Abstract
In this study, we evaluate the effect of participatory Ecohealth interventions on domestic reinfestation of the Chagas disease vector Triatoma dimidiata after village-wide suppression of the vector population using a residual insecticide. The study was conducted in the rural community of La Brea, Guatemala between 2002 and 2009 where vector infestation was analyzed within a spatial data framework based on entomological and socio-economic surveys of homesteads within the village. Participatory interventions focused on community awareness and low-cost home improvements using local materials to limit areas of refuge and alternative blood meals for the vector within the home, and potential shelter for the vector outside the home. As a result, domestic infestation was maintained at ≤ 3% and peridomestic infestation at ≤ 2% for 5 years beyond the last insecticide spraying, in sharp contrast to the rapid reinfestation experienced in earlier insecticide only interventions.
Introduction
Chagas disease, a parasitic infection caused by the protozoan Trypanosoma cruzi, remains a serious public health problem in many Latin American countries despite notable successes in vector control over the past two decades. The emergence of Chagas disease in regions where it was not previously known, its re-emergence in areas where control efforts have been in effect, the rapid reinfestation of insecticide-treated areas by the primary insect vectors (Triatominae), and declining control efforts because of budget limitations are of particular concern to long-term disease control.1 Endemic to Latin America, Chagas disease affects an estimated 8 million people in the Americas alone with ∼40,000 new infections each year.2 In Guatemala, 4 million children, women, and men that live in habitat suitable for the local vector, Triatoma dimidiata, are at risk.3 Control, however, has proven challenging in part because of the wide distribution, varied habitats, and adaptability of this vector.1,4 Spread of the disease is expected therefore to continue until effective vector control strategies can be implemented.
Traditional vector control has focused on eliminating the primary domestic insect vectors through intermittent spraying of residual insecticides. These measures, however, are costly and often only effective over a period of months. For example, in the Department of Jutiapa, an area in southeastern Guatemala with high infestation rates, multiple insecticide applications may be required each year to reduce infestation in villages where baseline T. dimidiata populations or reinfestation risk is high.5 Although Guatemala, like its neighbors, has realized significant progress in vector control through insecticide spraying, estimates of the annual incidence of Chagas disease remain among the highest in Central America, due largely to regional differences in the effectiveness of control strategies.3,5–9
In view of these health challenges, current global economic constraints, and the understanding gained through past control initiatives, interest in developing new or improved, sustainable, and cost effective long-term controls is increasing.10–12 Toward this end, Ecohealth approaches that integrate community participation, home improvements, and current ecological knowledge regarding the vector are being proposed as an alternative to, or in combination with, traditional insecticide controls. In this study, we evaluate the effectiveness of Ecohealth interventions to limit domestic reinfestation by the Chagas disease vector T. dimidiata after suppression of the village-wide vector population using traditional insecticide spraying. The study was conducted in the rural community of La Brea, Guatemala between 2002 and 2009 in a region where vector reinfestation has hindered vector control efforts.5,7,13 The success of the interventions was measured using standard entomological surveys before and after each intervention, entomological indices, and by assessing the spatial pattern of vector reinfestation within the village. Our analyses are based on public health metrics evaluated within a spatial data framework established using high spatial resolution satellite imagery and a geographic information system (GIS).14 Spatially explicit interpretation of these metrics was made in combination with information on socio-economic factors, housing quality, and domestic animals to assess observed vector infestation densities and distributions within the village over time. This article is a companion work to Pellecer and others,15 which analyzed blood meals in a different village in the same region.
Materials and Methods
Study site.
Located in the department of Jutiapa in the highlands region of Guatemala, La Brea (14.331° N, 90.063° W) is typical of the many small, rural villages throughout the region (Figure 1). Housing within the village ranges from adobe construction with few amenities to cement block houses with indoor conveniences and electrical appliances. Homes within the village did not have screens on the windows or doors during the study period. The surrounding terrain is rolling, reflecting a patchwork of forested and agricultural lands. The climate is temperate and dry with most precipitation falling between May and September.16
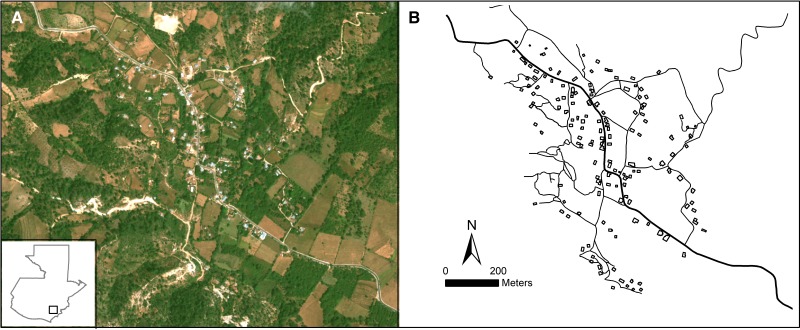
Figure 1.
The La Brea, Guatemala study area. (A) GeoEye satellite image shown as a natural color composite with the study area location shown in the inset map of Guatemala. (B) Houses and roads were digitized from the imagery and saved as separate geographic information system (GIS) data layers. The entomological and socio-economic survey data were then joined with the housing data layer using the survey global positioning system (GPS) coordinates.
Interventions.
A triatomine vector survey was conducted in La Brea in 2001 under the auspices of the National Vector Control Program administered by Guatemala's Ministry of Health (MoH) (Table 1) and infested homesteads (∼42% of those surveyed) sprayed with deltamethrin by MoH officials. All domestic and peridomestic habitats were then sprayed in March and September of 2003 and again in February 2005 in an effort to significantly reduce or eliminate the vector within the village.
