Abstract
A novel method using vector blood meal sources to assess the impact of control efforts on the risk of transmission of Chagas disease was tested in the village of El Tule, Jutiapa, Guatemala. Control used Ecohealth interventions, where villagers ameliorated the factors identified as most important for transmission. First, after an initial insecticide application, house walls were plastered. Later, bedroom floors were improved and domestic animals were moved outdoors. Only vector blood meal sources revealed the success of the first interventions: human blood meals declined from 38% to 3% after insecticide application and wall plastering. Following all interventions both vector blood meal sources and entomological indices revealed the reduction in transmission risk. These results indicate that vector blood meals may reveal effects of control efforts early on, effects that may not be apparent using traditional entomological indices, and provide further support for the Ecohealth approach to Chagas control in Guatemala.
Introduction
Triatoma dimidiata is the most important Chagas insect vector in Central America, and to interrupt Chagas transmission, a main goal of the Central American Control Initiative (IPCA) is to reduce T. dimidiata domiciliary infestation,1 although the traditional approach of application of residual insecticides has shown mixed results in Central America. In some localities it has successfully reduced infestation; however, in other localities insects rapidly reappear in houses after insecticide application.2,3 A new Ecosystem approach to human health (Ecohealth) has been proposed as a more sustainable method of control, where the communities actively participate in ameliorating the conditions identified as most important in Chagas transmission.4,5
Transmission of Chagas disease is dependent on a complex set of biological, environmental, sociological, and economic factors. House construction plays a particularly important role. First, houses are often constructed using forest materials, which, if they contain insect vectors, introduce the vectors into the domicile. Second, many rural houses have characteristics that make them attractive to the insect vectors. Cracks and crevices in the walls or clutter provide ideal hiding places, and the dust from dirt floors is used by some species as camouflage and often harbors eggs.6 Indeed, unplastered or deteriorated plastered walls and poor hygienic conditions have been shown to be the most important out of many factors in predicting T. dimidiata house infestation in Guatemala.7 We believe that encroachment and deforestation reduces sylvan blood meal sources and replaces those with human and domestic animal blood sources, which could support a higher insect density.
Traditionally, entomological indices have been used to assess the effects of vector control efforts. Because other factors besides simply the presence of vectors in the houses influence transmission (e.g., whether the vectors are feeding on humans or are infected with the parasite Trypanosoma cruzi, the causative agent of Chagas), we decided to test whether vector blood meals could be an indicator of the effectiveness of control efforts on the transmission risk. The purpose of this study is to test if the Ecohealth intervention reduces the risk of transmission of Chagas disease to humans by examining changes in the feeding patterns of vectors. We compare this novel assessment method to the traditional method of monitoring entomological indices. This article is a companion paper to Lucero and others,8 which addresses how the Ecohealth intervention limited triatomine reinfestation in another village within the same study area.
Materials and Methods
Study design.
We evaluated a new integrated Ecohealth intervention to determine its effectiveness in reducing the risk of Chagas transmission to humans by assessing changes in vector blood meals and compared this assessment to traditional entomological surveys. We applied the Ecohealth intervention to homesteads in El Tule, Jutiapa, Guatemala during four periods between 2004 and 2009, with a final assessment in 2011. Our approach consisted of four sequential interventions conducted in two phases: Phase 1 (2004–2006) included: 1) an initial insecticide application, followed by 2) wall plastering. Phase 2 (2008–2009) included: 3) “cementing” the dirt floor in the bedroom and 4) moving the domestic animals outside of the houses and into wire pens. A fifth intervention, 5) educating the community about Chagas disease and its vector, was ongoing throughout the study (Figure 1). More details of the intervention may be found in Monroy and others4,5; we compared the feeding patterns of the insect vectors to traditional entomological surveys to measure the transmission risk before and after each phase. We then conducted final entomological and vector blood meal surveys (in 2011) to assess long-term effects of interventions.
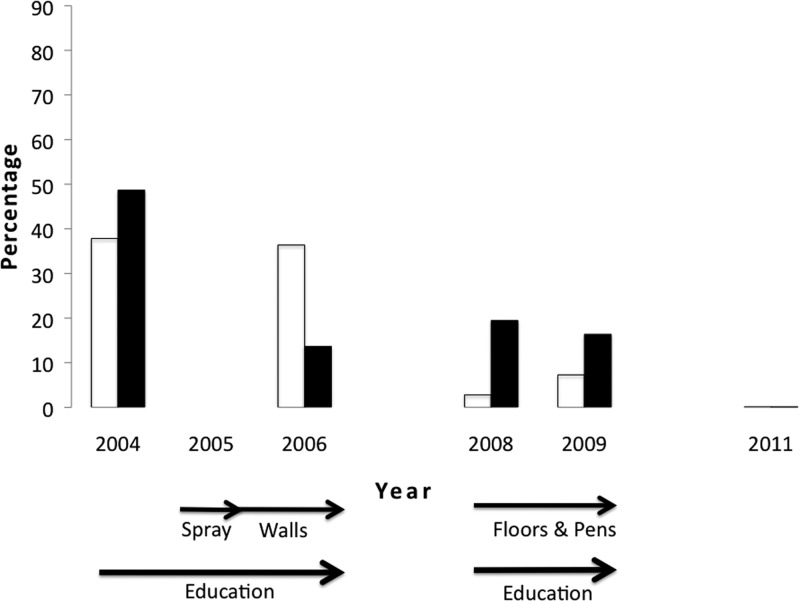
Figure 1.
