Abstract
Diurnal temperature fluctuations can fundamentally alter mosquito biology and mosquito-virus interactions in ways that impact pathogen transmission. We investigated the effect of two daily fluctuating temperature profiles on Aedes aegypti vector competence for dengue virus (DENV) serotype-1. A large diurnal temperature range of 18.6°C around a 26°C mean, corresponding with the low DENV transmission season in northwestern Thailand, reduced midgut infection rates and tended to extend the virus extrinsic incubation period. Dissemination was first observed at day 7 under small fluctuations (7.6°C; corresponding with high DENV transmission) and constant control temperature, but not until Day 11 for the large diurnal temperature range. Results indicate that female Ae. aegypti in northwest Thailand are less likely to transmit DENV during the low than high transmission season because of reduced DENV susceptibility and extended virus extrinsic incubation period. Better understanding of DENV transmission dynamics will come with improved knowledge of temperature effects on mosquito-virus interactions.
Introduction
In Mae Sot, northwestern Thailand, dengue virus (DENV) transmission is predictably seasonal, although the mean temperature across seasons varies minimally, remaining at approximately 26°C. The low transmission season in Thailand typically occurs during November–April and the high transmission season during June–October.1 Temperatures during February (low transmission) are associated with relatively large fluctuations in temperature (15–20°C), and during July–August (high transmission), there is a smaller daily temperature fluctuation (5–10°C).
The interaction between DENV and its principal mosquito vector, Aedes aegypti (Linnaeus), has been studied in detail in the laboratory under constant temperatures. Experimental transmission was observed at a range of constant temperatures from 13°C2 to 35°C.3 Increasing constant temperature reduced the extrinsic incubation period (EIP), increased mosquito mortality,3,4 and resulted in a higher proportion of infected mosquitoes.
There are relatively few studies that explored vector-virus interactions under variable, more environmentally realistic temperature regimens. Bates and Roca-Garcia5 in 1946 and Chamberlain and Sudia6 in 1955 examined the vector competence of Haemagogus sp. and Ae. triseriatus for yellow fever virus and eastern equine encephalitis virus, respectively, but found little difference between the constant and alternating temperature regimens. At low temperatures, a diurnal temperature range (DTR) of 14°C (10°C–24°C) around a mean of 17°C did not influence Ae. taeniorhynchus7 or Culex pipiens8 susceptibility to infection with Ockelbo virus.
More recently, Lambrechts and others9 examined the vector competence of Ae. aegypti for DENV under fluctuating temperatures, using small and large sinusoidal temperature fluctuations like the ones noted above for Mae Sot. Three temperature regimens were considered, all with a mean temperature of 26°C; i.e., a constant temperature, a DTR of 10°C (representing the high transmission season), and a DTR of 20°C (low transmission season). They found that mosquitoes exposed to DENV-1 and DENV-2 had reduced longevity and lower midgut infection rates when exposed to the large DTR, compared with small DTR or constant 26°C. Dissemination rates did not vary across temperature regimens, but as expected did increase with the duration of extrinsic incubation. Results from that study support the notion that large temperature fluctuations can contribute to a reduction in DENV transmission intensity during the low DENV transmission season in northwestern Thailand.
However, there are additional details in the DENV-Ae. aegypti interaction that were not addressed in the study of Lambrechts and others.9 study. First, as acknowledged by the authors, a symmetrical, sinusoidal temperature profile was used in their experiments, as opposed to a more realistic asymmetrical profile. Parton and Logan10 describe air temperatures that follow a truncated sinusoidal progression during the day with an exponential decrease at night. The symmetrical profile has increases in temperature above the mean that are directly offset by reciprocal decreases below, whereas that is not necessarily the case for the asymmetrical profile. Second, during larval development, all mosquitoes were held at a constant 26°C. They were not exposed to the same fluctuating temperatures as adults until three days before adult females were exposed to virus. Third, infection status of females used to estimate the survival curves was unknown (i.e., the survival of virus-exposed females was compared regardless of whether infection was successfully established or not). Results could be confounded by DENV infection status, which can alter female survival.11,12 Fourth, Lambrechts and others9 did not evaluate adult female size, which has been associated with Aedes spp. vector competence. Other investigators reported conflicting results regarding vector competence and mosquito size.13–15 Results from one study using DENV-2 indicated that smaller Ae. aegypti are more susceptible to the infection than larger ones.13 Rearing effects may have confounded the outcome of some previous experiments.
The study of Lambrechts and others9 also modeled the thermodynamics of virus infection and transmission by mosquitoes, under symmetric and asymmetric temperature profiles. Their model was based on empirical data from three flaviviruses (West Nile virus, St. Louis encephalitis virus, and Murray Valley encephalitis virus) and led to the following predictions. Temperature fluctuations were expected to alter the direction of the response, dependent upon the mean temperature. At a low mean temperature (< 18°C), fluctuations were predicted to increase the probabilities of infection and dissemination, and at higher mean temperatures (≥ 18°C) fluctuations reduce these probabilities. In addition, under an asymmetric temperature profile a larger DTR was predicted to shorten EIP. The expected EIP at a constant 26°C is 11–12 days, and ≤ 10 days with a DTR of 20°C.
Carrington and others16 recently assessed the effect of DTR on Ae. aegypti immature and reproductive traits. Using the same asymmetrical temperature regimens as those used in this study, they reported that a large DTR reduced immature survival and extended development time. However, a small DTR slightly increased reproduction.
In this study, we extended the experiments of Lambrechts and others9 by examining Ae. aegypti vector competence for DENV (midgut infection and dissemination of the virus to head tissue) and survival under asymmetrical temperature fluctuations (sinusoidal progression plus exponential decrease; the same temperature profiles as those used by Carrington and others16) that represent the high and low DENV transmission seasons in Mae Sot. Our aim was to include variables not examined in previous studies and to investigate the relationship between mosquito body size and vector competence using DENV-1 and Ae. aegypti. We hypothesized that females in the large DTR treatment are less susceptible to DENV-1 infection,9 and that smaller females are more likely to become infected with DENV-1 than larger conspecifics.13 As part of our analysis, we measured the proportion of mosquitoes with a midgut (body) and disseminated (head) infection, and the length of the EIP compared with predictions from the thermodynamic model of Lambrechts and others.9
Methods
Experimental design.
We determined the effect of three temperature regimens on adult Ae. aegypti survival and vector competence for DENV; one constant temperature and two with daily temperature fluctuations (see Figure 1 in Carrington and others16). Temperature profiles were intended to be representative of the high and low DENV transmission seasons in Mae Sot, Thailand. We used the average temperature of each hour of the day as measured over a three week period during the high and low DENV transmission seasons. Temperatures were recorded inside houses in villages near Mae Sot.17 The high transmission period was characterized by a 7.6°C fluctuation around a mean of 26.7°C (recorded over a 20-day period during July–August 2000). The low transmission season followed an 18.6°C fluctuation around a mean of 26.1°C (recorded over a 20-day period during February 2000). Fluctuating temperature profiles followed a sinusoidal increase during the day, and a negative exponential decrease at night, with minimum and maximum temperatures reached at 6:00 am and 2:00 pm, respectively. Experimental climatic conditions were maintained for experimental mosquitoes in KBF115 incubators (Binder, Tuttlingen, Germany). Internal data loggers recorded temperatures in all incubators. Additionally, HOBO data loggers recorded temperatures on an hourly basis in the two incubators with fluctuations. Actual air temperatures that mosquitoes were exposed to during experiments were consistent (±0.3°C) with that programmed at each point during the day. The mean temperature for the small DTR treatment incubator was 26.71°C, and for the large DTR temperature averaged at 26.34°C. Relative humidity was maintained at 70–80% across all treatments.