Table 1.
Entomological survey results for La Brea
| Year | No. homesteads surveyed | No. homesteads not surveyed | No. homesteads infested (%) | No. bugs/infested domestic habitat (mean ± SD) | No. bugs/infested peridomestic habitat (mean ± SD) | Total no. domestic bugs (range/homestead) | Total no. peridomestic bugs (range/homestead) |
|---|---|---|---|---|---|---|---|
| 2001 | 98 | 18 | 41 (42%) | 3.2 ± 2.5 | 17.7 ± 34.6 | 101 (1–9) | 173 (2–107) |
| 2002 | 122 | 22 | 31 (25%) | 3.7 ± 4.1 | 9.0 ± 8.5 | 68 (1–16) | 108 (1–26) |
| 2004 | 132 | 10 | 9 (6.8%) | 1.4 ± 0.8 | 1.0 ± 0 | 10 (1–3) | 2 (1) |
| 2006 | 128 | 11 | 4 (3.1%) | 2.0 | 14.0 ± 17.6 | 2 (2) | 42 (1–37) |
| 2008 | 152 | 10 | 5 (3.3%) | 3.0 ± 1.6 | 48 | 12 (1–5) | 48 (48) |
| 2009 | 152 | 14 | 7 (4.6%) | 4.0 ± 5.6 | 9.0 ± 7.5 | 20 (1–14) | 27 (1–16) |
Beginning in 2005, interventions also focused on community education regarding Chagas disease and on making houses resistant to reinfestation by T. dimidiata through hygienic and targeted home improvements.17,18 Interventions in 2008 focused on limiting vector access to alternative blood meal sources within the home and on limiting vector survival in peridomestic habitats.18 Home improvements included plastering of interior walls (2005) and replacing dirt floors with a cement-like floor material made of volcanic ash and lime (2008) to eliminate potential areas of refuge for the vector within the home, as well as limit vector access to soil particles often used for camouflage.19 Coordinated efforts in 2008 also focused on removing chicken coops from within the home and on replacing traditional adobe-constructed chicken coops with open-mesh wire coops to further limit vector refuge. Interventions were performed only upon approval of the head of household and were facilitated by local community leaders who, among other actions, coordinated access to building materials.
Survey data.
Five entomological and socio-economic surveys were completed over the 2002–2009 study period (Table 1) to evaluate the effectiveness of the combined insecticide spraying and Ecohealth intervention program and provide insight into the environmental and socio-economic factors associated with vector reinfestation.17,18 Five individuals from the Universidad de San Carlos and five health officials from the Guatemalan Chagas Program administered the questionnaire and performed the entomological surveys. All homesteads within the village were assigned a house number and the geographic coordinates of each home were recorded using a handheld global positioning system (GPS) receiver. Homesteads where the residents granted permission (85–95%) were then surveyed and the information recorded on standardized forms.
The entomological survey involved two individuals, one from the MoH and one from the Universidad de San Carlos, searching for T. dimidiata over a 30-minute period inside the house (intradomiciliary habitat) and an additional 30 minutes in the surrounding peridomestic habitat. Microenvironments potentially suitable as triatomine shelter (e.g., cracks in walls, behind furniture, in chicken coops, and in rock or woodpiles) were carefully examined using flashlights. All specimens collected were placed in labeled vials and returned to the Universidad de San Carlos, Guatemala where the life stage and number of vectors were recorded.
Upon completion of the entomological survey, housing and socio-economic information hypothesized to be linked to risk of infestation was recorded. In 2002 only basic information on house construction materials and number of residents was collected. From 2004 on, however, information regarding interior wall, floor and roof construction, hygiene within the home, socio-economic status, and domesticated animals within the house were also collected. Data on wall and floor condition, hygiene, and the presence of electric appliances were then used to categorize the risk (high, medium, or low) of triatomine infestation of each house.17 Houses having plastered walls, cement floors, good hygiene, and multiple electric appliances were characterized as low risk houses. Moderate risk houses showed some deterioration of the plaster, moderate hygiene, and fewer electric appliances. High risk houses had at least one interior wall without plaster or plaster in poor condition (e.g., peeling, cracked, or having open cavities), at least one room with dirt floors, poor hygiene within the home (e.g., piles of clothes, stored grain, boxes, wall hangings), few to no electric appliances, and chickens living within the home.
Imagery and derived GIS data layers.
High spatial resolution GeoEye satellite imagery (0.5 m panchromatic band and 2.0 m multispectral bands) centered over the village of La Brea was acquired December 24, 2009 (Figure 1). The data were then visually enhanced using a Brovey transform resolution merge to yield a 0.5 m multispectral image (Supplemental Figure S1. Digital integration of the panchromatic and multispectral data allowed detailed mapping of structures at a spatial resolution not possible with panchromatic or multispectral data alone. All structures, roadways, and drainages within or immediately adjacent to the village were screen digitized from the Brovey pan-sharpened image and saved as individual GIS data layers using the same map projection and datum as the imagery (UTM zone 15N NAD27). The GPS coordinate data from the field survey were then joined with the satellite-derived structures data layer to spatially combine the survey attribute data with each house location.
Statistical analyses.
Triatoma dimidiata infestation and crowding indices derived from the entomological surveys were examined before and after interventions and over time to assess the efficacy of the interventions. The infestation index was defined as the fraction of houses surveyed that tested positive for T. dimidiata, whereas crowding density was defined as the average number of vectors per infestation.20 Descriptive statistics and hypothesis testing were performed using JMP (ver. 9, SAS Institute Inc., Cary, NC). Spatial analyses were performed using ArcGIS ver. 10.0 (ESRI Inc., Redlands, CA).
