Abstract
Artemisinin-based combination therapies (ACTs) and trimethoprim-sulfamethoxazole (TS) prophylaxis are important tools for malaria control, but there are concerns about their effect on gametocytes, the stage of the parasite responsible for transmission. We conducted a longitudinal clinical trial in a cohort of HIV-infected and uninfected children living in an area of high malaria transmission intensity in Uganda. Study participants were randomized to artemether-lumefantrine (AL) or dihydroartemisinin-piperaquine (DP) for all treatments of uncomplicated malaria (N = 4,380) as well as TS prophylaxis for different durations. The risks of gametocytemia detected by microscopy in the 28 days after antimalarial therapy were compared using multivariate analyses. The risk of gametocyte detection was significantly higher in patients treated with DP compared with AL (adjusted relative risk = 1.85, P < 0.001) and among children prescribed TS prophylaxis (adjusted relative risk = 1.76, P < 0.001). The risk of gametocytemia and its potential for increasing transmission should be considered when evaluating different ACTs and TS prophylaxis for malaria control.
Introduction
The clinical manifestations of malaria are caused by proliferation of asexual parasites in human blood, but transmission of the parasite to the mosquito is dependent on the development of gametocytes—the sexual stage of infection. There is significant interest in understanding the impact of different malaria control interventions, including treatment with artemisinin-based combination therapies (ACTs) and chemoprevention regimens, on the development of gametocytes and their potential impact on malaria transmission.1
ACTs have been shown to be highly efficacious against asexual parasites,2–6 and several have been shown to be moderately effective against the gametocyte stage of infection and reduce transmission to mosquitoes.7–10 However, data comparing the effect of two leading ACTs in Africa—artemether-lumefantrine (AL) and dihyroartemesinin-piperaquine (DP)—on gametocytes have been mixed.2,4,11–16 Trimethoprim-sulfamethoxazole (TS), a drug routinely used to prevent opportunistic infections in human immunodeficiency virus (HIV) -infected individuals, has been shown to significantly reduce the incidence of malaria.17–21 However, there is limited data on the association between the use of TS prophylaxis and gametocytes, and several studies have reported an association between exposure to sulfadoxine-pyrimethamine (SP), another antifolate drug related to TS, and an increased prevalence and density of gametocytes.7,22–24
In Uganda, the Ministry of Health currently recommends AL and DP as first- and second-line drugs, respectively, for the treatment of uncomplicated malaria. Although both drugs are safe and efficacious, DP has gained increasing interest for treatment because of its one time per day dosing schedule and prolonged post-treatment prophylactic effect.4,11,25 Chemoprophylaxis with TS has also become an important tool for malaria control in Uganda and is currently recommend for all HIV-infected patients and HIV-exposed children born to HIV-infected mothers. A better understanding of the relationship between these antimalarial therapeutics and the risk of gametocytemia could have significant implications on malaria transmission and therefore, the development of antimalarial treatment and prevention policies in Uganda.
We compared the risk of gametocytemia after treatment with AL or DP for uncomplicated malaria in an open-label randomized trial in a cohort of HIV-infected, HIV-exposed (HIV-uninfected children born to HIV-infected mothers), and HIV-unexposed (HIV-uninfected children born to HIV-uninfected mothers) children living in an area of high malaria transmission of Uganda. We also assessed the association between other risk factors, including the use of TS prophylaxis, and gametocytemia after antimalarial therapy.
Materials and Methods
Study design, site, and participants.
This study was part of an open-label randomized controlled trial in Tororo, a district in Eastern Uganda with high malaria transmission intensity.26 Details of this study have been described elsewhere.2,21,27,28 Briefly, convenience sampling was used to enroll children referred to a dedicated study clinic from an adjacent post-natal clinic at Tororo District Hospital. Eligibility criteria included (1) age 6 weeks to 12 months, (2) documented HIV status of mother and child, (3) agreement to come to the study clinic for any febrile episode or other illness, (4) residence within a 30-km radius of the study clinic, (5) absence of active medical problem requiring in-patient evaluation at the time of screening, (6) provision of informed consent, and (7) currently breastfeeding if HIV-exposed. At enrollment, all study participants received a long-lasting insecticide-treated bed net (ITN), and all HIV-exposed and -infected children were given daily TS prophylaxis as per Uganda's Ministry of Health (MOH) guidelines.
Follow-up of study participants.
Subjects were followed for all of their medical problems at a dedicated study clinic open 7 days a week, and parents/guardians were encouraged to bring their children to the study clinic whenever they were ill. After-hours care was available through the adjacent hospital wards. Children who presented with new medical problems underwent a standardized medical evaluation using algorithms to guide therapy for common illnesses. Medications with antimalarial activity were avoided for the treatment of non-malarial illnesses. Monthly assessments were done in the study clinic to ensure adherence with the study protocol and perform routine blood smears. Study participants were withdrawn from the study for (1) movement out of the study area, (2) inability to be located for > 60 consecutive days, (3) withdrawal of informed consent, (4) inability to adhere to the study schedule and procedures, or (5) inability to tolerate the drugs used for malaria treatment.
Treatment allocation.
Children who were aged ≥ 4 months and weighing ≥ 5 kg were randomized to receive either AL or DP at the time that they got the first episode of uncomplicated malaria. Study participants received the same treatment regimen for all subsequent episodes of uncomplicated malaria. HIV-exposed uninfected children were retested for HIV 6–8 weeks after breastfeeding cessation. HIV-exposed children who remained HIV-uninfected were randomized at the cessation of breastfeeding to discontinue or continue TS prophylaxis until 2 years of age. At 2 years of age, those children who had continued TS were rerandomized to discontinue or continue TS prophylaxis. HIV-infected children, including those children who seroconverted during breastfeeding, were continued on TS prophylaxis for the duration of the study.
Malaria diagnosis and treatment.
