Abstract
Japanese encephalitis virus (JEV) infection in mosquitoes was monitored in Vietnam from 2006 to 2008. A total of 15,225 mosquitoes, identified as 26 species in five genera were collected and 12,621 were grouped into 447 pools for examination of JEV infection by assays for cytopathic effects in C6/36 cells and by RT-PCR to detect flavivirus RNA. Three JEV strains were isolated from Culex tritaeniorhynchus Giles collected in northern and southern Vietnam and two JEV strains were isolated from Culex vishnui Theobald collected in the highlands of Vietnam. Genetic and phylogenetic analyses, based on complete E gene nucleotide sequences, revealed that the five JEV strains were classified into the genotype I group and six amino acid differences were found in these five strains. These results indicated that multiple JEV genotype I populations are circulating countrywide in Vietnam, transmitted by bites of their Cx. tritaeniorhynchus and Cx. vishnui.
Introduction
Japanese encephalitis (JE) is a leading cause of viral encephalitis, an important human infectious disease, and is endemic in many Asian countries. Approximately 30,000–50,000 clinical JE cases, with 10,000 deaths, have been reported annually.1,2 Japanese encephalitis virus (JEV), a mosquito-borne flavivirus, has been found throughout Asia3,4 and relatively recently spread to south India, Sri Lanka, and northern Australia.5–7 The JEV is transmitted by paddy-breeding mosquitoes of the Culex vishnui subgroup, primarily Culex tritaeniorhynchus Giles, and amplified by infection of pigs and/or most likely Ardeidae birds in nature. Hence, JE distribution is significantly linked to irrigated rice production combined with pig rearing.
Several studies have previously analyzed the phylogentic relatedness of JEV strains isolated in Asian countries.8–10 The JEVs have been divided into five genotypes (genotype I to V) based on the nucleotide sequence of the viral envelope (E) gene.11 The only reported genotype V JEV is the Muar strain, first isolated from Malaysia in 1952, but genotype V JEV re-emerged in China in 2009.12 The JEV genotype I first appeared in Taiwan in 2008, but genotype III is still dominant in Taiwan.13,14 Genotype III was the major epidemic JEV genotype throughout the tropical and temperate regions of Asia before the 1990s.15 However, genotype III disappeared in northern Vietnam, Korea, and Japan during the early to mid-1990s and was replaced by genotype I.8,16 Genotype I has increased gradually and is now recognized as the dominant strain in most of the JEV-endemic areas in Asia.17 A molecular epidemiological study of JEV in Vietnam, China, and Japan divided genotype I into two subgenotypes (1-A and 1-B), and subgenotype 1-A into eight subclusters (1-A-1 to 1-A-8).18 These diverse JEV genotype I populations have been circulating in East Asia, with some being carried a long distance by migrating infected birds and/or mosquitoes.9 Vietnam has been suggested to be an important site on the flyway of birds and/or mosquitoes for the dispersal and consequent outbreaks of JEV throughout Asia.
In Vietnam, JEV has been circulating predominantly in rural environments and is recognized as one of the most significant mosquito-borne flaviviruses.19 The first JE case in Vietnam was reported in 1951 and JE epidemics then increased up to the late 1970s.20–22 Clinical cases subsequently decreased after vaccination was introduced in 1997,19 However, JE is still endemic countrywide in Vietnam with an annual incidence of 1,000–3,000 cases in 2002.2,23 Epidemics of acute encephalitis syndrome (AES), on the other hand, have been frequently reported in Vietnam since the 1960s, with reported incidences as high as 22/100,000 population.20 A large number of AES cases were reported recently, however laboratory tests showed that 52% of these were JEV infections.19 Both JE and AES infections have been recognized as important public health problems in Vietnam.
Mosquito surveys were carried out in Ha Tay province in northern Vietnam in 2003, and the distribution of important JE vectors, Culex vishnui subgroup and Culex gelidus Theobald, were investigated.24 Arbovirus and mosquito surveillance was subsequently conducted from 2004 to 2006 and four virus species (i.e., Sagiyama, Getah, Oya, and Akabane viruses), but not JEV, were isolated from mosquitoes.25 However, these surveillance studies were mostly conducted in northern Vietnam, around the city of Hanoi. Therefore, in the current study, we focused on JEV infection in mosquitoes throughout Vietnam, not only in northern areas, to investigate the current distribution of JEV in Vietnam. It has recently been reported that there are amino acid variations in the E protein among JEV genotype I strains26–29; we also carried out genetic and phylogenetic analyses of viral E gene sequences to study the relationships between Vietnamese JEVs and JEVs from other areas of Asia.
Materials and Methods
Study sites.
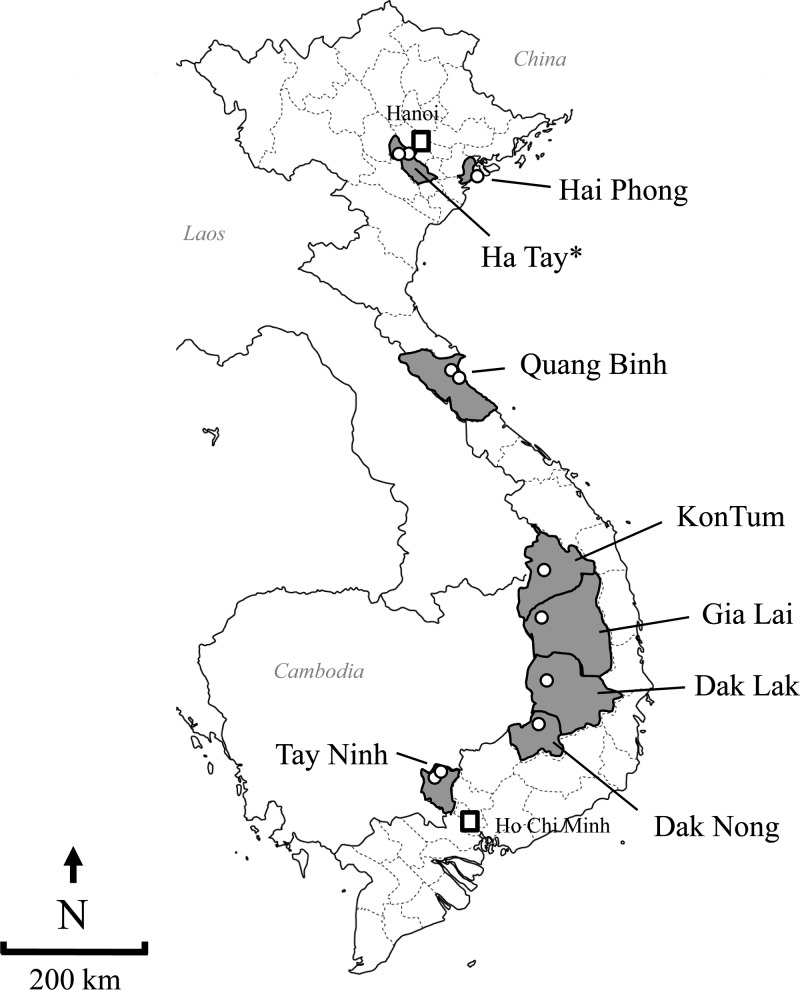
Mosquito collection was carried out at 11 sites in four districts of Vietnam from May 2006 to June 2008 (Figure 1). The sites were in Ha Tay province (the current administrative section is Hanoi city); Hai Phong city in northern Vietnam; Quang Binh province in central Vietnam; Kon Tum, Gia Lai, Dak Lak, and Dak Nong provinces in the highlands of Vietnam; and Tay Ninh province in southern Vietnam. Cat Que and Quang Trung communes in Ha Tay province (N21°13′ E105°49′ and N20°58′ E105°46′, respectively) are in mountainous and hilly areas, whereas Do Son town in Hai Phong city (N20°43′ E106°45′) is in a coastal area. Two communes, Tay Trach and Trung Trach, in Quang Binh province (N17°45′ E106°26′ and N17°31′ E106°31′, respectively) are near the coast. Each two collection sites in four provinces are located in the highlands, which are dry and cool during June and July. They were Hoa Binh in Kon Tum province (N14°16′ E107°59′), Jab Lang in Gia Lai province (N13°39′ E108°04′), Ebuk in Dak Lak province (N12°50′ E108°02′), and Tam Trang in Dak Nong province (N12°36′ E107°54′). Two communes in Tay Ninh province (N11°16′ E106°06′ and N11°21′ E106°11′, respectively) are in southwestern Vietnam, which has a tropical climate. Most commune inhabitants were rice farmers. Each site targeted for mosquito collection was located at a pig farm, a cattle farm, or a rice field within approximately a 10 m radius of a house with a confirmed human case of JEV.
