Abstract
Data on non-typhoidal Salmonella (NTS) infection in South Asia are limited. We used data gathered prospectively from 1996 to 2011 as part of a hospital surveillance system in Dhaka, Bangladesh, to identify diarrheal patients with NTS isolated from stool. NTS was isolated in 1.3% (468 of 37,439) of diarrheal patients; 47% of total cases of NTS were in children < 5 years of age, although older adults (≥ 60 years) had the highest isolation rates. NTS isolation peaked in the monsoon months of July and August. Over the study period, rates of multidrug resistance decreased, whereas rates of decreased susceptibility to ciprofloxacin increased. Compared with control patients, NTS patients were older and wealthier; however, no differences in type of housing or exposure to animals were found. NTS patients had increased inflammatory cells in stool and required more fluid resuscitation.
Introduction
Non-typhoidal Salmonella (NTS) are a group of Gram-negative bacteria known to cause disease in both animals and humans worldwide. In humans, NTS are responsible for an estimated 94 million cases of gastroenteritis each year globally, causing upward of 150,000 deaths.1 Although significant attention is put on food-borne outbreaks in high-income countries, the majority of the burden of diarrhea caused by NTS may rest in low- and middle-income countries. NTS is also associated with systemically invasive disease and bacteremia, especially in immunocompromised patients. In sub-Saharan Africa, NTS is a common cause of bacteremia in both adults and children, especially in areas of high human immunodeficiency virus (HIV) and malaria prevalence.2
Data on the epidemiology of NTS infections in South Asia are limited. An estimate of the burden of NTS disease in South Asia extrapolated from a single study of returning travelers to Sweden suggested an incidence of 470 per 100,000 person-years,3 but that number is likely not representative of the incidence within the local population. Most reports from South Asia have been limited to surveillance of antimicrobial resistance patterns4,5 and circulating serotypes.6 Studies on the burden of illness and risk factors for disease are limited.
In this study, we used prospectively collected data from the surveillance system of a diarrheal hospital in Dhaka, Bangladesh, to identify the demographic, seasonal, and antibiotic resistance patterns of diarrheal patients with NTS isolated in stool as well as identify risk factors and clinical presentation features specific to patients with NTS.
Methods
Setting.
The Dhaka Hospital of the International Centre for Diarrhoeal Disease Research, Bangladesh (icddr,b) is a diarrheal treatment center located in Dhaka, the capital city of Bangladesh. It provides care free of charge to approximately 120,000 patients per year who present with diarrhea. Most patients are self-referred, and many come from surrounding urban slums and periurban areas. Bangladesh has an HIV prevalence of < 0.1%,7 and the catchment area of the hospital is not endemic for malaria.
Surveillance.
The hospital has a longstanding Diarrheal Disease Surveillance system,8 in which every 50th patient undergoes detailed questioning, a physical examination, and culture and analysis of stool. The surveillance system has been described previously in detail.8 Using structured questions, trained personnel interview patients and/or their attendants to collect relevant information on symptoms, demographic and socioeconomic characteristics, housing and environmental conditions, feeding practices, and use of drugs and fluid therapy at home. All patients receive treatment according to the treatment guidelines of the hospital.
Laboratory methods.
Freshly collected stool or a rectal swab from each surveillance patient is immediately sent to the laboratory, where it is enriched in Selenite broth and inoculated onto MacConkey, taurocholate tellurite gelatin agar (TTGA) and Salmonella–Shigella agar plates. Testing for rotavirus is done by enzyme-linked immunosorbent assay (ELISA) method. Salmonella species are isolated and identified using standard bacteriological methods, including slide agglutination for serotyping with specific antisera. Antimicrobial susceptibility is determined by disc diffusion method on Mueller–Hinton agar as per Clinical Laboratory and Standard Institute (CLSI) guidelines. Stool samples also undergo microscopic examination for cell count and parasite detection from direct smear.
Case and control selection.
We extracted hospital surveillance data from January of 1996 to December of 2011 and reviewed records from patients whose stool culture grew any non-typhoidal Salmonella. To determine risk factors and differences in clinical presentation of NTS patients, we performed two comparisons.
-
(1)
We selected only those NTS cases with no copathogens identified and compared this cohort with a group of control patients that was determined by randomly selecting surveillance patients who had no pathogens identified in stool by the same assays matched only to month of admission at a 3:1 ratio of control to NTS patients (no-pathogen controls).
-
(2)
We selected all cases with NTS and compared them with a group of control patients, which was determined by randomly selecting surveillance patients matched only to month of admission at a 3:1 ratio of control to NTS patients (all-diarrhea controls).
Climate data.
We obtained climate data, including monthly rainfall and maximum temperature, from the Bangladesh Agricultural Research Council (http://www.barc.gov.bd) for the period of 1996 to 2008, the latest available year for Dhaka.
Statistics.
We analyzed yearly trends by the Mann–Kendall test, a non-parametric test to detect increasing or decreasing monotonic trends over time, and we estimated the magnitude of annual change using Sen's slope estimates. We performed both calculations using Microsoft Excel (Microsoft Corp., Redmond, WA) template application MAKESENS (Finnish Meteorological Institute, Helsinki, Finland). For comparisons between NTS and control groups, we used the student t test (for normally distributed data as assessed by Shapiro–Wilk test) or the Mann–Whitney U test (for non-parametric data) for continuous variables and the Fisher exact test for categorical variables. We adjusted for multiple comparisons using the Holm–Bonferroni correction.9 We used logistic regression to determine independent predictors of NTS infection: sex, age, family income, and demographic and housing variables with corrected P < 0.05 from univariate analysis were entered into the model. For these comparisons, we performed statistical analyses using SPSS 17.0 (SPSS Inc., Chicago, IL). Statistical significance was defined as a two-tailed P value < 0.05. We generated graphs using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA).
Results
NTS were isolated from the stool of a total of 468 patients over the 16-year study period from a total of 37,129 patients surveyed. Based on O-antigen grouping, the majority of isolates belonged to Groups B (32.9%) and C1 (28.4%) followed by Group E (12.6%) and Group C2 (10.9%). Group D, which includes S. enterica serotype Enteritidis, only accounted for 5.3% of all isolates. Nearly one-half (46%) of patients with NTS isolated from stool had another potential copathogen identified, with 26% having one or more bacterial copathogens (Table 1). Common copathogens included rotavirus (13%), Vibrio cholerae (12%), and helminths (6%).
