Abstract
We created a mobile phone microscope and assessed its accuracy for the diagnosis of soil-transmitted helminths compared with conventional microscopy. Mobile phone microscopy has a sensitivity of 69.4% for detecting any helminth egg and sensitivities of 81.0%, 54.4%, and 14.3% for the diagnosis of Ascaris lumbricoides, Trichuris trichiura and hookworm respectively.
Introduction
Soil-transmitted helminths (Ascaris lumbricoides, Trichuris trichiura, and hookworm) affect roughly 2 billion individuals worldwide and are a major public health issue in developing countries, predominantly rural settings with inadequate sanitation.1 Children are disproportionately affected, and chronic infection manifests as anemia and malnutrition, which consequently, results in poor physical and mental development.2
The diagnosis of soil-transmitted helminths traditionally involves stool examination under a microscope.3 The Kato–Katz method is frequently used,4 with increased sensitivity after multiple stool examinations.5 Novel methods of diagnosis are necessary, because many of the infected individuals live in underserviced rural areas and do not have access to appropriate diagnostic facilities or care.2 Here, we describe the proof of concept that a mobile phone can be converted into a microscope for the point-of-care diagnosis of soil-transmitted helminths. We compare the diagnostic accuracy of our mobile phone microscope with conventional light microscopy.
Methods
Ethics statement.
This proof-of-concept study was integrated into a clinical trial assessing the safety and efficacy of different anthelmintic drugs against soil-transmitted helminth infections in school-aged children on Pemba Island, Tanzania. Ethical approval was granted by the ethics committee of Basel (EKBB; reference no. 390/11) and the Ministry of Health and Social Welfare of Zanzibar (ZAMREC; reference no. 0001/Jan/11). This trial is registered with ClinicalTrials.gov (identifier ISRCTN54577342). Parents or legal guardians of children were asked for written informed consent, whereas children assented orally. At the end of the study, all participants were treated with albendazole (400 mg).
Procedures.
The clinical trial was conducted in September and October of 2012. For the purpose of this embedded study, stool samples were collected from children in the morning over a 5-day period and processed in the afternoon of the collection day using the Kato–Katz technique.4 Stool samples were analyzed by conventional light microscopy by trained laboratory technicians, and the eggs of A. lumbricoides, T. trichiura, and hookworm were counted and recorded separately. Data were double-entered into a database. For quality control, a random sample of 10% of the slides was re-examined by a senior microscopist to ensure internal validity.
Mobile phone microscope.
We transformed a mobile phone into a microscope by temporarily mounting a 3-mm ball lens (Edmund Optics, Barrington, NJ) to the camera of an iPhone 4S (Apple, Cupertino, CA) with double-sided tape (3M, St. Paul, MN) similar to the work described by Smith and others.6 A small hole was punctured in the middle of the double-sided tape, and the ball lens was positioned in this hole. The ball lens was then centered over the iPhone camera lens, with the tape holding the lens to the camera for stability. Kato–Katz thick smear slides were directly placed up against the double-sided tape, such that a small space less than 1 mm separated the lens from the slide (Figure 1). The mobile phone microscope was placed on top of a slide, which was illuminated from below by a generic, small, hand-held incandescent flashlight powered by one AA battery. Images were viewed on the mobile phone screen, and magnification was increased with the digital zoom function; we estimate that this method could achieve an equivalent of 50–60× magnification. The microscopist manually manipulated the slide underneath the mobile phone microscope to examine the entire area of stool on the slide. The thick double-sided tape (3M) that held the ball lens to the mobile phone provided a 1-mm buffer zone between the slide and ball lens. In addition, the cellophane strip placed over stool on the slide prevented the ball lens from becoming contaminated with stool.
Figure 1.

Mobile phone microscope apparatus with 3-mm ball lens embedded into double-sided tape and fixed over the iPhone camera lens. Note that the double-sided tape flanks the 3-mm ball lens to ensure a 1-mm space between the lens and glass slide.
Samples.
Kato–Katz thick smear slides (n = 199) were randomly selected and analyzed by mobile phone microscopy by a senior microscopist for the presence or absence of soil-transmitted helminth eggs within 2 hours of evaluation by conventional light microscopy. The mobile phone microscopist was blinded to results from conventional light microscopy.