Decrease in the percentage of Triatoma dimidiata feeding on human blood (clear bar) and infected with the parasite (black bar) after the interventions. Arrows indicate the timing of the different interventions. Results of the statistical tests that showed significant differences between years can be found in Table 2.
Study site, environmental and sociological surveys, and insect vector collections.
The Ecohealth intervention was implemented in 90% of the houses in the village of El Tule, Quesada, Jutiapa, Guatemala (14°19′35″ N 90°01′46″ W) in two phases, 2004–2006 and 2008–2009. El Tule is rural, largely agricultural with small areas of remnant dry forest, and a Spanish-speaking Ladino population that, although it is mixed Mayan-European, has lost the Mayan cultural practices. Some families' relatives work in the United States, and therefore have more resources for house improvements.
At the start of phase 1, before any intervention, all homesteads were surveyed where homeowners gave permission (∼90% of the houses in the village). The condition of the house, e.g., interior walls, floor, and roof construction, as well as living conditions, socio-economic status, animals within the house, and knowledge of villagers about Chagas disease and its insect vector were collected and recorded on standardized surveys. For the purposes of this study a house was classified as “at risk” for colonization if it had at least one indoor unplastered wall or plastered wall in poor condition. Following the house classification, T. dimidiata was collected inside houses (intradomiciliary) and in the areas surrounding the houses (peridomestic) by the traditional person-hour method (usually two people searching for half an hour per homestead). All insect vectors were placed in individual labeled vials and taken back to the laboratory at the University of San Carlos, Guatemala. Entomological indices were calculated following the World Health Organization (WHO) recommendations for Chagas vectors9: Infestation index = ([number of infested houses/number of houses investigated] × 100); intradomiciliary infestation index = ([number of infested intradomiciles/number of intradomiciles investigated] × 100); peridomiciliary infestation index = ([number of infested peridomiciles/number of peridomiciles investigated] × 100); colonization index = ([number of houses with triatomine nymphs/number of houses investigated] × 100); and density index = ([number of triatomines captured/number of houses examined] × 100).
The insect vectors were preserved at room temperature in 95% ethanol/5% glycerol (2004 and 2006) or directly frozen at −20°C (2008, 2009, and 2011) for later DNA isolation.
Interventions.
Phase 1 (Figures 1–4): Following the searches (latter part of 2004 into early 2005), all houses and peridomestic areas were sprayed with the insecticide Deltametrin (5% water-soluble powder at 25 mg of active ingredient per m2) by staff from the Guatemalan Ministry of Health.5 Villagers then improved their walls (2005–2006) by plastering them to cover cracks and crevices, thus eliminating hiding places for the insect vectors. The plaster was made from local materials (sand and clay). To assess the effects of insecticide application and wall improvements, a search for T. dimidiata in all houses and peridomestic areas was conducted in 2006 and 2008 as described previously and the vectors' blood meals and infection with T. cruzi determined.
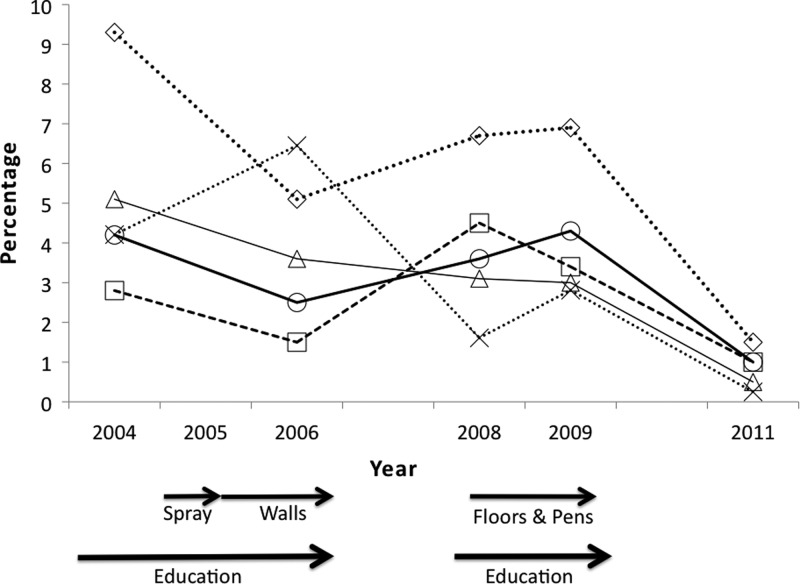
Figure 4.
Decrease in entomological indices ⋄-infestation and X-density (×10−1) after interventions (P < 0.01). Arrows indicate the timing of the different interventions. Results of the statistical tests that showed significant differences between years can be found in Table 3.
During phase 2 (Figures 1–4) villagers “cemented” their bedroom floor using mostly local materials. They covered the dirt floor with a mixture of volcanic ash, lime, and soil, and then a layer of sand containing a small amount of cement was used to seal the surface. The municipality provided transportation of the local materials to the houses. Once the floors were cemented, chicken wire was supplied to the villagers and they were asked to remove all domestic animals from the house. Many villagers built outdoor wire pens to house the chickens or other animals.
In 2009 and 2011 all houses and peridomestic areas were again searched for T. dimidiata to assess the results and long-term effects of all the interventions.
Determination of T. cruzi infection and blood meals present in insect vectors.
To determine the presence of different blood meals and T. cruzi in the T. dimidiata specimens, we extracted DNA from the last two segments of the abdomen from third stage nymphs through adults following the manufacturer's protocol (DNeasy Blood and Tissue kit, Qiagen, Inc., Valencia, CA). For years in which > 36 specimens were collected (2004, 2006, and 2009, Table 1) we randomly selected T. dimidiata specimens for blood meal analysis and T. cruzi testing. In 2008 and 2011, all specimens were tested. (only T. cruzi infection for 2011).
