Abstract
The FLOWERING LOCUS T (FT)/TERMINAL FLOWER 1 (TFL1) family proteins play an important role in the regulation of flowering time. In the Arabidopsis thaliana genome, there are six genes in the FT/TFL1 family. To determine how these FT/TFL1 family genes contribute to the regulation of flowering time, this study generated a comprehensive set of mutants (sixty-three multiple mutants in all combinations) of the FT/TFL1 family genes and analysed their flowering times at 23 and 16°C under long-day conditions. The analysis confirmed that FT and TFL1 are major determinants of flowering time under long-day conditions. At 23 °C, ft-10 tsf-1 mft-2 showed the latest flowering, whereas tfl1-20 atc-2 bft-2 showed the earliest flowering. Flowering occurred in the sextuple mutants. Introduction of tsf-1 led to reduced sensitivity to ambient temperature change. Introduction of tfl1-20 caused a stronger effect in accelerating flowering time at 16 °C than at 23 °C. Overexpression of miR156 did not block flowering of sextuple mutants, suggesting that there is a pathway to induce flowering independent of the FT/TFL1 pathway and miR156 pathway. This study proposes that this mutant population will be useful in further investigation of the functions of the FT/TFL1 family genes in plant development.
Key words: Arabidopsis thaliana, ATC, BFT, flowering time, FT, MFT, miR156, TFL1, TSF.
Introduction
The Arabidopsis life cycle is divided into vegetative and reproductive growth phases. Extensive molecular genetic analysis in Arabidopsis has provided considerable information on how plants integrate environmental and endogenous signals to transition from the vegetative phase to the reproductive phase (Srikanth and Schmid, 2011). Multiple, interdependent genetic pathways control the developmental transition to the flowering phase (Lee et al., 2006; Michaels, 2009); these pathways include the photoperiod, autonomous, vernalization, gibberellic acid, and thermosensory pathways. Under long-day conditions, genes that act within the photoperiod pathway play a major role in controlling flowering.
FLOWERING LOCUS T (FT) and TERMINAL FLOWER 1 (TFL1) belong to a small group of proteins that show structural similarities to mammalian phosphatidylethanolamine-binding protein (Kardailsky et al., 1999; Kobayashi et al., 1999; Ahn et al., 2006). In addition to FT and TFL1, four highly similar genes are present in the Arabidopsis thaliana genome, namely TWIN SISTER OF FT (TSF) (Yamaguchi et al., 2005), MOTHER OF FT AND TFL1 (MFT) (Yoo et al., 2004), BROTHER OF FT AND TFL1 (BFT) (Yoo et al., 2010), and ARABIDOPSIS THALIANA CENTRORADIALIS HOMOLOGUES (ATC) (Mimida et al., 2001). These six genes are found in many species and are commonly referred to as the FT/TFL1 family (Chardon and Damerval, 2005; Ahn et al., 2006; Karlgren et al., 2011; Harig et al., 2012).
A major function of FT/TFL1 family genes is the regulation of photoperiodic flowering. FT encodes a floral activator that integrates signal inputs from various pathways that regulate flowering time (Wigge, 2011; Pin and Nilsson, 2012). FT is a major target of CONSTANS in the photoperiod pathway (Valverde et al., 2004) and mediates signalling from the vernalization and autonomous pathways by the direct interaction with FLOWERING LOCUS C (Helliwell et al., 2006). Interestingly, despite its sequence similarities to the floral activator FT, TFL1 acts as a floral inhibitor, an opposite role to FT (Ratcliffe et al., 1998). In addition, TFL1 controls plant architecture by regulating the expression of LEAFY and APETALA1 (AP1) in the shoot apical meristem (Bradley et al., 1997; Ferrandiz et al., 2000). The opposite functions of FT and TFL1 proteins map to a single amino acid in the second exon (Hanzawa et al., 2005) and a small external loop domain in the 4th exon (Ahn et al., 2006). TSF is most similar to FT within the FT/TFL1 family. The tsf mutation on its own did not show any clear alteration of flowering time under long-day conditions, but it had an additive effect when combined with ft (Michaels et al., 2005; Yamaguchi et al., 2005). This indicated that TSF plays a redundant role with FT. However, the effect of tsf loss-of-function is apparent under short-day conditions, suggesting that TSF makes a major contribution to flowering under short-day conditions. Based on overexpression studies, it was suggested that MFT and ATC have weak FT- and TFL1-like activity, respectively (Mimida et al., 2001; Yoo et al., 2004). ATC was also shown to be a short-day-induced floral inhibitor (Huang et al., 2012). Finally, bft mutation produced more secondary inflorescences when combined with tfl1, suggesting that BFT has a TFL1-like activity and functions redundantly with TFL1 in inflorescence meristem development (Yoo et al., 2010). It was recently demonstrated that FT regulates stomatal opening (Kinoshita et al. 2011) and MFT regulates abscisic acid- and gibberellic acid-mediated seed germination (Xi et al., 2010), raising the possibility that FT/TFL1 family genes function in diverse aspects of plant development.
Flowering is also significantly affected by changes in the ambient temperature (Fitter and Fitter, 2002; Lee et al., 2008). Among flowering time mutants, a subset of mutants showed flowering that was insensitive to ambient temperature (23 and 16 °C), indicating that these genes mediate ambient temperature-responsive flowering; later, these genes were proposed to act within the thermosensory pathway (or ambient temperature pathway) (Blazquez et al., 2003; Fornara et al., 2010). A group of genes [FCA, FVE, HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1 (HOS1), PHYTOCHROME INTERACTING FACTOR4 (PIF4), SHORT VEGETATIVE PHASE (SVP), EARLY FLOWERING3 (ELF3), and TFL1] (Blazquez et al., 2003; Lee et al., 2007; Strasser et al., 2009; Kumar et al., 2012; Lee et al., 2012b) and ambient temperature-responsive miRNAs including miR156, miR172, and miR399 (Lee et al., 2010; Kim et al., 2011; Kim et al., 2012) are involved in this pathway. Increasing evidence points to a complex interplay of components within the thermosensory pathway. For instance, miR172 is subjected to multiple layers of regulation (at both transcriptional and biogenesis levels) (Cho et al., 2012; Jung et al., 2012b), which may allow plants to fine-tune their responses to changes in ambient temperature. In addition, the ambient temperature transcriptome is regulated by H2A.Z-containing nucleosomes (Kumar and Wigge, 2010). Although there are many components that affect ambient temperature signalling, the ambient temperature response is likely mediated by FT and TFL1 (Lee et al., 2007; Strasser et al., 2009; Kumar et al., 2012; Lee et al., 2012a).
Based on phenotypic analyses of single or double mutants of the FT/TFL1 family members, it was suggested that FT, TFL1, and TSF are the major players in the control of flowering time. However, the combinatorial effect of mutations of the FT/TFL1 family is unknown, due to the absence of a comprehensive set of mutants of the FT/TFL1 family. To determine how FT/TFL1 family genes contribute to the regulation of flowering time, this study generated a comprehensive set of mutants (63 multiple mutants in all combinations) of the FT/TFL1 family and analysed their genetic interactions. In addition, this study tested the hypothesis that ablation of FT/TFL1 family genes blocks flowering, since the FT/TFL1 family is suggested to play an important role in flowering. This study also tested whether miR156 overexpression in the sextuple mutant background inhibits flowering.
The analysis confirmed that FT and TFL1 are major determinants of flowering time under long-day conditions. A sextuple mutant, in which all the FT/TFL1 family genes are impaired, still flowered, indicating that the FT/TFL1 family genes are not essential to induce flowering. It was also found that tsf-1 caused reduced sensitivity to ambient temperature changes. Overexpression of miR156 delayed flowering of sextuple mutants, suggesting the possibility that there is an alternative pathway to induce flowering independent of the FT/TFL1 and miR156 pathways. This study proposes that this mutant population will be useful for further investigation of the functions of the FT/TFL1 family genes in plant development.
Materials and methods
Plant materials and growth conditions
All of the mutants used in this study were in the A. thaliana Columbia (Col) background. Single mutants of the FT/TFL1 family used to generate multiple mutants were described elsewhere (ft-10: Yoo et al., 2005; tsf-1: Yamaguchi et al., 2005; mft-2: Xi et al., 2010; tfl1-20: Yoo et al., 2010; atc-2: Huang et al., 2012; and bft-2: Yoo et al., 2010). The plants were grown in soil or MS medium at 23 °C or 16 °C in long-day conditions (16/8h light/dark cycle) at a light intensity of 120 μmol m–2 s–1.
PCR genotyping
The genomic DNA was extracted from fresh young leaves, which were homogenized in a tissue disrupter (Automill, Tokken, Japan) using metal beads. To increase accuracy of genotyping to isolate multiple mutants, two independent PCR reactions were used to detect mutant and wild-type alleles, instead of multiplex PCR. To amplify the mutant allele, a primer set (T-DNA primer and a gene-specific primer) was used. To amplify the wild-type allele, two gene-specific primers that hybridize adjacent to a T-DNA insertion site were used. The primers used for genotyping are described in Supplementary Table S1 (available at JXB online).
Measurement of flowering time
Flowering time was measured by scoring total leaf number (at least 10 plants) under long-day conditions (16 and 23 °C). The total leaf number was recorded when the primary inflorescence had reached a height of 5cm. The effect of each mutation is expressed as the difference in leaf numbers between two mutant combinations that contained or did not contain the mutation. The leaf number ratio (16 °C/23 °C, LNR) under long-day conditions was used as an indicator of ambient temperature-responsive flowering (Blazquez et al., 2003; Lee et al., 2007). A hypothetical ambient temperature-insensitive plant produces an identical total number of leaves at both 23 and 16°C; thus, its LNR is 1.0.
RT-qPCR and small RNA northern hybridization
For RNA extraction, whole seedlings were harvested at zeitgeber time (ZT) 16, at which point FT expression levels were high (Corbesier et al., 2007). Total RNA was extracted using Plant RNA Purification Reagent (Invitrogen), according to the manufacturer’s instructions. For real-time quantitative PCR (RT-qPCR), 1 μg of total RNA was treated with DNaseI (New England Biolabs) and used for cDNA synthesis with First-Strand cDNA Synthesis Kit (Roche).
Expression levels were analysed by RT-qPCR as described by Udvardi et al. (2008). RT-qPCR was performed in a 384-well plate with a LightCycler 480 using LightCycler 480 SYBR Green I Master Mix (Roche). For quantification, two stably expressed genes (At1G13320 and At2G28390) were used as reference genes (Hong et al., 2010). The threshold cycle (Ct) and PCR efficiency of the primers used were calculated using LinRegPCR (Ramakers et al., 2003). Oligonucleotide sequences used for RT-qPCR are given in Supplementary Table S2. All RT-qPCR experiments were performed in biological triplicate, and technical triplicates for each, with similar results. The results from a biological triplicate are shown.
