Abstract
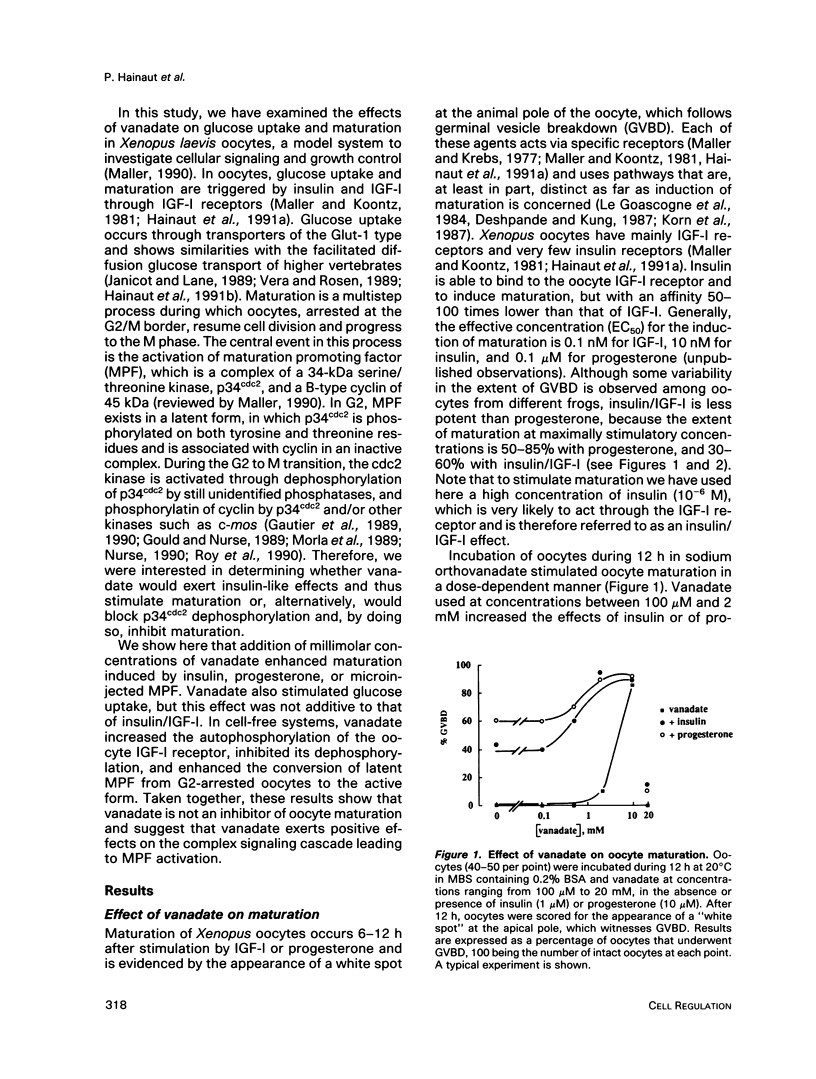
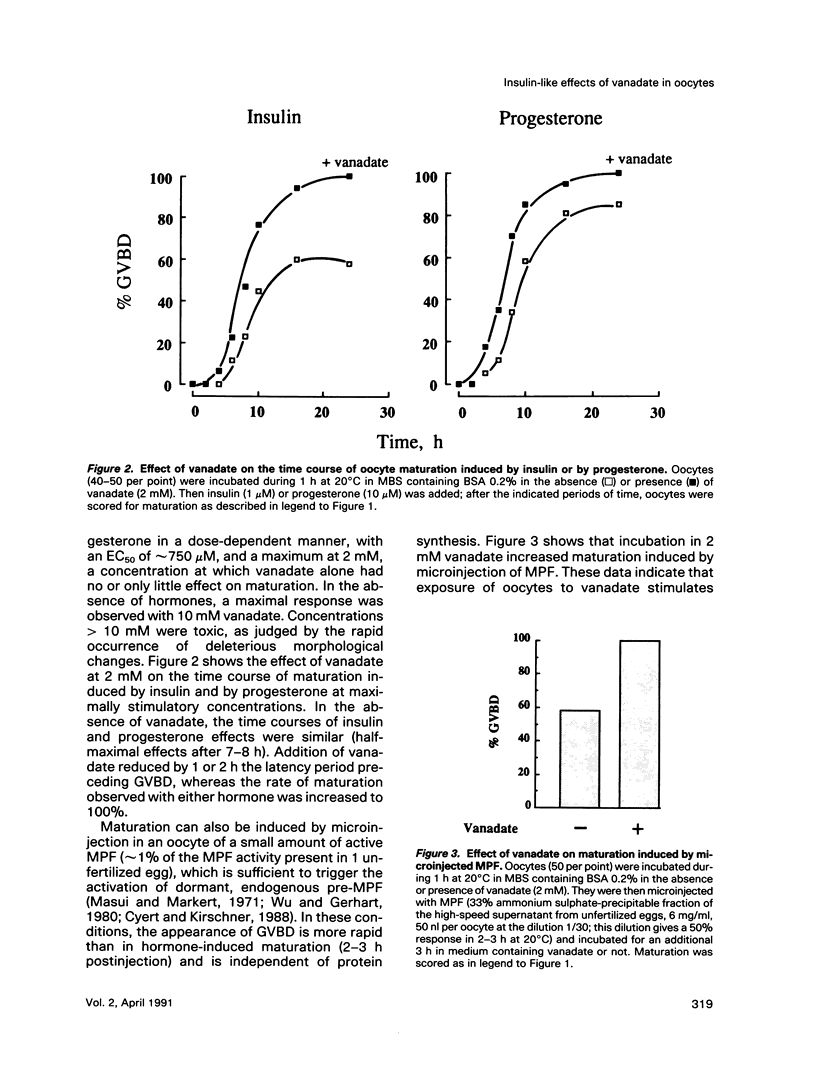
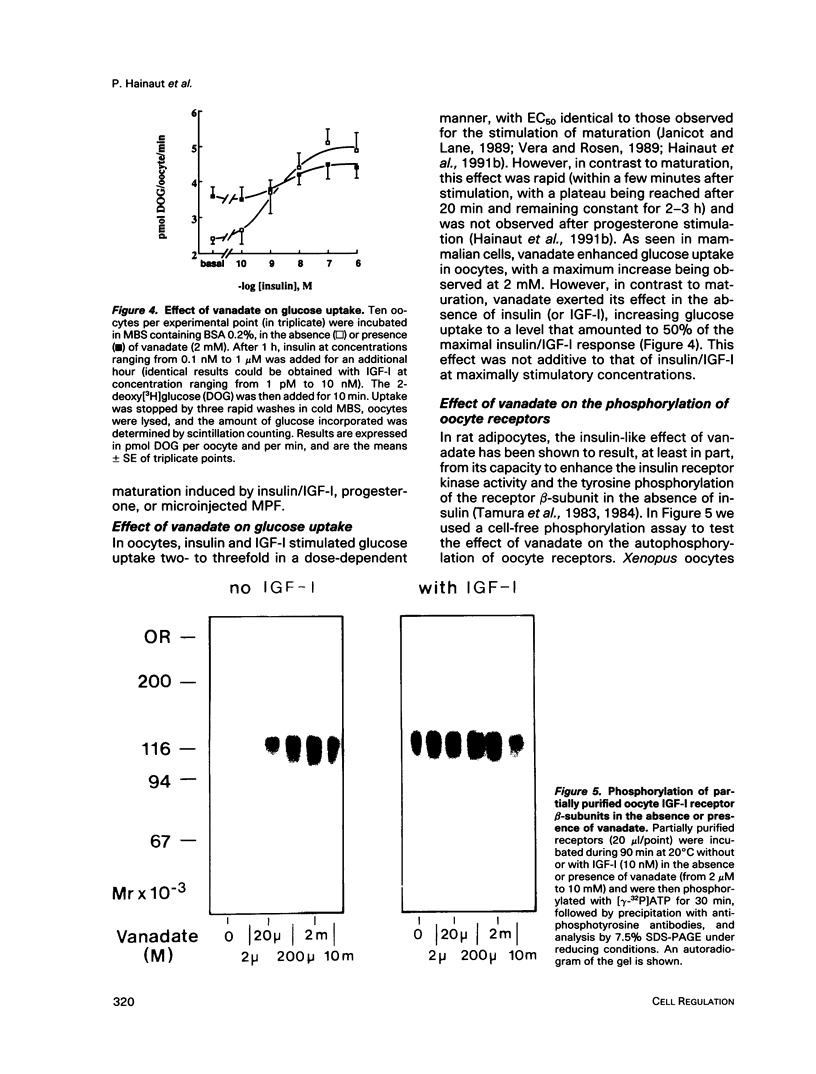
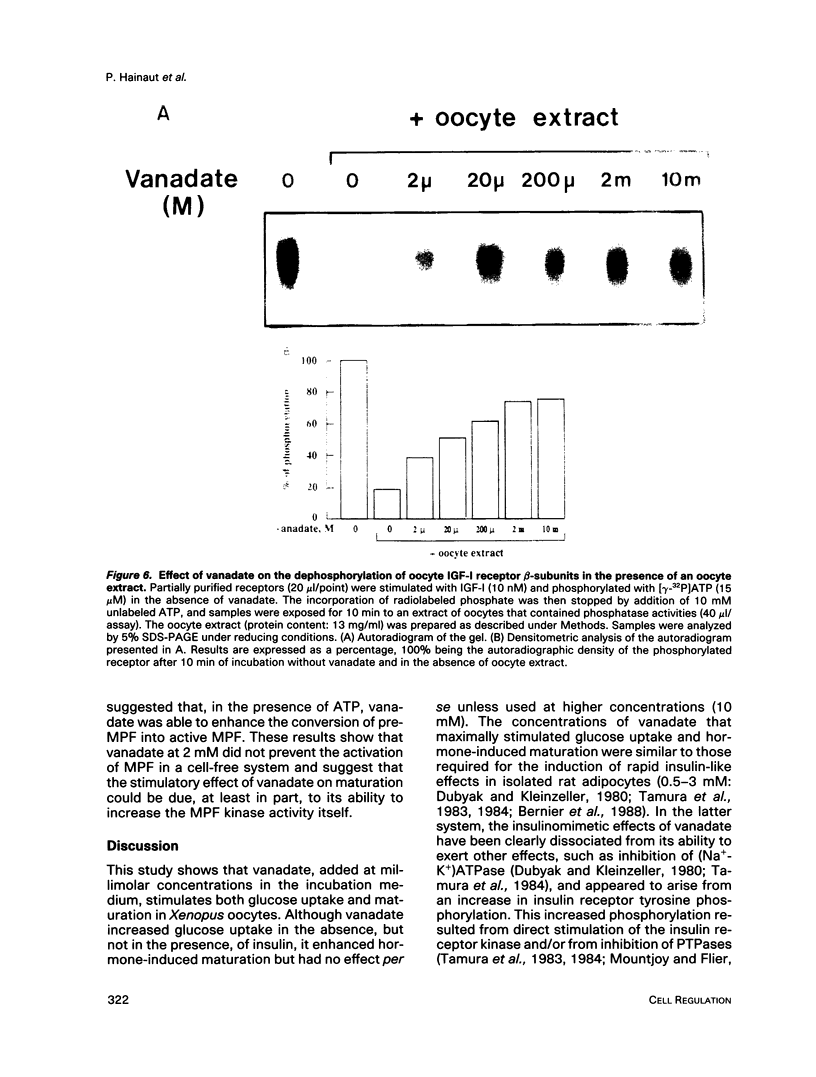
Vanadate, an inhibitor of phosphotyrosyl phosphatases that exerts insulin-like effects in intact cells, stimulated both maturation and glucose uptake in isolated Xenopus laevis oocytes. Vanadate enhanced the effects of insulin/IGF-I and progesterone on maturation in a dose-dependent manner, with an effective concentration of 750 microM and a maximum at 2 mM, whereas, in the absence of hormone, activation of maturation was seen at 10 mM vanadate. Further, vanadate at 2 mM increased glucose uptake, but this effect was not additive to that of the hormone. In cell-free systems, vanadate caused a 12-fold stimulation of autophosphorylation of the oocyte IGF-I receptor in the absence, but not in the presence, of IGF-I and inhibited largely, but not totally, receptor dephosphorylation induced by an extract of oocytes rich in phosphotyrosyl phosphatase activities. These effects were dose dependent, with effective concentrations of 50-100 microM and maxima at 2 mM. Moreover, using an acellular assay to study the effect of vanadate on the activation of maturation promoting factor (MPF), we found that vanadate at 2 mM stimulated the activation of the MPF H1 kinase. This suggests that vanadate did not prevent dephosphorylation of p34cdc2 on tyrosine residues. Vanadate thus exerted insulin-like effects in oocytes, including stimulation of maturation. These effects might result from a direct or indirect action of vanadate on the IGF-I receptor kinase and on MPF activity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boivin P., Galand C. The human red cell acid phosphatase is a phosphotyrosine protein phosphatase which dephosphorylates the membrane protein band 3. Biochem Biophys Res Commun. 1986 Jan 29;134(2):557–564. doi: 10.1016/s0006-291x(86)80456-9. [DOI] [PubMed] [Google Scholar]
