Abstract
India is one of three countries that account for an estimated 300,000 of 500,000 cases of visceral leishmaniasis (VL) occurring annually. Bihar State is the most affected area of India, with more than 90% of the cases. Surveys were conducted in two villages within the Saran district of Bihar, India, from 2009 to July of 2011 to assess risk factors associated with VL. Forty-five cases were identified, and individuals were given an oral survey. The results indicated that men contracted the disease more than women (58%), and cases over the age of 21 years accounted for 42% of the total VL cases. April to June showed the highest number of new cases. Of 135 households surveyed for sleeping conditions, 95% reported sleeping outside, and 98% slept in beds. Proximity to VL cases was the greatest risk factor (cluster 1 relative risk = 11.89 and cluster 2 relative risk = 138.34). The VL case clustering observed in this study can be incorporated in disease prevention strategies to more efficiently and effectively target VL control efforts.
Introduction
Leishmaniasis is a parasitic disease that affects people in 88 countries, 72 of which are considered to be developing nations.1,2 Visceral leishmaniasis (VL) or kala-azar, meaning black fever in Hindi, is the most lethal form of leishmaniasis and is transmitted by the bite of female phlebotomine sand flies. Currently, India, Bangladesh, and Nepal account for an estimated 300,000 of 500,000 global cases annually. Approximately 90% of all VL cases occur in poor suburban parts of India, Bangladesh, Nepal, Sudan, and Brazil.1,3–8 In South Asia, the protozoan Leishmania donovani causes VL,9 and Phlebotomus argentipes is the vector responsible for disease transmission. The area has seen a resurgence of the disease since the 1990s after the malaria eradication efforts of the 1950s were relaxed and the spraying of dichlorodiphenyltrichloroethane (DDT) decreased.10,11
According to the World Health Organization,12 there is also a growing concern about the global urbanization of VL as infected people migrate from rural areas into cities. Cases have been reported in Patna, India, the capital city of Bihar State, and it is hypothesized that these cases are a result of the increased number of cows in the suburbs of the city as people migrate into the area. Additionally, Khanal and others13 report that domestic animals, particularly goats, play an important role in the spread of VL. Bihar State is the most affected area, and it accounts for more than 90% of the VL cases found in India, with 28 of the state's 37 districts endemic for the disease.5,6
VL is characterized by symptoms of fever, weight loss, splenomegaly, hepatomegaly, skin darkening, and anemia.5,14 Claborn15 reported that cured patients, those patients experiencing no symptoms, could test parasite-positive again at any point from 1 to 30 years after treatment of VL. After diagnosis and confirmation of VL, various treatment options exist. Typically, the course of treatment lasts approximately 28 days. However, treatment duration can be extended for extensive parasite loads or if patients have been previously diagnosed with VL.29
VL is associated with elements of poverty, including unsuitable housing materials, poor sanitary conditions, and malnutrition.16–20 Studies indicate that VL can be an opportunistic disease, and thus, people suffering from weakened immune systems caused by malnutrition are at a higher risk of developing symptoms of the disease.21 Bern and others22 found that, in Bangladesh, individuals with a lower meat intake exhibiting protein-energy malnutrition were at higher risk for contracting VL, whereas intake of beef or goat increased the likelihood that an infection would remain asymptomatic. In addition to malnutrition, there have been cases of coinfection with patients contracting both VL and acquired immunodeficiency syndrome (AIDS). Given that both VL and AIDS weaken the immune system, this combination becomes particularly lethal, because both infections mutually reinforce the deleterious effects on the body.12 VL, when left untreated, is nearly always fatal.14
The objective of the present study was to determine the number of current VL cases between 2009 and 2011 in two villages in the Saran district of Bihar state and evaluate any risk factors associated with the occurrence of the disease. Surveys were conducted in the villages of Sutihaar and Rasulpur targeting active VL cases and living conditions of the patients. Global positioning was used to determine the location of each active case within the village. Additionally, surveys were conducted regarding the sleeping conditions of active case households as well as randomly chosen households. Given the severity of the disease when untreated or improperly treated and the potential for people to serve as reservoirs for the Leishmania parasite, it is essential that research is done to establish a relationship between the sand fly vector and the presence of active cases of VL. Determining potentially correlated factors associated with contracting VL will aid in developing disease control strategies to reduce transmission.
Materials and Methods
Study area.