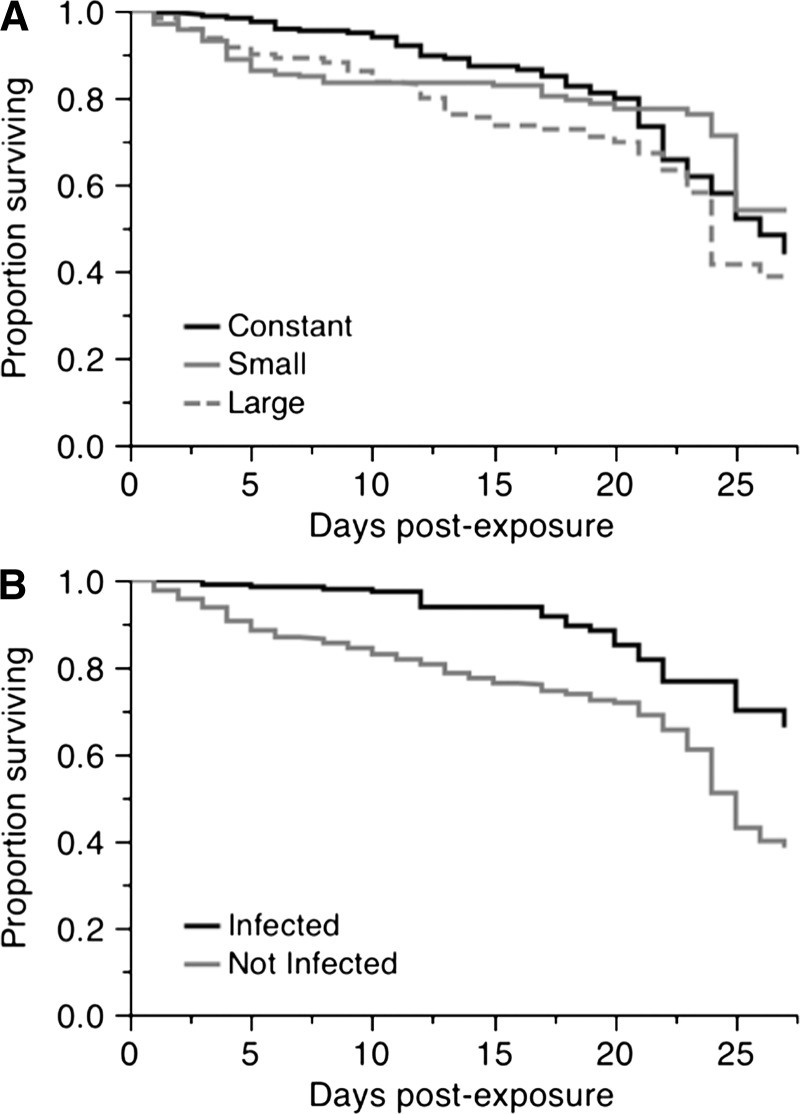
Figure 1.
Survival curves for female Aedes aegypti exposed to dengue virus serotype-1 from 0 to 27 days post exposure. A, Effect of temperature fluctuations on the survival of Ae. aegypti females (χ2 = 6.85, degrees of freedom [df] = 2, P = 0.032). B, Effect of infection status on survival (χ2 = 25.17, df = 1, P < 0.0001).
Mosquitoes.
Aedes aegypti used in our experiments were collected from Kamphaeng Phet Province, Thailand, which is less than 150 km southeast of Mae Sot and has a similar annual climate. Mosquitoes were collected as pupae during January 2011 and sent to the University of California at Davis as F1 eggs. After eggs were received, they were hatched and reared at a low density (1.3 larvae/10 mL) in 24 cm × 29 cm × 5 cm containers with 1.5 liters of deionized water. Colony maintenance was conducted under standard insectary conditions (constant 28°C ± 2°C, 70–80% relative humidity), and a 12:12 hour light:dark cycle, with > 500 females per generation. Mosquito rearing procedures for experiments are described below. Larvae were fed a 1:1 mix of bovine liver powder and puppy chow, with 0.1 grams per 200 larvae each day for the first four days, 0.2 grams on the fifth day, 0.3 grams on the sixth day, and then 0.2 grams on the remaining 2 days, at which time most larvae had pupated.
Experimental infections.
For each of the three treatments, F3 generation mosquitoes were reared at the temperature at which their vector competence was to be tested. Because of space limitations, rearing density was increased (4–5 larvae/10 mL) and food was increased proportionately. To limit variation in adult emergence time, we staggered the hatching of eggs on the basis of estimates from previous experiments of development time from egg hatch to adult emergence.16 Five to seven days after adult emergence, we exposed mosquitoes from each temperature regimen to an infectious DENV-1 blood meal using an artificial feeding system.
After having sugar removed for 30–36 hours, females were fed defibrinated sheep blood (QuadFive, Ryegate, MT), mixed with DENV-1 freshly grown in Ae. albopictus C6/36 cells before mosquito exposure. The virus supernatant was harvested after scraping and all cells were separated by centrifugation. Mosquitoes fed through a desalted porcine intestinal membrane stretched over the bottom of a warm water-filled jar to maintain a temperature of 37°C. The viral isolate used, SV2951 obtained from Ratchaburi, Thailand, had been passaged seven times in C6/36 cells before use in this study. Confluent cultures of C6/36 cells grown in 25-cm2 flasks were inoculated at a virus multiplicity of infection of 0.01 and left to grow for 10 days at 28°C in an atmosphere of 5% CO2. The infectious blood meal consisted of 45% viral supernatant harvested at day 10, 50% defibrinated sheep blood (Quadfive), and 5% sucrose solution (diluted 1:5 in water). An aliquot of the blood meal was taken at the end of the feed to determine the minimum viral titer of the blood that mosquitoes ingested.
We prepared two blood meals, but fed each female only once (feeding mosquitoes in one of two feeding sessions), and limited the feeding time to 30 minutes, in an effort to minimize the effect of virus degradation in the infectious blood meal. The titers of the two blood meals were 4.16×105 fluorescent focus units/mL and 3.09×105 fluorescent focus units/mL. After allowing 2–3 hours for mosquitoes to begin digestion, we sedated them with CO2, and retained only fully engorged females. Thirty-six replicate 1-pint paper cartons (Science Supplies WLE, Inc. Newark, NJ) with mesh tops, each containing 20 engorged females (12 cartons from each temperature regimen), were returned to their respective incubators.
Vector competence.
At 7, 11, 15, 19, 23, and 27 days post-exposure (DPE) to the infectious blood meal (i.e., days of EIP), we randomly selected and removed two cartons from each incubator at each time point. For all surviving mosquitoes in each carton, we measured two components of vector competence, midgut infection and virus dissemination from the midgut, using a qualitative indirect fluorescence assay.