- Brautigan D. L., Bornstein P., Gallis B. Phosphotyrosyl-protein phosphatase. Specific inhibition by Zn. J Biol Chem. 1981 Jul 10;256(13):6519–6522. [PubMed] [Google Scholar]
- Brautigan D. L., Sunwoo J., Labbé J. C., Fernandez A., Lamb N. J. Cell cycle oscillation of phosphatase inhibitor-2 in rat fibroblasts coincident with p34cdc2 restriction. Nature. 1990 Mar 1;344(6261):74–78. doi: 10.1038/344074a0. [DOI] [PubMed] [Google Scholar]
- Brown D. J., Gordon J. A. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem. 1984 Aug 10;259(15):9580–9586. [PubMed] [Google Scholar]
- Brunati A. M., Pinna L. A. Isolation and partial characterization of distinct species of phosphotyrosyl protein phosphatases from rat spleen. Biochem Biophys Res Commun. 1985 Dec 31;133(3):929–936. doi: 10.1016/0006-291x(85)91225-2. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Jr, Aisen P. The fate of cytoplasmic vanadium. Implications on (NA,K)-ATPase inhibition. J Biol Chem. 1979 Mar 25;254(6):1781–1784. [PubMed] [Google Scholar]
- Carpenter G. Vanadate, epidermal growth factor and the stimulation of DNA synthesis. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1115–1121. doi: 10.1016/s0006-291x(81)80127-1. [DOI] [PubMed] [Google Scholar]
- Cicirelli M. F., Pelech S. L., Krebs E. G. Activation of multiple protein kinases during the burst in protein phosphorylation that precedes the first meiotic cell division in Xenopus oocytes. J Biol Chem. 1988 Feb 5;263(4):2009–2019. [PubMed] [Google Scholar]
- Cicirelli M. F., Tonks N. K., Diltz C. D., Weiel J. E., Fischer E. H., Krebs E. G. Microinjection of a protein-tyrosine-phosphatase inhibits insulin action in Xenopus oocytes. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5514–5518. doi: 10.1073/pnas.87.14.5514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert M. S., Kirschner M. W. Regulation of MPF activity in vitro. Cell. 1988 Apr 22;53(2):185–195. doi: 10.1016/0092-8674(88)90380-7. [DOI] [PubMed] [Google Scholar]