For small RNA Northern blots, 10 μg of total RNA was separated on a denaturing 17% (w/v) polyacrylamide gel (8M urea) in TBE buffer and transferred to an N+ Hybond membrane (Amersham). Hybridization was carried out at 42°C using PerfectHyb Plus hybridization buffer (Sigma). DNA oligonucleotide probes specific to miR156 (Lee et al., 2010; Kim et al., 2012) were end labelled with γ32P-ATP using Optikinase (USB). U6 RNA was used to show an equal amount of loading in small RNA hybridization analyses.
Results
Generation of a comprehensive FT/TFL1 family mutant set
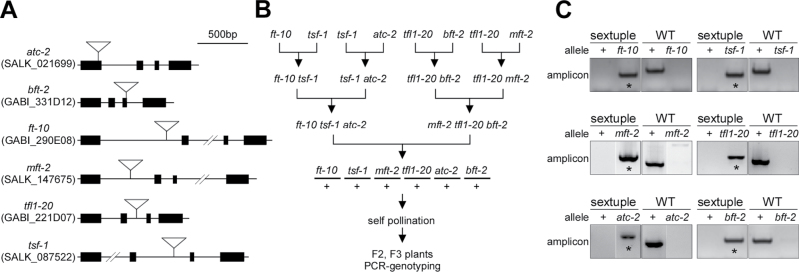
T-DNA insertion alleles of FT (ft-10) (Yoo et al., 2005), TSF (tsf-1) (Yamaguchi et al., 2005), MFT (mft-2) (Xi et al., 2010), TFL1 (tfl1-20) (Yoo et al., 2010), ATC (atc-2) (Huang et al., 2012), and BFT (bft-2) (Yoo et al., 2010) in the Columbia background were used to generate multiple mutants. All alleles were reported to be strong loss-of-function mutants with T-DNA insertions in the introns or exons: a single T-DNA was inserted in the first intron for ft-10 and mft-2, in the second intron for tsf-1 and tfl1-20, in the first exon for atc-2, and in the third exon for bft-2 (Fig. 1A).
Fig. 1.
Map of T-DNA insertions of mutants used in this study and strategy for generating the mutant population. (A) T-DNA insertions in the FT/TFL1 family mutants used in this work. Closed boxes indicate exons; solid lines indicate introns; inverted triangles indicate T-DNA insertion. Both the allele name and its public T-DNA library identifier (Alonso et al., 2003; Rosso et al., 2003) are presented. (B) The strategy for generating a comprehensive set of mutants of the FT/TFL1 family genes. (C) Confirmation of the genotype of sextuple (ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2) mutants by PCR. For example, ft-10 genotyping produced a single band of 926bp in size from the homozygous ft-10 allele (*), whereas the wild-type allele (WT, +) produced a single band of 1392bp in size. Genotyping primer information and the sizes of the expected amplicon of each mutant allele are provided in Table S1.
The FT/TFL1 family consists of six homologous genes in the Arabidopsis genome; thus, there are 63 possible combinations (6 single, 15 double, 20 triple, 15 quadruple, 6 quintuple, and 1 sextuple) in a comprehensive mutant set. All six genes are located on different chromosomes or far apart on the same chromosome in the Arabidopsis genome and therefore are unlikely to be linked. FT, MFT, ATC, TSF, TFL1, and BFT are located on chromsome I, I, II, IV, V, and V, respectively. The genes on the same chromosome, FT and MFT on chromosome I (18.1Mb apart) and TFL1 and BFT on chromosome V (23.9Mb apart) are located in different arms, according to the Arabidopsis Information Resource (TAIR, version 10). Therefore, linkage should not affect generation of mutant combinations.
Since introducing each mutation by repetitive crossing would be a time-consuming way to generate a complete mutant set, this study first generated two triple mutants that were complementary to each other (ft-10 tsf-1 atc-2 and mft-2 tfl1-20 bft-2) (Fig. 1B). These triple mutants were generated by introducing atc-2 and mft-2 mutations into ft-10 tsf-1 and tfl1-20 bft-2 double mutants, respectively, which have been previously reported (Yoo et al., 2010). The ft-10 tsf-1 atc-2 and mft-2 tfl1-20 bft-2 triple mutants were then crossed to generate a line heterozygous for all FT/TFL1 family genes (ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2). This line was self-pollinated and the resulting F2 and F3 plants were subjected to PCR genotyping to isolate individual triple, quadruple, quintuple, and sextuple homozygous mutants. For example, in the PCR genotyping of sextuple mutants (ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2), an amplicon corresponding to each mutant allele was detected (asterisks in Fig. 1C), but amplicons corresponding to wild-type alleles were not. This confirmed the successful isolation of a sextuple mutant.
Absence of cross-regulation among the FT/TFL1 family genes
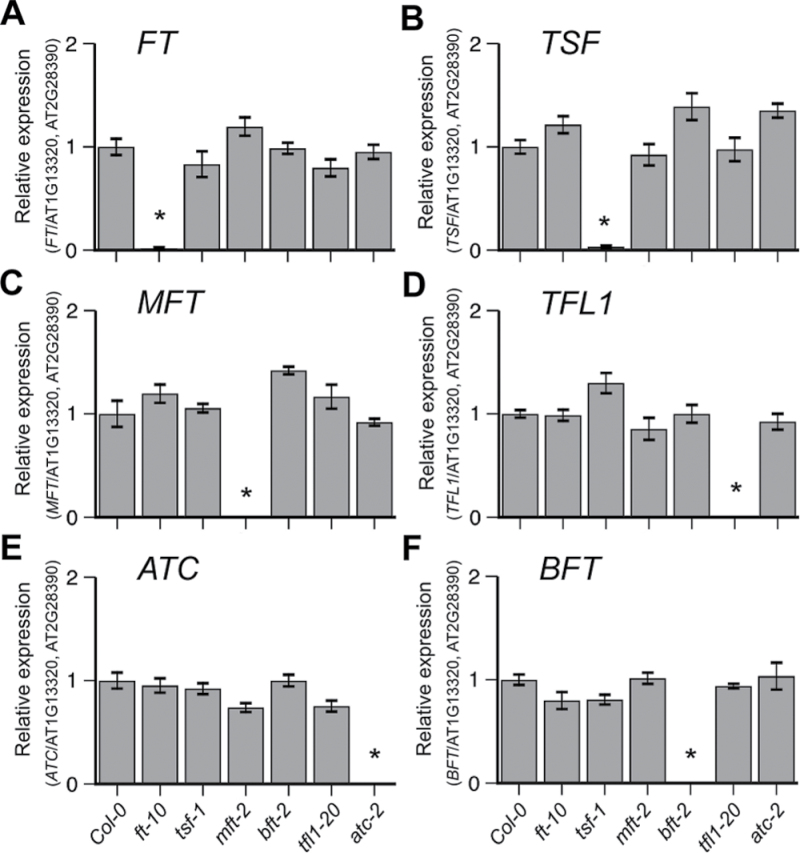
To determine whether the FT/TFL1 genes regulate each other, mRNA expression of the FT/TFL1 family genes was examined in each single mutant. There was no apparent reduction or increase (>2-fold) of mRNA levels of other FT/TFL1 family genes in any single mutant (Fig. 2). For instance, FT expression levels were unaltered in tsf-1, mft-2, tfl1-20, atc-2, and bft-2 mutants (Fig. 2A). This was also true for the other genes. These results indicated that a single mutation in a FT/TFL1 family gene did not affect the mRNA level of other members, excluding a possibility that transcriptional cross-regulation occurs among the FT/TFL1 family, which may have interfered with data obtained from this study of genetic interactions.
Fig. 2.
Expression levels of FT/TFL1 family genes in each single mutant determined via RT-qPCR: (A) FT, (B) TSF, (C) MFT, (D) TFL1, (E) ATC, and (F) BFT. Expression levels were normalized to At1G13320 and At2G28390 (Hong et al., 2010). Asterisks indicate near absence of transcript levels of the gene in the corresponding mutants.
Flowering time analysis at 23 °C under long-day conditions
The flowering time of all 63 mutants under long-day conditions at 23 °C (Table 1) was measured by counting the number of leaves at flowering. Among the six single mutants, only ft-10 and tfl1-20 plants showed late (ft-10, 36.1±2.6 leaves) and early (tfl1-20, 9.6±0.7 leaves) flowering, respectively, compared to wild-type plants (13.8±0.8 leaves). However, the flowering time of tsf-1 (14.3±0.6 leaves), mft-2 (15.3±0.5 leaves), bft-2 (13.8±1.1 leaves), and atc-2 (13.6±1.4 leaves) mutants was not significantly different from that of wild-type plants, consistent with previous observations (Mimida et al., 2001; Yoo et al., 2004; Yamaguchi et al., 2005; Yoo et al., 2010).
Table 1.