Statistical analysis.
Data were analyzed with Stata version 10.1 (StataCorp., College Station, TX). The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of mobile phone microscopy for the diagnosis of soil-transmitted helminths were calculated and compared with results from conventional microscopy. Although there is no true ‘gold standard’ for the diagnosis of soil-transmitted helminths, microscopy on multiple samples has been considered as a reasonable standard for diagnosis. Comparisons were made between any helminth egg visualized on mobile phone microscopy and conventional microscopy, regardless of the correct species diagnosis. We also assessed the diagnostic accuracy for each soil-transmitted helminth species separately. In addition, diagnostic accuracy was evaluated for low-, moderate-, and heavy-infection intensities using the cutoffs described by the World Health Organization (WHO).7 Moderate- and heavy-infection intensities were combined into a single category. Because we selected out positive slides in the evaluation of low- and moderate/heavy-intensity infections, we could not assess for specificity, PPV, and NPV, because by definition, there were no false-positive slides from conventional microscopy.
Results
A total of 199 Kato–Katz thick smears were evaluated by both mobile phone microscopy and conventional microscopy. Table 1 shows the sensitivity, specificity, PPV, and NPV for soil-transmitted helminth diagnosis. Of note, the mobile phone microscope revealed a sensitivity of 69.4% for detecting any soil-transmitted helminth infection. The highest diagnostic yield in terms of sensitivity was for A. lumbricoides (81.0%) followed by T. trichiura (54.4%). The sensitivity for detecting hookworm was very poor (14.3%). The mobile phone microscope showed higher sensitivities for the heavy-infection intensity category compared with lower fecal egg counts. All hookworm infections were of low intensity in this study (< 2,000 eggs per 1 g stool). There was a 61.5% concordance rate of the 26 slides with no visualized helminth eggs on conventional microscopy. Egg images with conventional and mobile phone microscopy can be viewed in Figure 2.
Table 1.
Operating characteristics of the mobile phone microscope compared with conventional microscopy for the diagnosis of soil-transmitted helminth infection (n = 199 Kato-Katz thick smears)
| Organism | Conventional microscope n (%) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|
| Any soil-transmitted helminth egg visualized | 173 (86.9) | 69.4 (61.8–76.0) | 61.5 (40.7–79.1) | 92.3 (85.9–96.0) | 23.2 (14.2–35.2) |
| All A. lumbricoides | 41 (20.6) | 81.0 (65.4–90.9) | 87.3 (80.7–91.9) | 63.0 (48.7–75.4) | 94.5 (89.1–97.4) |
| A. lumbricoides (light infection)* | 27 (13.6) | 74.5 (53.4–88.1) | – | – | – |
| A. lumbricoides (moderate/heavy infection)† | 15 (7.5) | 93.3 (66.0–99.7) | – | – | – |
| All T. trichiura | 158 (79.4) | 54.4 (46.3–62.3) | 63.4 (46.9–77.4) | 85.1 (76.4–91.2) | 26.5 (18.4–36.6) |
| T. trichiura (light infection)‡ | 107 (53.8) | 43.9 (34.5–53.8) | – | – | – |
| T. trichiura (moderate/heavy infection)§ | 51 (25.6) | 76.5 (62.2–86.7) | – | – | – |
| All hookworm¶ | 98 (49.2) | 14.3 (8.3–23.1) | 89.1 (81.0–94.2) | 56.0 (35.3–75.0) | 51.7 (44.1–59.3) |
CI = confidence interval; NPV = negative predictive value; PPV = positive predictive value.
Eggs per 1 g stool (EPG): 1–4,999.
EPG: ≥ 5,000.
EPG: 1–999.
EPG: ≥ 1,000.
Only WHO-defined low fecal egg counts available for analysis (1–1,999 EPG).