Table 1.
Copathogens identified in stool of diarrheal patients with NTS
| Copathogen | NTS (N = 468) |
|---|---|
| No copathogens identified | 254 (54%) |
| Rotavirus | 60 (13%) |
| Helminth | 30 (6%) |
| E. histolytica | 2 |
| Giardia | 2 |
| Bacterial copathogen present* | 123 (26%) |
| Aeromonas spp. | 13 |
| Campylobacter spp. | 19 |
| Hafnia alvei | 9 |
| Plesiomonas shigelloides | 5 |
| Shigella spp. | 16 |
| Vibrio parahaemolyticus | 15 |
| Vibrio cholerae | 57 |
Nine patients had more than one bacterial copathogen.
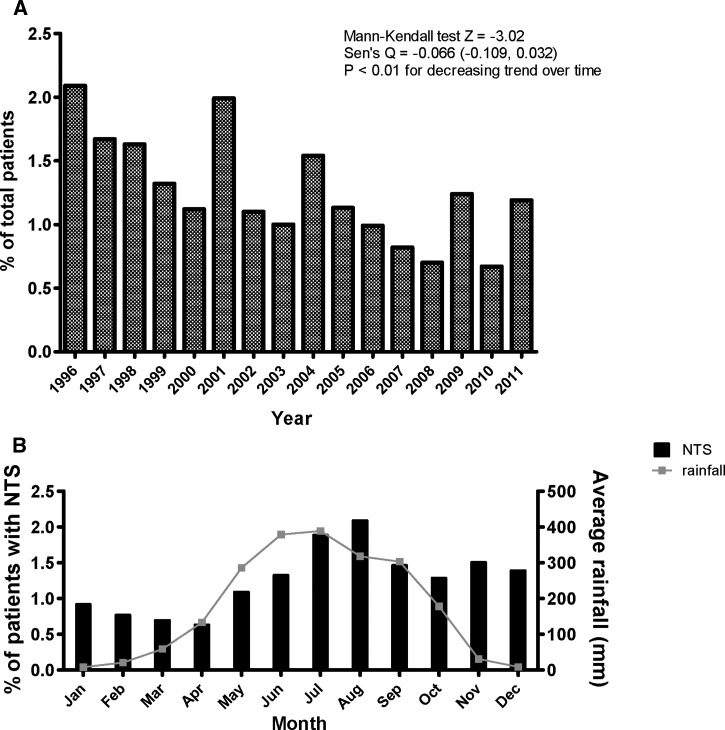
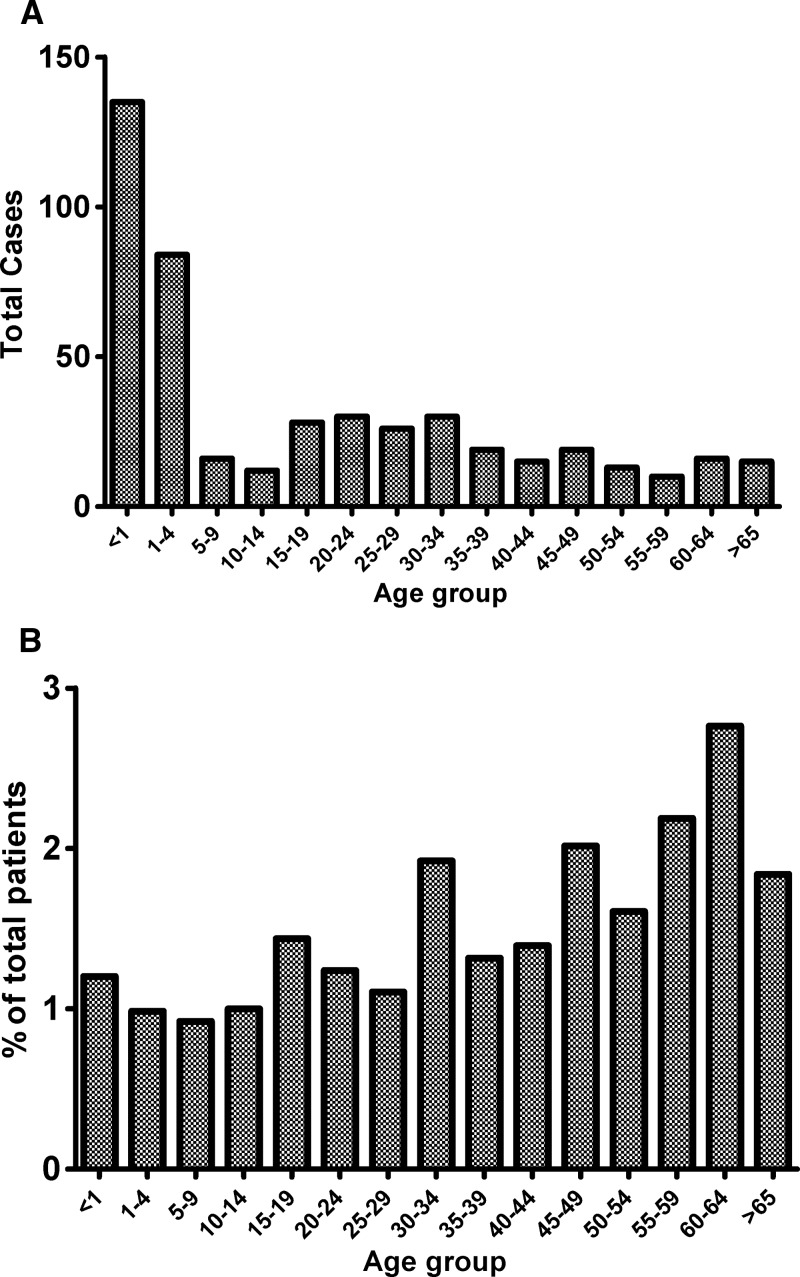
The percentage of total diarrheal patients with stool positive for NTS decreased over the study period (P < 0.01) (Figure 1A ). Nearly one-half (219, 47%) of all NTS cases were in children < 5 years of age (Figure 2A ). However, children < 5 years accounted for 53% of all cases presenting to Dhaka Hospital, and when expressed as a percentage of total patients of the same age group, patients ages ≥ 60 years had higher rates of NTS isolated in stool than those patients ages < 5 years (P < 0.001) (Figure 2B). Similar patterns existed even when patients with copathogens found in stool were excluded (data not shown). We show a seasonal variation for NTS, with the percentage of patients with NTS isolated in stool peaking in the hot and wet monsoon months of July and August and the lowest rates occurring in the hot but relatively dry spring months of March and April (Figure 1B).