Flowering time of the FT/TFL1 family mutants under long-day conditions. CL, cauline leaves; ND, not determined; RL, rosette leaves; TL, total number of leaves.
| Genotype | 23 °C | 16 °C | Leaf number ratio (16 °C/23 °C) | ||||
|---|---|---|---|---|---|---|---|
| RL | CL | TL | RL | CL | TL | ||
| Col-0 | 11.0±0.8 | 2.8±0.4 | 13.8±0.8 | 23.8±1.9 | 3.3±0.7 | 27.2±1.8 | 2.0 |
| ft-10 | 27.8±2.4 | 8.2±0.7 | 36.1±2.6 | 42.7±3.3 | 7.7±0.5 | 50.5±3.5 | 1.4 |
| tsf-1 | 11.4±0.7 | 2.8±0.6 | 14.3±0.6 | 21.7±1.2 | 5.8±0.7 | 27.4±1.6 | 1.9 |
| mft-2 | 12.3±0.5 | 3.0±0.0 | 15.3±0.5 | 24.1±2.7 | 7.5±1.2 | 31.6±3.9 | 2.1 |
| tfl1-20 | 8.6±0.7 | 1.0±0.0 | 9.6±0.7 | 11.7±1.3 | 0.6±0.5 | 12.4±1.1 | 1.3 |
| atc-2 | 11.1±1.1 | 2.5±0.5 | 13.6±1.4 | 20.3±1.4 | 5.1±0.9 | 25.5±2.1 | 1.9 |
| bft-2 | 11.2±0.8 | 2.6±0.7 | 13.8±1.0 | 19.3±1.9 | 6.3±1.2 | 25.6±2.6 | 1.9 |
| ft-10 tsf-1 | 43.2±3.5 | 13.0±2.3 | 56.2±4.4 | 47.7±2.2 | 11.7±2.5 | 59.5±3.2 | 1.1 |
| ft-10 mft-2 | 28.6±2.8 | 8.5±1.7 | 37.1±4.5 | 37.8±3.0 | 7.6±1.1 | 45.5±3.8 | 1.2 |
| ft-10 tfl1-20 | 25.4±2.2 | 7.5±1.0 | 32.9±2.9 | 29.0±1.7 | 6.7±0.9 | 35.7±2.0 | 1.1 |
| ft-10 atc-2 | 28.8±3.0 | 7.7±1.3 | 36.5±3.8 | 34.0±1.6 | 7.1±1.4 | 41.1±2.4 | 1.1 |
| ft-10 bft-2 | 24.8±3.3 | 8.7±1.6 | 33.5±4.3 | 33.4±2.6 | 8.6±1.5 | 42.0±3.9 | 1.3 |
| tsf-1 mft-2 | 13.6±1.7 | 4.1±0.8 | 17.7±2.4 | 23.6±3.4 | 6.7±0.5 | 30.3±3.9 | 1.7 |
| tsf-1 tfl1-20 | 9.5±0.7 | 1.3±0.7 | 10.8±0.9 | 13.5±1.6 | 0.3±0.5 | 13.8±1.7 | 1.3 |
| tsf-1 atc-2 | 11.4±0.7 | 2.8±0.6 | 14.2±0.6 | 21.3±1.8 | 5.1±0.6 | 26.4±1.9 | 1.9 |
| tsf-1 bft-2 | 11.4±0.7 | 2.7±0.6 | 14.2±1.2 | 20.1±1.2 | 5.4±0.8 | 25.6±1.6 | 1.8 |
| mft-2 tfl1-20 | 8.6±0.9 | 1.3±0.6 | 10.0±1.5 | 15.3±1.4 | 0.5±0.5 | 15.8±1.3 | 1.6 |
| mft-2 atc-2 | 11.9±1.0 | 2.6±0.5 | 14.5±1.3 | 28.8±1.7 | 8.4±1.4 | 37.3±2.7 | 2.6 |
| mft-2 bft-2 | 11.5±0.7 | 2.2±0.4 | 13.7±1.0 | 23.5±1.2 | 6.9±0.5 | 30.4±1.2 | 2.2 |
| tfl1-20 atc-2 | 9.1±0.6 | 1.4±0.5 | 10.5±0.8 | 12.8±0.8 | 1.3±0.7 | 14.1±0.9 | 1.3 |
| tfl1-20 bft-2 | 8.3±0.6 | 1.0±0.4 | 9.3±0.6 | 12.5±1.1 | 0.7±0.8 | 13.3±1.1 | 1.4 |
| atc-2 bft-2 | 10.9±0.6 | 2.6±0.7 | 13.5±0.5 | 23.0±1.3 | 5.9±0.7 | 28.8±1.7 | 2.1 |
| ft-10 tsf-1 mft-2 | 46.1±2.8 | 13.5±1.5 | 59.6±3.3 | 56.7±2.1 | 11.3±0.5 | 68.0±2.6 | 1.1 |
| ft-10 tsf-1 tfl1-20 | 37.6±2.6 | 10.7±2.8 | 48.3±3.5 | 39.8±2.6 | 7.1±0.6 | 47.0±2.8 | 1.0 |
| ft-10 tsf-1 atc-2 | 42.5±5.4 | 8.0±1.8 | 50.5±6.4 | 51.7±4.0 | 9.5±1.0 | 61.2±4.8 | 1.2 |
| ft-10 tsf-1 bft-2 | 40.1±1.6 | 12.0±1.3 | 52.1±2.2 | 40.8±1.3 | 11.5±1.5 | 52.4±1.6 | 1.0 |
| ft-10 mft-2 tfl1-20 | 28.5±0.6 | 7.8±1.0 | 36.3±2.3 | 29.8±0.7 | 7.0±1.1 | 36.8±1.2 | 1.0 |
| ft-10 mft-2 atc-2 | 26.4±1.7 | 9.0±1.0 | 35.4±2.4 | 39.8±2.6 | 9.2±0.9 | 49.1±3.0 | 1.4 |
| ft-10 mft-2 bft-2 | 26.6±0.6 | 7.9±1.2 | 34.5±3.4 | 47.0±1.0 | 7.0±0.0 | 54.0±1.0 | 1.6 |
| ft-10 tfl1-20 atc-2 | 21.0±2.2 | 6.6±1.0 | 27.6±2.8 | 34.5±2.5 | 7.0±1.1 | 41.5±1.9 | 1.5 |
| ft-10 tfl1-20 bft-2 | 23.2±2.2 | 6.2±0.7 | 29.4±2.4 | 29.1±2.0 | 4.7±2.0 | 33.9±2.7 | 1.1 |
| ft-10 atc-2 bft-2 | 26.0±1.7 | 8.7±0.6 | 34.7±2.3 | 33.7±1.5 | 8.7±1.2 | 42.4±2.2 | 1.2 |
| tsf-1 mft-2 tfl1-20 | 9.1±1.2 | 0.9±0.3 | 10.0±1.1 | 13.1±1.7 | 1.9±0.7 | 15.0±2.3 | 1.5 |
| tsf-1 mft-2 atc-2 | 12.5±1.2 | 2.9±0.3 | 15.3±1.3 | 23.8±2.2 | 6.2±0.4 | 30.0±2.3 | 1.9 |
| tsf-1 mft-2 bft-2 | 12.6±1.1 | 2.9±0.7 | 15.6±1.5 | 21.8±2.1 | 5.6±0.8 | 27.5±2.9 | 1.8 |
| tsf-1 tfl1-20 atc-2 | 9.8±1.0 | 0.7±0.5 | 10.5±1.2 | 10.9±0.7 | 0.7±0.8 | 11.6±0.8 | 1.1 |
| tsf-1 tfl1-20 bft-2 | 9.5±3.3 | 1.3±0.6 | 10.8±3.2 | 10.0±1.2 | 0.1±0.3 | 10.1±1.3 | 0.9 |
| tsf-1 atc-2 bft-2 | 12.2±1.2 | 3.1±0.8 | 15.3±1.8 | 21.3±0.9 | 4.8±0.6 | 26.1±0.8 | 1.7 |
| mft-2 tfl1-20 atc-2 | 8.2±1.4 | 0.9±0.3 | 9.1±1.7 | 14.1±0.9 | 1.7±0.5 | 15.8±1.1 | 1.7 |
| mft-2 tfl1-20 bft-2 | 8.4±0.7 | 1.1±0.6 | 9.5±1.0 | ND | ND | ||
| mft-2 atc-2 bft-2 | 14.0±1.4 | 3.4±0.9 | 17.4±1.9 | 25.2±1.5 | 7.3±0.9 | 32.4±2.2 | 1.9 |
| tfl1-20 atc-2 bft-2 | 7.8±1.0 | 0.7±0.5 | 8.5±1.5 | 11.3±0.9 | 1.3±0.5 | 12.6±1.1 | 1.5 |
| ft-10 tsf-1 mft-2 tfl1-20 | 39.3±2.5 | 5.4±0.6 | 44.7±2.4 | ND | ND | ||
| ft-10 tsf-1 mft-2 atc-2 | 40.1±2.3 | 12.4±1.9 | 52.5±4.2 | 48.1±1.4 | 12.7±0.8 | 60.8±1.3 | 1.2 |
| ft-10 tsf-1 mft-2 bft-2 | 40.1±2.6 | 11.7±2.3 | 51.8±4.9 | 47.1±1.6 | 11.2±0.7 | 58.3±1.5 | 1.1 |
| ft-10 tsf-1 tfl1-20 atc-2 | 37.8±2.9 | 11.0±2.8 | 48.8±5.7 | 39.3±1.8 | 9.7±0.7 | 49.0±1.7 | 1.0 |
| ft-10 tsf-1 tfl1-20 bft-2 | 41.8±1.6 | 9.4±0.8 | 51.2±2.4 | 41.8±1.6 | 9.4±0.8 | 51.3±2.1 | 1.0 |
| ft-10 tsf-1 atc-2 bft-2 | 35.6±4.7 | 11.6±1.7 | 47.2±5.4 | 34.5±4.4 | 6.1±1.2 | 40.7±5.2 | 0.9 |
| ft-10 mft-2 tfl1-20 atc-2 | 21.7±1.6 | 6.5±0.8 | 28.2±2.3 | 30.0±3.6 | 6.2±0.9 | 36.2±4.1 | 1.3 |
| ft-10 mft-2 tfl1-20 bft-2 | 23.2±1.4 | 6.9±1.0 | 30.2±2.1 | 27.5±1.7 | 5.7±1.6 | 33.2±2.7 | 1.1 |
| ft-10 mft-2 atc-2 bft-2 | 28.6±4.1 | 5.5±1.9 | 34.1±4.5 | ND | ND | ||
| ft-10 tfl1-20 atc-2 bft-2 | 20.0±1.8 | 6.5±0.8 | 26.5±2.3 | 29.3±1.7 | 8.0±0.6 | 37.3±2.1 | 1.4 |
| tsf-1 mft-2 tfl1-20 atc-2 | 9.2±0.6 | 1.3±0.5 | 10.5±0.8 | 14.1±1.3 | 1.4±0.5 | 15.5±1.1 | 1.5 |
| tsf-1 mft-2 tfl1-20 bft-2 | 8.9±0.8 | 1.0±0.0 | 9.9±0.8 | 14.2±1.5 | 1.4±0.5 | 15.6±1.4 | 1.6 |
| tsf-1 mft-2 atc-2 bft-2 | 15.5±1.0 | 4.2±1.3 | 19.7±1.0 | 23.2±2.7 | 5.0±1.6 | 28.2±4.0 | 1.4 |
| tsf-1 tfl1-20 atc-2 bft-2 | 8.9±0.6 | 1.0±0.0 | 9.9±0.6 | 12.7±1.4 | 1.3±0.5 | 14.0±1.6 | 1.4 |
| mft-2 tfl1-20 atc-2 bft-2 | 8.1±1.1 | 1.0±0.4 | 9.1±1.0 | 12.9±1.6 | 1.4±0.7 | 14.2±1.5 | 1.6 |
| ft-10 tsf-1 mft-2 tfl1-20 atc-2 | 40.8±4.3 | 10.8±1.5 | 51.6±5.8 | 39.2±2.8 | 7.6±0.9 | 46.8±2.9 | 0.9 |
| ft-10 tsf-1 mft-2 tfl1-20 bft-2 | 34.7±4.5 | 10.0±3.4 | 44.7±7.4 | 41.2±2.3 | 9.8±1.0 | 51.1±2.7 | 1.1 |
| ft-10 tsf-1 mft-2 atc-2 bft-2 | 33.1±2.6 | 11.4±2.1 | 44.5±3.3 | 47.1±1.4 | 9.5±1.9 | 56.7±1.6 | 1.3 |