Two villages, Sutihaar and Rasulpur, were selected from the Saran district north of Patna in Bihar, India. Sutihaar and Rasulpur have similar climates, with temperatures during 2010 ranging from 11°C in the winter to 36°C in the summer, average minimum and maximum humidity of 50.5% and 76.8%, respectively, and an average annual rainfall of 52.3 mm. July had the greatest amount of rainfall, whereas no rainfall was recorded between November and April. The two villages are approximately 1.5 km apart and do not vary geographically. According to a 2010 census performed by Genesis Laboratories, Rasulpur had 239 homes, a population of 1,752, and a study area perimeter of approximately 3,900 m. Although Sutihaar was not included in the census, it is more densely populated within the smaller VL survey perimeter of approximately 2,500 m.
Study design.
VL surveys.
Questionnaires were given to families with confirmed cases of VL in both Sutihaar and Rasulpur. For each house with a confirmed case of VL, the head of the household was asked to answer questions regarding the number and ages of all individuals inhabiting the home, structural materials of the house and roof, and type and number of livestock owned. In addition to general questions about the household, information was gathered pertaining specifically to the individual(s) of that household who contracted the disease from 2009 to 2011. All VL cases were confirmed by medical documentation and treatment records from the local hospital where patients received care. Hospital type and treatment regimen (antiparasitic used and oral or intravenous injection administration) were also noted. The coordinates of each household with a confirmed case were acquired using a Trimble Geo XM global positioning system (GPS). Aerial maps of active cases were then generated using Google Earth. Verbal consent was given from all survey participants who voluntarily participated in the study.
Sleeping condition surveys.
An additional questionnaire regarding sleeping conditions was distributed to households with confirmed cases of VL in Sutihaar and Rasulpur as well as 100 randomly selected houses without VL in Rasulpur. As with the VL surveys, the sleeping conditions surveys contained basic demographic questions such as household name, number of occupants, ages of occupants, housing and roofing material type, and if applicable, number of family members with VL from 2009 to 2011. These questionnaires also inquired about the sleeping location, bedding type, use of covers, seasonal changes in sleeping pattern, and age and sex of the individuals in each category.
Analysis.
Aerial maps of active cases in each village generated from Google Earth were used to locate non-case households by latitude and longitude. Each house represented only one point recorded in decimal degrees. Cluster analysis was then done using SatScan v9.0.30 A Bernoulli test was run to allow small and large clusters to be detected with an upper limit of 50%, ensuring that clusters with over 50% of the total population were not included in the results. Odds ratios (ORs) were calculated using SigmaPlot software package (Systat Software, San Jose, CA) comparing cases versus non-cases in Rasulpur, with the P values calculated using the χ2 statistic with the Yates correction and 95% confidence intervals (95% CIs) computed using the Taylor Series methodology.
Results
VL case surveys.
Total results from both Rasulpur and Sutihaar.
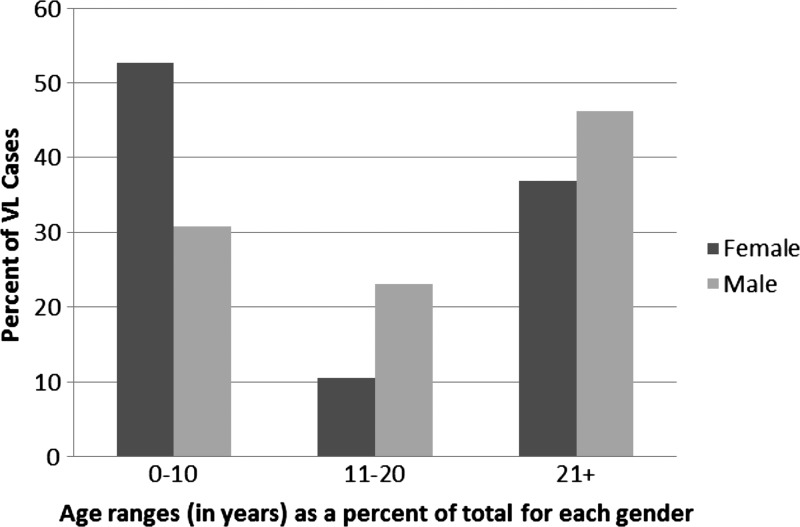
During 2009–2011, there was a total of 45 cases of VL in both Sutihaar and Rasulpur; 42% were females, and 58% were males. The distributions of total cases categorized by age are shown in Figure 1. Of the youngest age range (0–10 years), 55.6% (10/18) of females and 44.4% (8/18) of males were infected. The age range from 11 to 20 years had 25.0% (2/8) female cases and 75.0% (6/8) male cases, and finally, there were 36.8% (7/19) female and 63.2% (12/19) male cases in the oldest age range of 21 years and over. When examining the total cases of VL by livestock number, there were 68.9% (31/45) of cases with one to three livestock in the family, 20.0% (9/45) of cases with zero livestock, and 11.1% (5/45) of cases where the family owned four or more livestock.
Figure 1.