Subjects who presented to the study clinic with a documented fever (tympanic temperature ≥ 38.0°C) or history of fever in the previous 24 hours had blood obtained by finger prick for a thick smear. If the thick smear was positive, the patient was diagnosed with malaria regardless of parasite density. Children who had uncomplicated malaria were treated with the treatment to which they were randomized. A nurse administered study drugs according to weight-based guidelines for fractions of tablets as follows: AL (tablets of 20 mg artemether and 120 mg lumefantrine; Coartem; Novartis Pharmaceuticals Corporation, Suffern, NY) administered as one (5–14 kg) or two (15–24 kg) tablets given two times daily for 3 days; DP (tablets of 40 mg dihydroartemisinin and 320 mg piperaquine; Duocotecxin; Zhejiang Holley Nanhu Pharmaceutical Co. Ltd., Jiaxing City, P.R. China) targeting a total dose of 6.4 and 51.2 mg/kg dihydroartemisinin and piperaquine, respectively, given in three equally divided daily doses to the nearest one-quarter tablet. Patients were given a glass of milk or asked to breastfeed after each dose of study medication. The first daily dose of study drugs was directly observed at the study clinic. After each dose, children were observed for 30 minutes, and the dose was readministered if vomiting occurred. For patients randomized to receive AL, parents or guardians were instructed to give the second daily dose at home. Episodes of uncomplicated malaria in children less than 4 months of age or weighing less than 5 kg as well as episodes of complicated malaria and treatment failures occurring within 14 days of initiating treatment were treated with quinine. All children with malaria were followed up on days 1, 2, 3, 7, 14, 21, and 28 after the diagnosis. Thick blood smears were collected on all malaria follow-up days apart from day 1.
Laboratory methods.
Thick and thin blood smears were stained with 2% Giemsa for 30 minutes and read by trained laboratory technologists who were not involved in direct patient care. Gametocytemia was determined from thick smears using microscopy and recorded as present or absent. Parasite densities were calculated from thick blood smears by counting the number of asexual parasites per 200 leukocytes (or per 500 leukocytes if the count was < 10 asexual parasites/200 leukocytes), assuming a leukocyte count of 8,000/μL. A blood smear was considered negative when the examination of 100 high-power fields did not reveal asexual parasites. For quality control, all slides were read by a second reader, and a third reader would settle any readings with discrepancies. Laboratory technicians were blinded to the study participants' treatment assignments. Thin smears were used to determine the parasite species.
Statistical methods.
Data were entered by two independent data entrants into an Access database. Data analysis was done using Stata version 11 (Stata Corp, College Station, TX). The observation period began 1 day after enrollment and ended when the child turned 4 years of age or on the day the child was prematurely withdrawn from the study. The primary outcome of interest was whether gametocytes were detected by microscopy on the day a new episode of malaria was diagnosed and during the 28-day follow-up period after each episode of malaria. The primary exposure variable of interest was antimalarial treatment with AL or DP. Secondary exposure variables of interest included TS use, age at the time of malaria treatment, residence (rural versus urban), and whether an episode of malaria resulted in recurrent parasitemia within 4–28 days. Associations between risk factors of interest and the presence or absence of gametocytes were measured using generalized estimating equations with exchangeable correlation and robust standard errors to account for repeated measures in the same child. Cumulative risks of detecting gametocytes during malaria follow-up not detected at the time of diagnosis or microscopic clearance of gametocytes detected at the time of diagnosis after treatment with DP or AL were estimated using the Kaplan–Meier product limit formula. Kaplan–Meier survival curves were compared using the log-rank test. Associations between risk factors of interest and the hazard of either detecting gametocytes during malaria follow-up not detected at the time of diagnosis or microscopic clearance of gametocytes detected at the time of diagnosis were made using a Cox proportional hazards controlling for repeated measures in the same patient. In all analyses, a two-tailed P value < 0.05 was considered to be statistically significant.
Ethical approval.
Informed consent was obtained from the parents or legal guardians of all study participants. The study protocol was approved by the Uganda National Council of Science and Technology and the institutional review boards of the University of California, San Francisco, Makerere University, the University of Washington, and the Centers for Disease Control and Prevention.
Results
Trial profile and characteristics of malaria treatments.
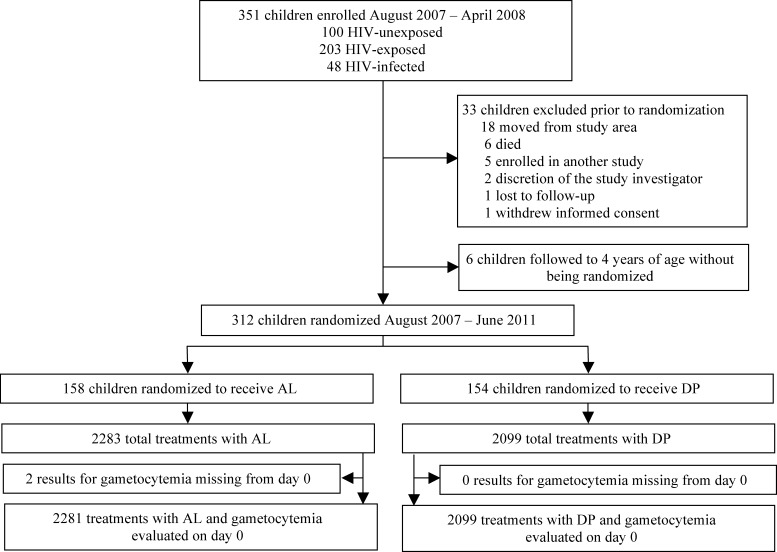
A total of 351 children were enrolled between August of 2007 and April of 2008, of which 100 children were HIV-unexposed, 203 children were HIV-exposed, and 48 children were HIV-infected. Thirty-three children were excluded before randomization to antimalarial therapy, and six children were followed to 4 years of age without being randomized; 158 children were randomized to AL, and 154 children were randomized to DP, resulting in 2,283 treatments with AL and 2,099 treatments with DP (Figure 1 ). The mean age in months, TS use, and residence of children at the time of antimalarial therapy were similar between both antimalarial treatment groups (Table 1). The risk of recurrent parasitemia within 4–28 days of follow-up was significantly higher in malaria episodes treated with AL compared with episodes treated with DP, as previously reported (relative risk [RR] = 4.74, P < 0.001).2 Of 4,380 episodes of uncomplicated malaria, 253 (5.8%) had microscopically detected gametocytes on the day of malaria diagnosis. The prevalence of gametocytemia on the day of malaria diagnosis was similar for those children randomized to DP (6.2%) versus AL (5.4%) after adjusting for TS use, age, and time since the last episode of malaria (P = 0.40).