- Deshpande A. K., Kung H. F. Insulin induction of Xenopus laevis oocyte maturation is inhibited by monoclonal antibody against p21 ras proteins. Mol Cell Biol. 1987 Mar;7(3):1285–1288. doi: 10.1128/mcb.7.3.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyak G. R., Kleinzeller A. The insulin-mimetic effects of vanadate in isolated rat adipocytes. Dissociation from effects of vanadate as a (Na+-K+)ATPase inhibitor. J Biol Chem. 1980 Jun 10;255(11):5306–5312. [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gautier J., Minshull J., Lohka M., Glotzer M., Hunt T., Maller J. L. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990 Feb 9;60(3):487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Goris J., Hermann J., Hendrix P., Ozon R., Merlevede W. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett. 1989 Mar 13;245(1-2):91–94. doi: 10.1016/0014-5793(89)80198-x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Hainaut P., Kowalski A., Giorgetti S., Baron V., Van Obberghen E. Insulin and insulin-like-growth-factor-I (IGF-I) receptors in Xenopus laevis oocytes. Comparison with insulin receptors from liver and muscle. Biochem J. 1991 Feb 1;273(Pt 3):673–678. doi: 10.1042/bj2730673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchon D., Ozon R., Demaille J. G. Protein phosphatase-1 is involved in Xenopus oocyte maturation. Nature. 1981 Nov 26;294(5839):358–359. doi: 10.1038/294358a0. [DOI] [PubMed] [Google Scholar]
- Janicot M., Lane M. D. Activation of glucose uptake by insulin and insulin-like growth factor I in Xenopus oocytes. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2642–2646. doi: 10.1073/pnas.86.8.2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Korn L. J., Siebel C. W., McCormick F., Roth R. A. Ras p21 as a potential mediator of insulin action in Xenopus oocytes. Science. 1987 May 15;236(4803):840–843. doi: 10.1126/science.3554510. [DOI] [PubMed] [Google Scholar]
