Abstract
Transmission by the oral route of Coxiella burnetii is controversial. Our objective was to evaluate dairy products in the transmission of Q fever. Pasteurized, unpasteurized, and thermized dairy products were tested for C. burnetii by using a quantitative polymerase chain reaction specific for IS1111 and IS30A spacers, culturing in human embryonic lung fibroblasts cells, and inoculation into BALB/c mice. We tested 201 products and C. burnetii was identified in 64%. Cow milk origin products were more frequently positive than goat or ewe products (P = 0.006 and P = 0.0001, respectively), and industrial food was more frequently positive than artisanal food (P < 0.0001). Food made from unpasteurized milk contained higher bacteria concentrations than food made from pasteurized milk (P = 0.02). All cultures were negative and mice did not show signs of illness. Farm animals are highly infected in France but consumption of cheese and yogurt does not seem to pose a public health risk for transmission of Q fever.
Introduction
Q fever is a zoonotic disease caused by Coxiella burnetii, an obligate intracellular gram-negative bacterium that is found worldwide.1 Current epidemiologic studies indicate that Q fever should be considered a public health problem in many countries and mostly in persons in contact with domestic animals such as cattle, sheep, and, less frequently, goats.1 Human infections occur mainly through aerosol dispersal. Humans can become infected by coming in contact with newborn animals, placentas, or wool that are contaminated with parturient fluids from infected animals.2 Coxiella burnetii shedding in dairy products seems to play a smaller role in the spread and transmission of disease than shedding in the vaginal mucus, birth products, or feces.3
The hypothesis that ingestion of C. burnetii can cause Q fever is controversial.1,2 Huebner and Bell reported recovery of C. burnetii from raw milk from four dairies in southern California and observed that seroconversion occurred in guinea pigs after subcutaneous injections of butter made from contaminated milk.4 Marmion and Stoker found that more persons who consumed raw milk in two towns near Kent, England, became ill with Q fever than persons in a control group.5 Beck and Bell showed that 32% of 300 Q fever cases in Los Angeles were found in persons who consumed raw milk.6 Fishbein and Raoult described an outbreak of Q fever in a psychiatric institution in southern France where seropositivity rates for C. burnetii were significantly higher among patients who consumed unpasteurized milk products.7 During a Q fever outbreak in 2009 in the Netherlands, De Bruin and others found high levels of C. burnetii DNA in environmental samples from bulk milk–positive farms, and 73% of these farms had a recent history of human infections with Q fever.8
Nevertheless, experiments involving human volunteers who consumed raw milk have generated contradictory results, and serologic analysis showed that persons who consumed raw milk had higher rates of Q fever than persons in control groups.9 Conversely, Krumbiegel and Wisniewski did not observe serologic conversion or clinical illness in a group of 34 human volunteers who consumed infected raw milk.10 Children who reported consumption of cheese from rural areas in Greece11 and goat workers who ate cheese made from pasteurized goat milk in Newfoundland had increased risk for Q fever.12 We tested commercially available cheese and yogurt to estimate rates of C. burnetii contamination and their eventual role in transmission of Q fever.
Materials and Methods
Sample collection and treatment.
We collected 201 dairy products (178 cheeses, 10 yogurts, 9 creams, and 7 butters) including 103 samples from supermarkets, 84 samples from cheese dairies, and 14 samples from artisanal producers located in the Alps and Provence in France. Among the artisans products, 40 (34%) were made from pasteurized milk, 70 (60%) from unpasteurized milk, and 7 (6%) from thermized milk (a process that uses a lower temperature than pasteurization). All 84 samples produced industrially were pasteurized. Twenty-two products were from Italy, 4 from Greece, and all others from France. One hundred twenty-five products were produced from cow milk, 41 from goat milk, 26 from ewe milk, 3 from goat and ewe milk, 3 from goat and cow milk, and 3 from ewe and cow milk. All samples were stored at 4°C before analysis.
Molecular analysis.