We separated and tested bodies (comprised of the thorax and abdomen) for midgut infection, and heads for disseminated infection, independently. Samples were placed into 1 mL of viral transport medium (77.2% low glucose Dulbecco's modified Eagle medium, 18.5% heat-inactivated fetal bovine serum, 3.8% penicillin/streptomycin, and 0.15% gentamicin and nystatin) with approximately ten 2-mm glass beads (Fisher Scientific, Pittsburgh, PA) in a screw-top plastic vial. After collection, all samples were frozen at –80°C for later analysis by indirect fluorescence assay. We also collected the whole bodies (without separation of heads) of dead females daily and tested them for infection status. Results from analysis of dead mosquitoes were included in our survival analyses.
Wing lengths.
Female wings were removed at the same time as heads and bodies were separated, and mounted on microscope slides with double-sided tape. Photographs of the wings were taken and measurements of each wing were made, in millimeters, using AutoMontage (Syncroscopy, Frederick, MD) software. Wings were measured from the distal end of the alula to the most posterior point of the R3 vein, minus the fringe. Damaged wings or wings that were mounted imperfectly were excluded from analysis.
Data analysis.
All data was analyzed by using JMP software version 10 (SAS Institute Inc., Cary, NC). Vector competence was analyzed by nominal logistic regression of the infection or dissemination status as a full-factorial function of temperature and DPE, and carton nested within temperature and DPE. To improve the statistical model, factors with a P value > 0.5 were removed sequentially from the model, except for the nested carton effect, which was required to test the effects of temperature and DPE. Survival was analyzed by assessing the longevity of females exposed to an infectious blood meal by using deaths throughout the duration of the experiment, as well as right-censored mosquitoes that were killed on the days we collected mosquitoes for testing midgut infection and dissemination (sampling days). We used a Kaplan-Meier analysis to test for differences in survival curves between different DTR and infection status of recently dead mosquitoes. Carton effects were not considered in survival analyses. We corrected for multiple comparisons between treatment groups for logistic regression and Kaplan-Meier analyses using a Bonferroni correction. Wing length was analyzed as a function of infection status and DTR. Replicate carton was nested within DTR, and we tested for an interaction between infection and DTR.
Fluorescent focus assay.
We used an infectious fluorescent focus assay to titrate viral samples.18 One-day-old confluent monolayers of Vero (green monkey kidney) cells in eight-well chamber slides (Nunc, Rochester, NY) were inoculated with serial 10-fold dilutions of virus and blood meal samples. Dilutions were prepared in 2% fetal bovine serum (FBS) maintenance medium in duplicate and inoculum was allowed to infect the cells for 1 hour. A negative control (the maintenance medium used for the dilutions) was included in all titrations. The overlay applied to the cells after the incubation was made of a 1:1 mixture of 2% FBS maintenance medium:carboxymethyl cellulose (2% in phosphate-buffered saline [PBS]). We then allowed 2 days for virus to replicate in the monolayer, then the medium was removed and the cells were washed carefully. In each washing step, PBS was added to cells three times, allowed to rest for 3–5 minutes, before PBS was again removed. The cells were fixed with 3.7% formaldehyde and washed again before being stained with 75 μL of a 1:250 dilution of primary mouse anti-DENV monoclonal antibody (MAB8705; Millipore, Billerica, MA) at 37°C for 1 hour. The cells were again washed to minimize background fluorescence, and then stained with 75 μL of a 1:100 dilution of fluorescein isothiocyanate–conjugated secondary goat anti-mouse antibody (AP124F; Millipore) for 30 minutes, which was used to detect and count the number of fluorescent foci under a fluorescent microscope at 20× magnification.
Qualitative indirect fluorescence assay.
To test mosquito samples for DENV infection, we homogenized the tissue samples for 2 minutes in a Retsch Mixer Mill 400, at 30 Hz. We filtered 300 μL of the sample through 0.22-μm cellulose acetate centrifuge filters (Costar Spin-X; Corning, Tokyo, Japan), and 50 μL of the filtered supernatant was inoculated in duplicate onto a one-day-old confluent monolayer of Vero cells, seeded at a density of 2.5 × 105 cells/well in a 96-well culture plate. The inoculum was allowed to infect the cells for 1 hour at 28°C, before a standard maintenance media containing 2% FBS overlay was applied to the cells in each well, and the plate was incubated 28°C for 4 days. Positive and negative controls were used in each plate. We then removed the overlay, washed the cells, and fixed them in 3.7% formaldehyde for 20 minutes. The washing and staining steps that followed were exactly the same as for the fluorescent focus assay, except that the volumes used for antibody staining were 50 μL for each of the primary and secondary antibodies. We viewed cells under a fluorescein isothiocyanate–fitted fluorescence anisotropy microscope at a magnification of 10× to screen for the presence or absence of green fluorescence, which was indicative of a sample being either infected or uninfected by DENV, respectively.
Results
Vector competence.
Over the entire time course of the experiment, 28.1% of the mosquitoes had a detectable DENV-1 midgut (body) infection, from a total of 712 mosquitoes that imbibed the infectious blood meal. Of the 200 infected females, we tested 174 for a disseminated infection, of which 69.5% (121) had positive head tissue samples. The 26 mosquitoes that were not tested for dissemination died before the scheduled sampling period and were only assayed for infection.
Using a nominal logistic regression to analyze samples collected from the six scheduled sampling days of the experiment, we found a highly significant effect of DTR on DENV-1 infection in mosquito bodies (Table 1). A small DTR resulted in the highest mean infection rates, and large DTR had more than a 50% relative reduction in the proportion of infected females (Table 2). Pairwise analyses demonstrated that constant temperature and small DTR treatments had significantly higher infection rates than the large DTR at two time points. After correcting for multiple comparisons, we determined that the constant DTR had higher infection rates than the large DTR at day 27, and the small DTR produced significantly more infected females than the large DTR at days 15 and 27. Levels of infection did not differ at any time point between the constant temperature and small DTR treatments (Table 3). A strong effect of DPE was also observed. An increase in DPE was associated with higher infection rates. Replicate carton (nested within DTR and DPE) did not significantly influence body infection rates. There was no detectable effect of DTR × DPE, and this term was removed from the final statistical model.
Table 1.
Test statistics of DTR and DPE effects on Aedes aegypti vector competence for dengue virus serotype-1*
| Factor | Midgut (body) infection | Dissemination (head infection) | ||||
|---|---|---|---|---|---|---|
| df | χ2 | P | df | χ2 | P | |
| DTR | 2 | 26.316 | < 0.0001 | 2 | 1.28 × 10−5 | 1.0000 |
| DPE | 5 | 16.291 | 0.0061 | 5 | 65.985 | < 0.0001 |
| DTR × DPE | – | NS | NS | 10 | 23.278 | 0.0098 |
| Carton (within DTR, DPE) | 18 | 23.868 | 0.1594 | 17 | 14.693 | 0.6176 |
Midgut (body) infection refers to the proportion of females with infected bodies tested at the sampling days. Dissemination (head infection) was tested for infected females only. df = degrees of freedom; DTR = daily fluctuating temperature; DPE = days post-exposure. NS indicates that the factor was highly non-significant and therefore the factor was removed from the final statistical model.
Table 2.