Geospatial analyses.
Measures of the spatial distribution of vector infestation within the village were standardized using the area enclosed by the village boundary, operationally defined as lying 50 m beyond that required to encompass all homesteads within the village. A homestead was defined as including both the domestic and associated peridomestic habitats. The observed spatial distribution of all homesteads and of only T. dimidiata-infested homesteads before and after the insecticide interventions (2002 versus 2006–2009) were compared with a hypothesized random distribution of those homesteads over the same area using the ArcGIS AVERAGE NEAREST NEIGHBOR function (ArcGIS, ver. 10.0, ESRI Inc.). To assess whether infested homesteads were concentrated along the village boundary and thus near potential sylvan source areas, we used a bootstrap approach to compare the number of infested homesteads observed along the perimeter to the number that would be realized if the infestations were randomly distributed among all homesteads in the village.21 This was accomplished by repeatedly drawing (N = 50) a random sample from all homesteads with replacement equal in size to the number of infested homesteads. The observed number of infested homesteads along the perimeter was then tested against the mean and confidence interval of the randomly allocated empirical sample distribution (z-score). A similar approach was also used to evaluate whether infestations were concentrated along the main road traversing the village to suggest passive transport as a possible factor influencing infestation. A sampled homestead was defined on the village perimeter if it were located within 51 m of the village boundary as defined previously or along the main road if it were located ≤ 30 m from that road. Distances between domiciles and the boundary or main road, respectively, were computed using the ArcGIS NEAR command.
The spatial distribution of infested homesteads in 2002 was evaluated using Hot Spot Analysis based on the Getis-Ord Gi* statistic to identify neighborhoods centered around each homestead having significantly higher or lower vector abundances than the village as a whole.14 We defined the radius of the neighborhoods as the average distance over which the vector abundances were spatially autocorrelated as determined using semi-variogram analysis coded in MATLAB R2010a (ver. 2010b, The MathWorks, Natick, MA).22
Semi-variance, γ(h), is defined as the spatial dissimilarity between a parameter measured at two points separated by distance, h:
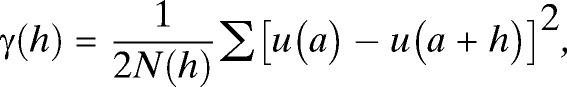 |
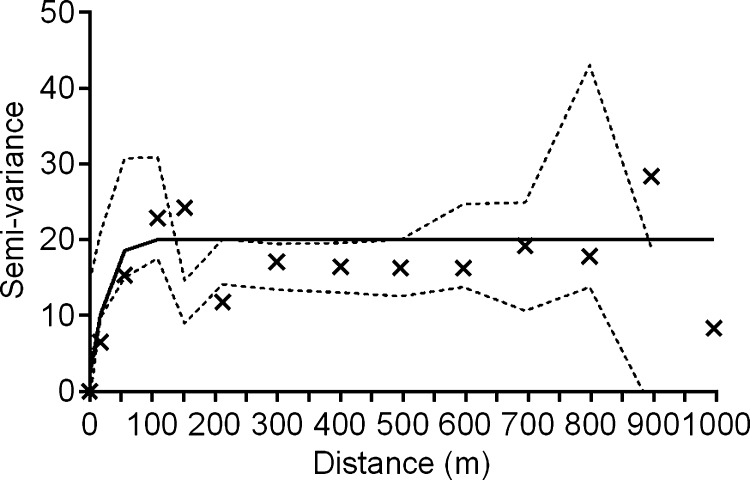
where N(h) is the number of data pairs separated by the distance, h, and u(a) and u(a + h) represent the number of T. dimidiata collected at location, a, at some distance, a + h, away. These data are assembled into bins defined by the ranges of separation distance between homestead pairs (e.g., in Figure 2 all pairs of homesteads separated by distances between 0 and 50 m are included in the first bin). The corresponding average variance associated with the number of T. dimidiata collected per homestead is calculated (“X” in Figure 2) creating an experimental semi-variogram. The model that best fits the experimental semi-variogram describes the spatial structure of the data using three parameters—the nugget, sill, and range.22 Discontinuity at the origin of the plot, i.e., the nugget, represents measurement error or the general variability within the measured parameter that is not spatially dependent. For the data shown in Figure 2, the nugget is 2.09. The semi-variogram range (also referred to as the range of decorrelation) defines the distance beyond which the variable (in our case, vector abundance per homestead) is no longer correlated. In this study vector abundances were spatially autocorrelated over a range of decorrelation of about 100 m. The sill represents the average variance in vector abundance for homesteads separated by distances greater than the range of decorrelation.
Figure 2.
Best-fit semi-variogram describing the spatial correlation between pairs of homesteads separated by distance. The average variance for each group of binned data is represented by an “X”. Each bin summarizes 369 pairwise comparisons of homesteads weighted by Triatoma dimidiata abundance for 2002 in La Brea, Guatemala with a 95% confidence envelope (dashed lines). After a range of decorrelation (∼100 m in this study) the data are no longer spatially correlated.
Results
Pre-intervention.
When surveyed in 2002, nearly 1 in 4 homesteads (31 of 122 surveyed) in the village of La Brea were infested by the Chagas vector T. dimidiata (Figure 3 ) despite that all known infested homesteads (42% of all homesteads) had been sprayed the previous year (Table 1). In all, 62% of the households surveyed in 2002 were classified as being at “high risk” of infestation based on the housing quality and socio-economic information collected and, in fact, all domestic infestations observed were in adobe houses lacking well-maintained interior plaster walls and cement floors. Vector crowding densities in peridomestic habitats were significantly higher than domestic habitats (P < 0.02; t test), averaging 9.0 ± 2.4 (SE) and 3.8 ± 1.0 (SE) vectors per infested homestead, respectively (Figure 4 ).