Figure 1.
Percentage of patients presenting to a diarrheal hospital with NTS isolated in stool as percent of total diarrheal patients by (A) year and (B) month with average rainfall.
Figure 2.
NTS isolated in stool by age group as (A) total patients and (B) percentage of diarrheal patients of the same age group.
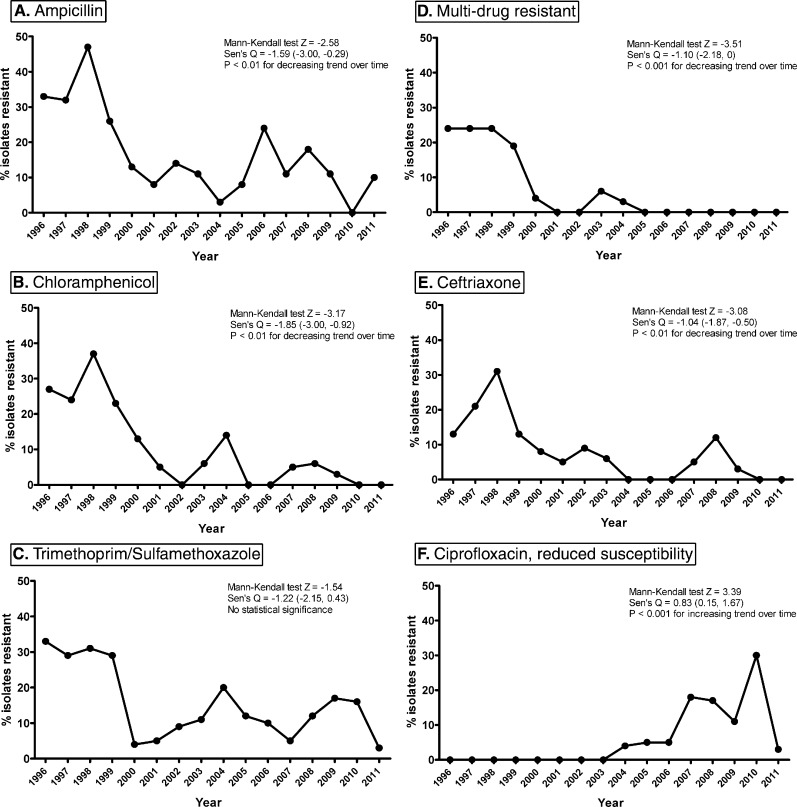
We examined patterns of antimicrobial resistance to NTS isolated over the study period. We found that rates of resistance to ampicillin, chloramphenicol, and ceftriaxone significantly decreased over time (Figure 3A, B, and E), and rates of resistance to trimethoprim/sulfamethoxazole (TMP/SMX) remained constant (Figure 3C). Rates of multidrug resistance (MDR), defined as isolates resistant to ampicillin, chloramphenicol, and TMP/SMX, decreased over time, with no MDR strain isolated since 2004 (Figure 3D). However, rates of reduced susceptibility to ciprofloxacin significantly increased (Figure 3F), and since 2006, one ciprofloxacin-resistant strain of NTS has been isolated per year.
Figure 3.
Antibiotic resistance trends of NTS stool isolates from diarrheal patients from 1996 to 2011 for ampicillin (A), chloramphenicol (B), trimethoprim/sulfamethoxazole (C), multi-drug resistant (D, defined as resistant to ampicillin, chloramphenicol, and trimethoprim/sulfamethoxazole), ceftriaxone (E), and reduced susceptibility to ciprofloxacin (F).
To determine risk factors and clinical characteristics specific to NTS-associated diarrhea, we first selected the 254 surveillance patients with NTS who did not have any other potential pathogen identified in the stool (Table 1) and compared them with control diarrheal surveillance patients with no pathogens identified matched in a 3:1 (control:NTS) ratio by month of admission. In this analysis, we found that NTS patients were older and more likely to own a television (Table 2). Comparing all NTS cases with controls selected regardless of pathogen identification (all-diarrhea controls), we found that NTS patients were more likely to have a higher family income (> 5,000 Taka/month), own a luxury cot, and have fewer family members sharing a drinking water source (Table 3). NTS patients were also older in this analysis, although this finding did not meet significance after Holm–Bonferroni adjustment (P = 0.07). In both comparisons, we did not find any differences in presence of animals in the home or type of home construction. In multivariate analysis, age, number of family members using the same drinking water source (only in all-diarrhea analysis), and luxury item ownership were independent predictors of NTS infection.
Table 2.
Demographics, housing, and animal exposures of 254 diarrheal patients with NTS identified as the only pathogen in stool compared with control diarrheal surveillance patients with no pathogen identified matched by month of admission
| NTS only (N = 254) | No pathogen (N = 762) | Adjusted P value* | |
|---|---|---|---|
| Demographic variable | |||
| Age (years) median (mean) | 20 (22) | 3 (16) | 0.02 |
| Sex (female; %) | 103 (41) | 318 (42) | NS |
| Father's education ≥ 6 years (%) | 77 (30) | 271 (36) | NS |
| Mother's education ≥ 6 years (%) | 59 (23) | 210 (28) | NS |
| Family monthly income > 5,000 Tk/month (%) | 151 (59) | 415 (55) | NS |
| Housing variable | |||
| Has own house | 89 (35) | 245 (32) | NS |
| Number of family members | 5 (6.1) | 5 (5.6) | NS |
| Drinking water source (%) | |||
| Tap | 142 (56) | 484 (63) | NS |
| Tube well | 112 (44) | 278 (37) | NS |
| Place of defecation (%) | |||
| Sanitary or semisanitary | 162 (64) | 494 (65) | NS |
| Other (dug hole, open pit, hanging) | 92 (36) | 268 (35) | NS |
| Time to hospital (minutes) median (mean) | 50 (73) | 45 (62) | NS |
| Distance to source of drinking water (feet) median (mean) | 20 (25) | 18 (26) | NS |
| Number family using water source, median (mean) | 4 (7) | 4 (9) | NS |
| Floor structure, cemented (%) | 172 (68) | 526 (69) | NS |
| Roof structure | |||
| Concrete | 66 | 211 | NS |
| Corrugated tin | 185 | 533 | NS |
| Others | 2 | 18 | NS |
| Fan ownership (%) | 217 (85) | 600 (79) | NS |
| TV ownership (%) | 146 (57) | 353 (46) | 0.04 |
| Luxury cot ownership (%) | 172 (68) | 447 (59) | NS |
| Gas for cooking | 142 (56) | 478 (63) | NS |
| Chicken enters kitchen | 51 (20) | 128 (17) | NS |
| Goat enters kitchen | 90 (35) | 275 (36) | NS |
| Any chicken/goat in home mentioned | 116 (46) | 342 (45) | NS |
Holm–Bonferroni-corrected. NS = P > 0.05.