Overall Aedes aegypti dengue virus serotype-1 infection rates in female bodies, separated by carton*
| Day | Carton | Constant | Small | Large | |||
|---|---|---|---|---|---|---|---|
| % | No. | % | No. | % | No. | ||
| 7 | 1 | 15 | (20) | 43 | (14) | 25 | (16) |
| 2 | 38 | (16) | 10 | (10) | 0 | (20) | |
| 11 | 1 | 30 | (20) | 47 | (15) | 37 | (19) |
| 2 | 47 | (15) | 47 | (19) | 14 | (14) | |
| 15 | 1 | 50 | (16) | 65 | (17) | 37 | (19) |
| 2 | 53 | (15) | 61 | (18) | 8 | (13) | |
| 19 | 1 | 11 | (18) | 33 | (15) | 24 | (17) |
| 2 | 42 | (19) | 41 | (17) | 30 | (10) | |
| 23 | 1 | 20 | (10) | 38 | (16) | 21 | (14) |
| 2 | 27 | (15) | 50 | (14) | 9 | (11) | |
| 27 | 1 | 54 | (13) | 38 | (8) | 13 | (8) |
| 2 | 25 | (12) | 50 | (14) | 0 | (6) | |
For each carton, the percentage of infected females is given, along with the number of females tested in parentheses.
Table 3.
Pairwise comparisons between DTR treatments for body infection of dengue virus serotype-1 in Aedes aegypti at each time-point*
| Day | Source | df | Constant vs. small | Constant vs. large | Small vs. large | |||
|---|---|---|---|---|---|---|---|---|
| χ2 | P | χ2 | P | χ2 | P | |||
| 7 | DTR | 1 | 0.044 | 0.833 | 5.043 | 0.025 | 3.104 | 0.078 |
| Carton [DTR] | 2 | 5.511 | 0.064 | 9.280 | 0.010 | 10.473 | 0.005 | |
| 11 | DTR | 1 | 0.561 | 0.454 | 1.453 | 0.228 | 3.677 | 0.055 |
| Carton [DTR] | 2 | 1.019 | 0.601 | 3.199 | 0.202 | 2.183 | 0.336 | |
| 15 | DTR | 1 | 1.422 | 0.233 | 5.639 | 0.018 | 12.513 | < 0.001 |
| Carton [DTR] | 2 | 0.083 | 0.959 | 3.965 | 0.138 | 3.979 | 0.137 | |
| 19 | DTR | 1 | 1.098 | 0.295 | 0.028 | 0.867 | 0.719 | 0.397 |
| Carton [DTR] | 2 | 5.043 | 0.080 | 4.969 | 0.083 | 0.345 | 0.841 | |
| 23 | DTR | 1 | 2.219 | 0.136 | 0.600 | 0.439 | 5.171 | 0.023 |
| Carton [DTR] | 2 | 0.708 | 0.702 | 1.112 | 0.574 | 1.355 | 0.508 | |
| 27 | DTR | 1 | 0.137 | 0.711 | 5.856 | 0.016 | 7.146 | 0.008 |
| Carton [DTR] | 2 | 1.586 | 0.453 | 2.439 | 0.295 | 1.500 | 0.472 | |
DTR = daily fluctuating temperature; df = degree of freedom. Values in boldface indicate that the effect was significant (P < 0.016) after Bonferroni correction for multiple comparisons.
For dissemination of the virus into head tissue (Table 1), no effect of DTR was observed, but we found a strong effect of DPE. Dissemination into the head tissue was first observed at day 7 for the constant 26°C treatment and small DTR, but not until day 11 for the large DTR, although an effect of DTR at day 7 was statistically insignificant (sample sizes and pairwise comparisons are shown in Tables 4 and 5, respectively). Rates of dissemination increased sharply until day 15, after which almost all infected females had a disseminated infection (Table 4). An interaction between DPE and DTR was observed (Table 1). Differences between treatments were observed at days 11 and 27. There was no effect of carton within DTR and DPE. Pairwise comparisons at each age (Table 5) demonstrated that the constant temperature regimen had significantly lower dissemination at day 11 than both other treatments, but higher dissemination rate than the small DTR at day 15. At day 27, there was only a single mosquito tested from the large DTR (which was uninfected). Therefore, we did not consider this data point in the pairwise analyses for that time point.
Table 4.
Overall Aedes aegypti dengue virus serotype-1 dissemination rates, as assayed by testing heads of infected females, separated by carton*
| Day | Carton | Constant | Small | Large | |||
|---|---|---|---|---|---|---|---|
| % | No. | % | No. | % | No. | ||
| 7 | 1 | 33 | (3) | 50 | (2) | 0 | (4) |
| 2 | 0 | (6) | 0 | (1) | – | (0) | |
| 11 | 1 | 17 | (6) | 57 | (7) | 71 | (7) |
| 2 | 0 | (7) | 44 | (9) | 50 | (2) | |
| 15 | 1 | 100 | (8) | 80 | (10) | 71 | (7) |
| 2 | 100 | (7) | 73 | (11) | 100 | (1) | |
| 19 | 1 | 100 | (2) | 100 | (5) | 100 | (4) |
| 2 | 63 | (8) | 100 | (7) | 100 | (3) | |
| 23 | 1 | 100 | (2) | 83 | (6) | 100 | (3) |
| 2 | 100 | (4) | 100 | (7) | 0 | (1) | |
| 27 | 1 | 100 | (6) | 100 | (3) | 0 | (1) |
| 2 | 100 | (3) | 100 | (7) | – | (0) | |
For each carton, the percentage of infected females is given, along with the number of females tested in parentheses.
Table 5.
Pairwise comparisons between DTR treatments for dengue virus serotype-1 dissemination in Aedes aegypti*
| Day | Source | Constant vs. small | Constant vs. large | Small vs. large | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | P | χ2 | df | P | χ2 | df | P | ||
| 7 | DTR | 2.374 | 1 | 0.123 | 0.000 | 1 | 0.998 | 2.993 | 1 | 0.084 |
| Carton [DTR] | 2.634 | 2 | 0.268 | 2.460 | 1 | 0.292 | 0.174 | 1 | 0.917 | |
| 11 | DTR | 7.855 | 1 | 0.005 | 6.600 | 1 | 0.010 | 0.194 | 1 | 0.660 |
| Carton [DTR] | 1.899 | 2 | 0.387 | 1.953 | 2 | 0.377 | 0.564 | 2 | 0.754 | |
| 15 | DTR | 5.804 | 1 | 0.016 | 0.000 | 1 | 0.999 | 0.370 | 1 | 0.543 |
| Carton [DTR] | 0.154 | 2 | 0.926 | 0.622 | 2 | 0.733 | 0.775 | 2 | 0.679 | |
| 19 | DTR | 0.000 | 1 | 0.998 | 0.000 | 1 | 0.998 | – | ||
| Carton [DTR] | 1.632 | 2 | 0.442 | 1.632 | 2 | 0.442 | – | |||
| 23 | DTR | 0.000 | 1 | 0.999 | 0.000 | 1 | 0.996 | 0.000 | 1 | 0.998 |
| Carton [DTR] | 1.644 | 2 | 0.440 | 4.499 | 2 | 0.106 | 6.143 | 2 | 0.046 | |
| 27 | DTR | – | † | † | ||||||
| Carton [DTR] | – | † | † | |||||||
DTR = daily fluctuating temperature; df = degree of freedom. Dashes indicate that the test was not conducted because there was no difference in the response variables for these two DTR treatments (i.e., both cartons for both treatments had 100% infection).
indicates the test was not performed because there was only a single individual to test for the large DTR treatment. Values in boldface indicate that the effect was significant (P < 0.016) after Bonferroni correction for multiple comparisons.