Samples from dairy products were incubated for 12 hours at 56°C with lysis buffer and proteinase K. DNA was extracted by using the BioRobot MDx System (QIAGEN, Hilden, Germany) according to the manufacturer's instructions. The extracted DNA was handled under sterile conditions to avoid cross-contamination at –20°C until analyzed by polymerase chain reaction (PCR). For detection of C. burnetii, DNA was used as a template in a reported quantitative PCR (qPCR) assay specific for the IS1111 spacer region and the less sensitive IS30A spacer region.13,14 DNA extracted from the supernatant of a culture of C. burnetii L929 served as a positive control, and samples collected from livers of rats that were negative for C. burnetii infection (by culturing and qPCR analysis) were used as negative controls. A result was considered positive when a qPCR result was positive for at least one gene (IS1111 or IS30A). The cut-off value for the positive result was a cycle threshold value < 35.
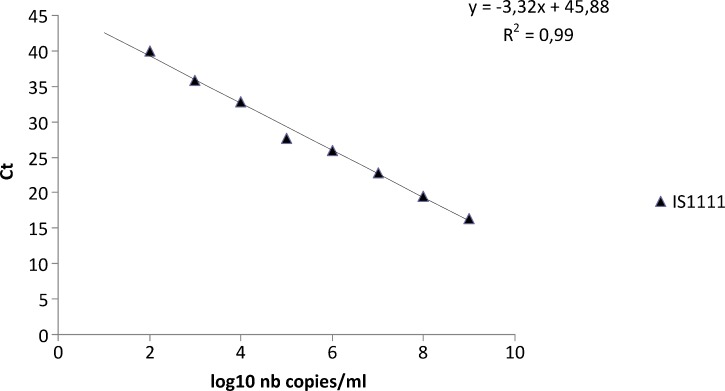
The IS1111 spacer region was used for quantification of C. burnetii. Serial 10-fold dilutions (from 10−1 to 10−11) of phase 2 antigens from C. burnetii L929 were tested. All samples were tested by qPCR assay specific for the IS1111 spacer region. Each dilution was tested by using an IS1111 qPCR to express Ct as number of bacteria/milliliter and copies of IS1111/mL of sample (Figure 1).15 For calculating the log10 bacteria copies from the number of IS1111 copies, we assumed that the number of IS1111 elements in the genome of the Nine Mile strain of C. burnetii was 20.15 Diluted samples were also tested by indirect immunofluorescence assay for C. burnetii.16 The number of copies/milliliter was calculated from the lowest dilution down to the dilution that contained at least one bacterium per microscopic field. We assumed that one bacterium per microscopic field corresponded to 104 bacteria/mL.
Figure 1.
Comparison between cycle threshold (Ct) values and log10 values of the number (nb) of copies/mL of the Coxiella burnetii IS1111 spacer region, France.
Culture.
Only positive samples that contained more than 4,000 DNA copies/mL by qPCR were inoculated into human embryonic lung (HEL) fibroblasts as described.17 Microscopic examination of cell monolayers was performed to detect any cytopathic effects that C. burnetii had on the HEL cells, and Gimenez and immunofluorescence staining were performed on days 10, 20, and 30.15 Cultures were also tested for C. burnetii by qPCR on days 10 and 30.13,14
Inoculation into mice.
Nine qPCR-positive samples were used to inoculate mice. Samples were mixed with 3 mL of phosphate-buffered saline for 1 hour and centrifuged at 200 rpm. Nine BALB/c mice were injected intraperitoneally with 250 μL of this mixture. Mice were monitored for signs of illness and mortality daily. The mice were humanely killed seven days after the injection, and their spleens were collected. Cell monolayers in shell vials were inoculated with 1 mL of the spleen extract and centrifuged at 700 rpm for 1 hour. Inoculated monolayers were incubated at 37°C in an atmosphere of 5% CO2 for 30 days as described.17 Gimenez staining and immunofluorescence staining were used to examine cell monolayers on days 10, 20, and 30. In addition, a qPCR was performed on days 0, 10, and 30.13,14
Statistical analysis.
