kuc JA. 1992. Proposed definitions related to induced disease resistance. Biocontrol Science and Technology 2, 349–351 [Google Scholar]
- Lee SH, Reid DM. 1997. The role of endogenous ethylene in the expansion of Helianthus annuus leaves. Canadian Journal of Botany-Revue Canadienne De Botanique 75, 501–508 [DOI] [PubMed] [Google Scholar]
- Li JP, Ovakim DH, Charles TC, Glick BR. 2000. An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Current Microbiology 41, 101–105 [DOI] [PubMed] [Google Scholar]
- Liang XW, Abel S, Keller JA, Shen NF, Theologis A. 1992. The 1-aminocyclopropane-1-carboxylate synthase gene family of Arabidopsis thaliana . Proceedings of the National Academy of Sciences, USA 89, 11046–11050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bucio J, Campos-Cuevas JC, Hernandez-Calderon E, Velasquez-Becerra C, Farias-Rodriguez R, Macias-Rodriguez LI, Valencia-Cantero E. 2007. Bacillus megatenum rhizobacteria promote growth and alter root-system architecture through an auxin-and ethylene-independent signalling mechanism in Arabidopsis thaliana . Molecular Plant–Microbe Interactions 20, 207–217 [DOI] [PubMed] [Google Scholar]
- Lugtenberg B, Kamilova F. 2009. Plant-growth-promoting rhizobacteria. Annual Review of Microbiology 63, 541–556 [DOI] [PubMed] [Google Scholar]
- Lynch JP. 2007. Roots of the second green revolution. Australian Journal of Botany 55, 493–512 [Google Scholar]
- Madhaiyan M, Poonguzhali S, Ryu J, Sa T. 2006. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense . Planta 224, 268–278 [DOI] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR. 2004a. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiology and Biochemistry 42, 565–572 [DOI] [PubMed] [Google Scholar]
- Mayak S, Tirosh T, Glick BR. 2004b. Plant growth-promoting bacteria that confer resistance to water stress in tomatoes and peppers. Plant Science 166, 525–530 [Google Scholar]
- McDonnell L, Plett JM, Andersson-Gunneras S, Kozela C, Dugardeyn J, Van Der Straeten D, Glick BR, Sundberg B, Regan S. 2009. Ethylene levels are regulated by a plant encoded 1-aminocyclopropane-1-carboxylic acid deaminase. Physiologia Plantarum 136, 94–109 [DOI] [PubMed] [Google Scholar]
- Morgan PW, Drew MC. 1997. Ethylene and plant responses to stress. Physiologia Plantarum 100, 620–630 [Google Scholar]
- Penrose DM, Moffatt BA, Glick BR. 2001. Determination of 1-aminocycopropane-1-carboxylic acid (ACC) to assess the effects of ACC deaminase-containing bacteria on roots of canola seedlings. Canadian Journal of Microbiology 47, 77–80 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek L. 2006. The Janus face of ethylene: growth inhibition and stimulation. Trends in Plant Science 11, 176–183 [DOI] [PubMed] [Google Scholar]
- Plett JM, McDonnell L, Regan S. 2009. Plant-encoded 1-aminocyclopropane-1-carboxylic acid deaminase activity implicated in different aspects of plant development. Plant Signalling and Behavior 4, 1186–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. 1995. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: five novel mutant loci integrated into a stress response pathway. Genetics 139, 1393–1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka K, Ljung K, Vanneste S, Podhorska R, Beeckman T, Friml J, Benkova E. 2007. Ethylene regulates root growth through effects on auxin biosynthesis and transport-dependent auxin distribution. The Plant Cell 19, 2197–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp RE, LeNoble ME, Else MA, Thorne ET, Gherardi F. 2000. Endogenous ABA maintains shoot growth in tomato independently of effects on plant water balance: evidence for an interaction with ethylene. Journal of Experimental Botany 51, 1575–1584 [DOI] [PubMed] [Google Scholar]
- Sobeih WY, Dodd IC, Bacon MA, Grierson D, Davies WJ. 2004. Long-distance signals regulating stomatal conductance and leaf growth in tomato (Lycopersicon esculentum) plants subjected to partial root-zone drying. Journal of Experimental Botany 55, 2353–2363 [DOI] [PubMed] [Google Scholar]
- Solano R, Stepanova A, Chao QM, Ecker JR. 1998. Nuclear events in ethylene signaling: a transcriptional cascade mediated by ETHYLENE-INSENSITIVE3 and ETHYLENE-RESPONSE-FACTOR1. Genes and Development 12, 3703–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spollen WG, LeNoble ME, Samuels TD, Bernstein N, Sharp RE. 2000. Abscisic acid accumulation maintains maize primary root elongation at low water potentials by restricting ethylene production. Plant Physiology 122, 967–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova AN, Yun J, Likhacheva AV, Alonso JM. 2007. Multilevel interactions between ethylene and auxin in Arabidopsis roots. The Plant Cell 19, 2169–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmusk S, Paalme V, Pavlicek T, Bergquist J, Vangala A, Danilas T, Nevo E. 2011. Bacterial distribution in the rhizosphere of wild barley under contrasting microclimates. Plos One 6, e17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vessey JK. 2003. Plant growth promoting rhizobacteria as biofertilizers. Plant and Soil 255, 571–586 [Google Scholar]
- Viterbo A, Landau U, Kim S, Chernin L, Chet I. 2010. Characterization of ACC deaminase from the biocontrol and plant growth-promoting agent Trichoderma asperellum T203. FEMS Microbiology Letters 305, 42–48 [DOI] [PubMed] [Google Scholar]
- Wang KLC, Li H, Ecker JR. 2002. Ethylene biosynthesis and signalling networks. The Plant Cell 14, S131–S151 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.