Survival.
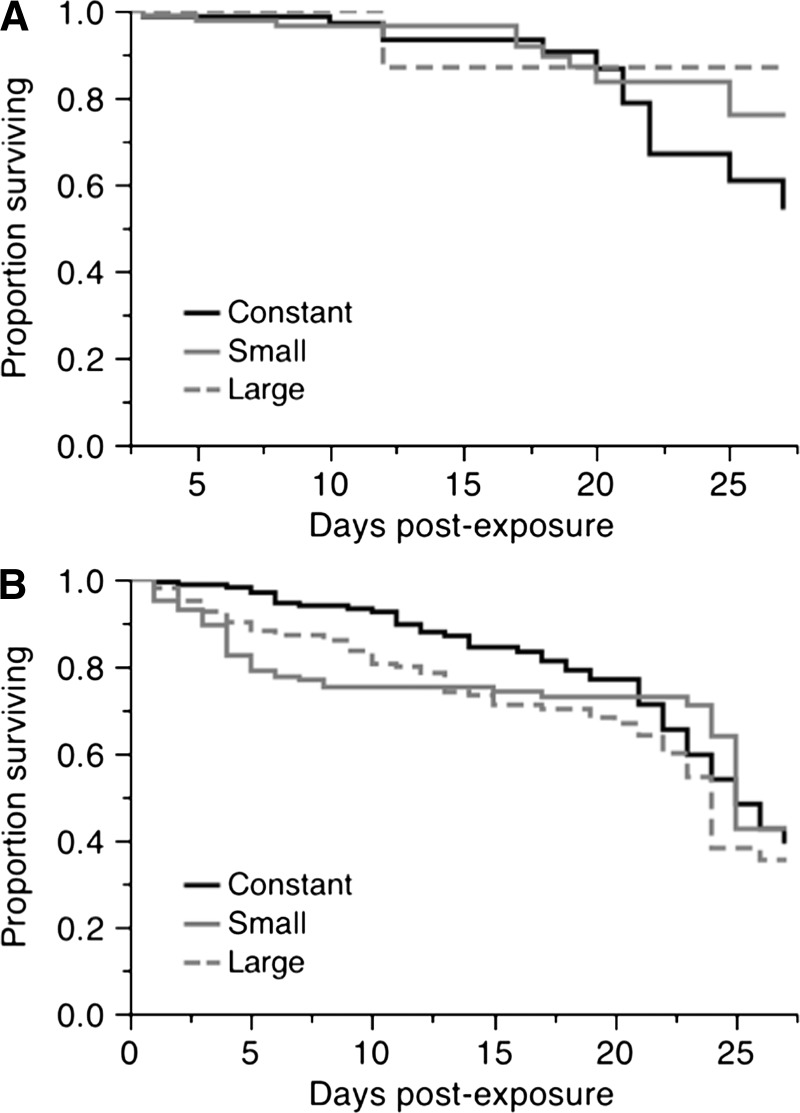
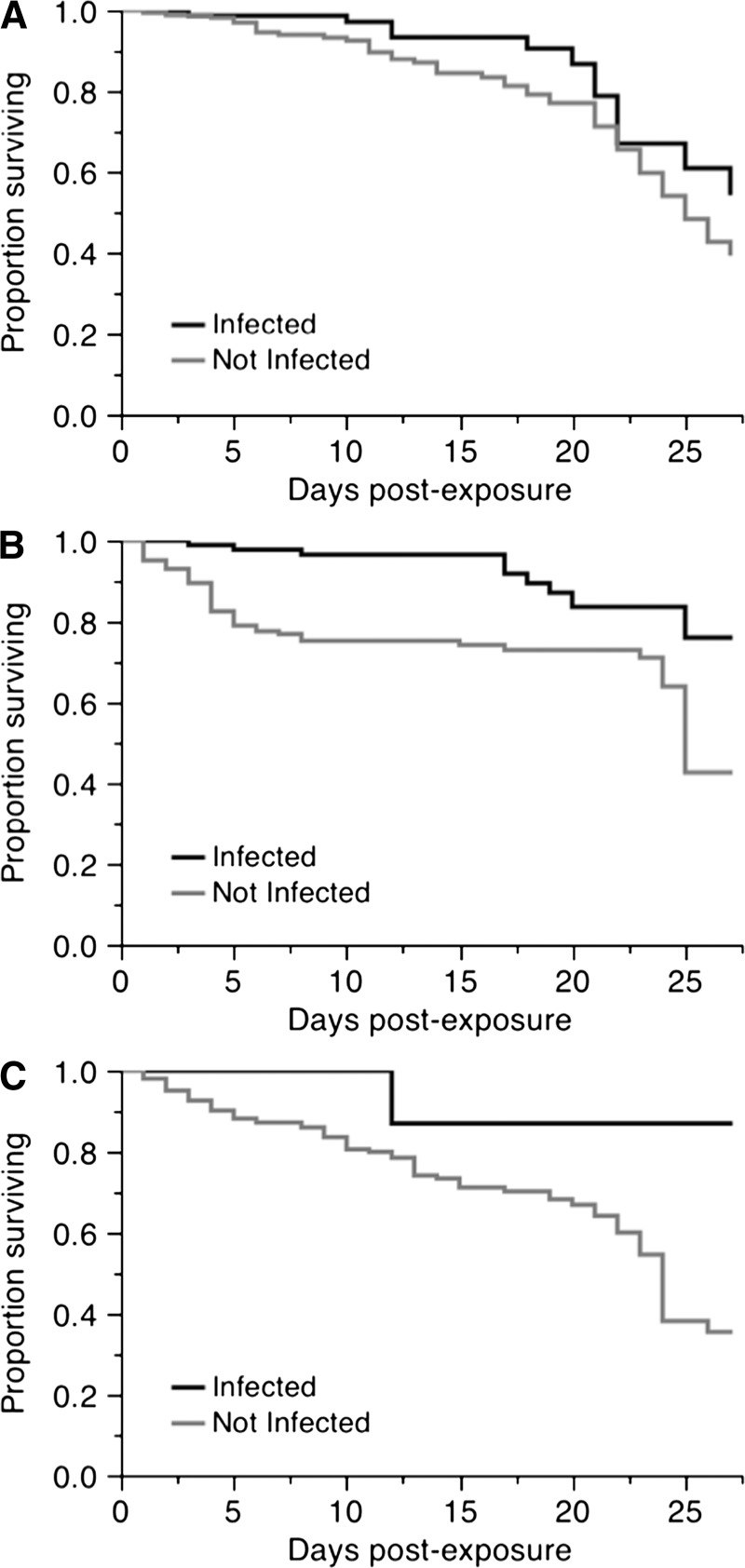
A total of 186 females died during the experiment and 526 were sampled at scheduled time points and were therefore right-censored. We identified an overall significant effect of DTR on survival (χ2 = 6.85, degrees of freedom [df] = 2, P = 0.032) (Figure 1A). Lowest survival was for females held under large fluctuations, with a mean adult lifespan of 20 days, relative to females under a constant temperature (23 days) and small fluctuations (21 days). We detected an overall effect of infection on survival (χ2 = 25.17, df = 1, P < 0.0001) (Figure 1B), in which females that were uninfected, although they had been exposed to the virus, had a lower survival than those that were infected. Uninfected females lived an average of approximately 4 days less (21 days) than females who had detectable infections (25 days). We separated infected and uninfected females and analyzed the effect of DTR for each, but found no significant effect for either (infected females: χ2 = 1.49, df = 2, P = 0.473; exposed but uninfected females: χ2 = 3.68, df = 2, P = 0.158) (Figure 2). We also assessed the effect of infection within each DTR. Although there was a consistent reduction in the lifespan of females that had been exposed to the virus but were uninfected (Figure 3), it was significant only for the small and large DTR regimens (χ2 = 13.93, df = 1, P < 0.001 and χ2 = 6.59, df = 1, P = 0.010, respectively). There were only three infected mosquitoes from the large DTR treatment that died through the duration of the experiment. There was no statistical difference in the survival curves of infected and uninfected females from the constant temperature regimen (χ2 = 3.31, df = 1, P = 0.068).