Figure 1.
Map of Vietnam showing locations of the 11 mosquito collection sites in this study.
Mosquito collection.
Adult mosquitoes were collected using several methods: Centers for Disease Control and Prevention (CDC) dry ice-baited traps,30 sweeping nets, aspirators, and animal nets24 based on the situation at each collection site. Sweeping nets and aspirators were used at all collection sites. In particular, animal net (cattle-baited) traps were used for mosquito collection in Quang Binh and Tay Ninh provinces, because it was difficult to supply dry ice for collection at these sites. Briefly, a stock stay was double covered with pieces of white nets to attract mosquitoes from livestock. Mosquitoes attracted to the outside of the inner net were caught by sweeping nets and/or aspirators from the area between the inner and outer nets. Female mosquitoes collected by this method did not have a blood meal in their body. Mosquito collection was generally carried out for 3 hours per night between 6:00 pm and 9:00 pm.
Identification of mosquito species.
All field-collected mosquitoes were transported on ice to the local provincial health office for species identification according to established identification keys.31–33 Blood-fed females were kept in mosquito cages for a few days before species identification, because blood in the mosquito gut must be digested before being used for virus isolation. After species identification, mosquitoes were sorted by species and sex, pooled in 2 mL microtubes (Eppendorf, Hamburg, Germany), with a maximum of 50 adults per pool, and kept in a vapor shipper (MVZ, Wheaton, CA) during transportation to the National Institute of Hygiene and Epidemiology (NIHE), Hanoi. For Anopheles mosquitoes, species identification was supplemented with molecular procedures using internal transcribed spacer (ITS) 2 sequences according to the previously described method.34,35 Mosquitoes were sorted into pools containing 20–50 adults and stored at −80°C
Virus isolation.
The mosquito C6/36 cell line, derived from Aedes albopictus Skuse (Health Science Research Resources Bank (HSRRB), Osaka, Japan), was used for virus isolation as described previously.36,37 Briefly, pools of mosquitoes were homogenized in 500 μL ice-cold Eagle's minimum essential medium (MEM, Sigma-Aldrich, St. Louis, MO) containing 2% heat-inactivated fetal bovine serum (FBS, MP Biomedicals, Costa Mesa, CA), 2% non-essential amino acids (NEAA, Sigma-Aldrich), 200 U penicillin/mL, 200 μg streptomycin/mL, and 10 μL Fungizone (Gibco BRL, Gaithersburg, MD)/mL using a Mixer Mill (Model MM300, Retsch GmbH, Haan, Germany). The homogenates were clarified by centrifugation, and the resulting supernatants passed through sterile 0.45 μm filters (Ultrafree MC, Millipore, Bedford, MA). The filtrates were diluted 10-fold with the same medium and 50 μL of these were inoculated onto monolayers of C6/36 cells in 24-well culture plates. The plates were incubated for 2 hr at 28°C to allow virus adsorption. After addition of 500 μL fresh medium, the cell cultures were incubated under the same conditions for ∼7 d. Observations were made daily by phase-contrast microscopy for cytopathic effects. Culture supernatants were collected after at least three blind passages and used as virus stocks. The virus stocks were stored at −80°C.
Reverse transcription-polymerase chain reaction (RT-PCR) and sequence analysis of viral RNA.
Viral RNA was extracted from cell culture supernatants using a High Pure Viral RNA Kit (Roche Diagnostics, Mannheim, Germany) or a QIAamp Viral RNA Mini Kit (QIAGEN Inc., Valencia, CA). The RT-PCR was conducted using a TaKaRa One Step RT-PCR Kit (Takara Bio, Shiga, Japan). Flavivirus universal primer sets for fragments of the NS3 gene (Fla-U5004/Fla-L5457)38 and NS5 gene (FU1/cFD2 and FU2/cFD3)39 were used. The RT-PCR was carried out according to the manufacturer's instructions. The amplified products were purified by agarose gel electrophoresis, followed by fragment extraction using a QIAEXII Gel Extraction Kit (QIAGEN). Purified DNA fragments were directly cycle-sequenced using an ABI PRISM BigDye Terminator Cycle Sequencing Kit v.1.1 (Applied Biosystems, Foster City, CA) and ABI PRISM 3130 Genetic Analyzer (Applied Biosystems). Sequence analyses were performed using the GENETYX-WIN v.10 program (Genetyx Corp., Tokyo, Japan).
Phylogenetic analysis of JEV.
To analyze the phylogenetic relationships of the JEV isolates, we reconstructed a phylogenetic tree based on complete E gene nucleotide sequences of the five JEV strains isolated in this study and 42 JEV strains in the GenBank database. The sequences were aligned by CLUSTALX ver. 2.0.840 and the aligned matrix data were analyzed by a neighbor-joining (NJ) algorithm using MEGA ver. 4.1.41 The statistical significance of the resulting NJ tree was evaluated using a bootstrap test with 1,000 replications.
Results
Mosquito collection.
A total of 15,225 mosquitoes were collected during a 3-year survey in Vietnam (data not shown). Of the 26 species collected in Vietnam in this study, Cx. tritaeniorhynchus was the predominant species (34.4% of the mosquitoes collected), followed by Cx. quinquefasciatus Say (28.2%), Cx. vishnui Theobald (11.0%), and Cx. gelidus (7.0%). Because 497 mosquitoes in the Cx. vishnui subgroup, consisting of Cx. tritaeniorhynchus, Cx. vishnui, and Cx. pseudovishnui Colless, were unidentified (3.3%), the actual prevalence of both Cx. tritaeniorhynchus and Cx. vishnui could be higher. Of these 496 Anopheles mosquitoes collected (3.3%), a total of 485 specimens were classified (3.2%) in five species: An. peditaeniatus Leicester, An. sinensis Wiedemann, An. subpictus Grassi, An. tessellates Theobald, and An. vagus Doenitz. The determined sequences by ITS2 sequence analysis were deposited in the GenBank database (accession nos.: AB731654–AB731658, respectively).