Figure 1.
Trial profile.
Table 1.
Characteristics of malaria treatments
| Characteristic | Antimalarial therapy | P | |
|---|---|---|---|
| AL (N = 2,281) | DP (N = 2,099) | ||
| Age, mean (SD) | 28.2 (11.0) | 28.2 (11.4) | 0.63 |
| Prescribed TS, n (%) | 500 (21.9) | 458 (21.8) | 0.30 |
| Live in rural residence, n (%) | 2,119 (92.8) | 1,840 (87.7) | 0.49 |
| Recurrent parasitemia,* n (%) | 1,066 (46.7) | 195 (9.3) | < 0.001 |
| Gametocyte prevalence at day of diagnosis, n (%) | 124 (5.4) | 129 (6.2) | 0.40 |
Defined as whether recurrent parasitemia occurred between days 4 and 28 of malaria follow-up.
Risk factors for gametocytemia after antimalarial treatment.
Of 25,767 blood smears obtained during malaria follow-up, gametocytes were detected in 766 (3.0%) by microscopy. Treatment with DP was associated with an 85% increase (RR = 1.85, 95% confidence interval [CI] = 1.34–2.54, P < 0.001) in the risk of any gametocytemia during malaria follow-up compared with AL after controlling for TS prophylaxis, age, and development of recurrent parasitemia. TS prophylaxis was associated with a 76% increase (RR = 1.76, 95% CI = 1.29–2.40, P < 0.001) in the risk of any gametocytemia during malaria follow-up by multivariate analysis. Increased age and recurrent parasitemia were also associated with an increased risk of any gametocytemia during malaria follow-up by multivariate analysis (Table 2). HIV status showed no significant association with the risk of gametocytemia after adjustment for TS prophylaxis, age, treatment arm, and development of recurrent parasitemia (RR = 1.29, 95% CI = 0.74–2.24, P = 0.373).
Table 2.
Risk factors for any gametocytemia 1–28 days after antimalarial therapy
| Risk factors | Risk of gametocytemia | Univariate | Multivariate | |||
|---|---|---|---|---|---|---|
| Exposed (%) | Unexposed (%) | RR (95% CI) | P | RR (95% CI) | P | |
| Treatment with DP vs. AL | 10.2 | 6.2 | 1.64 (1.21–2.22) | 0.002 | 1.85 (1.34–2.54) | < 0.001 |
| On TS prophylaxis vs. no TS | 10.3 | 7.5 | 1.47 (1.08–1.99) | 0.01 | 1.76 (1.29–2.40) | < 0.001 |
| Age (per 1 year increase) | N/A | N/A | 1.18 (1.04–1.33) | 0.008 | 1.24 (1.10–1.40) | < 0.001 |
| Developed recurrent parasitemia* | 8.2 | 8.1 | 1.12 (0.89–1.41) | 0.34 | 1.36 (1.05–1.76) | 0.02 |
Defined as whether recurrent parasitemia occurred between days 4 and 28 of malaria follow-up.
Risk factors for the first detection of gametocytes after antimalarial therapy.
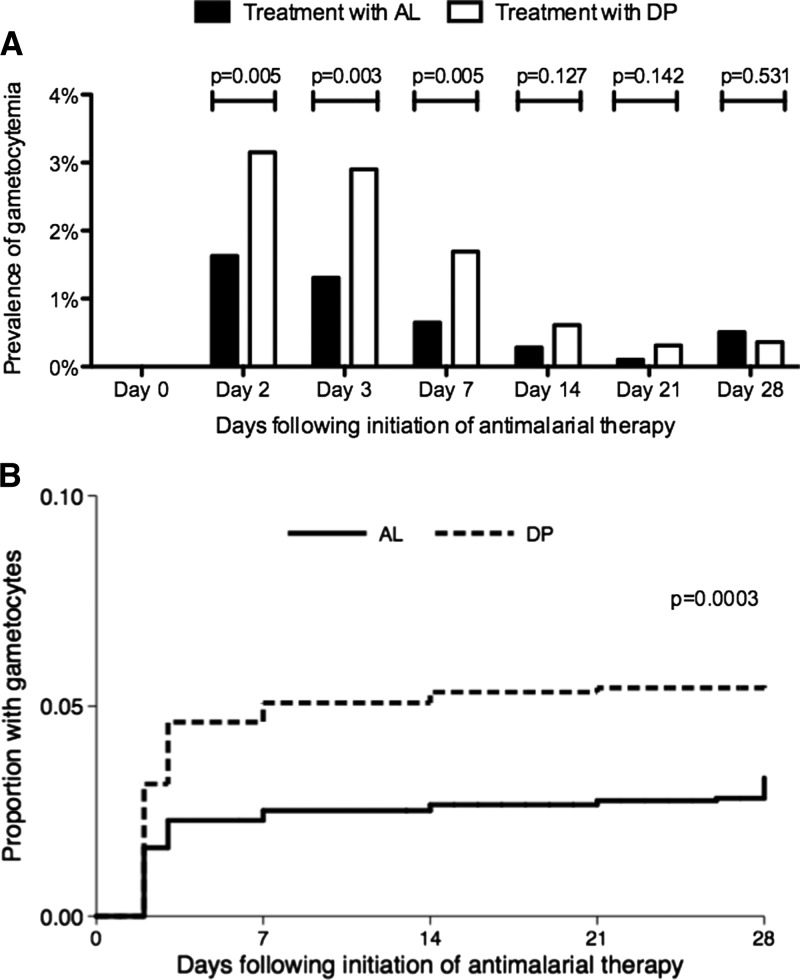
To further explore associations between risk factors of interest and gametocytemia after antimalarial therapy, results were stratified based on whether gametocytes were first detected after therapy or the clearance of gametocytes was detected before initiation of antimalarial therapy. Considering episodes of malaria where gametocytes were not detected before antimalarial therapy, the prevalence of gametocytes on days 2, 3, and 7 after antimalarial therapy was significantly higher after treatment with DP compared with AL, but the prevalence was not significantly different on days 14, 21, and 28 (Figure 2A ). The cumulative risk of first detection of gametoctyes was significantly higher after treatment with DP compared with AL (log rank test P < 0.001) (Figure 2B). Using a Cox proportional hazards model, there was over a twofold increase (hazard ratio [HR] = 2.12, 95% CI = 1.42–3.17, P < 0.001) in the rate of first detection of gametocytes after treatment with DP compared with AL after adjusting for TS prophylaxis, age, and recurrent parasitemia (Table 3). TS prophylaxis was associated with a trend to an increase in the rate of first detection of gametocytes, but this trend did not reach statistical significance (HR = 1.46, 95% CI = 0.95–2.25, P = 0.08). Increasing age and recurrent parasitemia were also associated with a significant increase in the rate of first detection of gametocytes (Table 3).