| ft-10 tsf-1 tfl1-20 atc-2 bft-2 | 31.0±1.9 | 7.4±1.1 | 38.4±2.4 | 39.1±1.1 | 8.0±1.0 | 47.1±1.3 | 1.2 |
| ft-10 mft-2 tfl1-20 atc-2 bft-2 | 21.5±1.8 | 6.9±1.1 | 28.3±2.2 | 35.8±2.7 | 7.5±1.1 | 43.3±2.8 | 1.5 |
| tsf-1 mft-2 tfl1-20 atc-2 bft-2 | 8.1±1.1 | 0.6±0.7 | 8.7±1.1 | 11.0±1.4 | 1.0±0.7 | 12.0±1.4 | 1.4 |
| ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 | 39.3±4.5 | 9.4±1.9 | 48.7±6.5 | 38.0±1.1 | 7.4±2.0 | 45.1±2.4 | 1.0 |
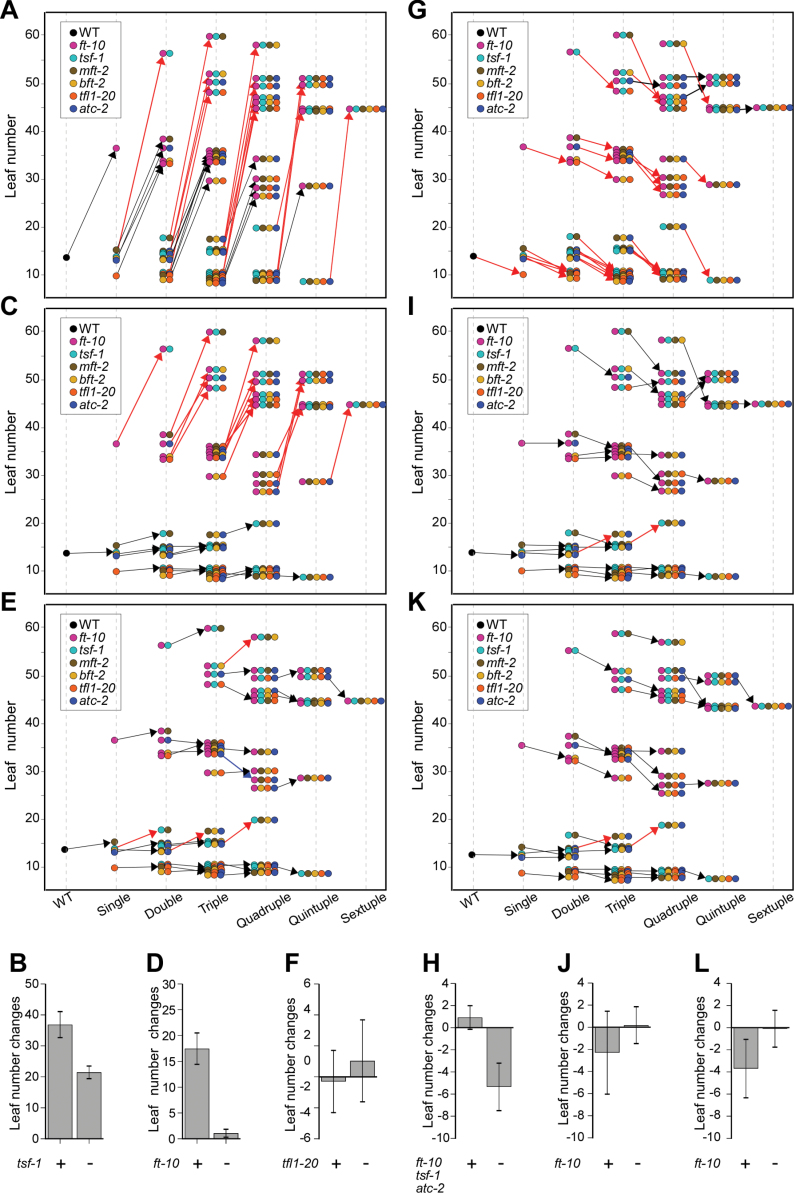
The effect of introducing each mutation to another mutation on flowering time was analysed by measuring the difference in leaf numbers at flowering between mutants with or without the mutation: for instance, the effect of the introduction of ft-10 into tfl1-20 was calculated thus: ft-10 tfl1-20 (32.9 leaves) – tfl1-20 (9.6 leaves) = 23.3 leaves. Introducing ft-10 generally delayed flowering time regardless of the genotype (Fig. 3A); however, a group of mutants apparently exhibited even more delayed flowering (red arrows in Fig. 3A). For instance, among double mutants, ft-10 tsf-1 mutants (56.2±4.4 leaves) flowered significantly later than other double mutants (ft-10 mft-2, 37.1±4.5 leaves; ft-10 tfl1-20, 32.9±2.9 leaves; ft-10 atc-2, 36.5±3.8 leaves; and ft-10 bft-2, 33.5±4.3 leaves). Among triple mutants, ft-10 tsf-1 mft-2 (59.6±3.3 leaves), ft-10 tsf-1 bft-2 (52.1±2.2 leaves), ft-10 tsf-1 tfl1-20 (48.3±3.5 leaves), and ft-10 tsf-1 atc-2 (50.5±6.4 leaves) mutants flowered significantly later than other triple mutants. The same is also true for quadruple and quintuple mutants. These results demonstrated that ft-10 caused a severe delay when combined with tsf-1, which strongly supports the observation that an additive delay was seen in ft-1 tsf-1 double mutants (Yamaguchi et al., 2005). Interestingly, the increase in the number of leaves at flowering caused by the introduction of ft-10 is similar in all mutant combinations. The introduction of ft-10 into any genotype with tsf-1 or without tsf-1 caused a flowering time delay of 36.7±4.3 and 21.2±2.5 leaves, respectively (Fig. 3B).
Fig. 3.
Leaf number changes caused by the introduction of each mutation at 23 °C under long-day conditions. (A, C, E, G, I, and K) The effect of introducing ft-10 (A), tsf-1 (C), mft-2 (E), tfl1-20 (G), atc-2 (I), and bft-2 (K); the arrows indicate leaf number changes after addition of a certain mutation to a genotype. (B, D, F, H, J, and L) Leaf number changes caused by the introduction of a single mutation in mutants containing (+) or not containing (–) another mutation: (B) introduction of ft-10 into mutants with or without tsf-1; (D) introduction of tsf-1 into mutants with or without ft-10; (F) introduction of mft-2 into mutants with or without tfl1-20; (H) introduction of tfl1-20 into mutants with or without ft-10 tsf-1 atc-2; (J) introduction of atc-2 into mutants with or without ft-10; (L) introduction of bft-2 into mutants with or without ft-10.
Introducing tsf-1 generally showed no effect or only weak effect (black arrows in Fig. 3C). However, the introduction of tsf-1 into a genotype that already contained ft-10 dramatically delayed flowering (red arrows in Fig. 3C). As already mentioned, increase in the number of leaves caused by the introduction of tsf-1 into a genotype containing ft-10 was similar. The introduction of tsf-1 into any genotype with or without ft-10 caused a flowering time delay of 16.1±3.2 and 1.0±0.9 leaves, respectively (Fig. 3D).
The introduction of mft-2 did not induce a dramatic alternation in flowering time. Although MFT is suggested to act as a flowering activator based on an overexpression study (Yoo et al., 2004), a slight increase in leaf number caused by introduction of mft-2 was only observed in tsf-1 mft-2, mft-2 atc-2 bft-2, ft-10 tsf-1 mft-2 bft-2, and tsf-1 mft-2 atc-2 bft-2 mutants (red arrows in Fig. 3E). The significant decrease of leaf number by mft-2 was observed only in ft-10 mft-2 tfl1-20 atc-2 mutants (blue arrows in Fig. 3E). The introduction of mft-2 in some mutants containing tfl1-20 appeared to have a weak effect; however, an analysis of leaf number changes by mft-2 revealed an insignificant difference between mutants that did or did not contain tfl1-20 (Fig. 3F).
Introduction of tfl1-20 caused a general decrease in leaf number (red arrows in Fig. 3G). For instance, introducing tfl1-20 into tsf-1 mft-2 bft-2 mutants caused slightly earlier flowering (from 15.6 to 9.2 leaves). Interestingly, introduction of tfl1-20 into the ft-10 tsf-1 atc-2 mutants failed to accelerate flowering (black arrows in Fig. 3E). Flowering time of ft-10 tsf-1 atc-2 and ft-10 tsf-1 tfl1-20 atc-2 mutants was similar (50.5 versus 48.8 leaves). The introduction of tfl1-20 into any genotype without ft-10 tsf-1 atc-2 reduced flowering time by 5.7±2.8 leaves, whereas the introduction of tfl1-20 into any genotype with ft-10 tsf-1 atc-2 produced no significant change in leaf number (Fig. 3H).
The effect of the introduction of atc-2 and bft-2 was similar (Fig. 3I, K) and generally caused weak acceleration of flowering only in the mutants containing ft-10. In mutants without ft-10, the introduction of atc-2 and bft-2 had only a minor acceleration of flowering. The introduction of atc-2 into the genotypes with ft-10 caused a slight decrease in leaf number (2.3±3.8 leaves) (Fig. 3J). Similarly, the introduction of bft-2 into the genotypes with ft-10 caused a slight decrease in leaf number (3.7±2.7 leaves) (Fig. 3L). In contrast, the introduction of atc-2 or bft-2 into the genotypes without ft-10 did not cause an apparent alteration in flowering time.
Under long-day conditions at 23 °C, ft-10 tsf-1 mft-2 mutants flowered the latest (59.6±3.3 leaves), and tfl1-20 atc-2 bft-2 mutants flowered the earliest (8.5±1.5 leaves) (Table 1).