Species composition of Culex mosquitoes in the highlands of Vietnam.
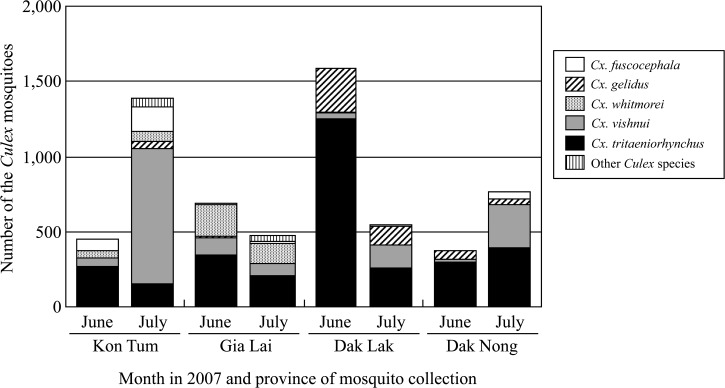
A total of 6,724 adult mosquitoes were collected in the four highlands provinces, with 93.2% identified as belonging to eight species of Culex mosquitoes (Figure 2). Culex tritaeniorhynchus was the most abundant species in the four highlands provinces followed by Cx. vishnui (50.8% and 26.5% of the total, respectively) (data not shown). After Cx. tritaeniorhynchus and Cx. vishnui, Cx. fuscocephala Theobald was the most abundant species in Kon Tum province, Cx. whitmorei Giles in Gia Lai province and Cx. gelidus in Dak Lak and Dak Nong provinces. Culex whitmorei was also collected in Kon Tum and Gia Lai provinces, and Cx. gelidus was also collected in Dak Lak and Dak Nong provinces. Significant differences in species compositions were found between June and July. The number of Cx. tritaeniorhynchus collected was significantly higher in June than in July in Kon Tum, Gia Lai, and Dak Lak provinces (χ2 test, P < 0.0001), but not in Dak Nong province. In contrast, the number of Cx. vishnui collected was significantly higher in July than in June (χ2 test, P < 0.0001) in Kon Tum, Dak Lak, and Dak Nong provinces, but not in Gia Lai province, although the significance of the difference was low (χ2 test, P > 0.4). The number of Cx. fuscocephala collected was higher in July than in June in Kon Tum, Gia Lai, and Dak Nong provinces (χ2 test, P < 0.0001), but not in Dak Lak province.
Figure 2.
Distribution of the eight species of Culex mosquitoes collected in four provinces in the highlands of Vietnam in June and July 2007. The “other Culex species” group contains three species: Cx. bitaeniorhynchus, Cx. quinquefasciatus and Cx. pseudovishnui.
Virus isolation from mosquitoes.
A total of 12,621 mosquitoes (10,407 females and 2,214 males) in 447 pools were analyzed for flaviviruses using C6/36 cells (Table 1). Three of the 131 pools of Cx. tritaeniorhynchus collected from Ha Tay province in May 2006 and from Tay Ninh province in June 2008, and two of the 46 pools of Cx. vishnui collected from Kon Tum province in July 2007 were positive for JEV. All five JEV isolates were confirmed by their nucleotide sequences.
Table 1.
Mosquitoes from Vietnam processed for JEV isolation from 2006 to 2008
| Species | No. mosquitoes tested | No. pools tested | No. JEV isolates | ||
|---|---|---|---|---|---|
| Total | Female | Male | |||
| Aedes aegypti | 452 | 230 | 222 | 23 | |
| Ae. albopictus | 85 | 61 | 24 | 21 | |
| Anopheles vagus | 429 | 429 | 0 | 13 | |
| Armigeres spp. | 57 | 28 | 29 | 13 | |
| Culex bitaeniorhynchus | 4 | 4 | 0 | 2 | |
| Cx. fuscocephala | 472 | 472 | 0 | 20 | |
| Cx. gelidus | 1,017 | 977 | 40 | 45 | |
| Cx. infula | 1 | 1 | 0 | 1 | |
| Cx. pseudovishnui | 87 | 87 | 0 | 6 | |
| Cx. quinquefasciatus | 3,693 | 1,821 | 1,872 | 101 | |
| Cx. sitiens | 1 | 1 | 0 | 1 | |
| Cx. tritaeniorhynchus | 4,199 | 4,182 | 17 | 131 | 3 |
| Cx. vishnui | 1,542 | 1,542 | 0 | 46 | 2 |
| Cx. vishnui subgroup | 200 | 200 | 0 | 7 | |
| Cx. whitmorei | 345 | 345 | 0 | 11 | |
| Mansonia annulifera | 10 | 0 | 10 | 1 | |
| Ma. dives | 1 | 1 | 0 | 1 | |
| Ma. indiana | 7 | 7 | 0 | 1 | |
| Ma. uniformis | 19 | 19 | 0 | 3 | |
| Total | 12,621 | 10,407 | 2,214 | 447 | 5 |
The minimum infection rate (MIR) of JEV is defined as (number of JEV-positive pools/number of mosquitoes tested) × 1,000. From the data in Table 1, the MIR was 0.71 (3 positive pools/4,199 mosquitoes tested) for JEV in Cx. tritaeniorhynchus and 1.30 (2 positive pools/1,542 mosquitoes tested) in Cx. vishnui.
Genetic and phylogenetic analysis of JEV.
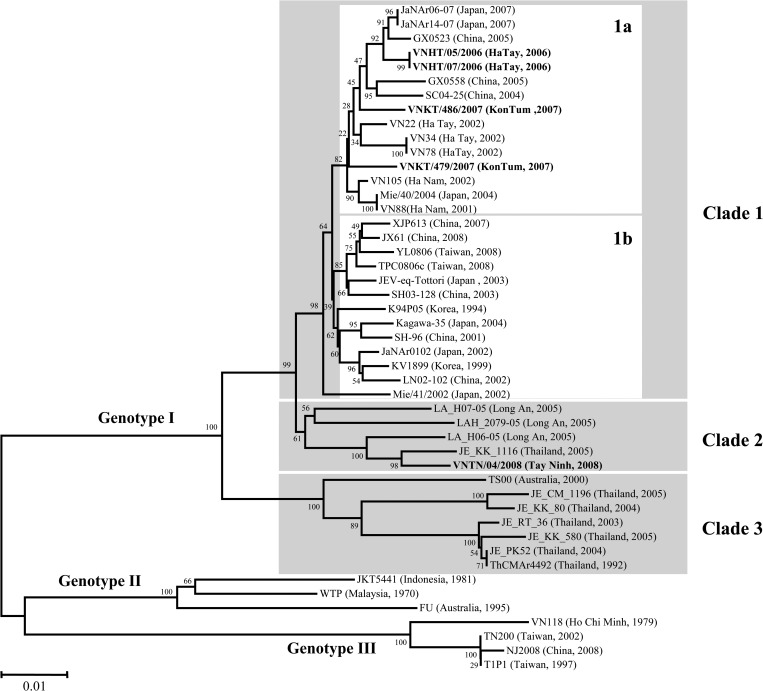
The complete nucleotide sequences of the E genes of the five JEVs isolated in this study have been submitted to the DNA Data Bank of Japan (DDBJ), European Molecular Biology Laboratory (EMBL), and GenBank databases (Table 2). The details of the three JEV strains isolated from Cx. tritaeniorhynchus (designated VNHT/05/2006, VNHT/07/2006, and VNTN/04/2008) and the two JEV strains isolated from Cx. vishnui (designated VNKT/479/2007 and VNKT/486/2007) are shown in Table 2. An NJ tree was constructed based on 1,500 complete nucleotide sequences of the E genes with 42 corresponding strains from the GenBank database (Table 2). The NJ tree showed that the five JEV strains isolated in this study were in the JEV genotype I branch, with four strains in clade 1 of genotype I, and one strain in clade 2 of genotype I (Figure 3 ). Clade 1 strains of JEV are widespread throughout China, Korea, and Japan, and in the northern and highlands areas of Vietnam. Clade 1 can be divided into two subclusters, clade 1a and 1b. The four JEV strains of clade 1 isolated in this study were in subcluster clade 1a. Clade 2 contained JEV strains from southern Vietnam and Thailand, including one of the JEV strains isolated in this study. Clade 3 contained JEV isolates from Thailand and Australia, but no Vietnamese JEV isolated in this study.