For data comparison, we used EpiInfo version 6.0 software (Centers for Disease Control and Prevention, Atlanta, GA). A P value < 0.05 was considered significant.
Results
Overall, 130 (64%) dairy products were positive for C. burnetii by qPCR specific for the IS1111 spacer region, and 84 (41%) were positive by qPCR specific for the IS30A spacer region. No samples were positive by qPCR for the IS30A spacer region and negative for the IS1111 spacer region. Positive and negative controls tested showed standard results.
Coxiella burnetii was identified in 117 cheeses (65%), 7 yogurts (70%), 5 creams (55%), and 1 butter (14%) (Table 1). Sixty-two (52%) artisanal products and 69 (82%) industrially produced products were positive. Moreover, 83 (66%) pasteurized products, 40 (575) unpasteurized products, and all (100%) thermized products were infected by C. burnetii. A total of 75% of the cow milk products (n = 94), 51% of the goat milk products (n = 21), and 34% of the ewe milk products (n = 9) were positive. Industrially produced products were positive more often than artisanal products (P < 0.0001). Cow milk origin products were more often infected by C. burnetii than goat or ewe milk origin products (P = 0.006 and P = 0.0001 respectively). No difference was found between pasteurized and unpasteurized (P = 0.21) or between pasteurized and thermized milk origin products (P = 0.09). Thermized milk origin products were positive more often than unpasteurized products (P = 0.038).
Table 1.
Coxiella burnetii–positive dairy products, France*
| Product | Animal milk origin | Pasteurized | Thermized | Unpasteurized | Artisanal | Industrial | Total | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Cow | Ewe | Goat | Mixed | |||||||
| Cheese | 83 (82) | 8 (32) | 20 (51) | 6 (66) | 71 (72) | 7 (100) | 39 (57) | 59 (59) | 58 (77) | 117 (67) |