Table 3.
Demographics, housing, and animal exposures of 468 diarrheal patients with NTS identified in stool compared with control diarrheal surveillance patients matched by month of admission
| All NTS (N = 468) | All-diarrhea (N = 1,404) | Adjusted P value* | |
|---|---|---|---|
| Demographic variable | |||
| Age (years) median (mean) | 8 (18) | 3 (14) | NS |
| Sex (female; %) | 190 (41) | 572 (41) | NS |
| Father's education ≥ 6 years (%) | 158 (34) | 494 (35) | NS |
| Mother's education ≥ 6 years (%) | 126 (27) | 371 (27) | NS |
| Family monthly income > 5,000 Tk/month (%) | 251 (54) | 629 (45) | 0.03 |
| Housing variable | |||
| Has own house (n; %) | 159 (34) | 383 (27) | NS |
| Number of family members median (mean) | 5 (5.8) | 5 (5.6) | NS |
| Drinking water source (%) | |||
| Tap | 273 (58) | 870 (62) | NS |
| Tube well | 273 (58) | 870 (62) | NS |
| Place of defecation (%) | |||
| Sanitary or semisanitary | 292 (62) | 850 (61) | NS |
| Other (dug hole, open pit, hanging) | 292 (62) | 850 (61) | NS |
| Time to hospital (minutes) median (mean) | 45 (79) | 40 (66) | NS |
| Distance to source of drinking water (feet) median (mean) | 20 (29) | 18 (32) | NS |
| Number of family using water source median (mean) | 5 (8) | 6 (11) | 0.02 |
| Floor structure, cemented (%) | 295 (63) | 902 (65) | NS |
| Roof structure, concrete (%) | 118 (25) | 340 (24) | NS |
| Fan ownership (%) | 372 (80) | 1,083 (77) | NS |
| TV ownership (%) | 230 (49) | 610 (44) | NS |
| Luxury cot ownership (%) | 285 (61) | 729 (52) | 0.02 |
| Gas for cooking (%) | 270 (58) | 857 (61) | NS |
| Chicken enters kitchen (%) | 102 (22) | 255 (18) | NS |
| Goat enters kitchen (%) | 187 (38) | 538 (39) | NS |
| Any chicken/goat in home mentioned (%) | 225 (48) | 650 (47) | NS |
Holm–Bonferroni-corrected. NS = P > 0.05.
We detected a number of differences in clinical presentation between NTS patients and controls, including significantly higher rates of abdominal pain (P = 0.02, compared with no-pathogen controls) and amount of intravenous rehydration required during admission (P = 0.002, compared with all-diarrhea controls) in NTS patients (Tables 4 and 5). We found no differences in measures of malnutrition, pre-admission oral rehydration solution (ORS) use, antibiotic use, degree of dehydration, use of intravenous rehydration, or duration of hospital stay. Although there were no differences in patient history regarding the self-reported character of the diarrhea (watery or bloody), microscopic examination of stool revealed that cases of NTS had stools with significantly more mucus, red blood cells, pus cells, and macrophages compared with stools in both control groups.
Table 4.
Clinical presentation, stool examination, and outcomes of 254 diarrheal patients with NTS identified as the only pathogen in stool compared with control diarrheal surveillance patients with no pathogen identified matched by month of admission
| Clinical variable at presentation | NTS only (N = 254) | No pathogen (N = 762) | Adjusted P value* |
|---|---|---|---|
| History | |||
| Watery stool (%) | 235 (93) | 713 (94) | NS |
| ORS use before presentation (%) | 232 (91) | 716 (94) | NS |
| Number of stools in past 24 hours > 10 times (%) | 116 (46) | 323 (42) | NS |
| Duration of diarrhea ≥ 7 days (%) | 25 (10) | 81 (11) | NS |
| Pre-admission antibiotic (%) | 194/459 (42) | 65/156 (42) | NS |
| Abdominal pain (%) | 132 (52) | 306 (40) | 0.02 |
| Vomiting in last 24 hours (%) | 202 (80) | 578 (76) | NS |
| Physical examination | |||
| Temperature > 37.8 °C (%) | 24 (9) | 35 (5) | NS |
| Drowsy or lethargic (%) | 149 (59) | 431 (57) | NS |
| Feeble or unpalpable radial pulse (%) | 43 (17) | 156 (20) | NS |
| Severe dehydration (%) | 44 (17) | 156 (20) | NS |
| HAZ < −2, stunted (%; for < 5 years of age) | 42/99 (42) | 137/384 (36) | NS |
| WAZ < −2, underweight (%; for < 5 years of age) | 52/99 (53) | 188/384 (49) | NS |
| WHZ < −2, wasted (%; for < 5 years of age) | 35/99 (35) | 126/384 (33) | NS |
| MUAC, median (mean; for < 5 years of age) | 12.8 (16.6) | 12.5 (15.9) | NS |
| Outcome | |||
| IV rehydration used | 64 (25) | 207 (27) | NS |
| Died | 1 (0.4) | 5 (0.7) | NS |
| Duration of hospital stay (hours) | 18 (31) | 16 (24) | NS |
| Stool examination | |||
| Consistency watery/liquid (%) | 162 (64) | 496 (65) | NS |
| Gross blood presence (%) | 8 (3) | 10 (1) | NS |
| Mucus moderate or heavy (%) | 82 (32) | 135 (18) | < 0.003 |
| RBC present on microscopy (%) | 128 (50) | 203 (27) | < 0.003 |
| Pus cells > 50 on microscopy (%) | 47 (18) | 54 (7) | < 0.003 |
| Macrophage present on microscopy (%) | 102 (40) | 140 (18) | < 0.003 |
HAZ = height-for-age Z score; MUAC = mid-upper arm circumference; RBC = red blood cell; WAZ = weight-for-age Z score; WHZ = weight-for-height Z score.