Figure 2.
(A) Prevalence of gametocytes during malaria follow-up if not present on day 0. Univariate comparisons between treatment arms (AL vs. DP) on each day of follow-up made using generalized estimating equations with robust standard errors. (B) Kaplan–Meier survival curves comparing appearance of new gametocytes between children treated with AL vs. DP. Survival curves compared using log-rank test.
Table 3.
Risk factors for the first detection of gametocytes after antimalarial therapy
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Treatment with DP vs. AL | 1.74 (1.18–2.56) | 0.005 | 2.12 (1.42–3.17) | < 0.001 |
| On TS prophylaxis vs. no TS | 1.21 (0.78–1.83) | 0.371 | 1.46 (0.95–2.25) | 0.08 |
| Age (per 1 year increase) | 1.32 (1.13–1.55) | < 0.001 | 1.38 (1.18–1.61) | < 0.001 |
| Developed recurrent parasitemia* | 1.09 (0.74–1.60) | 0.656 | 1.64 (1.10–2.43) | 0.02 |
Defined as whether recurrent parasitemia occurred between days 4 and 28 of malaria follow-up.
Clearance of gametocytes.
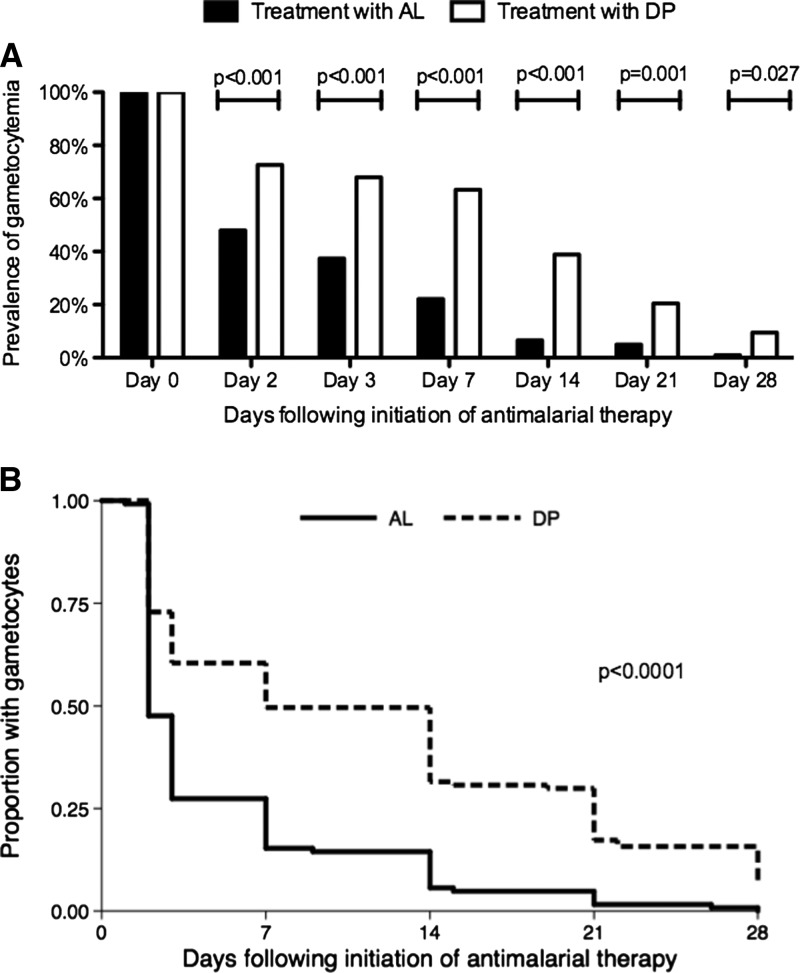
Considering episodes of malaria where gametocytes were detected before antimalarial therapy, the prevalence of gametocytes on all days after antimalarial therapy was significantly higher after treatment with DP compared with AL (Figure 3A ). The cumulative risk of continued detection of gametoctyes was significantly higher after treatment with DP compared with AL (log rank test P < 0.001) (Figure 3B). Using a Cox proportional hazards model, there was over a twofold increase (HR = 2.20, 95% CI = 1.73–2.79, P < 0.001) in the rate of clearance of gametocytes after treatment with AL compared with DP after adjusting for TS prophylaxis, age, and recurrent parasitemia (Table 4). Not being prescribed TS prophylaxis was also associated with a significant increase in the rate of clearance of gametocytes compared with being prescribed TS prophylaxis (HR = 1.32, 95% CI = 1.05–1.64, P = 0.02). Increasing age and recurrent parasitemia were not associated with a significant difference in the rate of clearance of gametocytes (Table 4).
Figure 3.
(A) Prevalence of gametocytes during malaria follow-up if present on day 0. Univariate comparisons between treatment arms (AL vs. DP) on each day of follow-up made using generalized estimating equations with robust standard errors. (B) Kaplan–Meier survival curves comparing clearance of gametocytes present on day 0 between children treated with AL vs. DP. Survival curves compared using log-rank test.
Table 4.