| Yogurt | 5 (71) | 1 (100) | 1 (50) | 0 (0) | 7 (70) | 0 (0) | 0 (0) | 1 (33) | 6 (85) | 7 (70) |
| Butter | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (14) | 0 (0) | 0 (0) | 0 (0) | 1 (100) | 1 (14) |
| Cream | 5 (55) | 0 (0) | 0 (0) | 0 (0) | 4 (57) | 0 (0) | 1 (50) | 1 (100) | 4 (50) | 5 (55) |
| Total | 94 (75) | 9 (34) | 21 (51) | 6 (66) | 83 (66) | 7 (100) | 40 (57) | 61 (52) | 69 (82) | 130 (65) |
Values are no. (%).
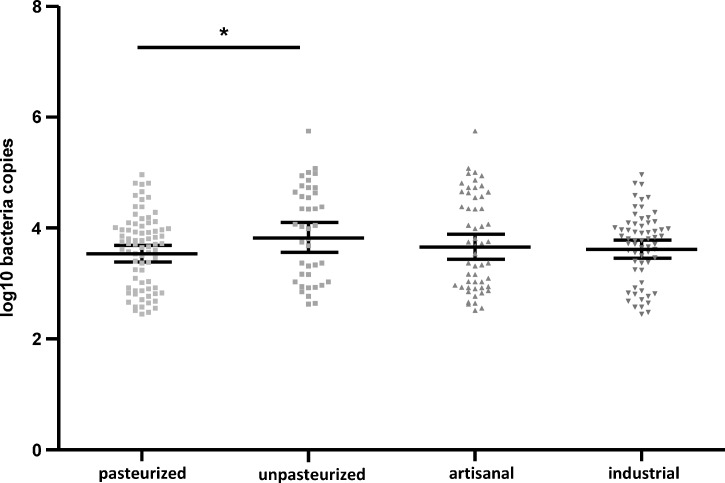
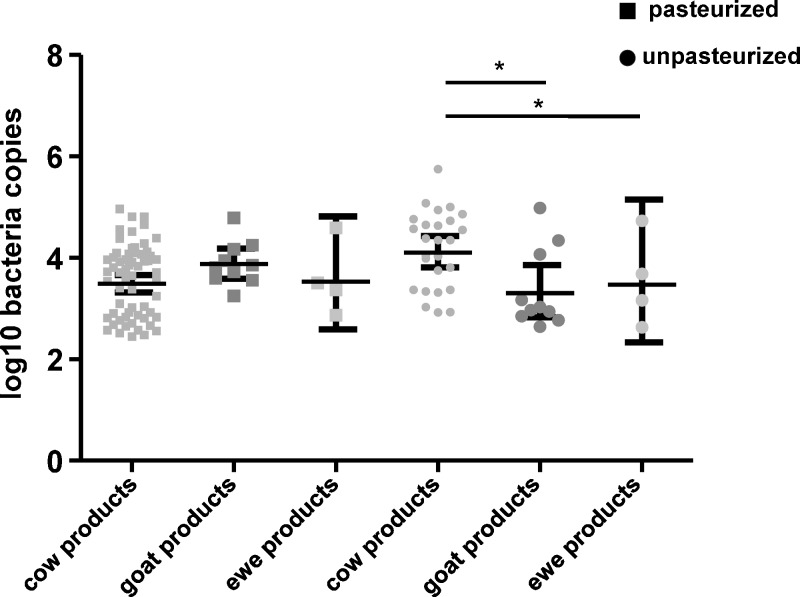
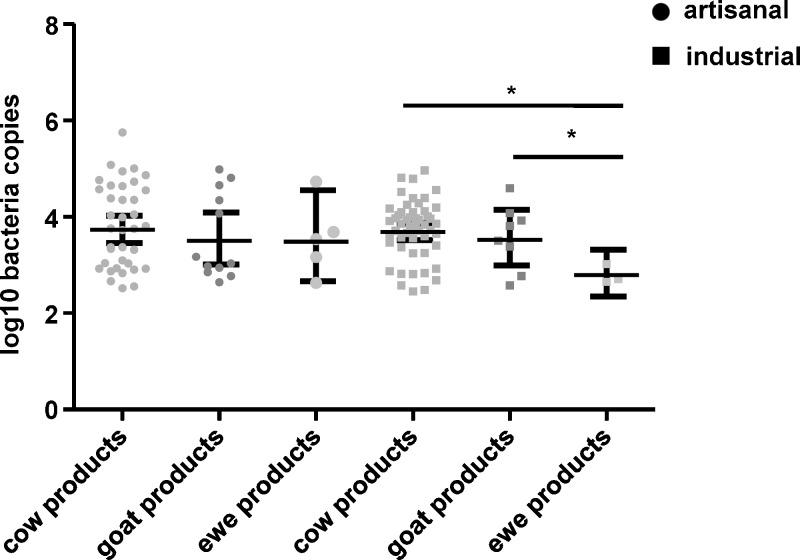
The geometric mean ± 95% confidence interval (CI) of log10 number of DNA copies/mL was significantly higher in unpasteurized products (mean ± SEM = 3.9 ± 0.13) than in pasteurized products (3.6 ± 0.07) (P = 0.02), but no difference was observed between artisanal (3.7 ± 0.12) and industrially produced (3.6 ± 0.07) samples (P = 0.57) (Figure 2). Among pasteurized products, no difference was observed in mean ± SEM log10 number DNA copies/mL between cow milk products (3.4 ± 0.62), ewe milk products (3.9 ± 0.71), and goat milk products (3.4 ± 1.12) (goat versus ewe: P = 0.8, cow versus goat: P = 0.8, and cow versus ewe: P = 0.4) (Figure 3). In unpasteurized products, more log10 number DNA copies/mL were found in cow milk (4.1 ± 0.42) than in goat milk (3.7 ± 1.33) or ewe milk (3.1 ± 0.63) or (P < 0.05) (Figure 3). Among artisanal samples, no significant difference was observed in log10 number DNA copies/mL between cow milk products (3.6 ± 0.46), goat milk products (3.4 ± 0.72) and ewe milk products (3.4 ± 1.31) (cow versus ewe: P = 0.4, cow versus goat: P = 0.8, ewe versus goat: P = 0.8) (Figure 4). In industrially produced samples, more DNA copies were found in cow milk products (3.6 ± 0.38) and in goat milk (3.4 ± 0.88) than in ewe milk products (3.0 ± 0.93) (P = 0.0001 and P = 0.01 respectively) (Figure 4).