- Labbé J. C., Picard A., Karsenti E., Dorée M. An M-phase-specific protein kinase of Xenopus oocytes: partial purification and possible mechanism of its periodic activation. Dev Biol. 1988 May;127(1):157–169. doi: 10.1016/0012-1606(88)90197-2. [DOI] [PubMed] [Google Scholar]
- Lau K. H., Farley J. R., Baylink D. J. Phosphotyrosyl protein phosphatases. Biochem J. 1989 Jan 1;257(1):23–36. doi: 10.1042/bj2570023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Goascogne C., Hirai S., Baulieu E. E. Induction of germinal vesicle breakdown in Xenopus laevis oocytes: synergistic action of progesterone and insulin. J Endocrinol. 1984 Apr;101(1):7–12. doi: 10.1677/joe.0.1010007. [DOI] [PubMed] [Google Scholar]
- Leis J. F., Kaplan N. O. An acid phosphatase in the plasma membranes of human astrocytoma showing marked specificity toward phosphotyrosine protein. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6507–6511. doi: 10.1073/pnas.79.21.6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtstein D., Mullikin-Kilpatrick D., Blume A. J. Modification of neuroblastoma x glioma hybrid NG108-15 adenylate cyclase by vanadium ions. Biochem Biophys Res Commun. 1982 Apr 14;105(3):1157–1165. doi: 10.1016/0006-291x(82)91091-9. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Koontz J. W. A study of the induction of cell division in amphibian oocytes by insulin. Dev Biol. 1981 Jul 30;85(2):309–316. doi: 10.1016/0012-1606(81)90262-1. [DOI] [PubMed] [Google Scholar]
- Maller J. L., Krebs E. G. Progesterone-stimulated meiotic cell division in Xenopus oocytes. Induction by regulatory subunit and inhibition by catalytic subunit of adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1977 Mar 10;252(5):1712–1718. [PubMed] [Google Scholar]
- Maller J. L. Xenopus oocytes and the biochemistry of cell division. Biochemistry. 1990 Apr 3;29(13):3157–3166. doi: 10.1021/bi00465a001. [DOI] [PubMed] [Google Scholar]