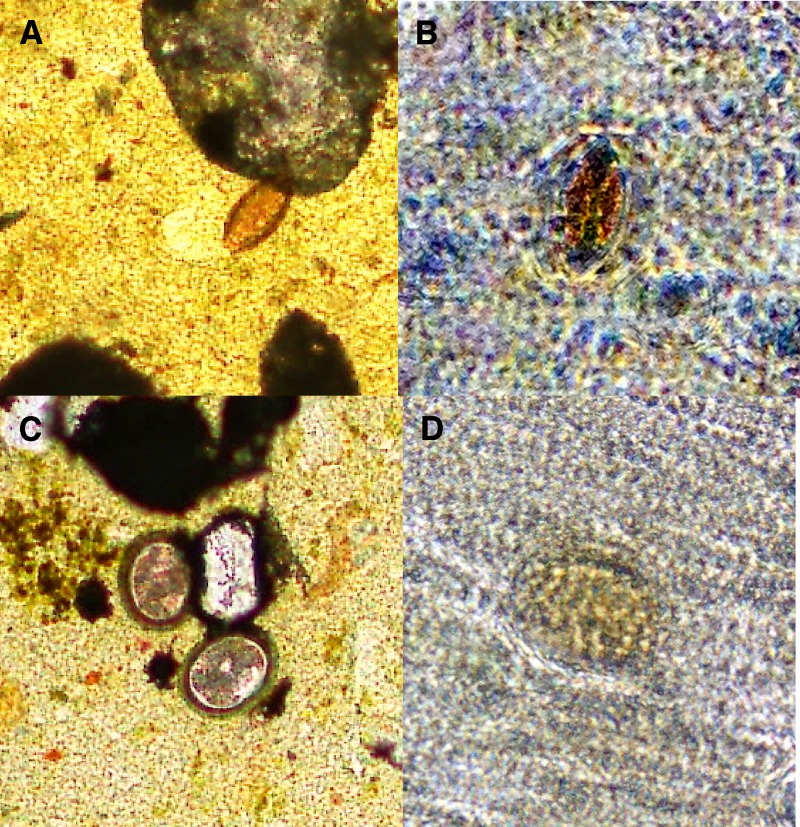
Figure 2.
Soil-transmitted helminth eggs visualized by conventional and mobile phone microscopy. T. trichiura is shown at 40× magnification by conventional microscopy in A and mobile phone microscopy in B. A. lumbricoides is shown at 40× magnification by conventional microscopy in C and mobile phone microscopy in D.
Discussion
We show the proof of concept of a first-generation mobile phone light microscope for the point-of-care diagnosis of soil-transmitted helminth infections among school-aged children on Pemba Island, where multiple species of helminth infection are the norm rather than the exception.8 Compared with conventional light microscopy, our simple mobile phone microscope achieved only modest diagnostic yield, with the highest sensitivity observed for moderate or heavy A. lumbricoides infection. However, we feel that the mobile phone microscope holds promise as a novel point-of-care test for intestinal helminth diagnosis, because it is portable, easy to construct and use, and relatively inexpensive. More importantly, mobile phones have become ubiquitous worldwide.9 These affordable products are reliable in field conditions, and prices are continuously declining. At the time of publication, a used iPhone 4S sells for US$250 and will likely decline in price as newer models come on the market.
The following points are offered for consideration regarding the modest performance of this mobile phone microscope for the diagnosis of soil-transmitted helminth infections. First, the image quality of our microscope, which uses a 3-mm ball lens, is not as sharp compared with conventional microscopy. Smaller lenses have greater resolution but a smaller field of view, which was seen with 1-mm ball lenses used on mobile phone microscopes in laboratory settings.6 We traded off a higher resolution for a larger field of view by using a 3-mm ball lens, because we sought to examine the entire Kato–Katz thick smear for helminth eggs in a field setting where a clinician would want to know if eggs were present anywhere on the slide. Image quality also seems to be superior when using a device to mount mobile phones onto a conventional light microscope; however, such a contraption is unsuitable to serve as a portable point-of-care test.10 Although helminth eggs can reliably be visualized with our mobile phone microscope, it is often a challenge to appropriately speciate the infecting organism. In addition, the mobile phone microscope detected several false-positive eggs that were likely stool artifact because of the relative lack in image quality. T. trichiura eggs are smaller than the eggs of A. lumbricoides and were more difficult to detect with mobile phone microscopy, which was reflected by the lower sensitivity for this helminth species. Hookworm diagnosis performed especially poorly; however, it must be noted that hookworm eggs are rapidly overcleared when using the Kato–Katz method,11 and despite our efforts to analyze these specimens quickly, the rapid overclearing and relatively poor image quality made diagnosis difficult.