Figure 2.
Effect of daily fluctuating temperature on survival curves of female Aedes aegypti, separated by dengue virus (DENV) serotype-1 infection status. A, Exposed, infected with DENV-1 (χ2 = 1.49, degrees of freedom [df] = 2, P = 0.473). B, Exposed, uninfected (χ2 = 3.68, df = 2, P = 0.158).
Figure 3.
Effect of dengue virus serotype-1 infection on female Aedes aegypti survival, for each of three temperature regimens. A, Constant 26°C (χ2 = 3.31, degrees of freedom [df] = 1, P = 0.068). B, small fluctuations (χ2 = 13.93, df = 1, P < 0.001). C, Large fluctuations (χ2 = 6.59, df = 1, P = 0.010).
Wing lengths.
Wing lengths were measured and analyzed for 95 mosquitoes of known infection status. There was no effect of carton nested within DTR (F27, 62 = 1.383, P = 0.146), or a main effect of DTR (F2, 62 = 2.548, P = 0.086) on mosquito wing length. Mean ± SE wing lengths were 2.95 ± 0.020 mm (n = 27) for mosquitoes reared under a constant temperature regimen, 2.90 ± 0.016 mm (n = 27) for mosquitoes reared under a small DTR, and 2.86 ± 0.021 mm (n = 35) for mosquitoes reared under a large DTR. No difference was observed between the wing lengths of infected and uninfected mosquitoes (F1, 62 = 0.242, P = 0.624). Mean ± SE wing length was 2.91 ± 0.018 mm (n = 39) for infected mosquitoes and 2.89 ± 0.013 mm (n = 63) for uninfected mosquitoes. There was no interaction between infection status and DTR (F2, 62 = 0.620, P = 0.541).
Discussion
Our results indicate that large DTRs can reduce DENV transmission probability by altering vector life history traits and vector-virus interactions. Depending on the location and climate (i.e., northwestern Thailand), our results may help explain seasonal changes in DENV transmission dynamics. During the high transmission season, mosquitoes are more likely to become infected than during the low transmission season and a larger number of infected mosquitoes would be expected to have enhanced survival. Conversely during the low transmission season, relatively fewer mosquitoes become infected with DENV. Additionally, a large DTR around of mean of 26°C has other negative effects, including reduced fecundity, extended immature development time and decreased survival.16
We found that a large DTR reduces the proportion of females that become infected with DENV-1 compared with either a constant 26°C or a set of small fluctuations around the same mean, which is consistent with results reported by Lambrechts and others.9 Although the shape of the temperature profile was different between the two studies (symmetrical versus asymmetrical fluctuations), results are similar across multiple collections of Ae. aegypti from two locations in Thailand. We found no difference in overall detectable mosquito DENV-1 infection rates between the constant temperature and small fluctuation regimens, which is the same result that Lambrechts and others9 reported for DENV-1 and DENV-2. However, unlike the study of Lambrechts and others,9 we observed a slight increase in detectable infection rates over time, which may be partly explained by the lower infection rate in our study compared with the previous study. Because the overall detectable infection rate in the study of Lambrechts and others9 was approximately 100%, a significant increase was not possible. Differences in infection rates between the two studies could be a result of the relatively low infectious titer of the blood meal used in the present experiment. Alternatively, viral- and/or mosquito-specific factors may have lead to variation in levels of infection.19 Despite this discrepancy, we are confident that the differences we detected are biologically meaningful because the infectious blood meal titers of this study fall within the range of observed viremia in humans.20,21
Lambrechts and others9 modeled the effect of fluctuating temperatures on infection and transmission of flaviviruses, using empirical data from West Nile, Murray Valley encephalitis, and St. Louis encephalitis viruses. The model predicted that transmission probability under an asymmetric temperature profile with a mean of 26°C and large daily fluctuations (15–20°C) would be reduced from approximately 95% to approximately 50%. Although we found a reduction in rates of midgut (body) infection when compared with the constant temperature, we did not detect an empirical difference between DTR treatments in rates of dissemination (head infections). These results are consistent with the empirical data reported by Lambrechts and others,9 in which a symmetrical temperature profile was used. It is likely that the discrepancy between the modeling outcome and the empirical data is a result of differences between vector-virus systems. However, it should be noted that dissemination probability may not adequately represent transmission, probability because a low titer of disseminated virus often does not correlate with successful transmission in laboratory assays.22
Previous studies have shown that the EIP is influenced by changes in mean temperature.2–4,23 In our experiment, the minimum EIP (i.e., the time when we first observed a disseminated infection in females), was not observed in any individual from the large DTR treatment until day 11, but was seen at day 7 for the constant and small DTR treatments. Again, the theoretical predictions made by Lambrechts and others9 are contrary to this finding, although resolution of their model below 10 days is limited by available data because in some cases our sample sizes were modest. Further investigation is required to obtain greater resolution of dissemination potential at these early time points.
Environmental temperatures during larval development phase may influence adult traits, such as vector competence.15 The present study was not designed to enable us to discern between the effects of temperature on immature and adult mosquitoes because it is highly unlikely that a mosquito would transition from one temperature regimen to another between the aquatic and adult phases. For this reason, we consider that our experiment to be more representative of field conditions than if we had standardized immature development temperatures, as was the case in the study of Lambrechts and others.9
Survival curves differed between mosquitoes in our three DTR treatments. We tested mosquitoes up to 27 DPE because we suspect that this is the most important time period for transmission, rather than later in life when mortality rates have increased, and we expect that most mosquitoes would have died24,25 and that external factors may skew the population age structure. As reported by Lambrechts and others,9 we detected a significant effect of DTR on mosquito survival. However, the magnitude of the observed response in our study was not as strong at that previously reported. The data show that exposing Ae. aegypti females to a large DTR reduces survival relative to a constant temperature. Under a smaller DTR, the magnitude of this effect is reduced but the biological significance of this is unclear. This difference in the magnitude of the effect could be caused by the difference in the shape of temperature profile (symmetric versus asymmetric) and perhaps the slight difference in magnitude of DTR between the two studies.