Flowering time analysis at 16 °C under long-day conditions
The flowering time also measured at 16 °C under long-day conditions. Among single mutants, altered flowering time was only seen in ft-10 (50.5±3.5 leaves) and tfl1-20 plants (12.4±1.1 leaves), compared to wild-type plants (27.2±1.8 leaves) (Table 1). Flowering time of tsf-1, mft-2, atc-2, and bft-2 single mutants was similar to that of wild-type plants, which was similar to that seen at 23 °C (Fig. 3).
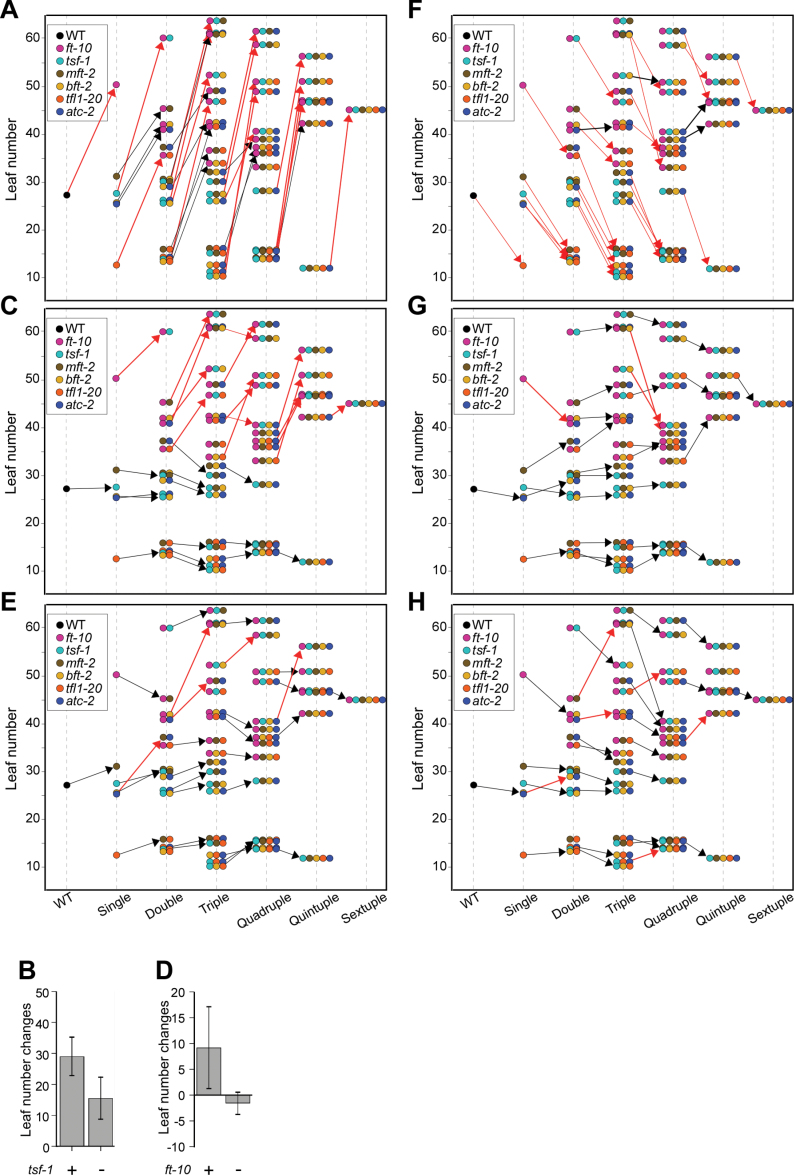
The effect of introducing each mutation on flowering time at 16 °C was analysed by calculating the difference of leaf numbers between mutants with or without the mutation at flowering (Fig. 4). Introducing ft-10 generally delayed flowering time regardless of genotype. Introduction of ft-10 into mutants containing tsf-1 generally led to very delayed flowering at 16 °C (red arrows in Fig. 4A), similar to flowering at 23 °C; however, its effect at 16 °C was not as distinct as at 23 °C. In spite of the absence of tsf-1, some mutants (e.g. ft-10 mft-2 atc-2) flowered as late as mutants carrying both ft-10 and tsf-1. Among double mutants, ft-10 tsf-1 mutants (59.5±3.2 leaves) flowered significantly later than other double mutants (ft-10 mft-2, 45.4±3.8 leaves; ft-10 tfl1-20, 35.7±2 leaves; ft-10 atc-2, 41.1±2.4 leaves; and ft-10 bft-2, 42±3.9 leaves). Among triple mutants, ft-10 tsf-1 mft-2 (68.0±2.6 leaves), ft-10 tsf-1 bft-2 (52.4±1.6 leaves), ft-10 tsf-1 atc-2 (61.2±4.8 leaves), and ft-10 mft-2 bft-2 (54.0±1.0 leaves) mutants flowered significantly later than other triple mutants.
Fig. 4.
Leaf number changes caused by the introduction of each mutation at 16 °C under long-day conditions. (A, C, E, F, G, and H) The effect of introducing ft-10 (A), tsf-1 (C), mft-2 (E), tfl1-20 (F), atc-2 (G), and bft-2 (H); the arrows indicate leaf number changes after addition of a certain mutation to a genotype. (B and D) Leaf number changes caused by the introduction of a single mutation in mutants containing (+) or not containing (–) another mutation: (B) introduction of ft-10 into mutants with or without tsf-1; (D) introduction of tsf-1 into ft-10.
The introduction of ft-10 into the mutants with tsf-1 caused a delay in flowering time (29.0±6.4 leaves), whereas the introduction of ft-10 into genotypes without tsf-1 caused a smaller delay in flowering time (15.5±7.0 leaves) (Fig. 4B). Considering that the introduction of ft-10 into any genotype led to a clearer difference depending on the presence of the tsf-1 mutation at 23 °C (Fig. 3B), this indicated that the additive effect of ft-10 and tsf-1 is diminished at 16 °C.
Introducing tsf-1 into a genotype that contained ft-10 delayed flowering with wide variation (red arrows in Fig. 4C). However, introducing tsf-1 into the genotypes without ft-10 showed slight acceleration of flowering or no clear effect (black arrows in Fig. 4C). The introduction of tsf-1 into a genotype with or without ft-10 caused a delay in flowering time (9.2±8.0 leaves) and no clear effect (–1.6±2.3 leaves), respectively (Fig. 4D). This result indicated that the additive effect of ft-10 and tsf-1 at 16 °C was not as clear as at 23 °C.
The introduction of mft-2 did not cause a dramatic change in flowering time at 16 °C (Fig. 4E). A significant delay in flowering caused by mft-2 was observed only in mft-2 atc-2, ft-10 mft-2 bft-2, ft-10 mft-2 atc-2, and ft-10 tsf-1 mft-2 atc-2 bft-2 mutants (red arrows in Fig. 4E), which showed no change from addition of mft-2 at 23 °C. Conversely, ft-10 mft-2 tfl1-20 atc-2 mutants, which showed a significant decrease in leaf number by addition of mft-2 at 23 °C, did not show an apparent alternation in flowering at 16 °C.
The introduction of tfl1-20 caused a dramatic decrease in leaf number (red arrows in Fig. 4F), except for the introduction of tfl1-20 into ft-10 atc-2, ft-10 tsf-1 bft-2, ft-10 tsf-1 atc-2 bft-2, and ft-10 mft-2 atc-2 bft-2 mutants (black arrows in Fig. 4F). Although introducing tfl1-20 into ft-10 mutants caused only slightly early flowering (from 36.1 to 32.9 leaves) at 23 °C, more apparent acceleration in flowering by the introduction of tfl1-20 was observed in ft-10 tfl1-20 double mutants (from 50.5 to 35.7 leaves) at 16 °C. The same is also true for ft-10 tsf-1 tfl1-20, ft-10 mft-2 tfl1-20 bft-2, and ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants. This suggested that the effect of tfl1-20 on the control of flowering time is ambient temperature dependent.
The introduction of atc-2 and bft-2 had little effect on flowering time at 16 °C. The acceleration of flowering time by introduction of atc-2 was seen only in some mutants (Fig. 4G). For instance, the introduction of atc-2 into the ft-10, ft-10 tsf-1 bft-2, and ft-10 mft-2 bft-2 backgrounds decreased leaf number at flowering by 9.4, 11.7, and 22.5 leaves, respectively. In contrast, introducing atc-2 into some mutants such as mft-2, ft-10 tfl1-20, and ft-10 mft-2 tfl1-20 bft-2 rather increased leaf number. The remaining mutants did not show a clear alteration (0.4±2.4 leaves). The introduction of bft-2 had only a minor effect in accelerating flowering at 16 °C. In spite of the suggested role of BFT as a flowering repressor, only some mutants, such as ft-10 mft-2 bft-2 (+15.7 leaves) and ft-10 mft-2 tfl1-20 atc-2 bft-2 (+7.1 leaves), showed a delay in flowering that was increased by addition of bft-2 (red arrows in Fig. 4H).
tfl1-20 strongly accelerated flowering at 16 °C
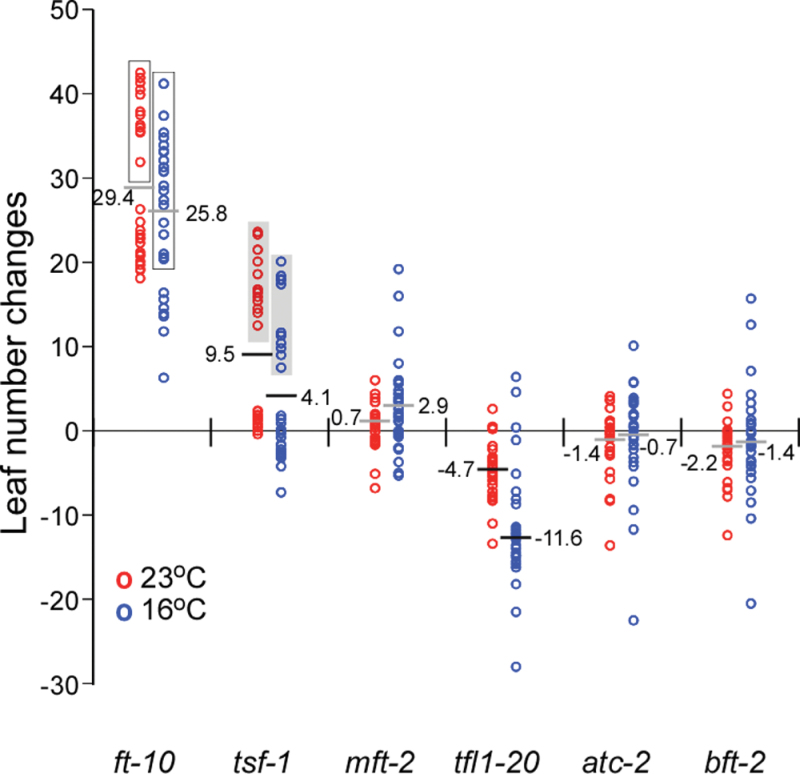
The change in leaf numbers in response to the introduction of each single mutation was next compared at 23 and 16°C to examine the effect at different ambient temperatures (Fig. 5). The introduction of ft-10 caused a severe delay in flowering time (ranging from 18.1 to 41.9 leaves) at 23 °C. The flowering response was clearly divided into two categories, namely with (open box in Fig. 5) or without tsf-1. However, at 16 °C, the delay in flowering by ft-10 mutation was attenuated and the additive delay by combination of ft-10 and tsf-1 was less distinct. The introduction of tsf-1 caused no delay on its own but significant flowering time delay when combined with ft-10 (grey box in Fig. 5) at both 23 and 16°C. mft-2 did not induce an apparent effect at either temperature, although there were a few mutants that showed a strong effect of mft-2 at 16 °C. The introduction of tfl1-20 caused a weak acceleration in flowering time at 23 °C (–4.7 leaves in average). A particularly interesting observation was that tfl1-20 had a stronger effect at 16 °C (–11.6 leaves in average) (horizontal bar in Fig. 5). Most mutants containing atc-2 or bft-2 did not show a clear alteration in flowering time at both temperatures. This comparison revealed that FT, TSF, and TFL1 play an important role in ambient temperature-responsive flowering and that the effect of tfl1-20 was stronger at 16 °C.