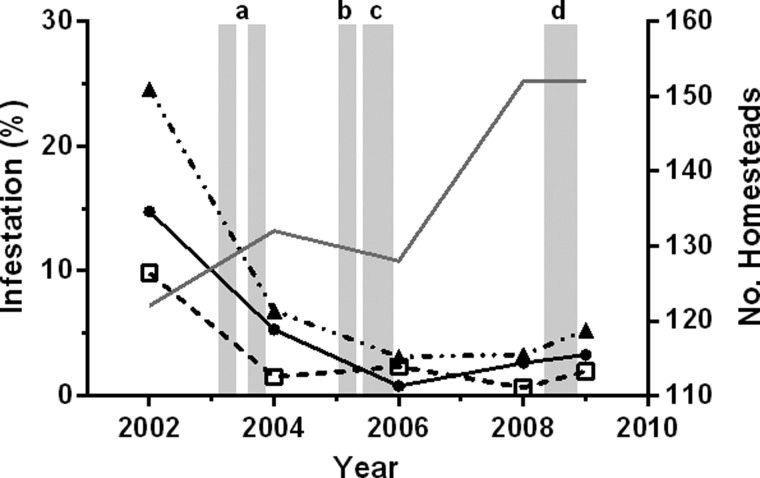
Figure 3.
Triatoma dimidiata infestation in La Brea, Guatemala before and after scheduled interventions. Data represent the fraction of houses surveyed within which the vector was found, summarized by habitat, i.e., domestic (•), peridomestic (□), and total (▴). The overlying shaded bars represent the scheduled interventions: a) insecticide applications (March and September), b) insecticide application (February), c) wall plastering, and d) cement-like flooring, exclusion of chicken coops from houses, and construction of coops using open-mesh wire. Also shown is the number of homesteads surveyed each survey year (grey solid line).
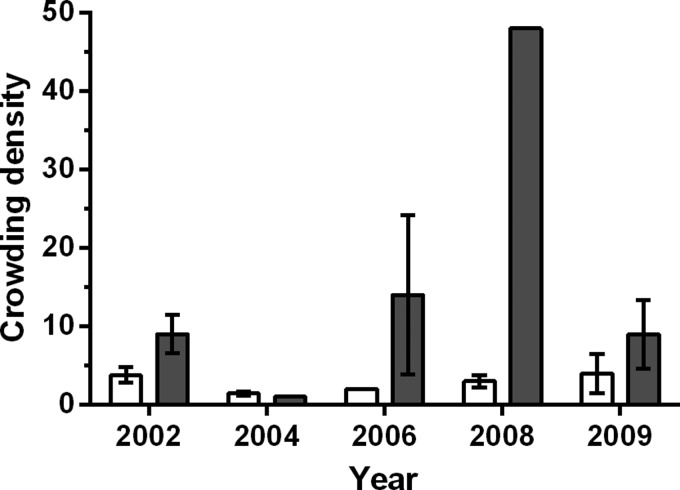
Figure 4.
Crowding densities (average vector abundance per infested homestead) for domestic (open bar) and peridomestic (shaded bar) Triatoma dimidiata populations in La Brea, Guatemala. The data shown represent the mean ± 1 SE.
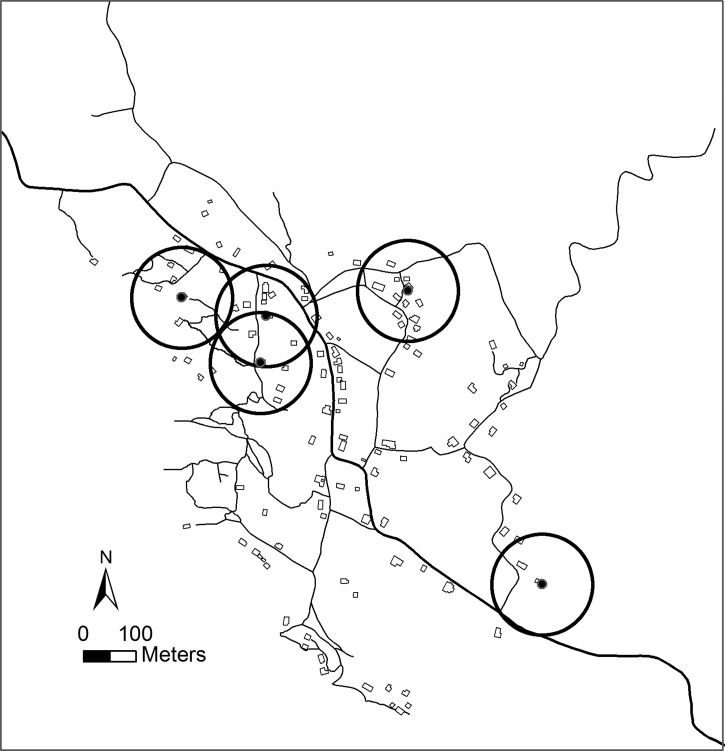
Although the spatial distribution of all houses within the village in 2002 was clustered, i.e., located statistically closer to one another than expected by chance alone (P < 0.0001; AVERAGE NEAREST NEIGHBOR), there was no evidence of clustering of T. dimidiata-infested homesteads (N = 31), i.e., the null hypothesis that infested homesteads were randomly distributed throughout the village could not be rejected (P = 0.39; Figure 5 ). The average distance between all homesteads was 32.8 m, whereas the distance between infested homesteads averaged 67.2 m. Analysis of vector abundances within neighborhoods using the Getis-Ord Gi* statistic, however, identified five hot spots that together accounted for 52% of all vectors sampled in 2002 (P < 0.05; Figure 6 ). In each case, the identified neighborhood was centered on a single homestead having an exceptionally high vector count, three representing peridomestic infestations (chicken coops) and two representing high risk domestic infestations. No homestead was observed in which the domestic and peridomestic habitats were simultaneously infested to suggest clustering within a homestead.