Table 1.
Survey and collection information
| Year | |||||
|---|---|---|---|---|---|
| 2004 | 2006 | 2008 | 2009 | 2011 | |
| Houses Surveyed, n | 214 | 197 | 223 | 231 | 200 |
| % “at risk” Houses | 38.8 | 22.8* | 17.9* | 17.8* | 20.5* |
| Chicken coops, n | nd | nd | 60 | 164† | nd |
| % “at risk” chicken coops | nd | nd | 16.7 | 3.7† | nd |
| Insects collected, n | 90 | 127 | 36 | 65 | 5 |
| Insects used for blood meal and Trypanosoma cruzi analyses, n (%) | 37 (41) | 44 (35) | 36 (100) | 55 (85) | 5 (100) |
| Female, n (%) | 12 (32) | 5 (11) | 6 (16) | 10 (18) | 1 (20) |
| Male, n (%) | 14 (38) | 4 (9) | 10 (28) | 13 (24) | 1 (20) |
| Nymphs, n (%) | 11 (30) | 35 (80) | 20 (56) | 32 (58) | 3 (60) |
P < 0.01 compared with baseline (2004).
P < 0.01 compared with 2008.
nd = no data.
The presence of different blood meals in 177 T. dimidiata was assessed using polymerase chain reaction (PCR).10 We tested for blood from humans, pigs, birds, rodents (primers amplify mouse and rat in one PCR reaction), dogs,11–13 and opossums (Walker J, personal communication) in individual PCR reactions. Samples that were negative for all seven PCR reactions (T. cruzi and six blood meals) were “spiked” with T. cruzi DNA to test for PCR inhibition, and none showed inhibition (data not shown). For the positive controls, human DNA was extracted from dried human blood spots on filter paper, pig and chicken meat was purchased commercially, rodent and opossum tissue was obtained from dead specimens donated by villagers, and the University of San Carlos of Guatemala Veterinary Hospital provided discarded dog tissue. The DNA was extracted from these samples according to manufacturer's instructions (DNeasy Blood and Tissue kit, Qiagen, Inc.).
The presence of T. cruzi in the extracted DNA was assessed by PCR in 177 T. dimidiata as in Reference 14, except we used primers TCZ1 and TCZ215 in this study. All PCR assays included a positive control (DNA extracted from the abdomen of a microscopy positive T. dimidiata specimen) and a negative control (water); all control reactions gave the expected results. Amplified products were visualized by agarose gel electrophoresis followed by UV transillumination.
Statistical analysis.
A logistic regression was used to model the odds of T. dimidiata infection with T. cruzi, and the odds of bugs having fed on a specific blood meal source (six in total) using the command glm in R.16 Year was used as the explanatory variable, and the proportion of bugs infected, or blood-fed with a specific source, was the response. The assumption of over dispersion was evaluated, and if found, a quasibinomial option was used to model the relationship between the mean and the variance.17
Because the database was used for multiple comparisons (T. cruzi and the six blood sources) we used the Bonferroni correction, and considered a corrected P value < 0.01 as indicating statistical significance.
We used a proportion test in R to determine if there was a significant difference among years in the proportion of “at risk” houses, the entomological indices, and the number of blood sources.16 A χ2 test was used to study the association between each blood source and infection with T. cruzi in the bugs.
For the rodent blood meals we used a Fisher exact test (JMP ver. 9, SAS Institute, Inc., Cary, NC) between years, because the zero result in 2009 interfered with the logistic regression.
Results
There was a high level of participation as most villagers implemented the Ecohealth changes (plastered their walls, cemented their floors, and removed the animals from the houses). This is reflected in a statistically significant reduction since 2004 in the percentage of “at risk” houses (Table 1). The number of chicken coops increased during the study because the construction of wire coops was promoted as part of the project; therefore, there was a significant reduction in the percentage of chicken coops “at risk” for harboring T. dimidiata (Table 1). The number of bugs collected in the village decreased drastically in 2011 (Table 1).
Blood meal analysis revealed a reduction in the risk of human transmission by a statistically significant decrease in the percentage of T. dimidiata feeding on humans in 2008 and 2009, in comparison to 2004 (Table 2). This reduction was evident following phase 1 interventions (Figure 1). Human blood was not found in any of the five bugs collected in 2011.
Table 2.
Logistic regression results indicating the years that showed statistically significant differences (P < 0.01) in the odds of Triatoma dimidiata feeding on human, pig, or dog, or infected with Trypanosoma cruzi*
| Model | Deviance; df | The odds of T. dimidiata feeding on human in: | Parameter estimate (OR) | Confidence interval |
|---|---|---|---|---|
| 1 | 173.79; 171 | 2008 vs. 2004 | 0.05 | 0.003–0.26 |
| 2009 vs. 2004 | 0.13 | 0.03–0.4 | ||
| 2008 vs. 2006 | 0.05 | 0.003–0.27 | ||
| 2009 vs. 2006 | 0.14 | 0.04–0.42 | ||
| The odds of T. dimidiata being infected with T. cruzi in: | ||||
| 2 | 186.57; 171 | 2006 vs. 2004 | 0.17 | 0.05–0.47 |
| 2009 vs. 2004 | 0.21 | 0.08–0.53 | ||
| The odds of T. dimidiata feeding on pig in: | ||||
| 3 | 188.92; 171 | 2009 vs. 2004 | 0.23 | 0.07–0.65 |
| 2008 vs. 2006 | 0.18 | 0.05–0.55 | ||
| 2009 vs. 2006 | 0.18 | 0.06–0.48 | ||
| The odds of T. dimidiata feeding on dog in: | ||||
| 4 | 237.30; 171 | 2009 vs. 2004 | 0.21 | 0.08–0.51 |
| 2009 vs. 2006 | 0.20 | 0.08–0.46 | ||
| The probability of T. dimidiata feeding on mice is different between: | ||||
| 2008 vs. 2004 | ||||
| Fisher's exact test | 2009 vs. 2004 | |||
| 2008 vs. 2006 | ||||
| 2009 vs. 2006 | ||||
The probabilities of T. dimidiata feeding on mice were compared with Fisher's exact test, and the years that showed statistically significant differences (P < 0.01) are shown.
df = degrees of freedom; OR = odds ratio.