Fig. 5.
Plotting of leaf number changes in all mutant combinations caused by introduction of each single mutation at 23 and 16°C. Open boxes indicate leaf number changes by ft-10 in the presence of tsf-1 (except ft-10 mft-2 bft-2 mutants at 16 °C). Grey boxes indicate leaf number changes caused by tsf-1 in the presence of ft-10 (except ft-10 tsf-1 mft-2 bft-2, ft-10 tsf-1 atc-2 bft-2, and ft-10 tsf-1 mft-2 tfl1-20 mutants at 16 °C). Horizontal bars indicate the average leaf number changes caused by the introduction of each mutation: note that tfl1-20 and tsf-1 caused a stronger effect at 16 °C than at 23 °C (black bars, P < 0.05), and that ft-10, mft-2, atc-2, and bft-2 did not show a clear effect (grey bars).
tsf-1 caused reduced sensitivity to ambient temperature-responsive flowering
The effect of introducing each mutation on the response to ambient temperature was analysed by measuring the leaf number ratio (LNR, 16 °C/23 °C). FT is suggested to be an important mediator of flowering time in the response to ambient temperature, since the LNR of ft-10 single mutants was reduced comparing to that of wild-type plants (Lee et al., 2007). Introducing ft-10 generally decreased LNR. The LNR of all mutants containing ft-10 was lower than that of wild-type plants (Fig. 6A). The average LNR of all mutant combinations containing ft-10 was 1.2. Noticeably, the LNR of ft-10 tsf-1, ft-10 tsf-1 bft-2, ft-10 tsf-1 tfl1-20, ft-10 mft-2 tfl1-20, ft-10 tsf-1 tfl-20 atc-2, and ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants was near 1.0, suggesting that they showed a flowering phenotype insensitive to ambient temperature changes. The hypersensitive flowering response to ambient temperature changes of mft-2 atc-2 mutants (LNR 2.6) was also significantly suppressed by the introduction of ft-10 (ft-10 mft-2 atc-2 LNR 1.4). This analysis demonstrated that ft-10 has a strong effect on reducing ambient temperature sensitivity.
Fig. 6.
Changes in leaf number ratios (16 °C/23 °C) by the introduction of mutations ft-10 (A), tsf-1 (B), mft-2 (C), tfl1-20 (D), atc-2 (E), and bft-2(F). Arrows indicate the changes in leaf number ratios after addition of a certain mutation into a genotype.
Interestingly, the introduction of tsf-1 into other mutants caused a general decrease in LNR (Fig. 6B), independent of the presence of ft-10, although tsf-1 on its own failed to change ambient temperature sensitivity (LNR 1.9). Introducing tsf-1 in some genotypes induced a dramatic decrease in LNR (more than –0.5). These include tsf-1 mft-2 bft-2 (change in LNR –0.5), ft-10 tsf-1 mft-2 bft-2 (–0.7), and ft-10 tsf-1 mft-2 tfl1-20 atc-20 (–0.5). The general reduction in LNR by tsf-1 suggested that TSF plays a role in the regulation of ambient temperature-responsive flowering.
Introducing mft-2 caused a slight increase in LNR (Fig. 6C). For instance, the increase in LNR of mft-2 atc-2 and ft-10 mft-2 bft-2 mutants by introduction of mft-2 was 0.7 and 0.5, respectively. An interesting observation was that introducing mft-2 even increased the LNR of mutants containing tfl1-20. The increase in LNR of mft-2 tfl1-20, mft-2 tfl1-20 atc-2, tsf-1 mft-2 tfl1-20 bft-2, and ft-10 tsf-1 mft-2 atc-2 bft-2 was 0.3, 0.4, 0.8, and 0.4, respectively. Although tfl1-20 is known to induce ambient temperature-insensitive flowering (Strasser et al., 2009), mft-2 weakly suppressed the effect of tfl1-20 in ambient temperature-responsive flowering.
Introducing tfl1-20 caused a general decrease in LNR (Fig. 6D). For instance, the decrease in LNR in tsf-1 tfl1-20, tsf-1 bft-2 tfl1-20, and tsf-1 mft-2 tfl1-20 atc-2 mutants by introduction of tfl1-20 was 0.6, 0.9, and 0.5, respectively. However, the introduction of tfl1-20 into a genotype that already contained ft-10 tsf-1 had a weak effect. The decrease in LNR in ft-10 tsf-1 tfl1-20 and ft-10 tsf-1 tfl1-20 bft-2 mutants by introduction of tfl1-20 was 0.1 and 0.1, respectively. This suggested that ambient temperature-insensitive flowering of mutants containing ft-10 and tsf-1 was not strongly enhanced by tfl1-20.
Introducing atc-2 and bft-2 did not produce a clear pattern in response to ambient temperature changes (Fig. 6E and F). Some mutants showed an increase in LNR caused by the introduction of atc-2 (mft-2 atc-2, LNR 0.5; tsf-1 mft-2 atc-2, LNR 0.3; and tsf-1 tfl1-20 atc-2 bft-2, LNR 0.5) (Fig. 6E). In contrast, some mutants showed a decrease in LNR caused by the introduction of atc-2 (ft-10 mft-2, LNR –0.3; mft-2 atc-2 bft-2, LNR –0.4; and tsf-1 mft-2 atc-2 bft-2, LNR –0.3). No significant change was observed by introducing bft-2 in any double mutants. But among triple mutants and other higher-order mutants, the effect of introducing bft-2 appeared to be dependent on ft-10 and tfl1-20, which caused weak temperature insensitivity. The introduction of bft-2 into a genotype containing ft-10 or tfl1-20 caused an increase in LNR (Fig. 6F). The increase of LNR in ft-10 mft-20 bft-2 and ft-10 mft-2 atc-2 bft-2 mutants by introduction of bft-2 was 0.6 and 0.3, respectively. However, the introduction of bft-2 into the other genotypes that were sensitive to ambient temperatures produced a decrease in LNR.
The LNR of ft-10 single mutants was 1.4, but the LNRs of the double mutants were even lower than that of ft-10 single mutants. The LNRs of ft-10 bft-2, ft-10 mft-2, ft-10 atc-2, ft-10 tfl1-20, and ft-10 tsf-1 double mutants were 1.3, 1.2, 1.1, 1.1, and 1.1, respectively (Table 1). This indicated that the addition of a mutation in any FT/TFL1 family member into the ft-10 mutant background reduced sensitivity to ambient temperature-responsive flowering. However, such an additive effect was not seen in other single-mutant backgrounds. This suggested that FT plays a redundant role with other FT/TFL1 family genes in ambient temperature-responsive flowering.
Taken together, these results show that, for ambient temperature-responsive flowering, the introduction of ft-10, tfl1-20, and tsf-1 caused flowering to be less sensitive to ambient temperatures. However, the introduction of mft-2, bft-2, and atc-2 did not show a clear general pattern.
miR156 overexpression in ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 sextuple mutants
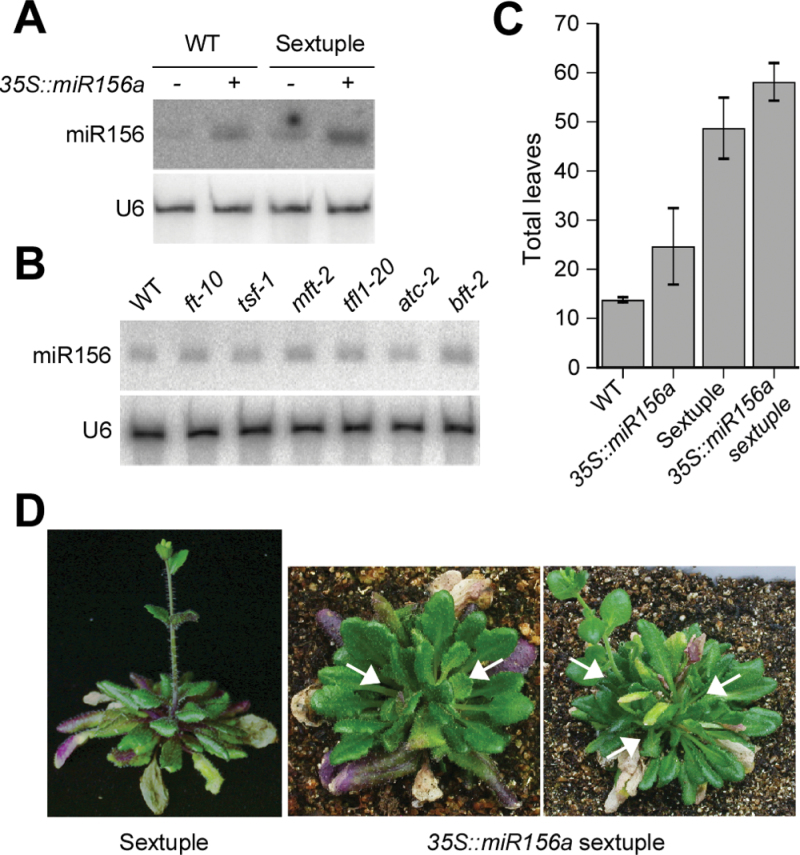
These genetic analyses revealed that Arabidopsis plants flowered in the absence of all FT/TFL1 family genes, suggesting that FT/TFL1 family genes are not essential for flowering. This further suggested a possibility that there is an FT/TFL1-independent pathway to induce flowering. One such pathway may include miR156, which delays flowering time by negatively regulating SPL genes (Wang et al., 2009; Jung et al., 2012a). Thus, this study tested whether the introduction of miR156 into our ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 sextuple mutants blocks flowering. For this experiment, the 35S::miR156a construct was introduced into the sextuple mutants and wild-type plants by Agrobacterium-mediated transformation. The introduction of 35S::miR156 into wild-type plants and sextuple mutants caused a general delay in flowering. The distribution of flowering times of 35S::miR156a and 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 plants in the T1 generation is shown (Supplementary Fig. S1). This study selected a line that showed strong late flowering and confirmed miR156 overexpression in transgenic plants (approximately 5-fold) (Fig. 7A).