Figure 5.
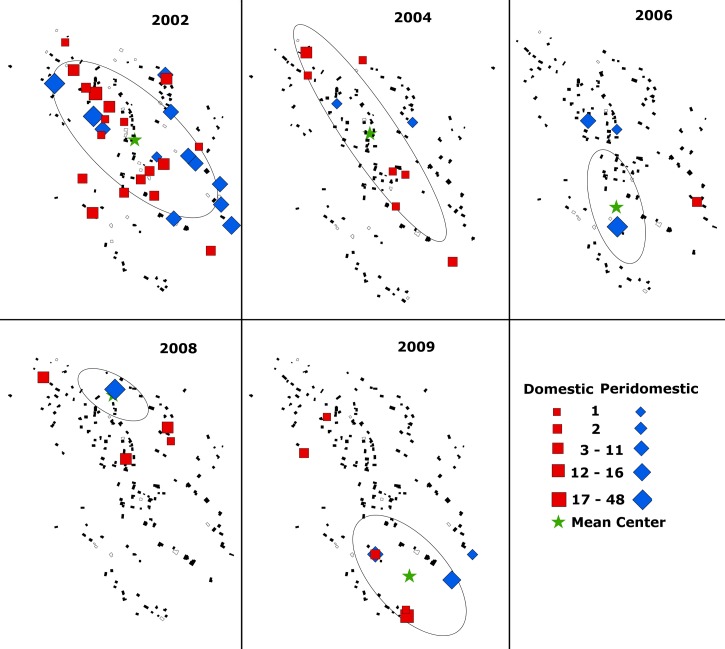
Spatial and temporal distribution of Triatoma dimidiata infestation within La Brea, Guatemala by survey year (2002–2009). Data represent vector densities in peridomestic (blue diamond) and domestic (red square) habitats. Symbols are sized in proportion to the vector density observed. Non-infested homesteads (black polygons) and homesteads that were not surveyed (grey polygons) are also represented. The geographic center of vector abundance (mean ± 1 SD) for each year is identified by a star and ellipse.
Figure 6.
Hot Spot analysis of Triatoma dimidiata infested homesteads in La Brea, Guatemala in 2002. Based on the Getis-Ord Gi* statistic, Hot Spots represent 100 m radius neighborhoods (circles) having significantly higher vector crowding densities than the village as a whole. The size (diameter) of the neighborhoods was chosen based on the range of decorrelation from the semi-variogram analysis (Figure 2).
Of the 31 infested homesteads observed at this time, 13 (42%) were located along the village perimeter. Our bootstrap analysis, however, suggested that nearly 50% of infested homesteads (15.4 ± 2.3) could be located along the perimeter by chance alone. As a consequence we cannot reject the null hypothesis (P = 0.16), that infested homesteads were not concentrated near potential sylvan source areas along the village periphery (Figure 5). We also observed seven infested homesteads within a 30 m corridor along the length of the main road traversing the village. However, if all infested homesteads were randomly distributed throughout the village ∼8.7 ± 2.4 would be expected to be located along the main road. The null hypothesis that infested homesteads were not concentrated along the main road was therefore not rejected (P = 0.24). In summary, therefore, we found no evidence of reinfestation due either to passive transport into the village along the main road or from sylvan sources beyond the village periphery.
Post-intervention.
The combined insecticide and Ecohealth interventions successfully reduced T. dimidiata homestead infestation in La Brea from 25% to < 5% and maintained it at this level over the 5-year period of study that followed the most recent insecticide application (Figure 3). Domestic infestation dropped from 14.8% to 5.3% in response to the two village-wide insecticide applications in 2003, and then to < 0.8% after the insecticide treatment in early 2005 and implementation of the first phase of the participatory interventions (interior wall plastering) through the remainder of 2005. Domestic infestation thereafter rose to 3.3% by 2009, but remained well within the target level of < 5% set by the National Chagas Disease Control Project.6 Peridomestic infestation similarly decreased from 9.8% to 1.5% after insecticide treatments in 2003 and then held steady near or below 2% for the remainder of the study.
Vector crowding densities in response to insecticide interventions in 2003 fell from a mean of 3.8 to 1.4 in domestic habitats and from 9.0 to 1.0 in peridomestic habitats (Figure 4). However, crowding densities soon began to rebound, reaching near pre-intervention levels in peridomestic habitats by 2006 and in domestic habitats by 2008. The large peridomestic crowding densities observed in 2006 (N = 3) and 2008 (N = 1) suggests that the vector is capable of rapidly colonizing when conditions are favorable, although the small number of homesteads infested over this period limits statistical analyses. We note, however, although no change in domestic or peridomestic infestation (Figure 3) was detected after the 2008 interventions (i.e., cementing floors, removing chicken coops from houses, wire construction of coops), the drop in the peridomestic vector crowding density in 2009 (N = 3) is consistent with expectations (Figure 4).