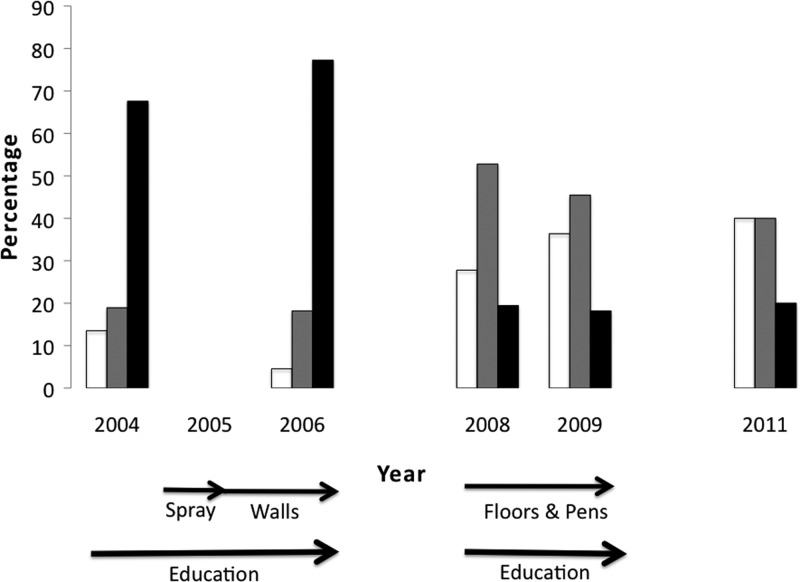
Before the interventions, T. dimidiata had fed on all vertebrates tested (Figures 1 and 2). Dog blood was found most frequently, followed by human, pig, bird, and rodent, the latter four with about the same frequency. Opossum blood was also found at a very low frequency. Following all interventions, there was a decrease in all vertebrate blood sources except birds. This reduction was significant following phase 1 interventions for all vertebrates except birds (Figure 2, Table 2). In the small number of bugs found and tested in 2011, dog blood was still the most frequent blood meal (3 of 5 bugs); bird, rodent, and opossum were found with equal frequency.
Figure 2.
Decrease in the percentage of Triatoma dimidiata feeding on pig (grey), dog (grid), and rodent blood (black) after the interventions. Arrows indicate the timing of the different interventions. Feeding on birds (clear) did not change significantly. Results of the statistical tests that showed significant differences between years can be found in Tables 2.
A statistically lower prevalence of T. cruzi infection in the vectors is also evident following all interventions (Figure 1); there was a significantly lower prevalence in 2006 compared with 2004 (Table 2). None of the five bugs collected in 2011 was positive for T. cruzi. The absence of T. cruzi in the T. dimidiata specimen was significantly correlated with the presence of human blood (P < 0.01). No other blood meal source showed a significant association with the presence or absence of T. cruzi.
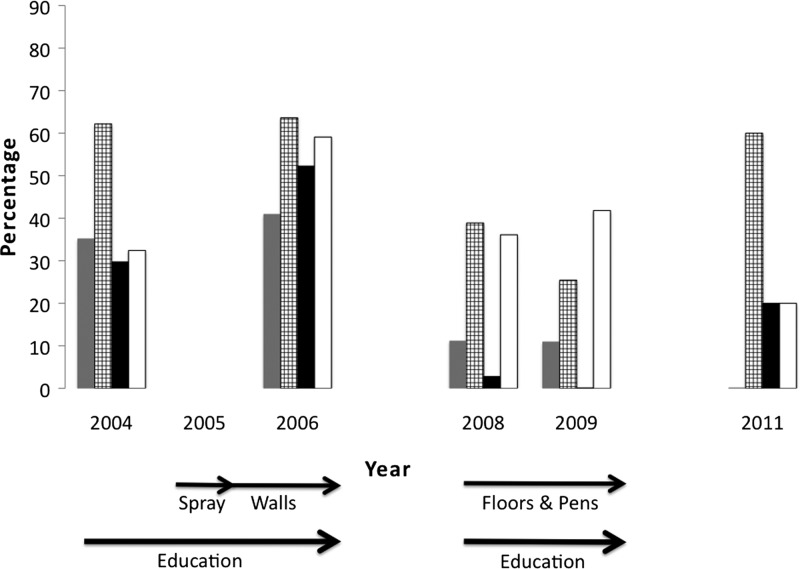
The number of different blood sources found in a single bug also decreased throughout the study period (Figure 3 ). A decrease in the frequency of multiple blood sources was statistically significant (Table 3) and was accompanied by an increase in the frequency of bugs with no detectable blood sources (Figure 3).
Figure 3.
Decrease in the number of blood sources found in Triatoma dimidiata after interventions. Percentage of T. dimidiata containing > 1 blood source (black), one (grey), or no detectable blood source (clear). Arrows indicate the timing of the different interventions. Results of the statistical tests that showed significant differences between years can be found in Table 3.