Fig. 7.
Flowering phenotype of 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants under long-day conditions. (A and B) Small RNA blots showing expression levels of miR156 in transgenic plants generated in this study (35S::miR156a plants and 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants) (A) and in single mutants (B); U6 RNA served as a loading control (Lee et al., 2010). (C and D) Flowering time (C) and morphology (D) of 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants under long-day conditions. Note multiple rosettes generated from 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants (arrows). Total leaf number of ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants in (C) came from Table 1.
To exclude the possibility that the FT/TFL1 family genes regulate miR156 expression, small RNA blot analysis was performed for each single mutant. The results indicated that miR156 expression was not altered in any single mutant (Fig. 7B). However, the miR156 level in the sextuple mutants was slightly higher than that of wild-type plants (Fig. 7A), suggesting a possibility that miR156 expression is negatively affected by combined mutations in the FT/TFL1 family.
Interestingly, 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 plants still flowered, but later than that of ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 and 35S::miR156a plants. 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 plants flowered with 58.1 leaves under long-day conditions, whereas 35S::miR156a control plants flowered with 24.7 leaves under the same conditions (Fig. 7C). Such late flowering was comparable to that seen in ft-10 tsf-1 mft-2 mutants (Table 1). This study also observed that 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 plants frequently generated multiple rosettes with many secondary leaves (Fig. 7D), although such phenotype was absent in the sextuple mutants.
Discussion
The FT/TFL1 family encodes six important regulators (FT, TSF, MFT, TFL1, ATC, and BFT) that control flower development in Arabidopsis. This study constructed a comprehensive mutant set for this family, including the sextuple mutant, and measured the flowering time of each mutant. Lesions in all six FT/TFL1 family genes and ectopic miR156 expression in the sextuple mutants did not inhibit flowering under long-day conditions. Also, tsf-1 reduced sensitivity to ambient temperature changes and tfl1-20 had a stronger effect at 16 °C than at 23 °C.
Flowering time studies of a subset of double and triple mutants have been reported (Hanzawa et al., 2005; Yamaguchi et al., 2005; Jang et al., 2009; Ahn et al., 2006; Yoo et al., 2010). The previous analyses using overexpression lines and a handful of mutants suggested that FT, TSF, and MFT are floral activators (Kardailsky et al., 1999; Kobayashi et al., 1999; Yoo et al., 2004) and that TFL1, ATC, and BFT are floral repressors (Bradley et al., 1997; Mimida et al., 2001; Yoo et al., 2010), and this study’s analysis of flowering time provided conclusive evidence to support the notion. Among them, FT and TFL1 exert a strong effect, whereas the contribution from other genes was minor. An excellent example was the flowering time of sextuple mutants. The ft-10 tsf-1 mft-2 mutants flowered latest (59.6±3.3 leaves) and the tfl1-20 atc-2 bft-2 mutants flowered earliest (8.5±1.5 leaves). The flowering time of ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 mutants (48.7±6.5 leaves) was intermediate (Table 1).
An important question is which gene activates flowering in the sextuple mutants, because the sextuple mutants still flower and ft-10 tsf-1 mft-2 mutants flowered later than tfl1-20 atc-2 bft-2 mutants. This study measured expression levels of SOC1, SPL3, FUL, and AP1 in the wild type, ft-10 tsf-1 mft-2, tfl1-20 atc-2 bft-2, and the sextuple mutants. Although SOC1 and FUL mRNA levels were lower in ft-10 tsf-1 mft-2 mutants, their mRNA levels in tfl1-20 atc-2 bft-2 mutants were not significantly higher than those of wild-type plants (Supplementary Fig. S2). This suggested the possibility that SOC1 and FUL did not activate flowering. SPL3 mRNA levels seemed to be unaffected by these mutations. However, AP1 transcript levels were significantly lower in ft-10 tsf-1 mft-2 mutants, but higher in tfl1-20 atc-2 bft-2 plants than in wild-type plants. These results suggested that increased AP1 expression is responsible for the early flowering of tfl1-20 atc-2 bft-2 plants. However, since this study tested a subset of flowering time genes, a genome-wide analysis would be necessary to identify the gene responsible for the flowering phenotype of the sextuple mutants.
One notable finding is that tsf-1 reduces sensitivity to ambient temperature changes. It was previously suggested that under long-day conditions, TSF on its own did not play a role in regulating flowering time (Michaels et al., 2005; Yamaguchi et al., 2005). However, the current data revealed that tsf-1 reduced the temperature response even without ft-10 (Fig. 6B). This finding is consistent with this study group’s previous proposal that the ambient temperature response is mediated by both FT and TSF, based on the weak effect of ft-10 single mutation in ambient temperature-responsive flowering (Lee et al., 2007). Indeed, the leaf number ratios of ft-10 tsf-1 double mutants and higher-order mutants containing both ft-10 and tsf-1 were close to 1.0. Thus, it seems likely that FT and TSF act downstream of the thermosensory pathway (Lee et al., 2007; Lee et al., 2010; McClung and Davis, 2010; Kumar et al., 2012). TSF plays a redundant role with FT under long-day conditions, mainly acts under short-day conditions (Yamaguchi et al., 2005) and acts in response to cytokinin treatment (D’Aloia et al., 2011). This group’s studies suggest an additional role for TSF under long-day conditions.
The observation that tfl1-20 had a stronger effect at 16 °C than at 23 °C further supports Cerdán group’s finding that TFL1 may be a positive regulator of the response to low temperature (Strasser et al., 2009). They performed a modified gene set enrichment analysis and found that TFL1 plays more general roles in the plant response to ambient temperature. How TFL1 regulates ambient temperature response is largely unknown, but its localization at the endomembrane compartment (Sohn et al., 2007) may provide a clue to its precise function. Membrane homeostasis including membrane thickness (Cybulski et al., 2010) and membrane integrity (Mansilla et al., 2004) is suggested to be a general cue for sensing temperature. Thus, the changes in biochemical properties of cell membrane lipids in response to ambient temperature changes may lead to alterations in the activity of a signalling molecule that regulates ambient temperature response. Considering that TFL1 is highly similar to an animal PEBP protein that encodes a Raf kinase inhibitor (Yeung et al., 1999), it is tempting to speculate that TFL1 may be involved in relay of temperature-responsive sensor kinase signalling (Mansilla et al., 2004), which is associated with thermal control of membrane lipid homeostasis.
Another interesting observation was that tfl1-20 did not accelerate flowering in the ft-10 tsf-1 atc-2 background, although tfl1-20 generally reduced the leaf number at flowering in almost all mutant combinations. This suggests that FT, TSF, and ATC are required for TFL1 function in the regulation of flowering time. ATC was recently described as a short-day-induced floral inhibitor that is graft transmissible (Huang et al., 2012). FT and TSF proteins interact with FD (Abe et al., 2005; Wigge et al., 2005; Jang et al., 2009), probably at the shoot apex. TFL1 protein also interacts with FD to transcriptionally repress flowering time genes that are induced by FT (Hanano and Goto, 2011). Thus, a possible scenario to explain the absence of the effect of tfl1-20 mutation is that recruitment of a coactivator or corepressor to the FD protein complex is inhibited in the ft-10 tsf-1 atc-2 background, as previously suggested (Ahn et al., 2006). Failure to recruit such cofactors may then render FD inactive or nearly inactive, explaining the absence of an effect of the addition of tfl1-20.
In summary, this study constructed a comprehensive set of mutants of the six A. thaliana FT/TFL1 family genes and analysed their genetic interactions in the regulation of flowering time. This mutant population will be useful to further define the role of the FT/TFL1 family genes in broad aspects of plant development. Further analyses using this population will provide strong genetic evidence of the functional roles and importance of the FT/TFL1 family.
Supplementary material
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Distribution of flowering time of wild type, 35S::miR156a and 35S::miR156a ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 plants at 23°C in the T1 generation.
Supplementary Fig. S2. Relative expression of SPL3, FUL, SOC1, and AP1 in WT, tfl1-20 atc-2 bft-2, ft-10 tsf-1 mft-2 tfl1-20 atc-2 bft-2 and ft-10 tsf-1 mft-2 plants.
Supplementary Table S1. Oligonucleotide sequences used for genotyping.
Supplementary Table S2. Oligonucleotide sequences used for RT-qPCR.