Viewed in combination with observed infestation levels, these results illustrate the effectiveness of the combined traditional insecticide and participatory interventions to make houses resistant to reinfestation and hold the village-wide T. dimidiata population in check over the 5-year period that followed the insecticide interventions. For example, the estimated village-wide vector population in 2009 (i.e. crowding density × infestation index × no. of homesteads) was only 26% of the pre-intervention (2002) population.
Spatial and temporal patterns of reinfestation.
Figure 5 shows the spatial and temporal pattern of T. dimidiata infestation within the village before and after the 2003 and 2005 insecticide interventions. Because of the limited number of reinfestations we combined the domestic and peridomestic observations between 2006 and 2009 to obtain a sufficient number of observations (N = 15) to test the spatial distribution of infestation within the village. Although based on a limited number of infestations, we saw no evidence of clustering of infested homesteads within the village after the insecticide interventions (i.e., the null hypothesis that infested homesteads were randomly distributed throughout the village was not rejected) to suggest the presence of one or more domestic foci from which reinfestation might have spread. Neither was there any evidence of a concentration of reinfested homesteads (8 of 15 total; P = 0.48) along the village perimeter or along the main road (4 of 15 total; P = 0.36) to suggest a measurable sylvan source or a passive source associated with traffic through the village. No repeat domestic infestation was observed between consecutive surveys over this period, although four non-consecutive repeat infestations were observed over the entire study period (2002–2009); three before the February 2005 insecticide spraying, and the fourth only after a 5-year interval (2005–2009).
Homestead improvements.
Nearly 80% of houses within the village of La Brea were improved to various degrees over the study period. Overall, the proportion of houses considered to be at “high risk” to T. dimidiata infestation was reduced from 62% of all houses surveyed in 2002 to 15% in 2009, despite a 25% increase in the number of houses (Table 2). An additional 40 houses were abandoned or razed and only nine occupied houses saw no improvement. By 2009, 56% of houses had cement-like floors and all homesteads with chickens (118 of 152 households in 2009) had outdoor coops, most of which were of wire construction. At least 20% of homesteads were improved on the residents own initiative; the remainder were completed under the auspices of this study.
Table 2.
Assigned household risk of Triatoma dimidiata infestation based on the quality of home construction and other socio-economic factors before and after scheduled interventions17
| Year | Risk of infestation (% of houses surveyed) | ||
|---|---|---|---|
| High | Moderate | Low | |
| 2002 | 62.0 | 23.0 | 15.0 |
| 2009 | 15.2 | 47.8 | 37.0 |
Discussion
The results of this study show that participatory Ecohealth interventions after suppression of the vector population successfully held domestic infestation by T. dimidiata to ≤ 3% and peridomestic infestation to ≤ 2% over a 5-year study period in a rural community in western Jutiapa where reinfestation had previously frustrated vector control efforts. The extended period over which the vector was controlled contrasts sharply to early control efforts using insecticides in La Brea and elsewhere in Jutiapa.5–7,13 Although a formal control was not present in this study, results from numerous studies have shown that residual insecticides alone cannot curtail reinfestation long term.5–9,18,19,23 The time scale over which residual insecticides are effective varies regionally but is typically on the order of weeks in peridomestic environments and months in domestic habitats. In La Brea, annual interventions were thought necessary because of rapid reinfestation following treatment. It would appear highly unlikely, therefore, that the low rates of T. dimidiata reinfestation observed from 2005 to 2009 after earlier insecticide applications was caused by the residual effects of the insecticide or to chance alone. Instead, we argue that these results suggest that a comprehensive vector control program combining traditional insecticide and participatory interventions appropriately tailored for the locality offers significant promise to not only limit vector-human contact, and thus the potential transmission of T. cruzi, but also reduce the costs and health risks associated with frequent insecticide applications.24
The limited reinfestation of both domestic and peridomestic habitats over the period of study after the insecticide interventions in La Brea is attributable not only to a low village-wide vector population, limited immigration from nearby sylvan habitats, and the slow reproductive rate (6–12 months) of T. dimidiata, but also to increased community awareness of Chagas disease and its vector and on community participation to render domestic and peridomestic environments more resistant to reinfestation. The latter was implemented through low-cost improvements in home construction to limit areas of refuge for the vector within the home, limit vector access to alternative blood meal sources within the home, and limit areas of refuge for the vector in peridomestic habitats. These improvements were completed by the village residents using local materials or materials (chicken wire) made available through efforts coordinated by local community leaders and health officials. Together, the house improvements cost about US$ 30/household and are expected to remain effective 3–5 years or more.18 The cost of insecticide spraying is about US$ 8.00/home,18 although multiple applications are often needed to effectively suppress the vector population.5 The total cost of vector control, therefore, will vary regionally depending upon the approach, the efficacy of the interventions, the period over which they are effective, and the projected period of control.
The higher infestation in domestic habitats compared with peridomestic habitats in four of the five surveys conducted before and after the initial home improvements (wall plaster and cement floors) may be attributable in part to the continued presence of chicken coops within houses until late 2008 when all remaining coops were removed. Alternatively, the rate at which T. dimidiata is reintroduced to domestic habitats may be greater than for peridomestic habitats. Various potential pathways of vector transport from sylvatic or peridomestic environments into the home have been suggested, including by domestic (e.g., chickens and dogs) or synanthropic (e.g., mice) animals or by the village residents themselves through gathering of firewood or accidentally in daily travels to and from nearby infested areas.8,25,26 The increased use of electrical lighting in rural communities also raises the possibility that the vector may be drawn not only to the village but also to homes when the lights are in use.9,27,28 Vector survival in peridomestic environments may also be lower than in domestic habitats. The diversity of potential blood meals and microhabitats offering shelter to the vector in peridomestic environments, however, suggest this is unlikely.23
An important component of any vector control program is continual surveillance. Spatial analysis of vector abundance in such efforts can provide important insight into vector distributions and abundance hot spots, reinfestation source areas, and change over time to complement traditional multivariate analyses. For example in this study, we identified five hot spots in vector abundance in 2002 that accounted for more than half of all vectors observed. The ability to identify neighborhoods having significantly higher vector abundances than the village as a whole could potentially aid vector control agencies to more effectively target intervention efforts to improve efficacy or limit costs. Although our results provide insight into Chagas disease risk, the ability to assess the spatial distribution of vector abundance in concert with information on human blood meals would allow researchers to also identify potential source areas and pathways of reinfestation and more directly assess disease risk to humans.23
Future efforts.