Our inability to identify a consistent effect of DTR on survival when accounting for infection status suggests an interaction between DTR and survival. Although we observed an overall effect of DTR on mosquito survival, when we separated mosquitoes by infection status, the effect was no longer detected in either group. This result can be most clearly seen by looking at the outcome from the large DTR treatment. There was only a small number of infected females that died during the course of the experiment (n = 3). Thus, the overall survival curve for the large DTR was lowered more by the larger number of uninfected females that died. This finding is compared with constant temperatures, in which there was less of a discrepancy in the numbers between infected/uninfected females, and the overall survival curve was weighted less by a single group of mosquitoes. Therefore, we suspect that the apparent low mortality rate of infected mosquitoes in the large DTR was caused primarily by the small sample size in that category.
Although the exact mechanisms for changes in survival and vector competence remain to be determined, a possible explanation is that for portions of the day temperatures exceed the optimal thermal range for adult mosquitoes, resulting in the diversion of resources from maintenance to thermal stress responses; e.g., induction of cellular chaperone proteins.26 Examining thermal tolerance at high and low extremes of infected and uninfected mosquitoes from this population would test a possible physiological basis for this interaction, and indicate the tolerance of the mosquito and virus populations to variable habitats and environments. We are currently testing the optimal thermal range and critical thermal limits for vector competence of this Ae. aegypti population in Kamphaeng Phet.
Across all temperature treatments, we observed higher mean survival estimates for females that were infected with DENV-1 compared with those that were uninfected. Our results do not support the expectation that there would be a relatively high cost of infection for Ae. aegypti once exposed. Instead, they suggest there may be a cost in mounting a successful antiviral immune response. An equivalent result was observed in both Ae. aegypti infected with DENV-211 and Cx. pipiens infected with West Nile virus,27 in which infected mosquitoes had higher survival rates than those that were exposed to the virus but remained uninfected. A cost of exposure to the virus could not be tested in our study because we did not include unexposed control mosquitoes. It is also possible that we did not detect infection in all dead mosquitoes. Dead females were collected every 24 hours, and virus may have degraded below the limits of detection between death and collection.
A number of researchers have investigated the relationship between vector competence and female mosquito size and their results are contradictory. We measured variation in size within each temperature regimen and could not attribute size as a factor influencing susceptibility to DENV infection. Alto and others13 reported that smaller Ae. aegypti and Ae. albopictus were more likely to become infected with DENV-2, with the effect being more prominent for Ae. aegypti. Westbrook and others14 and Muturi and Alto15 reported that larger mosquitoes were more likely to become infected with two alphaviruses (Chikungunya and Sindbis viruses). In the latter two studies, variation in the size of the adult females was produced through rearing larvae at different temperatures, which confounded size within developmental rearing conditions, rather than examining variation in size across different temperature treatments.
The lack of consistency between our study and the study of Alto and others13 may reflect our smaller sample size or that the increased susceptibility of small females was associated with the DENV isolates used in the respective experiments (DENV-1 versus DENV-2). Further work is required to resolve this discrepancy, including manipulation of immature rearing temperatures and adult exposure temperatures to determine whether smaller female size during the low DENV transmission season in Mae Sot is a contributing or confounding factor that alters mosquito susceptibility to DENV. Lambrechts and others9 reared mosquitoes at the same temperature of 26°C (therefore, we would expect wing size variation to be evenly distributed across temperature regimens) and then after feeding them virus infectious blood, exposed them to three different temperature treatments, and they still detected reduced infection rates under larger fluctuations. We detected some variation in mean wing length across different temperature regimens, but it was not statistically significantly different, perhaps because of the small number of replicates within DTR treatments.
Recent work on interactions between mosquitoes and malaria parasites similarly indicates that DTR influences parasite transmission, although the mechanism(s) appears to be different from the Ae. aegypti-DENV system. Compared with constant temperatures, Paaijmans and others28 showed that under low and a high mean temperature regimen, realistic magnitudes of fluctuations that would be observed in Africa altered the duration of the sporogony for Plasmodium chabaudi in Anopheles stephensi. Dependent upon whether the mean temperature was low or high, fluctuations altered the direction of the change in the time estimate for transmission to be achieved. Around lower mean temperatures, a DTR of 12°C accelerated parasite development. At higher mean temperatures, the same DTR slowed development. This finding is consistent with what we measured for DENV at a relatively high mean temperature of 26°C, in which an increased DTR slows the rate of virus dissemination in infected mosquitoes (i.e., a longer EIP).
Our results support two important points raised by Lambrechts and others.9 First, it is not always appropriate to assume in experimental studies that a constant mean temperature is adequate for representing the impact of natural fluctuating temperatures on mosquito-virus interactions. Second, the magnitude of DTR is important in determining the Ae. aegypti-DENV infection rate in mosquito bodies, and this can have epidemiologically relevant implications for our understanding of transmission dynamics in natural populations. In addition, our results indicate that under a realistic temperature profile, a large DTR may extend the EIP of mosquitoes, and points out that diurnal temperature fluctuations can influence multiple components of vectorial capacity. These results are consistent with the hypothesis that under environmentally realistic temperature conditions, female Ae. aegypti in northwestern Thailand would be less likely to transmit DENV-1 during the low than high transmission season because they have reduced susceptibility to DENV infection and an extended EIP.
ACKNOWLEDGMENTS
We thank W. K. Reisen for continued support of our use of containment facilities, laboratory space, and fruitful discussions; P. S. Ward for use of microscopes for wing measurements; A. Ponlawat for collecting and sending mosquito eggs; N. H. Willits for statistical advice; and two anonymous reviewers for constructive comments on an earlier version of this manuscript. This research benefited from discussions with M. B. Thomas, K. P. Paaijmans, and working group members in the Research and Policy for Infectious Disease Dynamics Program of the Science and Technology Directorate, Department of Homeland Security, and the Fogarty International Center, National Institutes of Health.
Footnotes
Financial support: The study was supported by grant EF-0914384 from the Ecology of Infectious Disease Program of the National Science Foundation. Thomas W. Scott acknowledges funding from the Research and Policy for Infectious Disease Dynamics Program.
Authors' addresses: Lauren B. Carrington, Nossal Institute for Global Health, University of Melbourne, Carlton, VIC, Australia, E-mail: lbcarrington@gmail.com. M. Veronica Armijos, Department of Entomology, University of California, Davis, CA, E-mail: mvarmijos@ucdavis.edu. Stephanie N. Seifert, Department of Biology, University of Pennsylvania, Philadelphia, PA, E-mail: seifst@sas.upenn.edu. Louis Lambrechts, Insects and Infectious Diseases, Centre National de la Recherche Scientifique, Unite de Recherche Associee 3012, Institut Pasteur, Paris Cedex 15, France, E-mail: louis.lambrechts@pasteur.fr. Thomas W. Scott, Department of Entomology, University of California, Davis, CA, and Fogarty International Center, National Institutes of Health, Bethesda, MD, E-mail: twscott@ucdavis.edu.