Table 3.
Years showing statistically significant differences (P < 0.01) in numbers of blood sources found in Triatoma dimidiata and entomological indices by proportion test*
| Years showing significant differences: | d, χ2, df | |
|---|---|---|
| T. dimidiata containing no detectable blood source: | 2006 vs. 2009 | −0.32, 12.54, 1 |
| T. dimidiata containing 1 blood source: | 2004 vs. 2008 | −0.34, 7.71, 1 |
| 2006 vs. 2008 | −0.35, 9.11, 1 | |
| 2006 vs. 2009 | −0.27, 7.00, 1 | |
| T. dimidiata containing > 1 blood source: | 2004 vs. 2008 | 0.48, 15.26, 1 |
| 2004 vs. 2009 | 0.49, 20.84, 1 | |
| 2006 vs. 2008 | 0.58, 24.24, 1 | |
| 2006 vs. 2009 | 0.59, 32.22, 1 | |
| Infestation index: | 2004 vs. 2011 | 0.08, 10.68, 1 |
| Density index: | 2004 vs. 2006 | −0.22, 19.78, 1 |
| 2004 vs. 2008 | 0.26, 34.48, 1 | |
| 2004 vs. 2011 | 0.40, 89.26, 1 | |
| 2006 vs. 2008 | 0.48, 100.83, 1 | |
| 2006 vs. 2009 | 0.36, 55.27, 1 | |
| 2006 vs. 2011 | 0.62, 168.93, 1 | |
| 2008 vs. 2011 | 0.14, 20.89, 1 | |
| 2009 vs. 2011 | 0.26, 49.93, 1 |
d = magnitude of the difference; df = degrees of freedom.
Entomological indices also decreased with the interventions (Figure 4). Initially 9.3% (20 of 214) of the houses were infested and this dropped to 5–7% between 2006 and 2009, and finally to 1.5% (3 of 200) in 2011. Of importance, both domestic and peridomestic infestation were reduced. However, the infestation index reduction was only significantly different between 2004 and 2011 (P < 0.001, Table 3). Although the interventions resulted in a reduction in all entomological indices except colonization, these reductions were not statistically significant except for the density index (P < 0.001, Table 3). The majority of the houses remaining infested are the ones where the homeowners did not make improvements, although overall infestation was reduced even in unimproved houses (data not shown).
Discussion
Our results show that the Ecohealth intervention is effective in reducing the risk of transmission of Chagas disease to humans as measured by a significant and sustained reduction in vectors feeding on humans, in T. cruzi infection prevalence in vectors, and in house infestation.
The impact of phase 1 interventions in reducing human–vector contact, clearly shown by vector blood meal analyses (Figure 1), would have been missed if only entomological indices were measured because most reductions in these indices were not significant until 2011 (Figure 3). This is important as most Chagas control programs are designed and evaluated based on infestation indices. Although these indices are an important piece of information, several studies have shown that they can be subject to substantial error18,19 and do not always correlate with T. cruzi infection in humans.20,21 It will be important in future triatomine control programs to include other measures of epidemiological risk, such as presence of human blood meals and the spatial analysis of reinfestaion patterns Lucero and others,8 in addition to traditional entomological indices. The lack of early reduction in the entomological indices after phase 1 could perhaps be caused by surviving eggs hatching, because they are not susceptible to insecticides. Furthermore, the high mobility of this species22 (Stevens L, unpublished data) could mean transient movement from the unimproved houses and peridomestic environments to the improved houses.5
The sustained reduction in infestation over the entire study is significant because many studies conducted in Central America and southern Mexico show that, without other interventions, T. dimidiata frequently reappears in houses after insecticide application, sometimes at pre-insecticide treatment levels.2,3,23 This is likely because persistence of even residual insecticides in houses is limited perhaps to as little as 4 months,24 probably less in the exposed peridomestic environment,25 which serves as a reservoir for domestic insects.26
The reduction in human–vector contact and domiciliary infestation is likely caused by removal of hiding places for the vectors and improved houses now refractory to insects.4 Previously, vectors could proliferate to high levels inside the house with abundant blood sources provided by domestic animals,27 but removing the animals from the houses reduces the blood sources available. Outdoor wire pens lack hiding places for the bugs making it easier for the chickens to catch the bugs, which may explain the reduction seen in peridomestic infestation; and, in turn, reduces the population that can reinfest the houses.26 Even the ∼20% of unimproved houses that remain “at risk” for T. dimidiata enjoy some risk reduction, which may be caused by the decreased number of vectors village-wide as seen in the reduced density index. The reduction in T. cruzi infection prevalence in the vectors may be correlated with a shift from feeding on mammals, which support T. cruzi replication, to birds, which cannot support replication.28