Cox proportional hazards model for gametocyte clearance after starting antimalarial treatment
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Treatment with AL vs. DP | 2.08 (1.68–2.58) | < 0.001 | 2.20 (1.73–2.79) | < 0.001 |
| No TS vs. TS prophylaxis | 1.26 (1.00–1.59) | 0.051 | 1.32 (1.05–1.64) | 0.02 |
| Age (per 1 year increase) | 1.02 (0.92–1.13) | 0.675 | 1.00 (0.90–1.11) | 0.99 |
| Developed recurrent parasitemia* | 1.21 (0.98–1.49) | 0.075 | 0.86 (0.68–1.07) | 0.18 |
Defined as whether recurrent parasitemia occurred between days 4 and 28 of malaria follow-up.
Discussion
In this large randomized trial of over 4,000 treatments for malaria in young Ugandan children living in a highly endemic setting, treatment of malaria with DP was associated with an increased risk of detection of Plasmodium falciparum gametocytes by microscopy during the 28 days after antimalarial therapy compared with treatment with AL. These differences were similar when considering the first detection of gametocytes after therapy and clearance of gametocytes detected before therapy. TS prophylaxis was also associated with a significantly increased risk of detection of gametocytes after antimalarial therapy. Increasing age and recurrent parasitemia were associated with an increased risk of first detection of gametocytes after therapy but not clearance of gametocytes.
Prior studies comparing the risk of gametocytes after treatment with AL versus DP have had mixed results. Several studies using microscopic detection of gametocytes have shown no difference,2,12,29 an increased risk of gametocyte detection after treatment with AL,4,11 or more recently, an increased risk of gametocyte detection after treatment with DP.14–16,30 These differences are likely caused by differences in analyses and the low sensitivity of microscopy for gametocytes.30 A small study performed in western Kenya found no difference in microscopic gametocyte carriage between treatment with AL or DP. However, when using a highly sensitive nucleic acid sequence-based amplification (NASBA) technique for detecting gametocytes, the authors found both a significantly higher proportion of individuals with gametocytes than detected by microscopy and a significantly increased risk of gametocyte detection after treatment with DP compared with treatment with AL.13 Membrane-feeding experiments have confirmed that both microscopic and subpatent gametocytemia result in infectivity to mosquitoes, with a positive association between gametocyte density and mosquito infection rates.7,31
The reason for an increased risk of detection of gametocytes with DP compared with AL is unclear. Dihydroartemisinin is the active metabolite of artemether, but it is not known if drug levels of dihydroartemisinin are different after administration of DP compared AL. In addition, the partner drugs (lumefantrine and piperaquine) may have differential gametocytidal effects, although classically, only the eight aminoquinolones (with primaquine most studied) are thought to have activity against mature, late-stage gametocytes.1 A recent in vitro study, however, has shown that lumefantrine may have moderate activity against late-stage gametocytes compared with piperaquine.32
We further found that the use of TS prophylaxis resulted in both an increased risk of gametocyte detection on the day of malaria diagnosis and delayed clearance of gametocytes after antimalarial therapy. Studies examining the effect of SP, another antifolate combination, when used for antimalarial treatment have long suggested an elevated risk of gametocytemia after treatment.22,24 There have been no studies that have looked at the effect of TS prophylaxis on gametocytemia, but one prior study examined the effect of TS on gametocytemia when used for malaria treatment. This study was conducted in Nigeria, and children were randomized to receive TS or SP for treatment of uncomplicated malaria. Children in both treatment arms had a significant increase in gametocyte carriage 7 days after treatment compared with pre-treatment levels. However, children treated with SP were significantly more likely to have gametocyte carriage on day 14 compared with children treated with TS (65.7% versus 35%, respectively, P = 0.018), suggesting a more marked effect of gametocytemia with SP than TS.33
The increase in the risk of gametocytemia in children receiving antifolates could be explained by decreased gametocydal activity, drug-induced release of sequestered gametocytes, or development of antifolate drug resistance. In a study of South African patients treated with SP for uncomplicated malaria, increases in the duration of gametocyte carriage after treatment were strongly associated with antifolate resistance markers dhfr and dhps.34 However, our group has reported no difference in the prevalence of these markers of antifolate resistance between children prescribed TS prophylaxis and those children not taking TS prophylaxis.35 Moreover, in our setting, widespread antifolate resistance is common, with some studies reporting prevalence of over 80% even in those individuals not taking TS prophylaxis.36
We also found that increasing age and development of recurrent parasitemia were associated with an increased risk of first detection of gametocytes. Several papers have noted that increasing age is inversely associated with the risk for gametocyte carriage thought to be caused by the development of immunity to asexual stages, which concomitantly limits the production of gametocytes.37–39 Notably, these other studies used cohorts much older than the cohort described in this paper, typically finding the decline in gametocyte prevalence occurring in individuals older than 10 years.37 Our findings suggest that children under 4 years may not yet have acquired immunity to asexual or sexual stages; the rise in gametocyte prevalence may, therefore, indicate a rise in the incidence of malaria in this setting.
There were several potential limitations to our study. Detection of gametocytes was done using microscopy, which likely underestimated the true prevalence of gametocytes because of failure to detect subpatent gametocytemia. Fortunately, the large number of malaria treatments observed provided us sufficient power to evaluate associations using microscopically detected gametocytes and supports the findings of other large studies that used microscopy.16 Although we may have only detected higher gametocyte densities because we used microscopy in our study, it has repeatedly been shown that microscopic gametocyte carriers are more infectious to mosquitoes than submicroscopic gametocyte carriers, adding to the public health significance of our findings.31 Although our study was an open-label randomized controlled trial, laboratory technicians who were reading the smears were blinded to the treatment arms. This process limited observer bias during reading of blood smears. Another important advantage of this study was that it was longitudinal; because children were treated for a repeated number of episodes, our study is the first to look at the effect of ACTs on gametocytemia when used repeatedly over nearly 4 years. Finally, although we describe a difference in gametocyte detection and clearance between the two arms, we cannot definitively describe differences in transmissibility without performing membrane- or direct-feeding experiments to assess infectivity.