Table 2.
JEV strains used for phylogenetic analysis*
| Strain | Year | Location | Source | Genotype | GenBank accession no. | |
|---|---|---|---|---|---|---|
| TS00 | 2000 | Australia | Badu Island | Swine serum | I | AF289814 |
| SH-96 | 2001 | China | Shanghai | Cx. tritaeniorhynchus | I | AY555760 |
| LN02-102 | 2002 | China | Liaoning | Cx. tritaeniorhynchus | I | DQ404085 |
| SH03-128 | 2003 | China | Shanghai | Cx. tritaeniorhynchus | I | DQ404102 |
| SC04-25 | 2004 | China | Sichuan | Culex spp. | I | DQ404094 |
| GX0523 | 2005 | China | Cx. tritaeniorhynchus | I | FJ161968 | |
| GX0558 | 2005 | China | Cx. tritaeniorhynchus | I | FJ161969 | |
| XJP613 | 2007 | China | Cx. tritaeniorhynchus | I | EU693899 | |
| JX61 | 2008 | China | Pig serum | I | GU556217 | |
| Mie/41/2002 | 2002 | Japan | Mie | Swine blood | I | AB112709 |
| JaNAr0102 | 2002 | Japan | Nagasaki | Swine blood | I | AY377577 |
| JEV-eq-Tottori | 2003 | Japan | Kurayoshi | Horse cerebrum | I | AB213007 |
| Mie/40/2004 | 2004 | Japan | Mie | Swine blood | I | AB231463 |
| Kagawa-35 | 2004 | Japan | Kagawa | Swine blood | I | AB231464 |
| JaNAr14-07 | 2007 | Japan | Nagasaki | Mosquito | I | FJ185147 |
| JaNAr06-07 | 2007 | Japan | Nagasaki | Mosquito | I | FJ185143 |
| K95P05 | 1994 | Korea | Nagasaki | Cx. tritaeniorhynchus | I | U34929 |
| KV1899 | 1999 | Korea | Gyeonggi | Swine blood | I | AY316157 |
| ThCMAr4492 | 1992 | Thailand | Chiang Mai | Mosquito | I | DQ084229 |
| JE_RT_36 | 2003 | Thailand | Ratchaburi | Swine blood | I | DQ087975 |
| JE_KK_80 | 2004 | Thailand | Khon Khen | Swine blood | I | DQ111784 |
| JE_PK52 | 2004 | Thailand | Phuket | Cx. quinquefasciatus | I | DQ084229 |
| JE_CM_1196 | 2005 | Thailand | Chiang Mai | Swine blood | I | DQ238602 |
| JE_KK_1116 | 2005 | Thailand | Khon Khen | Swine blood | I | DQ343290 |
| JE_KK_580 | 2005 | Thailand | Khon Khen | Swine blood | I | DQ238600 |
| TPC0806c | 2008 | Taiwan | Cx. tritaeniorhynchus | I | GQ260635 | |
| YL0806 | 2008 | Taiwan | Cx. tritaeniorhynchus | I | GQ260633 | |
| VN88 | 2001 | Vietnam | Ha Nam | Swine blood | I | AY376464 |
| VN22 | 2002 | Vietnam | Ha Tay | Swine blood | I | AY376465 |
| VN34 | 2002 | Vietnam | Ha Tay | Mosquito | I | AY376466 |
| VN78 | 2002 | Vietnam | Ha Tay | Mosquito | I | AY376467 |
| VN105 | 2002 | Vietnam | Ha Nam | Mosquito | I | AY376468 |
| LA_H07-05 | 2005 | Vietnam | Long An | Swine blood | I | FJ185154 |
| LAH_2079-05 | 2005 | Vietnam | Long An | Swine blood | I | FJ185155 |
| LA_H06-05 | 2005 | Vietnam | Long An | Swine blood | I | FJ185153 |
| VNHT/05/2006 | 2006 | Vietnam | Ha Tay | Cx. tritaeniorhynchus | I | AB728497 |
| VNHT/07/2006 | 2006 | Vietnam | Ha Tay | Cx. tritaeniorhynchus | I | AB728498 |
| VNKT/479/2007 | 2007 | Vietnam | Kon Tum | Cx. vishnui | I | AB728499 |
| VNKT/486/2007 | 2007 | Vietnam | Kon Tum | Cx. vishnui | I | AB728500 |
| VNKT/04/2008 | 2008 | Vietnam | Tay Ninh | Cx. tritaeniorhynchus | I | AB728501 |
| WTP | 1970 | Malaysia | Mosquito | II | U70421 | |
| JKT5441 | 1981 | Indonesia | Bali Island | An. vagus | II | U70406 |
| FU | 1995 | Australia | Human | II | AF217620 | |
| T1P1 | 1997 | Taiwan | Liu-Chiu islet | Ar. subalbatus | III | AF254453 |
| VN118 | 1979 | Vietnam | Ho Chin Minh | Cx. fatigans | III | U70420 |
| TN207 | 2002 | Taiwan | Mosquito | III | EU683895 | |
| NJ2008 | 2008 | China | III | GQ918133 |
The data in bold mark the five JEVs isolated in this study.
Figure 3.
Neighbor-joining dendrogram showing phylogenetic relationships of the nucleotide sequences of the E gene of 47 JEV strains. Bootstrap values correspond to 1,000 replications. Bar denotes the nucleotide similarity distance. The shaded area marks JEV genotype I strains. GenBank accession numbers for sequences used in the phylogenetic analysis are in Table 2. The five JEV strains isolated in this study (VNHT/05/2006, VNHT/07/2006, VNKT/479/2007, VNKT/486/2007, and VNTN/04/2008) are in bold.
The amino acid sequences of the E genes of 14 JEV strains isolated in Vietnam and one JEV strain (Mie/41/2002, AB241119) isolated in Japan were compared. There were nine amino acid differences among these JEVs, at residues 10, 34, 36, 65, 83, 123, 159, 363, and 469 (Table 3). For the five JEV strains isolated in this study, the two isolates from Ha Tay province (VNHT/05/2006 and VNHT/07/2006) had the same amino acid sequence, but the other three strains (VNKT/479/2007, VNKT/486/2007, and VNTN/04/2008) had several amino acid differences. Namely, six amino acid differences were found in these five Vietnamese JEV strains.