Holm–Bonferroni-corrected. NS = P > 0.05.
Table 5.
Clinical presentation, stool examination, and outcomes of 468 diarrheal patients with NTS identified in stool compared with control diarrheal surveillance matched by month of admission
| Clinical variable at presentation | All NTS (N = 468) | All-diarrhea (N = 1,404) | Adjusted P value* |
|---|---|---|---|
| History | |||
| Watery stool (%) | 438 (94) | 1,331 (95) | NS |
| ORS use before presentation (%) | 379 (81) | 1,131 (81) | NS |
| Number of stools in past 24 hours > 10 times (%) | 218 (47) | 521 (45) | NS |
| Duration of diarrhea ≥ 7 days (%) | 37 (8) | 109 (8) | NS |
| Pre-admission antibiotic (%) | 105 (36) | 303 (37) | NS |
| Abdominal pain (%) | 220 (47) | 574 (41) | NS |
| Vomiting in last 24 hours (%) | 377 (81) | 1,104 (79) | NS |
| Physical examination | |||
| Temperature > 37.8 °C (%) | 41 (9) | 75 (5) | NS |
| Drowsy or lethargic (%) | 269 (57) | 766 (55) | NS |
| Feeble or unpalpable radial pulse (%) | 87 (19) | 346 (25) | NS |
| Severe dehydration (%) | 87 (19) | 347 (25) | NS |
| HAZ < −2, stunted (%; for < 5 years of age) | 101 (46) | 284 (38) | NS |
| WAZ < −2, underweight (%; for < 5 years of age) | 116 (53) | 354 (48) | NS |
| WHZ < −2, wasted (%; for < 5 years of age) | 76 (35) | 228 (31) | NS |
| MUAC median (mean; for < 5 years of age) | 19 (29) | 15 (26) | NS |
| Outcome | |||
| IV rehydration used | 233 (50) | 465 (33) | 0.002 |
| Died | 2 (0.4) | 2 (0.1) | NS |
| Duration of hospital stay (hours) | 18 (31) | 15 (25) | NS |
| Stool examination | |||
| Consistency watery/liquid (%) | 321 (71) | 916 (70) | NS |
| Gross blood presence (%) | 13 (3) | 30 (2) | NS |
| Mucus moderate or heavy (%) | 119 (26) | 247 (19) | 0.02 |
| RBC present on microscopy (%) | 193 (42) | 387 (30) | < 0.003 |
| Pus cells > 50 on microscopy (%) | 71 (16) | 104 (8) | < 0.003 |
| Macrophage present on microscopy (%) | 149 (33) | 248 (19) | < 0.003 |
HAZ = height-for-age Z score; MUAC = mid-upper arm circumference; RBC = red blood cell; WAZ = weight-for-age Z score; WHZ = weight-for-height Z score.
Holm–Bonferroni-corrected. NS = P > 0.05.
Discussion
NTS infections are associated with significant morbidity and mortality worldwide; despite this fact, data on NTS in South Asia are limited. To our knowledge, this study is the first characterizing, in a longitudinal manner, the demographics, clinical characteristics, and risk factors for NTS gastroenteritis in South Asia.
We found that, as a percentage of diarrheal patients, NTS positivity in stools has decreased by approximately 7% per year over the 16-year study period. The reason for this observation is unclear, although it may be because of a combination of factors that could include changing socioeconomic conditions, better food handling practices, and increasing empiric antibiotic use in the community. The percentage of stools positive for NTS peaked in the summer months of July and August, months that are marked by high amounts of rainfall in the form of monsoons, flooding, and higher ambient temperature, and these conditions may be associated with higher rates of contamination of water and food. This seasonal peak during the summer months corresponds to reports from other Asian countries.10–12 Similarly, studies in Malawi13 and Kenya14,15 describe peaks of NTS disease associated with the rainy season, which may reflect, in part, the seasonal pattern of malaria in those areas, unlikely to be a factor at our site, as well as flooding. Although our data suggest that rainfall-associated contamination of water may be associated with disease transmission, it is likely that both environmental and host behavioral factors play a role in the seasonal variation of NTS, as they do for other enteric infections in Bangladesh.16,17
Although S. Typhimurium and S. Enteritidis are the predominant serotypes known to cause gastroenteritis and bacteremia in sub-Saharan Africa,18–20 limited data from studies in South and Southeast Asia show differences in serotype frequency, especially among stool isolates.6,12 In this analysis, we show that approximately 60% of NTS serogroups identified from stool were from Groups B and C1, whereas Group D, to which S. Enteritidis belongs, only represented 5% of isolates. This finding is consistent with a report from a hospital in Pakistan, where Groups B and C represented the majority of NTS isolated.5 It remains to be determined whether such differences in serogroup can explain differences in epidemiology and disease burden between Africa and Asia.
A recent study from Africa suggests that bacteremia-associated NTS infection has a bimodal age distribution, with one peak in children < 2 years and another peak in middle-aged adults associated with peak of HIV disease.21 Studies of NTS gastroenteritis have more heterogeneity in age patterns, but young children and older adults are thought to have higher risk of disease.22 In this study, we show that approximately one-half of all diarrhea cases associated with NTS were in children ages < 5 years, including nearly 30% in children < 1 year of age. This finding is likely a reflection of the demographic profile of patients presenting to our institution, with the majority (53%) of patients being < 5 years. In fact, when assessed as a percentage of those patients in the same age group presenting to the hospital with diarrhea, older adults have a higher likelihood of having NTS isolated from stool. Studies from developed countries have shown that the elderly with NTS infections have higher hospitalization and mortality rates.23,24
As opposed to reports of increasing resistance to traditional first-line agents in both Asia and Africa and increasing rates of multidrug resistance,13 we found a marked decrease in MDR and decreasing resistance to the traditional first-line agents ampicillin, chloramphenicol, and TMP/SMX but an increasing resistance to ciprofloxacin. This result may be because of a decreasing use of non-fluoroquinolone agents in favor of fluoroquinolones for the treatment of both gastroenteritis and undefined febrile illnesses. However, we also show decreasing resistance to ceftriaxone, which is in contrast with increasing rates of ceftriaxone resistance and emergence of extended spectrum β-lactamase–resistant (ESBL) organisms in Pakistan.5 Given recent increases in rates of S. Typhi with decreased susceptibility to fluorquinolones,25,26 third generation cephalosporins have been recommended as first-line agents for the treatment of enteric fever in India,27 and their use has increased in other parts of South Asia, including Bangladesh. We anticipate that this practice may lead to higher ceftriaxone resistance rates for Salmonella spp. as well as other organisms.