In conclusion, treatment of malaria with DP compared with treatment with AL and prophylaxis with TS compared with no prophylaxis were both independently associated with an increased risk of gametocyte detection after antimalarial therapy. We consider these findings of immediate public health relevance, because they suggest that these two interventions may be associated with an increased risk of transmission and may temper enthusiasm for their use as tools for antimalarial treatment and chemoprevention. There remains a need to assess the impact of malaria treatment and control interventions on the risk of gametocytemia and malaria transmission.
ACKNOWLEDGMENTS
We are grateful to all the parents and guardians for kindly giving their consent and the study participants for their cooperation. The authors thank all the members of the study team for their tireless effort and excellent work.
Disclaimer: The funders were not involved with study design, data analysis, or manuscript preparation. The contents of the manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the CDC or Doris Duke Charitable Foundation.
Footnotes
Financial support: Participants in this study were enrolled in programs supported by the US President's Emergency Plan for AIDS Relief and Cooperative Agreement No. U62P024421 from the Centers for Disease Control and Prevention (CDC); National Center for HIV, Viral Hepatitis, STD, and TB Prevention (NCHHSTP); and Global AIDS Program (GAP). P.J. is supported by National Institutes of Health T32 Training Grant 2 T32 AI 60530-6 and a Burroughs Wellcome Fund/American Society of Tropical Medicine and Hygiene Postdoctoral Fellowship. Funding was also provided by the Doris Duke Charitable Foundation (G.D. is a recipient of the Clinical Scientist Development Award).
Authors' addresses: Abel Kakuru, Emmanuel Arinaitwe, Humphrey Wanzira, Mary Muhindo, Victor Bigira, and Emmanuel Osilo, Infectious Diseases Research Collaboration, Kampala, Uganda, E-mails: abelkakuru@gmail.com, emmy3md@yahoo.com, wanzirah@yahoo.com, marymkakuru@gmail.com, vbigira@gmail.com, and goodbeads@yahoo.co.uk. Prasanna Jagannathan and Grant Dorsey, Department of Medicine, San Francisco General Hospital, University of California, San Francisco, CA, E-mails: jagannathanp@medsfgh.ucsf.edu and gdorsey@medsfgh.ucsf.edu. Jaco Homsy, Institute for Global Health, University of California, San Francisco, CA, E-mail: jhomsy@psg.ucsf.edu. Moses R. Kamya, Department of Medicine, Makerere University College of Health Sciences, Kampala, Uganda, E-mail: mkamya@infocom.co.ug. Jordan W. Tappero, Global AIDS Program, Centers for Disease Control and Prevention, Atlanta, GA, E-mail: jwt0@cdc.gov.
References
- 1.White NJ. The role of anti-malarial drugs in eliminating malaria. Malar J. 2008;7((Suppl 1)):S8. doi: 10.1186/1475-2875-7-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arinaitwe E, Sandison TG, Wanzira H, Kakuru A, Homsy J, Kalamya J, Kamya MR, Vora N, Greenhouse B, Rosenthal PJ, Tappero J, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for falciparum malaria: a longitudinal, randomized trial in young Ugandan children. Clin Infect Dis. 2009;49:1629–1637. doi: 10.1086/647946. [DOI] [PubMed] [Google Scholar]
- 3.Dorsey G, Staedke S, Clark TD, Njama-Meya D, Nzarubara B, Maiteki-Sebuguzi C, Dokomajilar C, Kamya MR, Rosenthal PJ. Combination therapy for uncomplicated falciparum malaria in Ugandan children: a randomized trial. JAMA. 2007;297:2210–2219. doi: 10.1001/jama.297.20.2210. [DOI] [PubMed] [Google Scholar]
- 4.Kamya MR, Yeka A, Bukirwa H, Lugemwa M, Rwakimari JB, Staedke SG, Talisuna AO, Greenhouse B, Nosten F, Rosenthal PJ, Wabwire-Mangen F, Dorsey G. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of malaria: a randomized trial. PLoS Clin Trials. 2007;2:e20. doi: 10.1371/journal.pctr.0020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faye B, Offianan AT, Ndiaye JL, Tine RC, Toure W, Djoman K, Sylla K, Ndiaye PS, Penali L, Gaye O. Efficacy and tolerability of artesunate-amodiaquine (Camoquin plus) versus artemether-lumefantrine (Coartem) against uncomplicated Plasmodium falciparum malaria: multisite trial in Senegal and Ivory Coast. Trop Med Int Health. 2010;15:608–613. doi: 10.1111/j.1365-3156.2010.02487.x. [DOI] [PubMed] [Google Scholar]
- 6.Zongo I, Dorsey G, Rouamba N, Tinto H, Dokomajilar C, Guiguemde RT, Rosenthal PJ, Ouedraogo JB. Artemether-lumefantrine versus amodiaquine plus sulfadoxine-pyrimethamine for uncomplicated falciparum malaria in Burkina Faso: a randomised non-inferiority trial. Lancet. 2007;369:491–498. doi: 10.1016/S0140-6736(07)60236-0. [DOI] [PubMed] [Google Scholar]
- 7.Bousema JT, Schneider P, Gouagna LC, Drakeley CJ, Tostmann A, Houben R, Githure JI, Ord R, Sutherland CJ, Omar SA, Sauerwein RW. Moderate effect of artemisinin-based combination therapy on transmission of Plasmodium falciparum. J Infect Dis. 2006;193:1151–1159. doi: 10.1086/503051. [DOI] [PubMed] [Google Scholar]
- 8.Okell LC, Drakeley CJ, Ghani AC, Bousema T, Sutherland CJ. Reduction of transmission from malaria patients by artemisinin combination therapies: a pooled analysis of six randomized trials. Malar J. 2008;7:125. doi: 10.1186/1475-2875-7-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bousema JT, Drakeley CJ, Mens PF, Arens T, Houben R, Omar SA, Gouagna LC, Schallig H, Sauerwein RW. Increased Plasmodium falciparum gametocyte production in mixed infections with P. malariae. Am J Trop Med Hyg. 2008;78:442–448. [PubMed] [Google Scholar]