Table 3.
Amino acid differences in the E genes of JEV strains from Vietnam*
| Year | Genotype | Strain | Amino acid at position in the E gene† | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 10 | 34 | 36 | 65 | 83 | 123 | 159 | 363 | 469 | |||
| 1979 | III | VN118 | D | M | D | V | E | R | A | T | W |
| 2002‡ | I | Mie/41/2002 | D | M | S | V | E | S | A | T | W |
| 2001 | I | VN88 | D | M | N | V | E | S | A | T | W |
| 2001 | I | VN78 | D | M | N | V | E | N | A | T | W |
| 2002 | I | VN105 | D | M | N | V | E | S | A | T | W |
| 2002 | I | VN22 | D | M | N | V | E | S | A | T | W |
| 2002 | I | VN34 | D | M | N | V | E | N | A | T | W |
| 2005 | I | LA_H06-05 | D | M | S | V | E | S | A | T | W |
| 2005 | I | LA_H07-05 | D | M | N | V | E | S | A | T | W |
| 2005 | I | LAH_2079-05 | D | M | S | I | D | N | V | T | W |
| 2006 | I | VNHT/05/2006 | D | M | N | V | E | N | A | T | W |
| 2006 | I | VNHT/07/2006 | D | M | N | V | E | N | A | T | W |
| 2007 | I | VNKT/479/2007 | D | M | N | V | E | S | A | A | W |
| 2007 | I | VNKT/486/2007 | N | S | N | V | E | S | A | T | R |
| 2008 | I | VNKT/04/2008 | D | M | S | V | E | S | A | T | W |
The data in bold mark the five JEVs isolated in this study.
Data for amino acids 1–500 in the E gene.
Data for the Mie/41/2002 strain has been submitted to the GenBank database (accession no. AB241119).
Discussion
The paddy-breeding mosquitoes, Culex vishnui subgroup, consists of Cx. tritaeniorhynchus, Cx. vishnui, and Cx. pseudovishnui, with Cx. tritaeniorhynchus being the primary vector of JEV throughout Asia.4 In this study, five JEV strains were isolated, two from pools of Cx. tritaeniorhynchus collected in Ha Tay province in northern Vietnam, one from a pool of Cx. tritaeniorhynchus in Tay Ninh province in southern Vietnam, and two from pools of Cx. vishnui collected in Kon Tum province in the highlands of Vietnam. A previous study carried out during June of 2002 and July–August 2004 in Ha Tay province found no JEV in any mosquito species,25 although the species composition in that report and this study were similar, except a difference in the investigation period. Because, our Ha Tay strains of JEV were obtained from Cx. tritaeniorhynchus collected in May. Therefore, we may conclude that JEV has been endemic and circulating throughout Vietnam, transmitted by Cx. tritaeniorhynchus and Cx. vishnui bites.
In the highland provinces of Vietnam, there was a seasonal difference between Cx. tritaeniorhynchus and Cx. vishnui, with Cx. tritaeniorhynchus predominant in June and Cx. vishnui predominant in July. Either species was in the majority depending on the season of the highlands of Vietnam. In particular, the MIR was 2.22 for the two JEV strains isolated from two pools containing a total of 899 Cx. vishnui collected in Kon Tum province, but was 1.3 for the two JEV strains isolated from two of the 46 pools containing a total of 1,542 Cx. vishnui collected throughout Vietnam (data not shown). However, JEV may be circulating in Vietnam by other Culex species. In Thailand, Cx. quinquefasciatus is an important JEV vector, probably because this species is predominantly an urban mosquito and the most common domestic mosquito species in semi-urban and rural areas.42 Hence, the two JEVs were probably isolated from Cx. vishnui in Kon Tum province in July 2007 because this was the most abundant Culex species in that province at that time. These results suggested that, in addition to Cx. tritaeniorhynchus, Cx. vishnui was an important vector for JEV transmission in Vietnam.
The JEV has been isolated from a number of mosquito species and Cx. tritaeniorhynchus to date; e.g., Cx. quinquefasciatus in Thailand; Cx. sitiens Wiedemann, Cx. rubithoracis Leicester, and Aedes vexans Theobald in Taiwan; Cx. annulirostris Skuse in Australia; and Armigeres subalbatus Coquillett in China.42–45 Among these species, at least Cx. tritaeniorhynchus, Cx. pseudovishnui, Cx. gelidus, Cx. annulirostris, Cx. sitiens, and Cx. fuscocephala have been suggested to be efficient vectors in the laboratory.46–48 One JEV isolate was recovered from Cx. vishnui captured in 1993 in northern Thailand.49 Culex vishnui should be recognized as the vector for JEV, although the vector competence of this species has been reported to date. In our study, a total of 497 mosquitoes that belonged in the Cx. vishnui subgroup have still not been unidentified. Distinguishing the three species in the Cx. vishnui subgroup has frequently been problematic,25,50,51 because there are too few morphological differences among them, in particular in the adult stage. Therefore, it has been necessary to distinguish these species using PCR-based techniques. Culex vishnui is endemic in all tropical regions and is the predominant species in most agricultural areas in Vietnam.24 The bionomics and vector competence of the members of the Cx. vishnui subgroup need to be closely monitored, because the vector competence of Cx. vishnui and Cx. pseudovishnui may be higher than has been recognized. The prevalence of Cx. quinquefasciatus has increased because of the urbanization surrounding Hanoi city and a large number of Cx. quinquefasciatus have been collected near Hanoi; e.g., in Ha Tay province and Hai Phong city (data not shown). Although Cx. quinquefasciatus carrying JEV have not been found, JEV transmission by this species should be monitored in Vietnam and in Thailand.42
The NJ phylogenetic tree reconstructed with the five JEV strains isolated in this study and 42 other JEV strains showed that the JEV genotype I strains were divided into three subgenotypes (clade 1, 2, and 3 in Figure 3). Clade 1 strains of JEV are widely spread throughout China, Korea, and Japan, and northern Vietnam and the highlands of Vietnam. In particular, the four JEV strains isolated from Ha Tay and Kon Tum provinces in this study were in the clade 1a subcluster. Clade 1a is probably the same as the previously reported subcluster 1-A-1 based on other criteria.18 Clade 1b probably includes five different subclusters, previously described as 1-A-2 to 1-A-6,18 but no JEV strain isolated in Vietnam in this study was in clade 1b. Subgenotype clade 2 contained JEV isolates from Thailand and southern Vietnam. Although three JEV isolates recovered from Long An province in southern Vietnam (LA_H07-05, LAH_2079-05, and LA_H06-05) were not previously classified in any subcluster,18 one isolate from Tay Ninh province in this study (VNTN/04/2008) formed a new subcluster with the three Long An isolates. The remaining subgenotype, clade 3, contained Australian and Thailand JEV strains, but no Vietnamese strain. Clade 3 has been suggested to be equivalent to subgenotype 1-B based on previous criteria.18 This phylogenetic tree showed that the classification of each JEV strain was not related to its vector species (Cx. tritaeniorhynchus or Cx. vishnui), but to the geography of its collection sites.