Previous studies identified human blood as a common food source for T. dimidiata in the domestic environment, and an important food source in peridomestic environments: 63.7% of bugs collected indoors and 21.9% of bugs collected outdoors in an endemic area of Costa Rica29; 37.7% of bugs collected outdoors in Chiriqui, Panama30; 30.6% of bugs collected indoors in Santa Rosa, Guatemala27; and 87.5% of bugs collected indoors and 25% of the bugs collected outdoors in Veracruz, Mexico.31 We detected human blood in almost 40% of bugs at the beginning of this study, and this was reduced considerably (< 10%) after the control interventions. These data suggest that T. dimidiata will often take human blood when it's available, and that the interventions have successfully reduced human–vector contacts. Preliminary results from studies currently being carried out in Chiquimula, Guatemala, showed no T. cruzi infection in children 5 years of age or younger, in five villages where only about 3% of T. dimidiata had fed on humans and about 12% are infected with T. cruzi (Monroy and others, unpublished data). This suggests that keeping human–vector contacts under a certain threshold could prevent parasite transmission to humans. Indeed, a recent study suggests that reduction of domestic infestation of T. dimidiata to < 8% correlates with interruption of transmission to humans.21
Blood meal sources also provide valuable information for understanding the movement and epidemiological importance of populations of triatomines. As described previously, T. dimidiata found in peridomestic environments contained human blood, indicating bug movement between houses and peridomestic environments. Domiciliary Triatoma infestans populations showed a wide range of human blood prevalence in South America, ranging from 15.4% to 91.7%.32 In contrast, other species like peridomestic Triatoma longipennis did not contain human blood, therefore was not considered epidemiologically important.33 Recently, human blood meals were detected in sylvan Triatoma rubida and Triatoma protracta in the United States,34 therefore it is now necessary to investigate transmission risk for humans in this region. Various factors such as season, developmental stage, and vector density, may affect the feeding patterns of vectors, thus it would be important to monitor blood sources at different times of the year if blood sources are to be used to evaluate control programs.32,33,35,36
Although there was a significant decrease in vectors containing dog blood meals, dogs remain an important reservoir animal in Guatemala, as they are elsewhere37–39; the majority of the blood meals found before the interventions were dog, and even after the interventions nearly 30% of vectors had fed on dog. Usually houses have many dogs and there is no breeding control. Future interventions should address reducing the dog population by spaying or neutering. Other authors have suggested that dogs could be used as sentinels or as “bug lethal traps” by “applying lotions or insecticide impregnated collars.”40
Another important result is the increase in the proportion of bugs where no blood sources were detected after the interventions (among the ones tested). This may suggest that the population is experiencing a worsening nutritional status and this could negatively affect the fecundity of females. In a controlled experiment, T. infestans females living in huts with the fewest hiding places had smaller blood meals and this lowered their survival and fecundity.41
In summary, our results show further evidence of the effectiveness of the Ecohealth intervention in reducing transmission of Chagas disease in Guatemala. A reduction in human–vector contact after just insecticide spraying and wall plastering, evident by vector blood source analysis, would have been missed by traditional entomological indices. The sustainability of this approach is evident as 2 years after the last intervention; entomological indices remain significantly below levels before the interventions. We recommend monitoring vector blood sources in addition to entomological indices to understand the true transmission risk of Chagas disease. A similar approach should be useful for understanding the epidemiology and control of many other vector-borne diseases involving hematophagous vectors.
ACKNOWLEDGMENTS
We thank the residents of El Tule, LENAP field teams, and Guatemala's Ministry of Health personnel for their crucial contributions and collaboration in this study. We also thank the anonymous reviewer for helpful comments, which helped us improve the manuscript.
Footnotes
Financial support: This research was funded by the International Development Research Center (Canada) Grants 101812 and 103696-005, by Network for Research and Training in Tropical Diseases in Central America (Sweden) Grant RO7-008, and the National Institutes of Health (USA) Grant 1R15 A1079672-01A.
Authors' addresses: Mariele J. Pellecer, Dulce M. Bustamante, and M. Carlota Monroy, Laboratorio de Entomología Aplicada y Parasitología, Escuela de Biología, Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos, Ciudad de Guatemala, Guatemala, E-mails: marielepellecer@gmail.com, dulce_mariab@hotmail.com, and mcarlotamonroy@gmail.com. Patricia L. Dorn, Department of Biological Sciences, Loyola University New Orleans, New Orleans, LA, E-mail: dorn@loyno.edu. Antonieta Rodas, Lcda, Laboratorio de Entomología Aplicada y Parasitología, Escuela de Biología, Facultad de Ciencias Químicas y Farmacia, Universidad de San Carlos, Ciudad de Guatemala, Guatemala, E-mail: antonieta55@yahoo.com.
References
- 1.Ponce C. Current situation of Chagas disease in Central America. Mem Inst Oswaldo Cruz. 2007;102((Suppl 1)):41–44. doi: 10.1590/s0074-02762007005000082. [DOI] [PubMed] [Google Scholar]