- 10.Sutherland CJ, Ord R, Dunyo S, Jawara M, Drakeley CJ, Alexander N, Coleman R, Pinder M, Walraven G, Targett GA. Reduction of malaria transmission to Anopheles mosquitoes with a six-dose regimen of co-artemether. PLoS Med. 2005;2:e92. doi: 10.1371/journal.pmed.0020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeka A, Dorsey G, Kamya MR, Talisuna A, Lugemwa M, Rwakimari JB, Staedke SG, Rosenthal PJ, Wabwire-Mangen F, Bukirwa H. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treating uncomplicated malaria: a randomized trial to guide policy in Uganda. PLoS ONE. 2008;3:e2390. doi: 10.1371/journal.pone.0002390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zongo I, Dorsey G, Rouamba N, Dokomajilar C, Sere Y, Rosenthal PJ, Ouedraogo JB. Randomized comparison of amodiaquine plus sulfadoxine-pyrimethamine, artemether-lumefantrine, and dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria in Burkina Faso. Clin Infect Dis. 2007;45:1453–1461. doi: 10.1086/522985. [DOI] [PubMed] [Google Scholar]
- 13.Mens PF, Sawa P, van Amsterdam SM, Versteeg I, Omar SA, Schallig HD, Kager PA. A randomized trial to monitor the efficacy and effectiveness by QT-NASBA of artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment and transmission control of uncomplicated Plasmodium falciparum malaria in western Kenya. Malar J. 2008;7:237. doi: 10.1186/1475-2875-7-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nambozi M, Van Geertruyden JP, Hachizovu S, Chaponda M, Mukwamataba D, Mulenga M, Ubben D, D'Alessandro U. Safety and efficacy of dihydroartemisinin-piperaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Zambian children. Malar J. 2011;10:50. doi: 10.1186/1475-2875-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bassat Q, Mulenga M, Tinto H, Piola P, Borrmann S, Menendez C, Nambozi M, Valea I, Nabasumba C, Sasi P, Bacchieri A, Corsi M, Ubben D, Talisuna A, D'Alessandro U. Dihydroartemisinin-piperaquine and artemether-lumefantrine for treating uncomplicated malaria in African children: a randomised, non-inferiority trial. PLoS ONE. 2009;4:e7871. doi: 10.1371/journal.pone.0007871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Four Artemisinin-Based Combinations (4ABC) Study Group A head-to-head comparison of four artemisinin-based combinations for treating uncomplicated malaria in African children: a randomized trial. PLoS Med. 2011;8:e1001119. doi: 10.1371/journal.pmed.1001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anglaret X, Chene G, Attia A, Toure S, Lafont S, Combe P, Manlan K, N'Dri-Yoman T, Salamon R. Early chemoprophylaxis with trimethoprim-sulphamethoxazole for HIV-1-infected adults in Abidjan, Cote d'Ivoire: a randomised trial. Cotrimo-CI Study Group. Lancet. 1999;353:1463–1468. doi: 10.1016/s0140-6736(98)07399-1. [DOI] [PubMed] [Google Scholar]
- 18.Wiktor SZ, Sassan-Morokro M, Grant AD, Abouya L, Karon JM, Maurice C, Djomand G, Ackah A, Domoua K, Kadio A, Yapi A, Combe P, Tossou O, Roels TH, Lackritz EM, Coulibaly D, De Cock KM, Coulibaly IM, Greenberg AE. Efficacy of trimethoprim-sulphamethoxazole prophylaxis to decrease morbidity and mortality in HIV-1-infected patients with tuberculosis in Abidjan, Cote d'Ivoire: a randomised controlled trial. Lancet. 1999;353:1469–1475. doi: 10.1016/s0140-6736(99)03465-0. [DOI] [PubMed] [Google Scholar]
- 19.Nunn AJ, Mwaba P, Chintu C, Mwinga A, Darbyshire JH, Zumla A. Role of co-trimoxazole prophylaxis in reducing mortality in HIV infected adults being treated for tuberculosis: randomised clinical trial. BMJ. 2008;337:a257. doi: 10.1136/bmj.a257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamya MR, Gasasira AF, Achan J, Mebrahtu T, Ruel T, Kekitiinwa A, Charlebois ED, Rosenthal PJ, Havlir D, Dorsey G. Effects of trimethoprim-sulfamethoxazole and insecticide-treated bednets on malaria among HIV-infected Ugandan children. AIDS. 2007;21:2059–2066. doi: 10.1097/QAD.0b013e3282ef6da1. [DOI] [PubMed] [Google Scholar]
- 21.Sandison TG, Homsy J, Arinaitwe E, Wanzira H, Kakuru A, Bigira V, Kalamya J, Vora N, Kublin J, Kamya MR, Dorsey G, Tappero JW. Protective efficacy of co-trimoxazole prophylaxis against malaria in HIV exposed children in rural Uganda: a randomised clinical trial. BMJ. 2011;342:d1617. doi: 10.1136/bmj.d1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.von Seidlein L, Drakeley C, Greenwood B, Walraven G, Targett G. Risk factors for gametocyte carriage in Gambian children. Am J Trop Med Hyg. 2001;65:523–527. doi: 10.4269/ajtmh.2001.65.523. [DOI] [PubMed] [Google Scholar]
- 23.von Seidlein L, Milligan P, Pinder M, Bojang K, Anyalebechi C, Gosling R, Coleman R, Ude JI, Sadiq A, Duraisingh M, Warhurst D, Alloueche A, Targett G, McAdam K, Greenwood B, Walraven G, Olliaro P, Doherty T. Efficacy of artesunate plus pyrimethamine-sulphadoxine for uncomplicated malaria in Gambian children: a double-blind, randomised, controlled trial. Lancet. 2000;355:352–357. doi: 10.1016/S0140-6736(99)10237-X. [DOI] [PubMed] [Google Scholar]