It has been reported that there are amino acid variations in the E protein among JEV genotype I strains.26–29 Single amino acid substitutions at several E protein positions have been suggested to be associated with JEV virulence. For example, a Glu to Lys substitution at amino acid 138 attenuates JEV virulence,26,27 and a Met to Lys substitution at amino acid 279 increases JEV virulence in mice.28 In addition, a Ser to Arg substitution at amino acid 123 affects JEV growth and pathogenicity.29 Alignment of JEV E gene nucleotide sequences in the GenBank database showed that the great majority of JEV strains had Ser, not Arg, at residue 123.29 The five Vietnamese JEV isolates in this study had amino acid differences at nine E protein positions. The amino acid differences in the three JEV strains from the highlands and southern Vietnam (VNKT/479/2007, VNKT/486/2007, and VNTN/04/2008) appeared similar to those in the JEV strains previously isolated in Long An province in southern Vietnam (LA_H06-05, LA_H07-05, and LAH_2079-05). A Ser to Asp substitution in amino acid 123 has been found in JEV isolated from mosquitoes in Vietnam since the 2000s. Both JEV strains with Ser at amino acid 123 and JEV strains with Asp at amino acid 123 have been isolated in Vietnam. Further data are needed to explain the function and mechanism of the E protein in JEV infection, and on other structural and non-structural JEV proteins (e.g., prM and NS4A52–54) and on the JEV 5′ and 3′ NTRs.29,55
This study has identified multiple JEV populations in Vietnam, and the nucleotide sequences of these JEVs were similar to those isolated in other area of Asia. This suggested that some JEV strains have migrated between different areas in Asia by vector mosquitoes. In addition, a large number of AES cases have been reported in Vietnam, and over 50% of these AES cases had laboratory evidence of recent JEV infection.19,23 The effects of nucleotide substitutions on JEV virulence and pathogenesis may be related to the high prevalence of AES cases in Vietnam. Accurate JE surveillance data has recently been reported from Vietnam, Japan, and other Asian countries.4,12,13,43,51 Hence, ecological and epidemiological JEV surveillance in humans and mosquitoes should be carried out in JEV-endemic areas to provide data for understanding JEV transmission and infection.
ACKNOWLEDGMENTS
We thank Trang Huynh T.T. of the Department of Medical Entomology and Zoology, Pasteur Institute in Ho Chi Minh City, Vietnam, the staff members of Department of Medical Entomology and Zoology, National Institute of Hygiene and Epidemiology, Vietnam, and the Department of Medical Entomology of National Institute of Infectious Diseases, Japan, for their kind arrangements for field work and helpful criticisms. We also thank K. Morita of the Department of Virology of Institute of Tropical Medicine, Nagasaki University, Japan, for his helpful discussion.
Footnotes
Financial support: This work was partially supported by grant-in-aids awarded by the Ministry of Health, Labor and Welfare (H21-Shinko-Ippan-005), JSPS KAKENHI Grant (no. 21406012) and by Japan initiative for global Research network on infectious diseases (J-grid) (Program of Founding Research Centers for Emerging and Reemerging Infectious Diseases (2005–2009).
Authors' addresses: Ryusei Kuwata, Keita Hoshino, Haruhiko Isawa, Toshinori Sasaki, Yoshio Tsuda, Mutsuo Kobayashi, and Kyoko Sawabe, Department of Medical Entomology, National Institute of Infectious Diseases, Tokyo, Japan, E-mails: ryusei@nih.go.jp, khoshino@nih.go.jp, hisawa@nih.go.jp, tsasaki@nih.go.jp, tsudayso@nih.go.jp, mutsuo@nih.go.jp, and sawabe@nih.go.jp. Phan Thi Nga, Department for Training and Research Management, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, E-mail: pnga_arboviruses@yahoo.com. Nguyen Thi Yen and Tran Vu Phong, Department of Medical Entomology and Zoology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, E-mails: yenanihe@yahoo.com and tranvuphong@yahoo.com. Yukiko Higa and Masahiro Takagi, Department of Medical Entomology and Zoology Institute of Tropical Medicine, Nagasaki University, Nagasaki, Japan, E-mails: yukko@nagasaki-u.ac.jp and mstakagi@nagasaki-u.ac.jp. Nguyen Vet Hoang, Bui Minh Trang, and Do Phuong Loan, Department of Virology, National Institute of Hygiene and Epidemiology, Hanoi, Vietnam, E-mails: hoangyhn2000@gmail.com, trangminhbui3007@gmail.com, and loankobe@yahoo.com.
Reprint requests: Kyoko Sawabe, Department of Medical Entomology, National Institute of Infectious Diseases, 1-23-1 Toyama, Shinjuku-ku, Tokyo 162-8640, Japan, Tel: 81-3-5285-1111, Fax: 81-3-5285-1178, E-mail: sawabe @nih.go.jp.
References
- 1.World Health Organization . Manual for the Laboratory Diagnosis of Japanese Encephalitis Virus Infection. 2007. pp. 1–52. [Google Scholar]
- 2.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. Past, present, and future of Japanese encephalitis. Emerg Infect Dis. 2009;15:1–7. doi: 10.3201/eid1501.080311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon T, Dung NM, Kneen M, Gainsborough M, Vaughn DW, Khanh VT. Japanese encephalitis. J Neurol Neurosurg Psychiatry. 2000;68:405–415. doi: 10.1136/jnnp.68.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Den Hurk AF, Ritchie SA, Mackenzie JS. Ecology and geographical expansion of Japanese encephalitis virus. Annu Rev Entomol. 2009;54:17–35. doi: 10.1146/annurev.ento.54.110807.090510. [DOI] [PubMed] [Google Scholar]
- 5.Tewari SC, Thenmozhi V, Arunachalam N, Samuel PP, Tyagi BK. Desiccated vector mosquitoes used for the surveillance of Japanese encephalitis virus activity in endemic southern India. Trop Med Int Health. 2008;13:286–290. doi: 10.1111/j.1365-3156.2008.02038.x. [DOI] [PubMed] [Google Scholar]
- 6.Hanna JN, Ritchie SA, Phillips DA, Shield J, Bailey MC, Mackenzie JS, Poidinger M, McCall BJ, Mills PJ. An outbreak of Japanese encephalitis in the Torres Strait, Australia, 1995. Med J Aust. 1996;165:256–260. doi: 10.5694/j.1326-5377.1996.tb124960.x. [DOI] [PubMed] [Google Scholar]