Figure 2.
Geometric mean and 95% confidence intervals of log10 number of Coxiella burnetii DNA copies/mL in pasteurized versus unpasteurized products and in artisanal versus industrial products, France.
Figure 3.
Geometric mean and 95% confidence intervals of log10 number of Coxiella burnetii DNA copies/mL in ewe, goat, and cow milk products from pasteurized and unpasteurized products, France.
Figure 4.
Geometric mean and 95% condidence intervals of log10 number of Coxiella burnetii DNA copies/mL in ewe, goat and cow milk products from artisanal and industrial products, France.
We found that 23 (23%) products were strongly positive and contained more than 3.0 log10 DNA copies/mL. Significantly more unpasteurized samples (13 [18%]) contained more than 3.0 log10 DNA copies/ml than pasteurized products (10 [8%]) (P = 0.05). No thermized samples contained more than 3.0 log10 DNA copies/mL. No significant difference was observed between the number of positive artisanal samples (14 [22%]) and industrial samples (9 [13%]) (P = 0.24). No significant difference was observed between the number of cow milk samples (16 [12%], goat milk samples (4 [9%]), and ewe milk samples (1 [3%]) (cow versus ewe: P = 0.33, cow versus goat: P = 0.8, ewe versus goat: P = 0.67).
Fourteen products containing more than 4.0 log10 DNA copies/mL (9 unpasteurized, 3 pasteurized, and 2 thermized) were inoculated into HEL cells. Gimenez and immunofluorescence staining and qPCR showed negative results during the 30 days that we observed cell cultures. Nine of these strongly positive samples (5 unpasteurized, 2 pasteurized, and 2 thermized) were inoculated into mice. All animals showed no signs of illness, and no deaths were observed. In cultures of spleens collected from mice, Gimenez and immunofluorescence staining did not identify C. burnetii during the 30 days that we observed cell cultures.
Discussion
We identified C. burnetii by qPCR in dairy products, and we tested bacteria viability by culture and inoculation into mice. Our qPCR was sensitive and versatile and has been evaluated for detection of C. burnetii.13,14 We confirmed that our qPCR specific for the IS30A spacer region was less sensitive than the qPCR specific for the IS1111 spacer region.
Furthermore, we routinely included large numbers of negative and positive controls in our assays, which were processed identically as the test samples. Although we have sufficient experience in culturing of C. burnetii,18 we did not obtain isolates of C. burnetii. We did not evaluate if mice showed a serologic response because our goal was to evaluate C. burnetii viability by culture. All mice showed no sign of illness and cultures of their spleens remained negative, leading us to conclude that live bacteria were not present.