From a practical standpoint, the sensitivity for diagnosing any soil-transmitted helminth egg with the mobile phone microscope was close to 70%, and although it is not sensitive enough for immediate application, it is getting close to acceptable diagnostic characteristics. For example, if a clinical decision rests on whether a patient has a soil-transmitted helminth infection, seeing an egg, regardless of the species, should prompt clinicians to treat with an anthelmintic drug with a broad spectrum of activity. In addition, appropriate microbiologic confirmation should be sought to ensure that an appropriate anthelmintic drug is used and guide proper follow-up care if assessing for response to therapy. Albendazole will not have efficacy against Schistosoma mansoni and because it is administered in a single dose, only shows moderate efficacy against T. trichiura.12 Of note, different helminth species coexist in much of sub-Saharan Africa.2,13,14 Because S. mansoni eggs are considerably larger than soil-transmitted helminth eggs and have other distinctive morphological features (i.e., lateral spine in S. mansoni eggs), these eggs can be easily distinguished from each other based on our experience with mobile phone microscopy. The mobile phone microscope would likely be of clinical use when sensitivities reach or exceed 80%.
We opted to use a simple first-generation ball-lens mobile phone microscope because of ease of creation and use. This device can be easily assembled in less than 5 minutes at a cost of approximately US$15, making it attractive for use in resource-limited settings. However, there are several other cell phone microscopy methods in development that may provide better resolution. Next-generation technology, such as lens-free devices, may offer exceptional diagnostic yield15–17; however, these methods have not yet been tested in the field. Other light-microscope attachments to mobile phones can also generate superior image quality that will likely enhance diagnostic yield.18 These technologies will likely have the capabilities required to function as effective point-of-care tests in resource-constrained settings,9 but first, they require rigorous testing outside of controlled laboratory settings to ensure that they have useful diagnostic capabilities in the field. In addition, the digitization of images may aid in diagnoses by enabling clinicians to transfer photos to centralized locations for diagnostic support.10,19
Mobile phone diagnostic technology will likely contribute to the diagnosis of infections and non-infectious etiologies in resource-constrained settings. A first-generation mobile phone microscope using a ball lens had modest diagnostic yield for soil-transmitted helminth infections; however, newer technologies may further improve mobile phone diagnostic capabilities, but they require additional field testing in different epidemiologic settings.
Disclaimer: The funders (Medicor Foundation and SNSF) had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Financial support: This work was supported by The Medicor Foundation. J.K. is financially supported by the Swiss National Science Foundation (SNSF; projects PPOOA-114941 and PPOOP3_135170).
Authors' addresses: Isaac I. Bogoch, Divisions of Internal Medicine and Infectious Diseases, Toronto General Hospital, Toronto, Ontario, Canada, E-mail: isaac.bogoch@uhn.ca. Jason R. Andrews, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, E-mail: jandrews6@partners.org. Benjamin Speich, Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: benjamin.speich@unibas.ch. Jürg Utzinger, Department of Epidemiology and Public Health, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: juerg.utzinger@unibas.ch. Shaali M. Ame and Said M. Ali, Public Health Laboratory, Ivo de Carneri, Pemba Island, Zanzibar, United Republic of Tanzania, E-mails: shaaliame@yahoo.com and saidmali2003@yahoo.com. Jennifer Keiser, Department of Medical Parasitology and Infection Biology, Swiss Tropical and Public Health Institute, Basel, Switzerland, and University of Basel, Basel, Switzerland, E-mail: jennifer.keiser@unibas.ch.