- 7.Pyke AT, Williams DT, Nisbet DJ, van den Hurk AF, Taylor CT, Johansen CA, Macdonald J, Hall RA, Simmons RJ, Mason RJ, Lee JM, Ritchie SA, Smith GA, Mackenzie JS. The appearance of a second genotype of Japanese encephalitis virus in the Australasian region. Am J Trop Med Hyg. 2001;65:747–753. doi: 10.4269/ajtmh.2001.65.747. [DOI] [PubMed] [Google Scholar]
- 8.Nga PT, del Carmen Parquet M, Cuong VD, Ma SP, Hasebe F, Inoue S, Makino Y, Takagi M, Nam VS, Morita K. Shift in Japanese encephalitis virus (JEV) genotype circulating in northern Vietnam: implications for frequent introductions of JEV from Southeast Asia to East Asia. J Gen Virol. 2004;85:1625–1631. doi: 10.1099/vir.0.79797-0. [DOI] [PubMed] [Google Scholar]
- 9.Nabeshima T, Hyunh Thi Kim Loan HT, Inoue S, Sumiyoshi M, Haruta Y, Nga PT, Huoung VT, del Carmen Parquet M, Hasebe F, Morit K. Evidence of frequent introductions of Japanese encephalitis virus from south-east Asia and continental East Asia to Japan. J Gen Virol. 2009;90:827–832. doi: 10.1099/vir.0.007617-0. [DOI] [PubMed] [Google Scholar]
- 10.Morita K. Molecular epidemiology of Japanese encephalitis in East Asia. Vaccine. 2009;27:7131–7132. doi: 10.1016/j.vaccine.2009.09.051. [DOI] [PubMed] [Google Scholar]
- 11.Solomon T, Ni H, Beasley DW, Ekkelenkamp M, Cardosa MJ, Barrett AD. Origin and evolution of Japanese encephalitis virus in Southeast Asia. J Virol. 2003;77:3091–3098. doi: 10.1128/JVI.77.5.3091-3098.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li MH, Fu SH, Chen WX, Wang HY, Guo YH, Liu QY, Li YX, Luo HM, Da W, Ji DZD, Ye XM, Liang GD. Genotype V Japanese encephalitis virus is emerging. PLoS Negl Trop Dis. 2011;5:e1231. doi: 10.1371/journal.pntd.0001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang JH, Lin TH, Teng HJ, Su CL, Tsai KH, Lu LC, Lin C, Yang CF, Chang SF, Liao TL, Yu SK, Cheng CH, Chang MC, Hu HC, Shu PY. Molecular epidemiology of Japanese encephalitis virus, Taiwan. Emerg Infect Dis. 2010;16:876–878. doi: 10.3201/eid1605.091055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen YY, Fan YC, Tu WC, Chang RY, Shin CC, Lu IH, Chien MS, Lee WC, Chen TH, Chang GJ, Chiou SS. Japanese encephalitis virus genotype replacement, Taiwan, 2009–2010. Emerg Infect Dis. 2011;17:2354–2356. doi: 10.3201/eid1712.110914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98–S109. doi: 10.1038/nm1144. [DOI] [PubMed] [Google Scholar]
- 16.Ma SP, Yoshida Y, Makino Y, Tadano M, Ono T, Ogawa M. A major genotype of Japanese encephalitis virus currently circulating in Japan. Am J Trop Med Hyg. 2003;69:151–154. [PubMed] [Google Scholar]
- 17.Pan XL, Liu H, Wang HY, Fu SH, Liu HZ, Zhang HL, Li MH, Gao XY, Wang JL, Sun XH, Lu XJ, Zhai YG, Meng WS, He Y, Wang HQ, Han N, Wei B, Wu YG, Feng Y, Yang DJ, Wang LH, Tang Q, Xia G, Kurane I, Rayner S, Liang GD. Emergence of genotype I of Japanese encephalitis virus as the dominant genotype in Asia. J Virol. 2011;85:9847–9853. doi: 10.1128/JVI.00825-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nabeshima T, Morita K. Phylogeographic analysis of the migration of Japanese encephalitis virus in Asia. Future Virol. 2010;5:343–354. [Google Scholar]
- 19.Yen NT, Duffy MR, Hong NM, Hien NT, Fischer M, Hills SL. Surveillance for Japanese encephalitis in Vietnam, 1998–2007. Am J Trop Med Hyg. 2010;83:816–819. doi: 10.4269/ajtmh.2010.10-0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuno T. An epidemiological review of Japanese encephalitis. World Health Stat Q. 1978;31:120–133. [PubMed] [Google Scholar]
- 21.Hinh LD. Clinical aspects of Japanese B encephalitis in North Vietnam. Clin Neurol Neurosurg. 1986;88:189–192. doi: 10.1016/s0303-8467(86)80027-0. [DOI] [PubMed] [Google Scholar]
- 22.Ha DQ, Hong VT, Loan HTK, Thong DQ, Deubel V. Current situation of Japanese encephalitis in the south of Vietnam, 1976–1992. Trop Med. 1994;36:202–214. [Google Scholar]
- 23.Tam NH, Yen NT. Japanese encephalitis in Vietnam 1985–1993. Southeast Asian J Trop Med Public Health. 1995;26((Suppl 3)):47–50. [Google Scholar]
- 24.Hasegawa M, Tuno N, Yen NT, Nam VN, Takagi M. Influence of the distribution of host species on adult abundance of Japanese encephalitis vectors-Culex vishnui subgroup and Culex gelidus- in a rice-cultivating village in northern Vietnam. Am J Trop Med Hyg. 2008;78:159–168. [PubMed] [Google Scholar]
- 25.Bryant J, Crabtree MB, Nam VS, Yen NT, Duc HM, Miller B. Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am J Trop Med Hyg. 2005;73:470–473. [PubMed] [Google Scholar]
- 26.Zhao Z, Date T, Li Y, Kato T, Miyamoto M, Yasui K, Wakita T. Characterization of the E-138 (Glu/Lys) mutation in Japanese encephalitis virus by using a stable, full-length, infectious cDNA clone. J Gen Virol. 2005;86:2209–2220. doi: 10.1099/vir.0.80638-0. [DOI] [PubMed] [Google Scholar]
- 27.Monath TP, Arroyo J, Levenbook I, Zhang ZX, Catalan J, Draper K, Guirakhoo F. Single mutation in the flavivirus envelope protein hinge region increases neurovirulence for mice and monkeys but decreases viscerotropism for monkeys: relevance to development and safety testing of live, attenuated vaccines. J Virol. 2002;76:1932–1943. doi: 10.1128/JVI.76.4.1932-1943.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tajima S, Nerome R, Nukui Y, Kato F, Takasaki T, Kurane I. A single mutation in the Japanese encephalitis virus E protein (S123R) increases its growth rate in mouse neuroblastoma cells and its pathogenicity in mice. Virology. 2010;396:298–304. doi: 10.1016/j.virol.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 29.Obara M, Yamauchi T, Watanabe M, Hasegawa S, Ueda Y, Matsuno K, Iwai M, Horimoto E, Kurane I, Takizawa T, Kariwa H, Takashima I. Continuity and change of Japanese encephalitis virus in Toyama prefecture, Japan. Am J Trop Med Hyg. 2011;84:695–708. doi: 10.4269/ajtmh.2011.10-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsuda Y, Higa Y, Kurahashi H, Hayashi T, Hoshino K, Komagata O, Isawa H, Kasai S, Sasaki T, Tomita T, Sawabe K, Nihei N, Kobayashi M. Dry-ice trap collection of mosquitoes at urban areas surrounding Tokyo, Japan in 2003 and 2004. Med Entomol and Zool. 2006;57:75–82. [Google Scholar]