Although NTS is a known cause of gastroenteritis in resource-rich environments, the data are incomplete regarding how its isolation from stool relates to presence of symptoms in low-income countries with frequent fecal–oral contamination. Case-controlled studies from the United States,28 Palestine,29 Denmark,30 and Brazil31 suggest an association with acute diarrhea, whereas those studies in Thailand32 and Bangladesh33 do not; however, all of these studies have been limited by the small numbers of NTS identified. As shown in our study and others, NTS are often isolated alongside other pathogenic organisms, a finding common for other enteropathogens. In a previous report, nearly 40% of children presenting with diarrhea to our institution with NTS had infections with two or more pathogens, including approximately 60% of those children with V. cholerae O1 or O139.33 Similarly, 29% of patients presenting to a diarrheal center in Kolkata, India, had multiple pathogens, including 43% of those children with V. cholerae O1.34 Thus, to determine whether the NTS isolated in stool are incidental isolates or true pathogens, we performed a comparison of patients with NTS as the sole pathogen isolated with a control group of patients who had no pathogen isolated with the assumption that, if NTS were incidental, then there would be no differences between these groups. We also compared all patients with NTS isolated from stool with control patients chosen regardless of number of pathogens isolated. Doing this comparison, we show that NTS isolation is associated with more abdominal pain and an increased need for intravenous rehydration, and there is evidence of increased gut inflammation based on stool examination, including higher amounts of mucus, red blood cells, pus cells, and macrophages. These data suggest pathogenicity of NTS in our study population.
We then used the same comparison groups to determine risk factors for presence of NTS in stool among diarrheal patients. We found that NTS patients were generally older than controls, had higher rates of household ownership of luxury items, such as a television and a luxury cot, and a higher family income. We hypothesize that these socioeconomic associations may reflect differences in diet, housing, resources, and/or behavior. Although in our all-diarrhea analysis, NTS patients had significantly fewer numbers of family members using the same water source, there were no differences in type of home construction, distance to water source, or type of water source.
Unlike S. Typhi and S. Paratyphi, NTS are not human-restricted pathogens, and animals are thought to be important reservoirs associated with human infection. Direct animal contact has been associated with an estimated 11% of NTS cases in the United States35; however, a study from The Gambia showed no overlap in serotypes or genotypes of NTS isolated from humans and animals from the same household.36 In our analysis, we also did not find any association of NTS infection with presence of animals in the patient's home. Combined, these data may suggest that, in Dhaka, transmission of NTS may occur largely through ingestion of contaminated food and water rather than through direct contact with animals.
The burden of bacteremia-associated invasive NTS disease in many areas of Asia is likely much less than the burden in sub-Saharan Africa. For instance, only six cases of invasive NTS were detected in a community-based surveillance of febrile episodes in multiple Asian countries that analyzed over 20,000 blood culture results.37 At our diarrheal hospital, NTS accounts for < 1% of all positive blood cultures (unpublished data; not part of systematic surveillance system; blood cultures were drawn at the clinical discretion of the attending physician). We hypothesize that the discrepancy in invasive disease between sub-Saharan Africa and South Asia may be because of several factors, including lower rates in South Asia of severely immunocompromising conditions, such as advanced HIV infection, different burdens of pertinent coinfections including malaria, and perhaps, differences in circulating serotypes of NTS. Unfortunately, rates of NTS bacteremia are increasing in Asia, most notably in HIV-positive populations.38,39 Additional studies are needed to investigate the cause of such intercontinental differences.
Our study has several limitations. First, we used data from a hospital-based surveillance system, and thus, our epidemiological findings are limited to those individuals in the population who have symptoms severe enough to prompt them to seek medical care. Second, the conventional assays used to detect enteropathogens in the surveillance system likely only detect a subset of cases. We do not have data on pathogens such as diarrheagenic Escherichia coli, Yersinia, astrovirus, norovirus, or adenovirus; however, it is likely that both cases and control groups in our study may have similar occurrences of such pathogens. Third, housing and animal exposures were self-reported. Fourth, the surveillance system does not subtype NTS, and therefore, we cannot comment on the causative Salmonella beyond the serogroup level. Despite these limitations, our study is the largest longitudinal analysis of NTS in Asia, has identified seasonal and behavioral risk factors, and suggests that NTS are a significant cause of gastroenteritis in Dhaka, Bangladesh.
ACKNOWLEDGMENTS
This work was supported by the ICDDR,B and its donors, which provide unrestricted support to ICDDR,B for its operations and research. Current donors providing unrestricted support include the Australian Agency for International Development (AusAID), the Government of the People's Republic of Bangladesh, the Canadian International Development Agency (CIDA), the Swedish International Development Cooperation Agency (Sida), and the Department for International Development, United Kingdom (DFID). This study was also supported by grants from the National Institutes of Health, including National Institute of Allergy and Infectious Diseases Grants AI100923 (to D.T.L), AI100023 (to E.T.R.), and AI077883 (to E.T.R.) and Fogarty International Center Grant TW005572 (to F.K., F.Q., and E.T.R.), and a Postdoctoral Fellowship in Tropical Infectious Diseases from the American Society of Tropical Medicine and Hygiene/Burroughs Wellcome Fund (to D.T.L.).
Footnotes
Authors' addresses: Daniel T. Leung, Farhana Khanam, and Firdausi Qadri, Centre for Vaccine Sciences, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh, E-mails: dleung@icddrb.org, farhanak@icddrb.org, and fqadri@icddrb.org. Sumon K. Das, M. A. Malek, and A. S. G. Faruque, Centre for Nutrition and Food Security, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh, E-mails: sumon@icddrb.org, mamalek@icddrb.org, and gfaruque@icddrb.org. Dilruba Ahmed, Clinical Microbiology Laboratory, International Centre for Diarrhoeal Disease Research, Bangladesh (ICDDR,B), Dhaka, Bangladesh, E-mail: dahmed@icddrb.org. Edward T. Ryan, Division of Infectious Diseases, Massachusetts General Hospital, Boston, MA, E-mail: etryan@partners.org.