Our results suggest that C. burnetii is highly prevalent in dairy products such as cheese and yogurt. Products made from cow milk were more frequently positive and showed significantly higher concentrations of C. burnetii than products made from goat or ewe milk. This result might be explained by the higher number of cattle or farms from which milk originates compared with that for goats or ewes. Moreover, the higher prevalence of C. burnetii in cow milk products may reflect an increased distribution of this pathogen in cattle, rather than in goats or ewes. The prevalence of C. burnetii has been found slightly higher in cattle than in small ruminants such as sheep and goats.19 In the United States, it was estimated that nearly 3 million lactating cattle are shedding C. burnetti daily.20 In Switzerland, 4.7% of the analyzed bovine milk samples were positive for C. burnetii but all ovine and caprine milk samples were negative.21
Industrially produced products were positive more often than artisanal products. A possible explanation for this finding is the higher number of animals or farms from which the milk originates. Milk from farms may be transported directly to the milk or yogurt processing plants or it may be held at a transfer station where it is pooled with other raw milk loads. In the United States, it was found that more than 90% of dairy herds sampled were infected with C. burnetii on the basis of bulk tank milk testing over a three-year period.20 In France, the within-herd prevalence of milk-shedder cows increased significantly with the estimated titer of C. burnetii in bulk tank milk samples.22 In Switzerland, C. burnetii was found in the milk samples from 29.6% of cow farms that supplied two cheese dairies.21 In Germany, bulk milk from farms producing raw milk cheese were also positive.23
Products made from unpasteurized milk had higher concentrations of bacteria than products mad from pasteurized milk. Pasteurization kills and reduces the number of viable C. burnetii in milk,24 although DNA is probably not fully destroyed by this process. All products from unpasteurized milk had artisanal origin and their more heavy contamination might have been caused by differences in hygienic conditions during the artisanal production. Difference in animal vaccination between farms might explain differences between artisanal products because the prevalence of Q fever and bacterial load of C. burnetii are reduced in vaccinated animals compared with unvaccinated animals.25 In addition, antibiotics such as oxytetracycline have been associated with a reduction of C. burnetii in milk, and some artisanal producers might have treated their animals with antibiotics.26 Thermized milk products were positive more often than unpasteurized products, although the reason for this finding is unknown. This finding might indicate a bias in product selection.
Despite the presence of C. burnetii DNA in many products, we could not isolate the bacterium from any dairy product. To confirm that bacteria were not viable, we tested the viability of C. burnetii by injection into mice.27,28 Although mice can quickly clear most contaminating microbes, C. burnetii can grow slowly in mice.27,28 Thus, analysis of mice after injection with C. burnetii-positive products can indicate whether viable C. burnetii were present and can greatly facilitate isolation. Our data confirmed that cheese and yogurt products we tested did not contain viable C. burnetii. Conversely, previous reports demonstrated that viable organisms may be variably shed in the milk of dairy cattle for extended periods of greater than one year and across multiple lactations,29 and that within a herd the proportion of cows shedding in milk may range from 15% to 62%.29,30 Also, Hirai and others detected C. burnetii in 19% of 147 commercially available cheese samples in Japan, but they did not isolate the bacterium by using tissue culture for inoculated mice.31
In conclusion, our results demonstrate that C. burnetii is commonly present in commercially available cheese and yogurt from France but is apparently not viable. Although additional studies are needed, we believe that C. burnetii does not survive in cheese and yogurt, which suggests that transmission of Q fever by consumption of these products does not occur. Additional studies are needed to determine whether nonviable C. burnetii in cheese and yogurt can induce a protective immune response in persons consuming these products.
We also showed that C. burnetii DNA is found in high copy numbers in dairy products from France, which may reflect the widespread distribution of this pathogen in domestic ruminants. The French National Reference Center for Q fever reported two outbreaks involving sheep, one outbreak involving goats, and one outbreak involving a cattle abattoir over the past 25 years.32 Despite the presence of a large amount of C. burnetii in dairy products in France, our results suggest that its presence in cheese and yogurt products does not pose a public health risk.
Footnotes
Authors' addresses: Carole Eldin, Emmanouil Angelakis, Aurélie Renvoisé, and Didier Raoult, Faculté de Médecine, Université de la Méditerranée, URMITE, Marseille, France, E-mails: carole.eldin@gmail.com, angelotasmanos@msn.com, aurelie.renvoise@gmail.com, and didier.raoult@gmail.com.