Understanding the ecological, socio-economic, and genetic factors most important to the transmission of Chagas disease is challenging because disease transmission is influenced by a complex interplay of ecological and evolutionary processes. Based on the results of this study, however, we recommend future interventions focus on long-term control and targeted Ecohealth interventions that limit domestic and peridomestic reinfestation after suppression of the vector by traditional methods. Approaches that combine insecticide use and community-based participatory approaches are expected to offer long-term benefits, although many uncertainties remain. For example, improved understanding of sylvatic, peridomestic, and domestic population interactions and dynamics is needed, particularly as it relates to vector immigration from external sources and vector survival in domestic and peridomestic environments. We also recommend that future surveillance and analyses efforts be conducted within a spatial framework to aid both identifying risk factors and targeting specific sites at both regional and village scales for subsequent intervention.
Supplementary Material
ACKNOWLEDGMENTS
We thank the residents of La Brea, Laboratorio de Entomología Aplicada y Parasitología (LENAP) de la Universidad de San Carlos de Guatemala personnel, and Guatemala's Ministry of Health personnel for their crucial contribution and collaboration in this study. We are grateful for the comments received from our anonymous reviewers and the assistance provided by Gerald P. Livingston that greatly improved this manuscript.
Footnotes
Financial support: This research was funded by WHO TDR (2001–2002), IDRC (2004–2009) and NETROPICA (2008–2009), and by the National Institutes of Health (USA) Grant 1R15 A1079672-01A1.
Authors' addresses: David E. Lucero, Leslie A. Morrissey, Donna M. Rizzo, and Lori Stevens, University of Vermont, Burlington, VT, E-mails: david.lucero@uvm.edu, leslie.morrissey@uvm.edu, drizzo@cems.uvm.edu, and lori.stevens@uvm.edu. Antonieta Rodas, Roberto Garnica, Dulce M. Bustamante, and Maria Carlota Monroy, Universidad de San Carlos, Ciudad de Guatemala, Guatemala, E-mails: antonieta55@yahoo.com, rogarnika@hotmail.com, dulce_mariab@hotmail.com, and mcarlotamonroy@gmail.com.
References
- 1.Schofield C. Challenges of Chagas Disease Vector Control in Central America. Global Collaboration for Development of Pesticides for Public Health; World Health Organization: 2000. [Google Scholar]
- 2.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa J, Cordon-Rosales C, Juarez J, Itzep C, Nonami T. Impact of residual spraying on Rhodnius prolixus and Triatoma dimidiata in the department of Zacapa in Guatemala. Mem Inst Oswaldo Cruz. 2003;98:277–281. doi: 10.1590/s0074-02762003000200019. [DOI] [PubMed] [Google Scholar]
- 4.Coura JR, Vinas PA. Chagas disease: a new worldwide challenge. Nature. 2010;465:S6–S7. doi: 10.1038/nature09221. [DOI] [PubMed] [Google Scholar]
- 5.Hashimoto K, Cordon-Rosales C, Trampe R, Kawabata M. Impact of single and multiple residual sprayings of pyrethroid insecticides against Triatoma dimidiata (Reduviiade; Triatominae), the principal vector of Chagas disease in Jutiapa, Guatemala. Am J Trop Med Hyg. 2006;75:226–230. [PubMed] [Google Scholar]
- 6.Manne J, Nakagawa J, Yamagata Y, Goehler A, Brownstein JS, Castro MC. Triatomine infestation in Guatemala: spatial assessment after two rounds of vector control. Am J Trop Med Hyg. 2012;86:446–454. doi: 10.4269/ajtmh.2012.11-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nakagawa J, Hashimoto K, Cordón-Rosales C, Abraham Juárez J, Trampe R, Marroquín Marroquín L. The impact of vector control on Triatoma dimidiata in the Guatemalan department of Jutiapa. Ann Trop Med Parasitol. 2003;97:288–297. doi: 10.1179/000349803235001895. [DOI] [PubMed] [Google Scholar]
- 8.Dumonteil E, Ruiz-Pina H, Rodriguez-Felix E, Barrera-Perez M, Ramirez-Sierra MJ, Rabinovich JE, Menu F. Re-infestation of houses by Triatoma dimidiata after intra-domicile insecticide application in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2004;99:253–256. doi: 10.1590/s0074-02762004000300002. [DOI] [PubMed] [Google Scholar]
- 9.Tabaru Y, Monroy MC, Rodas A, Mejia M, Rosales R. Chemical control of Triatoma dimidiata and Rhodnius prolixus (Reduviidae: Triatominae), the principal vectors of Chagas' disease in Guatemala. Medical Entomology and Zoology. 1998;49:6. [Google Scholar]
- 10.Charron DF. Ecosystem approaches to health for a global sustainability agenda. EcoHealth. 2012;9:256–266. doi: 10.1007/s10393-012-0791-5. [DOI] [PubMed] [Google Scholar]