References
- 1.Majowicz SE, Musto J, Scallan E, Angulo FJ, Kirk M, O'Brien SJ, Jones TF, Fazil A, Hoekstra RM. International Collaboration on Enteric Disease ‘Burden of Illness’ Studies The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis. 2010;50:882–889. doi: 10.1086/650733. [DOI] [PubMed] [Google Scholar]
- 2.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal Salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ekdahl K, de Jong B, Wollin R, Andersson Y. Travel-associated non-typhoidal salmonellosis: geographical and seasonal differences and serotype distribution. Clin Microbiol Infect. 2005;11:138–144. doi: 10.1111/j.1469-0691.2004.01045.x. [DOI] [PubMed] [Google Scholar]
- 4.Menezes GA, Khan MA, Harish BN, Parija SC, Goessens W, Vidyalakshmi K, Baliga S, Hays JP. Molecular characterization of antimicrobial resistance in non-typhoidal salmonellae associated with systemic manifestations from India. J Med Microbiol. 2010;59:1477–1483. doi: 10.1099/jmm.0.022319-0. [DOI] [PubMed] [Google Scholar]
- 5.Jabeen K, Zafar A, Irfan S, Khan E, Mehraj V, Hasan R. Increase in isolation of extended spectrum beta lactamase producing multidrug resistant non typhoidal salmonellae in Pakistan. BMC Infect Dis. 2010;10:101. doi: 10.1186/1471-2334-10-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar Y, Sharma A, Sehgal R, Kumar S. Distribution trends of Salmonella serovars in India (2001–2005) Trans R Soc Trop Med Hyg. 2009;103:390–394. doi: 10.1016/j.trstmh.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 7.UNAIDS . Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. Geneva: UNAIDS (Joint United Nations Programme on HIV/AIDS); 2010. [Google Scholar]
- 8.Stoll BJ, Glass RI, Huq MI, Khan MU, Holt JE, Banu H. Surveillance of patients attending a diarrhoeal disease hospital in Bangladesh. Br Med J (Clin Res Ed) 1982;285:1185–1188. doi: 10.1136/bmj.285.6349.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 10.Lal A, Hales S, French N, Baker MG. Seasonality in human zoonotic enteric diseases: a systematic review. PLoS One. 2012;7:e31883. doi: 10.1371/journal.pone.0031883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen PL, Li CY, Hsieh TH, Chang CM, Lee HC, Lee NY, Wu CJ, Lee CC, Shih HI, Ko WC. Epidemiology, disease spectrum and economic burden of non-typhoidal Salmonella infections in Taiwan, 2006–2008. Epidemiol Infect. 2012;140:2256–2263. doi: 10.1017/S0950268812000088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hendriksen RS, Bangtrakulnonth A, Pulsrikarn C, Pornruangwong S, Noppornphan G, Emborg HD, Aarestrup FM. Risk factors and epidemiology of the ten most common Salmonella serovars from patients in Thailand: 2002–2007. Foodborne Pathog Dis. 2009;6:1009–1019. doi: 10.1089/fpd.2008.0245. [DOI] [PubMed] [Google Scholar]
- 13.Gordon MA, Graham SM, Walsh AL, Wilson L, Phiri A, Molyneux E, Zijlstra EE, Heyderman RS, Hart CA, Molyneux ME. Epidemics of invasive Salmonella enterica serovar enteritidis and S. enterica serovar typhimurium infection associated with multidrug resistance among adults and children in Malawi. Clin Infect Dis. 2008;46:963–969. doi: 10.1086/529146. [DOI] [PubMed] [Google Scholar]
- 14.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Muyodi J, Githinji JW, Kagendo D, Munyalo A, Hart CA. Invasive multidrug-resistant non-typhoidal Salmonella infections in Africa: zoonotic or anthroponotic transmission? J Med Microbiol. 2006;55:585–591. doi: 10.1099/jmm.0.46375-0. [DOI] [PubMed] [Google Scholar]
- 15.Brent AJ, Oundo JO, Mwangi I, Ochola L, Lowe B, Berkley JA. Salmonella bacteremia in Kenyan children. Pediatr Infect Dis J. 2006;25:230–236. doi: 10.1097/01.inf.0000202066.02212.ff. [DOI] [PubMed] [Google Scholar]
- 16.Koelle K, Rodo X, Pascual M, Yunus M, Mostafa G. Refractory periods and climate forcing in cholera dynamics. Nature. 2005;436:696–700. doi: 10.1038/nature03820. [DOI] [PubMed] [Google Scholar]
- 17.Hashizume M, Armstrong B, Wagatsuma Y, Faruque AS, Hayashi T, Sack DA. Rotavirus infections and climate variability in Dhaka, Bangladesh: a time-series analysis. Epidemiol Infect. 2008;136:1281–1289. doi: 10.1017/S0950268807009776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brooks JT, Ochieng JB, Kumar L, Okoth G, Shapiro RL, Wells JG, Bird M, Bopp C, Chege W, Beatty ME, Chiller T, Vulule JM, Mintz E, Slutsker L. Surveillance for bacterial diarrhea and antimicrobial resistance in rural western Kenya, 1997–2003. Clin Infect Dis. 2006;43:393–401. doi: 10.1086/505866. [DOI] [PubMed] [Google Scholar]
- 19.Reddy EA, Shaw AV, Crump JA. Community-acquired bloodstream infections in Africa: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10:417–432. doi: 10.1016/S1473-3099(10)70072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart CA. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006;6:101. doi: 10.1186/1471-2180-6-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feasey NA, Archer BN, Heyderman RS, Sooka A, Dennis B, Gordon MA, Keddy KH. Typhoid fever and invasive nontyphoid salmonellosis, Malawi and South Africa. Emerg Infect Dis. 2010;16:1448–1451. doi: 10.3201/eid1609.100125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hohmann EL. Nontyphoidal salmonellosis. Clin Infect Dis. 2001;32:263–269. doi: 10.1086/318457. [DOI] [PubMed] [Google Scholar]