References
- 1.Maurin M, Raoult D. Q fever. Clin Microbiol Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angelakis E, Raoult D. Q fever. Vet Microbiol. 2010;140:297–309. doi: 10.1016/j.vetmic.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 3.Rodolakis A. Q fever in dairy animals. Ann N Y Acad Sci. 2009;1166:90–93. doi: 10.1111/j.1749-6632.2009.04511.x. [DOI] [PubMed] [Google Scholar]
- 4.Huebner RJ, Bell JA. Q fever studies in southern California; summary of current results and a discussion of possible control measures. J Am Med Assoc. 1951;145:301–305. doi: 10.1001/jama.1951.02920230025005. [DOI] [PubMed] [Google Scholar]
- 5.Marmion BP, Stoker MG. The epidemiology of Q fever in Great Britain; an analysis of the findings and some conclusions. BMJ. 1958;2:809–816. doi: 10.1136/bmj.2.5100.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beck MD, Bell JA. Q fever studies in southern California; an epidemiological study of 300 cases. Public Health Rep. 1949;64:41–56. [PMC free article] [PubMed] [Google Scholar]
- 7.Fishbein DB, Raoult D. A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40. doi: 10.4269/ajtmh.1992.47.35. [DOI] [PubMed] [Google Scholar]
- 8.de Bruin A, van der Plaats RQ, de Heer L, Paauwe R, Schimmer B, Vellema P, van Rotterdam BJ, van Duynhoven YT. Detection of Coxiella burnetii DNA on small ruminant farms during a Q fever outbreak in the Netherlands. Appl Environ Microbiol. 2012;78:1652–1657. doi: 10.1128/AEM.07323-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benson WW, Brock DW, Mather J. Serologic analysis of a penitentiary group using raw milk from a Q fever infected herd. Public Health Rep. 1963;78:707–710. [PMC free article] [PubMed] [Google Scholar]
- 10.Krumbiegel ER, Wisniewski HJ. Q fever in the Milwaukee area. II. Consumption of infected raw milk by human volunteers. Arch Environ Health. 1970;21:63–65. doi: 10.1080/00039896.1970.10667193. [DOI] [PubMed] [Google Scholar]
- 11.Maltezou HC, Constantopoulou I, Kallergi C, Vlahou V, Georgakopoulos D, Kafetzis DA, Raoult D. Q fever in children in Greece. Am J Trop Med Hyg. 2004;70:540–544. [PubMed] [Google Scholar]
- 12.Hatchette TF, Hudson RC, Schlech WF, Campbell NA, Hatchette JE, Ratnam S, Raoult D, Donovan C, Marrie TJ. Goat-associated Q fever: a new disease in Newfoundland. Emerg Infect Dis. 2001;7:413–419. doi: 10.3201/eid0703.010308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolain J-M, Raoult D. Molecular detection of Coxiella burnetii in blood and sera during Q fever. QJM. 2005;98:615–617. doi: 10.1093/qjmed/hci099. [DOI] [PubMed] [Google Scholar]
- 14.Mediannikov O, Fenollar F, Socolovschi C, Diatta G, Bassene H, Molez JF, Sokhna C, Trape JF, Raoult D. Coxiella burnetii in humans and ticks in rural Senegal. PLoS Negl Trop Dis. 2010;4:e654. doi: 10.1371/journal.pntd.0000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klee SR, Tyczka J, Ellerbrok H, Franz T, Linke S, Baljer G, Appel B. Highly sensitive real-time PCR for specific detection and quantification of Coxiella burnetii. BMC Microbiol. 2006;6:2. doi: 10.1186/1471-2180-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fournier PE, Marrie TJ, Raoult D. Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834. doi: 10.1128/jcm.36.7.1823-1834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raoult D, Vestris G, Enea M. Isolation of 16 strains of Coxiella burnetii from patients by using a sensitive centrifugation cell culture system and establishment of the strains in HEL cells. J Clin Microbiol. 1990;28:2482–2484. doi: 10.1128/jcm.28.11.2482-2484.