- 2.Hashimoto K, Cordon-Rosales C, Trampe R, Kawabata M. Impact of single and multiple residual sprayings of pyrethroid insecticides against Triatoma dimidiata (Reduviidae; Triatominae), the principal vector of Chagas disease in Jutiapa, Guatemala. Am J Trop Med Hyg. 2006;75:226–230. [PubMed] [Google Scholar]
- 3.Dumonteil E, Ruiz-Pina H, Rodriguez-Felix E, Barrera-Perez M, Ramirez-Sierra MJ, Rabinovich JE, Menu F. Re-infestation of houses by Triatoma dimidiata after intra-domicile insecticide application in the Yucatan peninsula, Mexico. Mem Inst Oswaldo Cruz. 2004;99:253–256. doi: 10.1590/s0074-02762004000300002. [DOI] [PubMed] [Google Scholar]
- 4.Monroy C, Castro X, Bustamante DM, Pineda SF, Rodas A, Moguel B, Ayala V, Quiñonez J. An ecosystem approach for the prevention of Chagas disease in rural Guatemala. In: Charron DF, editor. Ecohealth Research in Practice: Innovative Applications of an Ecosystem Approach to Health. New York: Springer; 2012. pp. 153–162. [Google Scholar]
- 5.Monroy C, Bustamante DM, Pineda S, Rodas A, Castro X, Ayala V, Quinones J, Moguel B. House improvements and community participation in the control of Triatoma dimidiata re-infestation in Jutiapa, Guatemala. Cad Saude Publica. 2009;25((Suppl 1)):S168–S178. doi: 10.1590/s0102-311x2009001300016. [DOI] [PubMed] [Google Scholar]
- 6.Starr MD, Rojas JC, Zeledon R, Hird DW, Carpenter TE. Chagas' disease: risk factors for house infestation by Triatoma dimidiata, the major vector of Trypanosoma cruzi in Costa Rica. Am J Epidemiol. 1991;133:740–747. doi: 10.1093/oxfordjournals.aje.a115949. [DOI] [PubMed] [Google Scholar]
- 7.Bustamante DM, Monroy C, Pineda S, Rodas A, Castro X, Ayala V, Quinones J, Moguel B, Trampe R. Risk factors for intradomiciliary infestation by the Chagas disease vector Triatoma dimidiata in Jutiapa, Guatemala. Cad Saude Publica. 2009;25((Suppl 1)):S83–S92. doi: 10.1590/s0102-311x2009001300008. [DOI] [PubMed] [Google Scholar]
- 8.Lucero DE, Morrissey LA, Rizzo DM, Rodas A, Garnica R, Stevens L, Bustamante DM, Monroy MC. Ecohealth interventions limit triatomine reinfestation following insecticide spraying in La Brea, Guatemala. Am J Trop Med Hyg. 2013;88:630–637. doi: 10.4269/ajtmh.12-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization . Control of Chagas disease (Second Report) In: WHO, editor. WHO Technical Report Series 905. Geneva: World Health Organization; 2002. p. 109. [PubMed] [Google Scholar]
- 10.Pizarro JC, Stevens L. A new method for forensic DNA analysis of the blood meal in Chagas disease vectors demonstrated using Triatoma infestans from Chuquisaca, Bolivia. PLoS ONE. 2008;3:e3585. doi: 10.1371/journal.pone.0003585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walker JA, Hughes DA, Anders BA, Shewale J, Sinha SK, Batzer MA. Quantitative intra-short interspersed element PCR for species-specific DNA identification. Anal Biochem. 2003;316:259–269. doi: 10.1016/s0003-2697(03)00095-2. [DOI] [PubMed] [Google Scholar]
- 12.Walker JA, Hughes DA, Hedges DJ, Anders BA, Laborde ME, Shewale J, Sinha SK, Batzer MA. Quantitative PCR for DNA identification based on genome-specific interspersed repetitive elements. Genomics. 2004;83:518–527. doi: 10.1016/j.ygeno.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 13.Walker JA, Kilroy GE, Xing J, Shewale J, Sinha SK, Batzer MA. Human DNA quantitation using Alu element-based polymerase chain reaction. Anal Biochem. 2003;315:122–128. doi: 10.1016/s0003-2697(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 14.Dorn P, Perniciaro L, Yabsley M, Roellig D, Balsamo G, Diaz J, Wesson DM. Autochthonous transmission of Trypanosoma cruzi, Louisiana. Emerg Infect Dis. 2007;13:605–607. doi: 10.3201/eid1304.061002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moser DR, Kirchhoff LV, Donelson JE. Detection of Trypanosoma cruzi by DNA amplification using the polymerase chain reaction. J Clin Microbiol. 1989;27:1477–1482. doi: 10.1128/jcm.27.7.1477-1482.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.R Development Core Group The R Project for Statistical Computing. 2010. http://www.r-project.org/ Available at. Accessed January 15, 2011.
- 17.Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. [Google Scholar]
- 18.Monroy C, Mejía M, Rodas A, Rosales R, Horio M, Tabaru Y. Comparison of indoor searches with whole house demolition collections of the vectors of Chagas disease and their indoor distribution. Med Entomol Zool. 1998;49:195–200. [Google Scholar]
- 19.Gürtler RE, Chuit R, Cecere MC, Castanera MB. Detecting domestic vectors of Chagas disease: a comparative trial of six methods in north-west Argentina. Bull World Health Organ. 1995;73:487–494. [PMC free article] [PubMed] [Google Scholar]
- 20.Piesman J, Sherlock IA, Mota E, Todd CW, Hoff R, Weller TH. Association between household triatomine density and incidence of Trypanosoma cruzi infection during a nine-year study in Castro Alves, Bahia, Brazil. Am J Trop Med Hyg. 1985;34:866–869. doi: 10.4269/ajtmh.1985.34.866. [DOI] [PubMed] [Google Scholar]