References
- 1.Nisalak A, Endy TP, Nimmannitya S, Kalayanarooj UT, Scott RM, Burke DS, Hoke CH, Innis BL, Vaughn DW. Serotype-specific dengue virus circulation and dengue disease in Bangkok, Thailand from 1973 to 1999. Am J Trop Med Hyg. 2003;68:191–202. [PubMed] [Google Scholar]
- 2.McLean DM, Miller MA, Grass PN. Dengue virus transmission by mosquitoes incubated at low temperatures. Mosq News. 1975;35:322–327. [Google Scholar]
- 3.Watts DM, Burke DS, Harrison BA, Whitmire RE, Nisalak A. Effect of temperature on the vector efficiency of Aedes aegypti for dengue 2 virus. Am J Trop Med Hyg. 1987;36:143–152. doi: 10.4269/ajtmh.1987.36.143. [DOI] [PubMed] [Google Scholar]
- 4.Rohani A, Wong YC, Zamre I, Lee HL, Zurainee MN. The effect of extrinsic incubation temperature on development of dengue serotype 2 and 4 viruses in Aedes aegypti (L.) Southeast Asian J Trop Med Public Health. 2009;40:942–950. [PubMed] [Google Scholar]
- 5.Bates M, Roca-Garcia M. The development of the virus of yellow fever in haemagogus mosquitoes. Am J Trop Med Hyg. 1946;26:585–605. doi: 10.4269/ajtmh.1946.s1-26.585. [DOI] [PubMed] [Google Scholar]
- 6.Chamberlain RW, Sudia WD. The effects of temperature upon the extrinsic incubation of eastern equine encephalitis in mosquitoes. Am J Hyg. 1955;62:295–305. doi: 10.1093/oxfordjournals.aje.a119780. [DOI] [PubMed] [Google Scholar]
- 7.Turell MJ, Lundstrom JO. Effect of environmental temperature on the vector competence of Aedes aegypti and Ae. taeniorhynchus for Ockelbo virus. Am J Trop Med Hyg. 1990;43:543–550. doi: 10.4269/ajtmh.1990.43.543. [DOI] [PubMed] [Google Scholar]
- 8.Lundstrom JO, Turell MJ, Niklasson B. Effect of environmental temperature on the vector competence of Culex pipiens and Cx. torrentium for Ockelbo virus. Am J Trop Med Hyg. 1990;43:534–542. doi: 10.4269/ajtmh.1990.43.534. [DOI] [PubMed] [Google Scholar]
- 9.Lambrechts L, Paaijmans KP, Fansiri T, Carrington LB, Kramer LD, Thomas MB, Scott TW. Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti. Proc Natl Acad Sci USA. 2011;108:7460–7465. doi: 10.1073/pnas.1101377108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parton WJ, Logan JE. A model for diurnal variation in soil and air temperature. Agric Meteorol. 1981;23:205–216. [Google Scholar]
- 11.Maciel-de-Freitas R, Koella JC, Lourenço-de-Oliveira R. Lower survival rate, longevity and fecundity of Aedes aegypti (Diptera: Culicidae) females orally challenged with dengue virus serotype 2. Trans R Soc Trop Med Hyg. 2009;105:452–458. doi: 10.1016/j.trstmh.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 12.Lambrechts L, Scott TW. Mode of transmission and the evolution of arbovirus virulence in mosquito vectors. Proc R Soc Lond B Biol Sci. 2009;276:1369–1378. doi: 10.1098/rspb.2008.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alto BW, Reiskind MH, Lounibos LP. Size alters susceptibility of vectors to dengue virus infection and dissemination. Am J Trop Med Hyg. 2008;79:688–695. [PMC free article] [PubMed] [Google Scholar]
- 14.Westbrook CJ, Reiskind MH, Pesko K, Greene KE, Lounibos LP. Larval environmental temperature and the susceptibility of Aedes albopiictus Skuse (Diptera: Culicidae) to Chikungunya virus. Vector Borne Zoonotic Dis. 2010;10:241–247. doi: 10.1089/vbz.2009.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muturi EJ, Alto BW. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector Borne Zoonotic Dis. 2011;11:1157–1163. doi: 10.1089/vbz.2010.0209. [DOI] [PubMed] [Google Scholar]
- 16.Carrington LB, Seifert SN, Willits NH, Lambrechts L, Scott TW. Large diurnal temperature fluctuations negatively influence Aedes aegypti (Diptera: Culicidae) life history traits. J Med Entomol. 2013;50:43–51. doi: 10.1603/me11242. [DOI] [PubMed] [Google Scholar]
- 17.Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, Edman JD. Dispersal of the dengue vector Aedes aegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- 18.Payne AF, Binduga-Gajewska I, Kauffman EB, Kramer LD. Quantitation of falviviruses by fluorescent focus assay. J Virol Methods. 2006;134:183–189. doi: 10.1016/j.jviromet.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Lambrechts L, Chevillon C, Albright RG, Thaisomboonsuk B, Richardson JH, Jarman RG, Scott TW. Genetic specificity and potential for local adaptation between dengue viruses and mosquito vectors. BMC Evol Biol. 2009;9:160. doi: 10.1186/1471-2148-9-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gubler DJ, Suharyono W, Lubis I, Eram S, Gunarso S. Epidemic dengue 3 in central Java, associated with low viremia in man. Am J Trop Med Hyg. 1981;30:1094–1099. doi: 10.4269/ajtmh.1981.30.1094. [DOI] [PubMed] [Google Scholar]
- 21.Gubler DJ, Suharyono W, Tan R, Abidin M, Sie A. Viraemia in patients with naturally acquired dengue infection. Bull World Health Organ. 1981;59:623–630. [PMC free article] [PubMed] [Google Scholar]
- 22.Lambrechts L, Fansiri T, Pongsiri A, Thaisomboonsuk B, Klungthong C, Richardson JH, Ponlawat A, Jarman RG, Scott TW. Dengue-1 virus clade replacement in Thailand associated with enhanced mosquito transmission. J Virol. 2012;86:1853–1861. doi: 10.1128/JVI.06458-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McLean DM, Clarke AM, Coleman JC, Montalbetti CA, Skidmore AG, Walters TE, Wise R. Vector capability of Aedes aegypti mosquitoes for California encephalitis and dengue viruses at various temperatures. Can J Microbiol. 1974;20:255–262. doi: 10.1139/m74-040. [DOI] [PubMed] [Google Scholar]
- 24.Styer LM, Carey JR, Wang J-L, Scott TW. Mosquitoes do senesce: departure from the paradigm of constant mortality. Am J Trop Med Hyg. 2007;76:111–117. [PMC free article] [PubMed] [Google Scholar]
- 25.Harrington LC, Vermeylen F, Jones JJ, Kitthawee S, Sithiprasasna R, Edman JD, Scott TW. Age-dependent survival of the dengue vector Aedes aegypti (Diptera: Culicidae) demonstrated by simultaneous release-recapture of different age cohorts. J Med Entomol. 2008;45:307–313. doi: 10.1603/0022-2585(2008)45[307:asotdv]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Feder ME, Hoffman GE. Heat shock proteims, molecular chaperones and the heat shock response: evolutionary and ecological physiology. Annu Rev Physiol. 1999;61:243–282. doi: 10.1146/annurev.physiol.61.1.243. [DOI] [PubMed] [Google Scholar]
- 27.Ciota AT, Styer LM, Meola MA, Kramer LD. The costs of resistance and infection as determinants of West Nile virus susceptibility in Culex mosquitoes. BMC Ecol. 2011;11:23. doi: 10.1186/1472-6785-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paaijmans KP, Blanford S, Bell AS, Blanford JI, Read AF, Thomas MB. Influence of climate on malaria transmission depends on daily temperature variation. Proc Natl Acad Sci USA. 2010;107:15135–15139. doi: 10.1073/pnas.1006422107. [DOI] [PMC free article] [PubMed] [Google Scholar]