- 31.Stojanovich CJ, Scott HG. Illustrated Key to Mosquitoes of Vietnam. Atlanta, GA: U.S. Department of Health, Education, and Welfare, Public Health Service; 1966. [Google Scholar]
- 32.Reuben R, Tewari SC, Hiriyan J, Akiyama J. Illustrated keys to species of Culex (Culex) associated with Japanese encephalitis in Southeast Asia (Diptera: Culicidae) Mosq Systematics. 1994;26:75–96. [Google Scholar]
- 33.Reinert JF. New classification for the composite genus Aedes (Diptera: Culicidae: Aedini), elevation of subgenus Ochlerotatus to generic rank, reclassification of the other subgenera, and notes on certain subgenera and species. J Am Mosq Control Assoc. 2000;16:175–188. [PubMed] [Google Scholar]
- 34.Sawabe K, Takagi M, Tsuda Y, Tuno N. Molecular variation and phylogeny of the Anopheles minimus complex (Diptera: Culicidae) inhabiting Southeast Asian countries, based on ribosomal DNA internal transcribed spacers, ITS1 and 2, and the 28S D3 sequences. Southeast Asian J Trop Med Public Health. 2003;34:771–780. [PubMed] [Google Scholar]
- 35.Sawabe K, Isawa H, Hoshino K, Sasaki T, Roychoudhury S, Higa Y, Kasai S, Tsuda Y, Nishiumi I, Hisai N, Hamao S, Kobayashi M. Host-feeding habits of Culex pipiens and Aedes albopictus (Diptera: Culicidae) collected at the urban and suburban residential areas of Japan. J Med Entomol. 2010;47:442–450. doi: 10.1603/ME09256. [DOI] [PubMed] [Google Scholar]
- 36.Hoshino K, Isawa H, Tsuda Y, Sawabe K, Kobayashi M. Genetic characterization of a new insect flavivirus isolated from Aedes mosquitoes in Japan. Virology. 2009;391:119–129. doi: 10.1016/j.virol.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 37.Hoshino K, Isawa H, Tsuda Y, Yano K, Sasaki T, Yuda M, Takasaki T, Kobayashi M, Sawabe K. Genetic characterization of a new insect flavivirus isolated from Culex pipiens mosquitoes in Japan. Virology. 2007;359:405–414. doi: 10.1016/j.virol.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 38.Briese T, Jia XY, Huang C, Grady LJ, Lipkin WI. Identification of a Kunjin/West Nile-like flavivirus in brains of patients with New York encephalitis. Lancet. 1999;354:1261–1262. doi: 10.1016/s0140-6736(99)04576-6. [DOI] [PubMed] [Google Scholar]
- 39.Kuno G, Chang GJ, Tsuchiya KR, Karabatsos N, Cropp CB. Phylogeny of the genus Flavivirus. J Virol. 1998;72:73–83. doi: 10.1128/jvi.72.1.73-83.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 41.Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.1. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- 42.Nitatpattana N, Apiwathnasorn C, Barbazan P, Leemingsawat S, Yoksan S, Gonzalez JP. First isolation of Japanese encephalitis from Culex quinquefasciatus in Thailand. Southeast Asian J Trop Med Public Health. 2005;36:875–878. [PubMed] [Google Scholar]
- 43.Weng MH, Lien JC, JI DD. Monitoring Japanese encephalitis virus infection in mosquitoes, (Diptera: Culicidae) at Guandu Nature Park, Taipei, 2002–2004. J Med Entomol. 2005;42:1085–1088. doi: 10.1093/jmedent/42.6.1085. [DOI] [PubMed] [Google Scholar]
- 44.Ritchie SA, Phillips D, Broom A, Mackenzie J, Poidinger M, van den Hurk A. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80. [DOI] [PubMed] [Google Scholar]
- 45.Sun X, Fu S, Gong Z, Ge J, Meng W, Feng Y, Wang J, Zhai Y, Wang H, Nasci R, Wang H, Tang Q, Liang G. Distribution of arboviruses and mosquitoes in northwestern Yunnan Province, China. Vector Borne Zoonotic Dis. 2009;9:623–630. doi: 10.1089/vbz.2008.0145. [DOI] [PubMed] [Google Scholar]
- 46.Gould DJ, Barnett HC, Suyemoto W. Transmission of Japanese encephalitis virus by Culex gelidus Theobald. Trans R Soc Trop Med Hyg. 1962;56:429–435. doi: 10.1016/0035-9203(62)90018-4. [DOI] [PubMed] [Google Scholar]
- 47.Muangman D, Edelman R, Sullivan MJ, Gould DJ. Experimental transmission of Japanese encephalitis virus by Culex fuscocephala. Am J Trop Med Hyg. 1972;21:482–486. doi: 10.4269/ajtmh.1972.21.482. [DOI] [PubMed] [Google Scholar]
- 48.Doi R, Shirasaka A, Sasa M, Oya A. Studies on the susceptibility of three species of mosquitoes to Japanese encephalitis virus. J Med Entomol. 1977;13:591–594. doi: 10.1093/jmedent/13.4-5.591. [DOI] [PubMed] [Google Scholar]
- 49.Ali A, Igarashi A, Paneru LR, Hasebe F, Morita K, Takagi M, Sumonkerd W, Tsuda Y, Wada Y. Characterization of two Japanese encephalitis virus strains isolated in Thailant. Arch Virol. 1995;140:1557–1575. doi: 10.1007/BF01322530. [DOI] [PubMed] [Google Scholar]
- 50.Toma T, Miyagi I, Crabtree MB, Miller BR. Identification of Culex vishnui subgroup (Diptera: Culicidae) mosquitoes from the Ryukyu Archipelago, Japan: development of a species-diagnostic polymerase chain reaction assay based on sequence variation in ribosomal DNA spacers. J Med Entomol. 2000;37:554–558. doi: 10.1603/0022-2585-37.4.554. [DOI] [PubMed] [Google Scholar]
- 51.Hiscox A, Winter CH, Vongphrachanh P, Sisouk T, Somoulay V, Phompida S, Kaul S, Sananikhom P, Yen NT, Paul RE, Brey P, Bryant JE. Serological investigations of flavivirus prevalence in Khammouane Province, Lao People's Democratic Republic, 2007–2008. Am J Trop Med Hyg. 2010;83:1166–1169. doi: 10.4269/ajtmh.2010.09-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lindenbach BD, Rice CM. Genetic interaction of flavivirus nonstructural proteins NS1 and NS4A as a determinant of replicase function. J Virol. 1999;73:4611–4621. doi: 10.1128/jvi.73.6.4611-4621.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi Y, Nukui Y, Tajima S, Nerome R, Kato F, Watanabe H, Takasaki T, Kurane I. An amino acid substitution (V3I) in the Japanese encephalitis virus NS4A protein increases its virulence in mice, but not its growth rate in vitro. J Gen Virol. 2011;92:1601–1606. doi: 10.1099/vir.0.031237-0. [DOI] [PubMed] [Google Scholar]
- 54.Lindenbach BD, Thiel HJ, Rice CM. Flaviviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Fifth edition. Philadelphia, PA: Lippincott Williams & Wilkins; 2007. pp. 1101–1151. [Google Scholar]
- 55.Sumiyoshi H, Tignor GH, Shope RE. Characterization of a highly attenuated Japanese encephalitis virus generated from molecularly cloned cDNA. J Infect Dis. 1995;171:1144–1151. doi: 10.1093/infdis/171.5.1144. [DOI] [PubMed] [Google Scholar]