- 11.Dumonteil E, Gourbiere S, Barrera-Perez M, Rodriguez-Felix E, Ruiz-Pina H, Banos-Lopez O, Ramirez-Sierra MJ, Menu F, Rabinovich JE. Geographic distribution of Triatoma dimidiata and transmission dynamics of Trypanosoma cruzi in the Yucatan peninsula of Mexico. Am J Trop Med Hyg. 2002;67:176–183. doi: 10.4269/ajtmh.2002.67.176. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovich JE, Kitron UD, Obed Y, Yoshioka M, Gottdenker N, Chaves LF. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae) Mem Inst Oswaldo Cruz. 2011;106:479–494. doi: 10.1590/s0074-02762011000400016. [DOI] [PubMed] [Google Scholar]
- 13.Tabaru Y, Monroy MC, Rodas A, Mejia M, Rosales R. The geographical distribution of vectors of Chagas' disease and populations at risk of infection in Guatemala. Medical Entomology and Zoology. 1999;50:9–17. [Google Scholar]
- 14.Getis A, Ord JK. Local spatial statistics: an overview. In: Longley P, Batty M, editors. Spatial Analysis: Modeling in a GIS Environment. Cambridge, UK: Geoinformation International; 1996. pp. 261–277. [Google Scholar]
- 15.Pellecer M, Dorn P, Bustamante D, Rodas A, Monroy M. Vector blood meals are an early indicator of the effectiveness of the Ecohealth approach in halting Chagas transmission in Guatemala. Am J Trop Med Hyg. 2013;88:638–644. doi: 10.4269/ajtmh.12-0458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Weather Online Jutiapa Weather, Guatemala Weather Averages. 2012. http://www.worldweatheronline.com/ Available at. Accessed December 3, 2012.
- 17.Bustamante DM, Monroy C, Pineda S, Rodas A, Castro X, Ayala V, Quiñónes J, Moguel B, Trampe R. Risk factors for intradomiciliary infestation by the Chagas disease vector Triatoma dimidiata in Jutiapa, Guatemala. Cad Saude Publica. 2009;25:S83–S92. doi: 10.1590/s0102-311x2009001300008. [DOI] [PubMed] [Google Scholar]
- 18.Monroy MC, Bustamante DM, Pineda S, Rodas A, Castro X, Ayala V, Quinones J, Moguel B. House improvements and community participation in the control of Triatoma dimidiata re-infestation in Jutiapa, Guatemala. Cad Saude Publica. 2009;25((Suppl 1)):S168–S178. doi: 10.1590/s0102-311x2009001300016. [DOI] [PubMed] [Google Scholar]
- 19.Zeledon R, Vargas LG. The role of dirt floors and of firewood in rural dwellings in the epidemiology of Chagas' disease in Costa Rica. Am J Trop Med Hyg. 1984;33:232–235. doi: 10.4269/ajtmh.1984.33.232. [DOI] [PubMed] [Google Scholar]
- 20.World Health Organization Control of Chagas Disease. 1991;811:1–95. Report of a WHO Expert Committee. WHO Technical Report Series. [PubMed] [Google Scholar]
- 21.Mooney CZ, Duval RD. Bootstrapping: A Nonparametric Approach to Statistical Inference. Newbury Park, CA: Sage; 1993. Sage University Paper series on Quantitative Applications in the Social Sciences, 07-095. [Google Scholar]
- 22.Isaaks EH, Srivastava RM. Applied Geostatistics. New York: Oxford University Press; 1989. [Google Scholar]
- 23.Cecere MC, Vazquez-Prokopec GM, Gurtler RE, Kitron U. Reinfestation sources for Chagas disease vector, Triatoma infestans, Argentina. Emerg Infect Dis. 2006;12:1096–1102. doi: 10.3201/eid1207.051445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villarini M, Moretti M, Pasquini R, Scassellati-Sforzolini G, Fatigoni C, Marcarelli M, Monarca S, Rodriguez AV. In vitro genotoxic effects of the insecticide deltamethrin in human peripheral blood leukocytes: DNA damage (‘comet’ assay) in relation to the induction of sister-chromatid exchanges and micronuclei. Toxicology. 1998;130:129–139. doi: 10.1016/s0300-483x(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 25.Coutinho CF, Souza-Santos R, Lima MM. Combining geospatial analysis and exploratory study of triatomine ecology to evaluate the risk of Chagas disease in a rural locality. Acta Trop. 2012;121:30–33. doi: 10.1016/j.actatropica.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Vazquez-Prokopec GM, Cecere MC, Kitron U, Gurtler RE. Environmental and demographic factors determining the spatial distribution of Triatoma guasayana in peridomestic and semi-sylvatic habitats of rural northwestern Argentina. Med Vet Entomol. 2008;22:273–282. doi: 10.1111/j.1365-2915.2008.00746.x. [DOI] [PubMed] [Google Scholar]
- 27.Pacheco-Tucuch FS, Ramirez-Sierra MJ, Gourbiere S, Dumonteil E. Public street lights increase house infestation by the Chagas Disease Vector Triatoma dimidiata. PLoS ONE. 2012;7:e36207. doi: 10.1371/journal.pone.0036207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monroy MC, Bustamante DM, Rodas AG, Enriquez ME, Rosales RG. Habitats, dispersion and invasion of sylvatic Triatoma dimidiata (Hemiptera: Reduviidae: Triatominae) in Peten, Guatemala. J Med Entomol. 2003;40:800–806. doi: 10.1603/0022-2585-40.6.800. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.