Acknowledgements
This work was supported by the Creative Research Initiatives of the National Research Foundation for the Ministry of Education, Science and Technology (R16-2008-106-01000-0 to JHA), the Korea University (WK), and the BK 21 Program (to WK, TIP, SJY, and ARJ). The authors thank SY Yoo for her initial support and MH Lee for his technical assistance.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. 2005. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309, 1052–1056 [DOI] [PubMed] [Google Scholar]
- Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel D. 2006. A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. The EMBO Journal 25, 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso JM, Stepanova AN, Leisse TJ, et al. 2003. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301, 653–657 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Ahn JH, Weigel D. 2003. A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nature Genetics 33, 168–171 [DOI] [PubMed] [Google Scholar]
- Bradley D, Ratcliffe O, Vincent C, Carpenter R, Coen E. 1997. Inflorescence commitment and architecture in Arabidopsis. Science 275, 80–83 [DOI] [PubMed] [Google Scholar]
- Chardon F, Damerval C. 2005. Phylogenomic analysis of the PEBP gene family in cereals. Journal of Molecular Evolution 61, 579–590 [DOI] [PubMed] [Google Scholar]
- Cho HJ, Kim JJ, Lee JH, Kim W, Jung JH, Park CM, Ahn JH. 2012. SHORT VEGETATIVE PHASE (SVP) protein negatively regulates miR172 transcription via direct binding to the pri-miR172a promoter in Arabidopsis. FEBS Letters 586, 2332–2337 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, et al. 2007. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316, 1030–1033 [DOI] [PubMed] [Google Scholar]
- Cybulski LE, Martin M, Mansilla MC, Fernandez A, de Mendoza D. 2010. Membrane thickness cue for cold sensing in a bacterium. Current Biology 20, 1539–1544 [DOI] [PubMed] [Google Scholar]
- D’Aloia M, Bonhomme D, Bouche F, Tamseddak K, Ormenese S, Torti S, Coupland G, Perilleux C. 2011. Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. The Plant Journal 65, 972–979 [DOI] [PubMed] [Google Scholar]
- Ferrandiz C, Gu Q, Martienssen R, Yanofsky MF. 2000. Redundant regulation of meristem identity and plant architecture by FRUITFULL, APETALA1 and CAULIFLOWER. Development 127, 725–734 [DOI] [PubMed] [Google Scholar]
- Fitter AH, Fitter RS. 2002. Rapid changes in flowering time in British plants. Science 296, 1689–1691 [DOI] [PubMed] [Google Scholar]
- Fornara F, de Montaigu A, Coupland G. 2010. SnapShot: control of flowering in Arabidopsis. Cell 141, 550, 550 e551–552 [DOI] [PubMed] [Google Scholar]
- Hanano S, Goto K. 2011. Arabidopsis TERMINAL FLOWER1 is involved in the regulation of flowering time and inflorescence development through transcriptional repression. The Plant Cell 23, 3172–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa Y, Money T, Bradley D. 2005. A single amino acid converts a repressor to an activator of flowering. Proceedings of the National Academy of Sciences, USA 102, 7748–7753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harig L, Beinecke FA, Oltmanns J, et al. 2012. Proteins from the FLOWERING LOCUS T -like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. The Plant Journal 72, 908–921 [DOI] [PubMed] [Google Scholar]
- Helliwell CA, Wood CC, Robertson M, James Peacock W, Dennis ES. 2006. The Arabidopsis FLC protein interacts directly in vivo with SOC1 and FT chromatin and is part of a high-molecular-weight protein complex. The Plant Journal 46, 183–192 [DOI] [PubMed] [Google Scholar]
- Hong SM, Bahn SC, Lyu A, Jung HS, Ahn JH. 2010. Identification and testing of superior reference genes for a starting pool of transcript normalization in Arabidopsis. Plant Cell Physiology 51, 1694–1706 [DOI] [PubMed] [Google Scholar]
- Huang NC, Jane WN, Chen J, Yu TS. 2012. Arabidopsis thaliana CENTRORADIALIS homologue (ATC) acts systemically to inhibit floral initiation in Arabidopsis. The Plant Journal 72, 175–184 [DOI] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G. 2009. Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. The Plant Journal 60, 614–625 [DOI] [PubMed] [Google Scholar]
- Jung JH, Ju Y, Seo PJ, Lee JH, Park CM. 2012a. The SOC1-SPL module integrates photoperiod and gibberellic acid signals to control flowering time in Arabidopsis. The Plant Journal 69, 577–588 [DOI] [PubMed] [Google Scholar]
- Jung JH, Seo PJ, Ahn JH, Park CM. 2012b. The Arabidopsis RNA-binding protein FCA regulates microRNA172 processing in thermosensory flowering. Journal of Biological Chemistry 287, 16007–16016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. 1999. Activation tagging of the floral inducer FT. Science 286, 1962–1965 [DOI] [PubMed] [Google Scholar]
- Karlgren A, Gyllenstrand N, Kallman T, Sundstrom JF, Moore D, Lascoux M, Lagercrantz U. 2011. Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiology 156, 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Lee JH, Kim W, Jung HS, Huijser P, Ahn JH. 2012. The microRNA156-SQUAMOSA PROMOTER BINDING PROTEIN-LIKE3 module regulates ambient temperature-responsive flowering via FLOWERING LOCUS T in Arabidopsis. Plant Physiology 159, 461–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Ahn HJ, Chiou TJ, Ahn JH. 2011. The role of the miR399-PHO2 module in the regulation of flowering time in response to different ambient temperatures in Arabidopsis thaliana. Molecules and Cells 32, 83–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, et al. 2011. FLOWERING LOCUS T regulates stomatal opening. Current Biology 21, 1232–1238 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. 1999. A pair of related genes with antagonistic roles in mediating flowering signals. Science 286, 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alos E, Alvey E, Harberd NP, Wigge PA. 2012. Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484, 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar SV, Wigge PA. 2010. H2A.Z-containing nucleosomes mediate the thermosensory response in Arabidopsis. Cell 140, 136–147 [DOI] [PubMed] [Google Scholar]
- Lee H, Yoo SJ, Lee JH, Kim W, Yoo SK, Fitzgerald H, Carrington JC, Ahn JH. 2010. Genetic framework for flowering-time regulation by ambient temperature-responsive miRNAs in Arabidopsis. Nucleic Acids Research 38, 3081–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Hong SM, Yoo SJ, Park OK, Lee JS, Ahn JH. 2006. Integration of floral inductive signals by flowering locus T and suppressor of overexpression of Constans 1. Physiologia Plantarum 126, 475–483 [Google Scholar]
- Lee JH, Lee JS, Ahn JH. 2008. Ambient temperature signaling in plants: an emerging field in the regulation of flowering time. Journal of Plant Biology 51, 321–326 [Google Scholar]
- Lee JH, Kim JJ, Ahn JH. 2012a. Role of SEPALLATA3 (SEP3) as a downstream gene of miR156-SPL3-FT circuitry in ambient temperature-responsive flowering. Plant Signaling and Behavior 7, 1151–1154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Kim JJ, Kim SH, Cho HJ, Kim J, Ahn JH. 2012b. The E3 ubiquitin ligase HOS1 regulates low ambient temperature-responsive flowering in Arabidopsis thaliana. Plant and Cell Physiology 53, 1802–1814 [DOI] [PubMed] [Google Scholar]
- Lee JH, Yoo SJ, Park SH, Hwang I, Lee JS, Ahn JH. 2007. Role of SVP in the control of flowering time by ambient temperature in Arabidopsis. Genes and Development 21, 397–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansilla MC, Cybulski LE, Albanesi D, de Mendoza D. 2004. Control of membrane lipid fluidity by molecular thermosensors. Journal of Bacteriology 186, 6681–6688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR, Davis SJ. 2010. Ambient thermometers in plants: from physiological outputs towards mechanisms of thermal sensing. Current Biology 20, R1086–R1092 [DOI] [PubMed] [Google Scholar]
- Michaels SD. 2009. Flowering time regulation produces much fruit. Current Opinion in Plant Biology 12, 75–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM. 2005. Integration of flowering signals in winter-annual Arabidopsis. Plant Physiology 137, 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimida N, Goto K, Kobayashi Y, Araki T, Ahn JH, Weigel D, Murata M, Motoyoshi F, Sakamoto W. 2001. Functional divergence of the TFL1-like gene family in Arabidopsis revealed by characterization of a novel homologue. Genes to Cells 6, 327–336 [DOI] [PubMed] [Google Scholar]
- Pin PA, Nilsson O. 2012. The multifaceted roles of FLOWERING LOCUS T in plant development. Plant, Cell and Environment 35, 1742–1755 [DOI] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RH, Moorman AF. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neuroscience Letters 339, 62–66 [DOI] [PubMed] [Google Scholar]
- Ratcliffe OJ, Amaya I, Vincent CA, Rothstein S, Carpenter R, Coen ES, Bradley DJ. 1998. A common mechanism controls the life cycle and architecture of plants. Development 125, 1609–1615 [DOI] [PubMed] [Google Scholar]
- Rosso MG, Li Y, Strizhov N, Reiss B, Dekker K, Weisshaar B. 2003. An Arabidopsis thaliana T-DNA mutagenized population (GABI-Kat) for flanking sequence tag-based reverse genetics. Plant Molecular Biology 53, 247–259 [DOI] [PubMed] [Google Scholar]
- Sohn EJ, Rojas-Pierce M, Pan S, Carter C, Serrano-Mislata A, Madueno F, Rojo E, Surpin M, Raikhel NV. 2007. The shoot meristem identity gene TFL1 is involved in flower development and trafficking to the protein storage vacuole. Proceedings of the National Academy of Sciences, USA 104, 18801–18806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanth A, Schmid M. 2011. Regulation of flowering time: all roads lead to Rome. Cellular and Molecular Life Sciences 68, 2013–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser B, Alvarez MJ, Califano A, Cerdan PD. 2009. A complementary role for ELF3 and TFL1 in the regulation of flowering time by ambient temperature. The Plant Journal 58, 629–640 [DOI] [PubMed] [Google Scholar]
- Udvardi MK, Czechowski T, Scheible WR. 2008. Eleven golden rules of quantitative RT-PCR. The Plant Cell 20, 1736–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. 2004. Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303, 1003–1006 [DOI] [PubMed] [Google Scholar]
- Wang JW, Czech B, Weigel D. 2009. miR156-regulated SPL transcription factors define an endogenous flowering pathway in Arabidopsis thaliana. Cell 138, 738–749 [DOI] [PubMed] [Google Scholar]
- Wigge PA. 2011. FT, a mobile developmental signal in plants. Current Biology 21, R374–R378 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. 2005. Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309, 1056–1059 [DOI] [PubMed] [Google Scholar]
- Xi W, Liu C, Hou X, Yu H. 2010. MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. The Plant Cell 22, 1733–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. 2005. TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant and Cell Physiology 46, 1175–1189 [DOI] [PubMed] [Google Scholar]
- Yeung K, Seitz T, Li S, et al. 1999. Suppression of Raf-1 kinase activity and MAP kinase signalling by RKIP. Nature 401, 173–177 [DOI] [PubMed] [Google Scholar]
- Yoo SJ, Chung KS, Jung SH, Yoo SY, Lee JS, Ahn JH. 2010. BROTHER OF FT AND TFL1 (BFT) has TFL1-like activity and functions redundantly with TFL1 in inflorescence meristem development in Arabidopsis. The Plant Journal 63, 241–253 [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH. 2005. CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiology 139, 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SY, Kardailsky I, Lee JS, Weigel D, Ahn JH. 2004. Acceleration of flowering by overexpression of MFT (MOTHER OF FT AND TFL1). Molecules and Cells 17, 95–101 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.