- 24.Tjitra E, Suprianto S, Currie BJ, Morris PS, Saunders JR, Anstey NM. Therapy of uncomplicated falciparum malaria: a randomized trial comparing artesunate plus sulfadoxine-pyrimethamine versus sulfadoxine-pyrimethamine alone in Irian Jaya, Indonesia. Am J Trop Med Hyg. 2001;65:309–317. doi: 10.4269/ajtmh.2001.65.309. [DOI] [PubMed] [Google Scholar]
- 25.Cisse B, Cairns M, Faye E, NDiaye O, Faye B, Cames C, Cheng Y, NDiaye M, Lo AC, Simondon K, Trape JF, Faye O, NDiaye JL, Gaye O, Greenwood B, Milligan P. Randomized trial of piperaquine with sulfadoxine-pyrimethamine or dihydroartemisinin for malaria intermittent preventive treatment in children. PLoS ONE. 2009;4:e7164. doi: 10.1371/journal.pone.0007164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okello PE, Van Bortel W, Byaruhanga AM, Correwyn A, Roelants P, Talisuna A, D'Alessandro U, Coosemans M. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–225. [PubMed] [Google Scholar]
- 27.Katrak S, Gasasira A, Arinaitwe E, Kakuru A, Wanzira H, Bigira V, Sandison TG, Homsy J, Tappero JW, Kamya MR, Dorsey G. Safety and tolerability of artemether-lumefantrine versus dihydroartemisinin-piperaquine for malaria in young HIV-infected and uninfected children. Malar J. 2009;8:272. doi: 10.1186/1475-2875-8-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vora N, Homsy J, Kakuru A, Arinaitwe E, Wanzira H, Sandison TG, Bigira V, Kamya MR, Tappero JW, Dorsey G. Breastfeeding and the risk of malaria in children born to HIV-infected and uninfected mothers in rural Uganda. J Acquir Immune Defic Syndr. 2010;55:253–261. doi: 10.1097/QAI.0b013e3181eb4fd7. [DOI] [PubMed] [Google Scholar]
- 29.Ratcliff A, Siswantoro H, Kenangalem E, Maristela R, Wuwung RM, Laihad F, Ebsworth EP, Anstey NM, Tjitra E, Price RN. Two fixed-dose artemisinin combinations for drug-resistant falciparum and vivax malaria in Papua, Indonesia: an open-label randomised comparison. Lancet. 2007;369:757–765. doi: 10.1016/S0140-6736(07)60160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bousema T, Drakeley C. Epidemiology and infectivity of Plasmodium falciparum and Plasmodium vivax gametocytes in relation to malaria control and elimination. Clin Microbiol Rev. 2011;24:377–410. doi: 10.1128/CMR.00051-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ouedraogo AL, Bousema T, Schneider P, de Vlas SJ, Ilboudo-Sanogo E, Cuzin-Ouattara N, Nebie I, Roeffen W, Verhave JP, Luty AJ, Sauerwein R. Substantial contribution of submicroscopical Plasmodium falciparum gametocyte carriage to the infectious reservoir in an area of seasonal transmission. PLoS ONE. 2009;4:e8410. doi: 10.1371/journal.pone.0008410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adjalley SH, Johnston GL, Li T, Eastman RT, Ekland EH, Eappen AG, Richman A, Sim BK, Lee MC, Hoffman SL, Fidock DA. Quantitative assessment of Plasmodium falciparum sexual development reveals potent transmission-blocking activity by methylene blue. Proc Natl Acad Sci USA. 2011;108:E1214–E1223. doi: 10.1073/pnas.1112037108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sowunmi A, Fateye BA, Adedeji AA, Fehintola FA, Bamgboye AE, Babalola CP, Happi TC, Gbotosho GO. Effects of antifolates–co-trimoxazole and pyrimethamine-sulfadoxine–on gametocytes in children with acute, symptomatic, uncomplicated, Plasmodium falciparum malaria. Mem Inst Oswaldo Cruz. 2005;100:451–455. doi: 10.1590/s0074-02762005000400019. [DOI] [PubMed] [Google Scholar]
- 34.Barnes KI, Little F, Mabuza A, Mngomezulu N, Govere J, Durrheim D, Roper C, Watkins B, White NJ. Increased gametocytemia after treatment: an early parasitological indicator of emerging sulfadoxine-pyrimethamine resistance in falciparum malaria. J Infect Dis. 2008;197:1605–1613. doi: 10.1086/587645. [DOI] [PubMed] [Google Scholar]
- 35.Gasasira AF, Kamya MR, Ochong EO, Vora N, Achan J, Charlebois E, Ruel T, Kateera F, Meya DN, Havlir D, Rosenthal PJ, Dorsey G. Effect of trimethoprim-sulphamethoxazole on the risk of malaria in HIV-infected Ugandan children living in an area of widespread antifolate resistance. Malar J. 2010;9:177. doi: 10.1186/1475-2875-9-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malamba S, Sandison T, Lule J, Reingold A, Walker J, Dorsey G, Mermin J. Plasmodium falciparum dihydrofolate reductase and dihyropteroate synthase mutations and the use of trimethoprim-sulfamethoxazole prophylaxis among persons infected with human immunodeficiency virus. Am J Trop Med Hyg. 2010;82:766–771. doi: 10.4269/ajtmh.2010.08-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouedraogo AL, Schneider P, de Kruijf M, Nebie I, Verhave JP, Cuzin-Ouattara N, Sauerwein RW. Age-dependent distribution of Plasmodium falciparum gametocytes quantified by Pfs25 real-time QT-NASBA in a cross-sectional study in Burkina Faso. Am J Trop Med Hyg. 2007;76:626–630. [PubMed] [Google Scholar]
- 38.Boudin C, Lyannaz J, Bosseno MF, Carnevale P, Ambroise-Thomas P. Epidemiology of Plasmodium falciparum in a rice field and a savanna area in Burkina Faso: seasonal fluctuations of gametocytaemia and malarial infectivity. Ann Trop Med Parasitol. 1991;85:377–385. doi: 10.1080/00034983.1991.11812580. [DOI] [PubMed] [Google Scholar]
- 39.Bousema JT, Gouagna LC, Drakeley CJ, Meutstege AM, Okech BA, Akim IN, Beier JC, Githure JI, Sauerwein RW. Plasmodium falciparum gametocyte carriage in asymptomatic children in western Kenya. Malar J. 2004;3:18. doi: 10.1186/1475-2875-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]