References
- 1.Hotez PJ, Brindley PJ, Bethony JM, King CH, Pearce EJ, Jacobson J. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–1321. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, Hotez PJ. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–1532. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 3.Knopp S, Steinmann P, Keiser J, Utzinger J. Nematode infections: soil-transmitted helminths and Trichinella. Infect Dis Clin North Am. 2012;26:341–358. doi: 10.1016/j.idc.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in schistosomiasis mansoni. Rev Inst Med Trop São Paulo. 1972;14:397–400. [PubMed] [Google Scholar]
- 5.Knopp S, Mgeni AF, Khamis IS, Steinmann P, Stothard JR, Rollinson D, Marti H, Utzinger J. Diagnosis of soil-transmitted helminths in the era of preventive chemotherapy: effect of multiple stool sampling and use of different diagnostic techniques. PLoS Negl Trop Dis. 2008;2:e331. doi: 10.1371/journal.pntd.0000331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith ZJ, Chu K, Espenson AR, Rahimzadeh M, Gryshuk A, Molinaro M, Dwyre DM, Lane S, Matthews D, Wachsmann-Hogiu S. Cell-phone-based platform for biomedical device development and education applications. PLoS One. 2011;6:e17150. doi: 10.1371/journal.pone.0017150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Ser. 2002;912:1–57. [PubMed] [Google Scholar]
- 8.Speich B, Ame SM, Ali SM, Alles R, Hattendorf J, Utzinger J, Albonico M, Keiser J. Efficacy and safety of nitazoxanide, albendazole, and nitazoxanide-albendazole against Trichuris trichiura infection: a randomized controlled trial. PLoS Negl Trop Dis. 2012;6:e1685. doi: 10.1371/journal.pntd.0001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pai NP, Vadnais C, Denkinger C, Engel N, Pai M. Point-of-care testing for infectious diseases: diversity, complexity, and barriers in low- and middle-income countries. PLoS Med. 2012;9:e1001306. doi: 10.1371/journal.pmed.1001306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuijn CJ, Hoefman BJ, van Beijma H, Oskam L, Chevrollier N. Data and image transfer using mobile phones to strengthen microscopy-based diagnostic services in low and middle income country laboratories. PLoS One. 2011;6:e28348. doi: 10.1371/journal.pone.0028348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin LK, Beaver PC. Evaluation of Kato thick-smear technique for quantitative diagnosis of helminth infections. Am J Trop Med Hyg. 1968;17:382–391. doi: 10.4269/ajtmh.1968.17.382. [DOI] [PubMed] [Google Scholar]
- 12.Keiser J, Utzinger J. Efficacy of current drugs against soil-transmitted helminth infections: systematic review and meta-analysis. JAMA. 2008;299:1937–1948. doi: 10.1001/jama.299.16.1937. [DOI] [PubMed] [Google Scholar]
- 13.Hürlimann E, Schur N, Boutsika K, Stensgaard AS, Laserna de Himpsl M, Ziegelbauer K, Laizer N, Camenzind L, Di Pasquale A, Ekpo UF, Simoonga C, Mushinge G, Saarnak CF, Utzinger J, Kristensen TK, Vounatsou P. Toward an open-access global database for mapping, control, and surveillance of neglected tropical diseases. PLoS Negl Trop Dis. 2011;5:e1404. doi: 10.1371/journal.pntd.0001404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pullan RL, Brooker SJ. The global limits and population at risk of soil-transmitted helminth infections in 2010. Parasit Vectors. 2012;5:81. doi: 10.1186/1756-3305-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Isikman SO, Greenbaum A, Lee M, Bishara W, Mudanyali O, Su TW, Ozcan A. Lensfree computational microscopy tools for cell and tissue imaging at the point-of-care and in low-resource settings. Anal Cell Pathol (Amst) 2012;35:229–247. doi: 10.3233/ACP-2012-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee M, Yaglidere O, Ozcan A. Field-portable reflection and transmission microscopy based on lensless holography. Biomed Opt Express. 2011;2:2721–2730. doi: 10.1364/BOE.2.002721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenbaum A, Sikora U, Ozcan A. Field-portable wide-field microscopy of dense samples using multi-height pixel super-resolution based lensfree imaging. Lab Chip. 2012;12:1242–1245. doi: 10.1039/c2lc21072j. [DOI] [PubMed] [Google Scholar]
- 18.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS One. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linder E, Lundin M, Thors C, Lebbad M, Winiecka-Krusnell J, Helin H, Leiva B, Isola J, Lundin J. Web-based virtual microscopy for parasitology: a novel tool for education and quality assurance. PLoS Negl Trop Dis. 2008;2:e315. doi: 10.1371/journal.pntd.0000315. [DOI] [PMC free article] [PubMed] [Google Scholar]