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouriet F, Fenollar F, Patrice J-Y, Drancourt M, Raoult D. Use of shell-vial cell culture assay for isolation of bacteria from clinical specimens: 13 years of experience. J Clin Microbiol. 2005;43:4993–5002. doi: 10.1128/JCM.43.10.4993-5002.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guatteo R, Seegers H, Taurel A-F, Joly A, Beaudeau F. Prevalence of Coxiella burnetii infection in domestic ruminants: a critical review. Vet Microbiol. 2011;149:1–16. doi: 10.1016/j.vetmic.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 20.Kim SG, Kim EH, Lafferty CJ, Dubovi E. Coxiella burnetii in bulk tank milk samples, United States. Emerg Infect Dis. 2005;11:619–621. doi: 10.3201/eid1104.041036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fretz R, Schaeren W, Tanner M, Baumgartner A. Screening of various foodstuffs for occurrence of Coxiella burnetii in Switzerland. Int J Food Microbiol. 2007;116:414–418. doi: 10.1016/j.ijfoodmicro.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Guatteo R, Beaudeau F, Joly A, Seegers H. Assessing the within-herd prevalence of Coxiella burnetii milk-shedder cows using a real-time PCR applied to bulk tank milk. Zoonoses Public Health. 2007;54:191–194. doi: 10.1111/j.1863-2378.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 23.Kloppert B, Wolter W, Zschöck M, Kabisch D, Hamann HP, Frost JW. Coxiella burnetii as zoonotic pathogen with special regard to food hygiene. Dtsch Tierarztl Wochenschr. 2004;111:321–323. [PubMed] [Google Scholar]
- 24.Cerf O, Condron R. Coxiella burnetii and milk pasteurization: an early application of the precautionary principle? Epidemiol Infect. 2006;134:946–951. doi: 10.1017/S0950268806005978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogerwerf L, van den Brom R, Roest HI, Bouma A, Vellema P, Pieterse M, Dercksen D, Nielen M. Reduction of Coxiella burnetii prevalence by vaccination of goats and sheep, The Netherlands. Emerg Infect Dis. 2011;17:379–386. doi: 10.3201/eid1703.101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Astobiza I, Barandika JF, Hurtado A, Juste RA, García-Pérez AL. Kinetics of Coxiella burnetii excretion in a commercial dairy sheep flock after treatment with oxytetracycline. Vet J. 2010;184:172–175. doi: 10.1016/j.tvjl.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Tamrakar SB, Haluska A, Haas CN, Bartrand TA. Dose-response model of Coxiella burnetii (Q fever) Risk Anal. 2011;31:120–128. doi: 10.1111/j.1539-6924.2010.01466.x. [DOI] [PubMed] [Google Scholar]
- 28.Kersh GJ, Wolfe TM, Fitzpatrick KA, Candee AJ, Oliver LD, Patterson NE, Self JS, Priestley RA, Loftis AD, Massung RF. Presence of Coxiella burnetii DNA in the environment of the United States, 2006 to 2008. Appl Environ Microbiol. 2010;76:4469–4475. doi: 10.1128/AEM.00042-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biberstein EL, Behymer DE, Bushnell R, Crenshaw G, Riemann HP, Franti CE. A survey of Q fever (Coxiella burnetii) in California dairy cows. Am J Vet Res. 1974;35:1577–1582. [PubMed] [Google Scholar]
- 30.Guatteo R, Beaudeau F, Berri M, Rodolakis A, Joly A, Seegers H. Shedding routes of Coxiella burnetii in dairy cows: implications for detection and control. Vet Res. 2006;37:827–833. doi: 10.1051/vetres:2006038. [DOI] [PubMed] [Google Scholar]
- 31.Hirai A, Nakama A, Chiba T, Kai A. Development of a method for detecting Coxiella burnetii in cheese samples. J Vet Med Sci. 2012;74:175–180. doi: 10.1292/jvms.11-0023. [DOI] [PubMed] [Google Scholar]
- 32.Frankel D, Richet H, Renvoisé A, Raoult D. Q fever in France, 1985–2009. Emerg Infect Dis. 2011;17:350–356. doi: 10.3201/eid1703.100882. [DOI] [PMC free article] [PubMed] [Google Scholar]