- 23.Gradel KO, Schonheyder HC, Dethlefsen C, Kristensen B, Ejlertsen T, Nielsen H. Morbidity and mortality of elderly patients with zoonotic Salmonella and Campylobacter: a population-based study. J Infect. 2008;57:214–222. doi: 10.1016/j.jinf.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Kennedy M, Villar R, Vugia DJ, Rabatsky-Ehr T, Farley MM, Pass M, Smith K, Smith P, Cieslak PR, Imhoff B, Griffin PM. Emerging Infections Program FoodNet Working Group Hospitalizations and deaths due to Salmonella infections, FoodNet, 1996–1999. Clin Infect Dis. 2004;38((Suppl 3)):S142–S148. doi: 10.1086/381580. [DOI] [PubMed] [Google Scholar]
- 25.Chuang CH, Su LH, Perera J, Carlos C, Tan BH, Kumarasinghe G, So T, VAN PH, Chongthaleong A, Hsueh PR, Liu JW, Song JH, Chiu CH. Surveillance of antimicrobial resistance of Salmonella enterica serotype typhi in seven Asian countries. Epidemiol Infect. 2009;137:266–269. doi: 10.1017/S0950268808000745. [DOI] [PubMed] [Google Scholar]
- 26.Holt KE, Dutta S, Manna B, Bhattacharya SK, Bhaduri B, Pickard DJ, Ochiai RL, Ali M, Clemens JD, Dougan G. High-resolution genotyping of the endemic Salmonella typhi population during a Vi (typhoid) vaccination trial in Kolkata. PLoS Negl Trop Dis. 2012;6:e1490. doi: 10.1371/journal.pntd.0001490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kundu R, Ganguly N, Ghosh TK, Yewale VN, Shah RC, Shah NK, IAP Task Force. IAP task force report: management of enteric fever in children. Indian Pediatr. 2006;43:884–887. [PubMed] [Google Scholar]
- 28.Nataro JP, Mai V, Johnson J, Blackwelder WC, Heimer R, Tirrell S, Edberg SC, Braden CR, Glenn Morris J, Jr, Hirshon JM. Diarrheagenic Escherichia coli infection in Baltimore, Maryland, and New Haven, Connecticut. Clin Infect Dis. 2006;43:402–407. doi: 10.1086/505867. [DOI] [PubMed] [Google Scholar]
- 29.Al Jarousha AM, El Jarou MA, El Qouqa IA. Bacterial enteropathogens and risk factors associated with childhood diarrhea. Indian J Pediatr. 2011;78:165–170. doi: 10.1007/s12098-010-0249-0. [DOI] [PubMed] [Google Scholar]
- 30.Olesen B, Neimann J, Bottiger B, Ethelberg S, Schiellerup P, Jensen C, Helms M, Scheutz F, Olsen KE, Krogfelt K, Petersen E, Molbak K, Gerner-Smidt P. Etiology of diarrhea in young children in denmark: a case-control study. J Clin Microbiol. 2005;43:3636–3641. doi: 10.1128/JCM.43.8.3636-3641.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreno AC, Filho AF, Gomes Tdo A, Ramos ST, Montemor LP, Tavares VC, Filho Ldos S, Irino K, Martinez MB. Etiology of childhood diarrhea in the northeast of Brazil: significant emergent diarrheal pathogens. Diagn Microbiol Infect Dis. 2010;66:50–57. doi: 10.1016/j.diagmicrobio.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 32.Bodhidatta L, McDaniel P, Sornsakrin S, Srijan A, Serichantalergs O, Mason CJ. Case-control study of diarrheal disease etiology in a remote rural area in western Thailand. Am J Trop Med Hyg. 2010;83:1106–1109. doi: 10.4269/ajtmh.2010.10-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Albert MJ, Faruque AS, Faruque SM, Sack RB, Mahalanabis D. Case-control study of enteropathogens associated with childhood diarrhea in Dhaka, Bangladesh. J Clin Microbiol. 1999;37:3458–3464. doi: 10.1128/jcm.37.11.3458-3464.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindsay B, Ramamurthy T, Sen Gupta S, Takeda Y, Rajendran K, Nair GB, Stine OC. Diarrheagenic pathogens in polymicrobial infections. Emerg Infect Dis. 2011;17:606–611. doi: 10.3201/eid1704100939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hale CR, Scallan E, Cronquist AB, Dunn J, Smith K, Robinson T, Lathrop S, Tobin-D'Angelo M, Clogher P. Estimates of enteric illness attributable to contact with animals and their environments in the United States. Clin Infect Dis. 2012;54((Suppl 5)):S472–S479. doi: 10.1093/cid/cis051. [DOI] [PubMed] [Google Scholar]
- 36.Dione MM, Ikumapayi UN, Saha D, Mohammed NI, Geerts S, Ieven M, Adegbola RA, Antonio M. Clonal differences between non-typhoidal Salmonella (NTS) recovered from children and animals living in close contact in the gambia. PLoS Negl Trop Dis. 2011;5:e1148. doi: 10.1371/journal.pntd.0001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan MI, Ochiai RL, von Seidlein L, Dong B, Bhattacharya SK, Agtini MD, Bhutta ZA, Do GC, Ali M, Kim DR, Favorov M, Clemens JD. Non-typhoidal Salmonella rates in febrile children at sites in five Asian countries. Trop Med Int Health. 2010;15:960–963. doi: 10.1111/j.1365-3156.2010.02553.x. [DOI] [PubMed] [Google Scholar]
- 38.Nga TV, Parry CM, Le T, Lan NP, Diep TS, Campbell JI, Hoang NV, Dung le T, Wain J, Dolecek C, Farrar JJ, Chau NV, Hien TT, Day JN, Baker S. The decline of typhoid and the rise of non-typhoid salmonellae and fungal infections in a changing HIV landscape: bloodstream infection trends over 15 years in southern vietnam. Trans R Soc Trop Med Hyg. 2012;106:26–34. doi: 10.1016/j.trstmh.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Kiratisin P. Bacteraemia due to non-typhoidal Salmonella in Thailand: clinical and microbiological analysis. Trans R Soc Trop Med Hyg. 2008;102:384–388. doi: 10.1016/j.trstmh.2008.01.019. [DOI] [PubMed] [Google Scholar]