- 21.Aiga H, Sasagawa E, Hashimoto K, Nakamura J, Zuniga C, Chevez JE, Hernandez HM, Nakagawa J, Tabaru Y. Chagas disease: assessing the existence of a threshold for bug infestation rate. Am J Trop Med Hyg. 2012;86:972–979. doi: 10.4269/ajtmh.2012.11-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorn PL, Melgar S, Rouzier V, Gutierrez A, Combe C, Rosales R, Rodas A, Kott S, Salvia D, Monroy CM. The Chagas vector, Triatoma dimidiata (Hemiptera: Reduviidae), is panmictic within and among adjacent villages in Guatemala. J Med Entomol. 2003;40:436–440. doi: 10.1603/0022-2585-40.4.436. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira Filho A. Using new tools to control triatomines in different entomological situations in the Americas. Rev Soc Bras Med Trop. 1997;30:41–46. doi: 10.1590/s0037-86821997000100008. [DOI] [PubMed] [Google Scholar]
- 24.Tabaru Y, Monroy C, Rodas A, Mejía M, Rosales R. Chemical control of Triatoma dimidiata and Rhodnius prolixus (Reduviidae: Triatominae), the principal vectors of Chagas Disease in Guatemala. Med. Entomol. Zoo. 1998;49:87–92. [Google Scholar]
- 25.Gürtler RE, Canale DM, Spillmann C, Stariolo R, Salomon OD, Blanco S, Segura EL. Effectiveness of residual spraying of peridomestic ecotopes with deltamethrin and permethrin on Triatoma infestans in rural western Argentina: a district-wide randomized trial. Bull World Health Organ. 2004;82:196–205. [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens L, Dorn PL, Schmidt JO, Klotz JH, Lucero D, Klotz SA. Kissing bugs. The vectors of chagas. Adv Parasitol. 2011;75:169–192. doi: 10.1016/B978-0-12-385863-4.00008-3. [DOI] [PubMed] [Google Scholar]
- 27.Sasaki H, Rosales R, Tabaru Y. Host feeding profiles of Rhodnius prolixus and Triatoma dimidiata in Guatemala (Hemiptera: Reduviidae: Triatominae) Med. Entomol. and Zoo. 2003;54:283–289. [Google Scholar]
- 28.Gürtler RE, Cohen JE, Cecere MC, Lauricella MA, Chuit R, Segura EL. Influence of humans and domestic animals on the household prevalence of Trypanosoma cruzi in Triatoma infestans populations in northwest Argentina. Am J Trop Med Hyg. 1998;58:748–758. doi: 10.4269/ajtmh.1998.58.748. [DOI] [PubMed] [Google Scholar]
- 29.Zeledon R, Solano G, Zuniga A, Swartzwelder JC. Biology and ethology of Triatoma dimidiata (Latreille, 1811). 3. Habitat and blood sources. J Med Entomol. 1973;10:363–370. doi: 10.1093/jmedent/10.4.363. [DOI] [PubMed] [Google Scholar]
- 30.Christensen HA, Sousa OE, de Vasquez AM. Host feeding profiles of Triatoma dimidiata in peridomestic habitats of western Panama. Am J Trop Med Hyg. 1988;38:477–479. doi: 10.4269/ajtmh.1988.38.477. [DOI] [PubMed] [Google Scholar]
- 31.Torres-Montero J, Lopez-Monteon A, Dumonteil E, Ramos-Ligonio A. House infestation dynamics and feeding sources of Triatoma dimidiata in central Veracruz, Mexico. Am J Trop Med Hyg. 2012;86:677–682. doi: 10.4269/ajtmh.2012.11-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gürtler RE, Cecere MC, Castanera MB, Canale D, Lauricella MA, Chuit R, Cohen JE, Segura EL. Probability of infection with Trypanosoma cruzi of the vector Triatoma infestans fed on infected humans and dogs in northwest Argentina. Am J Trop Med Hyg. 1996;55:24–31. [PubMed] [Google Scholar]
- 33.Breniere SF, Pietrokovsky S, Gastelum EM, Bosseno MF, Soto MM, Ouaissi A, Kasten FL, Wisnivesky-Colli C. Feeding patterns of Triatoma longipennis Usinger (Hemiptera, Reduviidae) in peridomestic habitats of a rural community in Jalisco State, Mexico. J Med Entomol. 2004;41:1015–1020. doi: 10.1603/0022-2585-41.6.1015. [DOI] [PubMed] [Google Scholar]
- 34.Stevens L, Dorn PL, Hobson J, de la Rua NM, Lucero DE, Klotz JH, Schmidt JO, Klotz SA. Vector blood meals and Chagas disease transmission potential, United States. Emerg Infect Dis. 2012;18:646–649. doi: 10.3201/eid1804.111396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gürtler RE, Ceballos LA, Ordonez-Krasnowski P, Lanati LA, Stariolo R, Kitron U. Strong host-feeding preferences of the vector Triatoma infestans modified by vector density: implications for the epidemiology of Chagas disease. PLoS Negl Trop Dis. 2009;3:e447. doi: 10.1371/journal.pntd.0000447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceballos LA, Vazquez-Prokopec GM, Cecere MC, Marcet PL, Gürtler RE. Feeding rates, nutritional status and flight dispersal potential of peridomestic populations of Triatoma infestans in rural northwestern Argentina. Acta Trop. 2005;95:149–159. doi: 10.1016/j.actatropica.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Gürtler RE, Cecere MC, Lauricella MA, Cardinal MV, Kitron U, Cohen JE. Domestic dogs and cats as sources of Trypanosoma cruzi infection in rural northwestern Argentina. Parasitology. 2007;134:69–82. doi: 10.1017/S0031182006001259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rabinovich JE, Kitron UD, Obed Y, Yoshioka M, Gottdenker N, Chaves LF. Ecological patterns of blood-feeding by kissing-bugs (Hemiptera: Reduviidae: Triatominae) Mem Inst Oswaldo Cruz. 2011;106:479–494. doi: 10.1590/s0074-02762011000400016. [DOI] [PubMed] [Google Scholar]
- 39.Cohen JE, Gurtler RE. Modeling household transmission of American trypanosomiasis. Science. 2001;293:694–698. doi: 10.1126/science.1060638. [DOI] [PubMed] [Google Scholar]
- 40.Gürtler RE, Ceballos LA, Stariolo R, Kitron U, Reithinger R. Effects of topical application of fipronil spot-on on dogs against the Chagas disease vector Triatoma infestans. Trans R Soc Trop Med Hyg. 2009;103:298–304. doi: 10.1016/j.trstmh.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cecere MC, Canale DM, Gürtler RE. Effects of refuge availability on the population dynamics of Triatoma infestans in central Argentina. J Appl Ecol. 2003;40:742–756. [Google Scholar]