- Masui Y., Markert C. L. Cytoplasmic control of nuclear behavior during meiotic maturation of frog oocytes. J Exp Zool. 1971 Jun;177(2):129–145. doi: 10.1002/jez.1401770202. [DOI] [PubMed] [Google Scholar]
- Meyerovitch J., Farfel Z., Sack J., Shechter Y. Oral administration of vanadate normalizes blood glucose levels in streptozotocin-treated rats. Characterization and mode of action. J Biol Chem. 1987 May 15;262(14):6658–6662. [PubMed] [Google Scholar]
- Morgan D. O., Kaplan J. M., Bishop J. M., Varmus H. E. Mitosis-specific phosphorylation of p60c-src by p34cdc2-associated protein kinase. Cell. 1989 Jun 2;57(5):775–786. doi: 10.1016/0092-8674(89)90792-7. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norbury C. J., Nurse P. Control of the higher eukaryote cell cycle by p34cdc2 homologues. Biochim Biophys Acta. 1989 Jul 28;989(1):85–95. doi: 10.1016/0304-419x(89)90036-x. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Rime H., Ozon R. Protein phosphatases are involved in the in vivo activation of histone H1 kinase in mouse oocyte. Dev Biol. 1990 Sep;141(1):115–122. doi: 10.1016/0012-1606(90)90106-s. [DOI] [PubMed] [Google Scholar]
- Roy L. M., Singh B., Gautier J., Arlinghaus R. B., Nordeen S. K., Maller J. L. The cyclin B2 component of MPF is a substrate for the c-mos(xe) proto-oncogene product. Cell. 1990 Jun 1;61(5):825–831. doi: 10.1016/0092-8674(90)90192-h. [DOI] [PubMed] [Google Scholar]
- Russell P., Nurse P. The mitotic inducer nim1+ functions in a regulatory network of protein kinase homologs controlling the initiation of mitosis. Cell. 1987 May 22;49(4):569–576. doi: 10.1016/0092-8674(87)90459-4. [DOI] [PubMed] [Google Scholar]
- Ryder J. W., Gordon J. A. In vivo effect of sodium orthovanadate on pp60c-src kinase. Mol Cell Biol. 1987 Mar;7(3):1139–1147. doi: 10.1128/mcb.7.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy S., Choi J. K., Bagrodia S., Copeland T. D., Maller J. L., Shalloway D. Purified maturation promoting factor phosphorylates pp60c-src at the sites phosphorylated during fibroblast mitosis. Cell. 1989 Jun 2;57(5):763–774. doi: 10.1016/0092-8674(89)90791-5. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Dubler R. E., Larner J. Insulin-like effect of vanadate on adipocyte glycogen synthase and on phosphorylation of 95,000 dalton subunit of insulin receptor. Biochem Biophys Res Commun. 1983 May 31;113(1):80–86. doi: 10.1016/0006-291x(83)90434-5. [DOI] [PubMed] [Google Scholar]
- Tamura S., Brown T. A., Whipple J. H., Fujita-Yamaguchi Y., Dubler R. E., Cheng K., Larner J. A novel mechanism for the insulin-like effect of vanadate on glycogen synthase in rat adipocytes. J Biol Chem. 1984 May 25;259(10):6650–6658. [PubMed] [Google Scholar]
- Tonks N. K., Cicirelli M. F., Diltz C. D., Krebs E. G., Fischer E. H. Effect of microinjection of a low-Mr human placenta protein tyrosine phosphatase on induction of meiotic cell division in Xenopus oocytes. Mol Cell Biol. 1990 Feb;10(2):458–463. doi: 10.1128/mcb.10.2.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Functional expression of mammalian glucose transporters in Xenopus laevis oocytes: evidence for cell-dependent insulin sensitivity. Mol Cell Biol. 1989 Oct;9(10):4187–4195. doi: 10.1128/mcb.9.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitto A., Jr, wallace R. A. Maturation of Xenopus oocytes. I. Facilitation by ouabain. Exp Cell Res. 1976 Jan;97:56–62. doi: 10.1016/0014-4827(76)90654-6. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Misulovin Z. The role of zinc and follicle cells in insulin-initiated meiotic maturation of Xenopus laevis oocytes. Science. 1980 Nov 21;210(4472):928–930. doi: 10.1126/science.7001631. [DOI] [PubMed] [Google Scholar]
- Wu M., Gerhart J. C. Partial purification and characterization of the maturation-promoting factor from eggs of Xenopus laevis. Dev Biol. 1980 Oct;79(2):465–477. doi: 10.1016/0012-1606(80)90131-1. [DOI] [PubMed] [Google Scholar]