Sex and age for cases of VL in 2009–2011 in both Rasulpur and Sutihaar. Percentages add up to 100% for each sex.
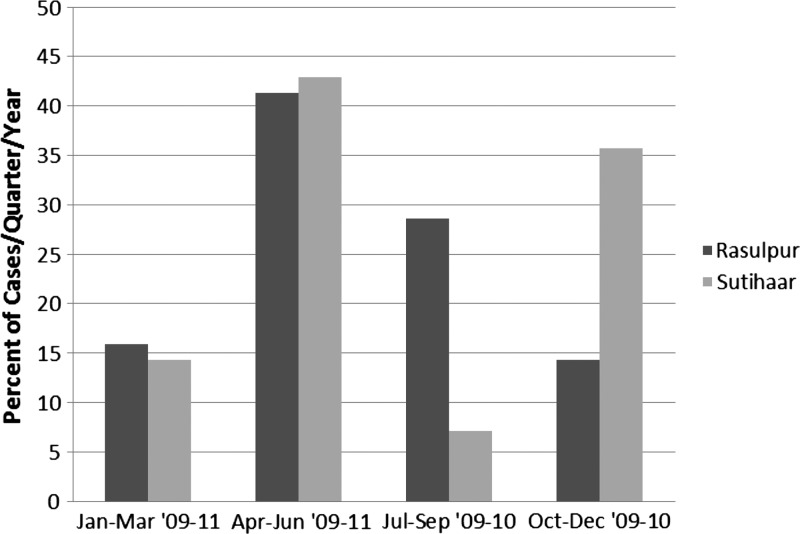
The respondents in all 45 cases were asked to identify the month in which they first experienced symptoms (Figure 2). The fewest identified cases were from January to March, with a total of 2.7 cases/quarter, whereas the greatest number reported was from April to June, with 7.3 cases/quarter. Both the July through September and October through December quarters had 3.5 cases/quarter. The time from the onset of symptoms until the individuals sought treatment varied (median = 30 days, interquartile range [IQR] = 23, quartile 1 [Q1] = 30, Q3 = 53), although most reported seeking treatment within 30–60 days. However, one individual reported waiting 1 year before seeking treatment. All cases were given miltefosine as treatment against the infection (40.0% by injection and 57.8% orally), whereas one case received both methods of administration. The treatment duration was 28 days or less for 62.2% of the cases. However, 35.6% of individuals received miltefosine for up to 56 days, and in one instance, a case was treated for 150 days (the same case who received miltefosine orally and through injection). Eighty percent of the cases reported that their 2009–2011 VL infection was their first time diagnosed with the disease, whereas four people had been diagnosed two times; two people suffered from symptoms of the disease four separate times (three people did not respond). Of 45 cases, 62.2% of the patients sought treatment at a government facility, whereas only 35.6% chose a private hospital. One person reported having gone to both during the course of treatment.
Figure 2.
The total number as a percent of VL cases per quarter per year in Rasulpur (N = 27) and Sutihaar (N = 18) from 2009 to July of 2011. Each village's total cases adds to 100%.
For all VL cases, material used for houses and roofs was documented. Of 45 households with VL patients, 51.1% of them used thatch as a housing construction material. Twenty-two percent of the active case households (six in Rasulpur and four in Sutihaar) had mud plastering. One hundred percent of the households interviewed with confirmed VL cases reported their economic status as poor or agri-labor/poor, except one, which reported their status as farmer.
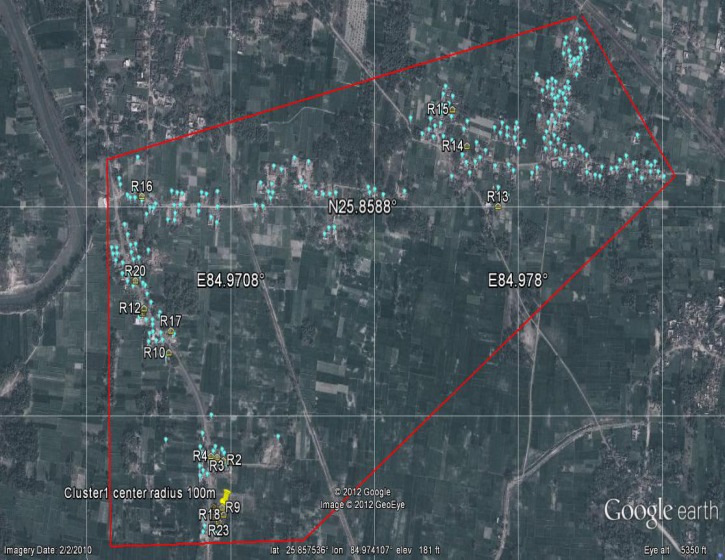
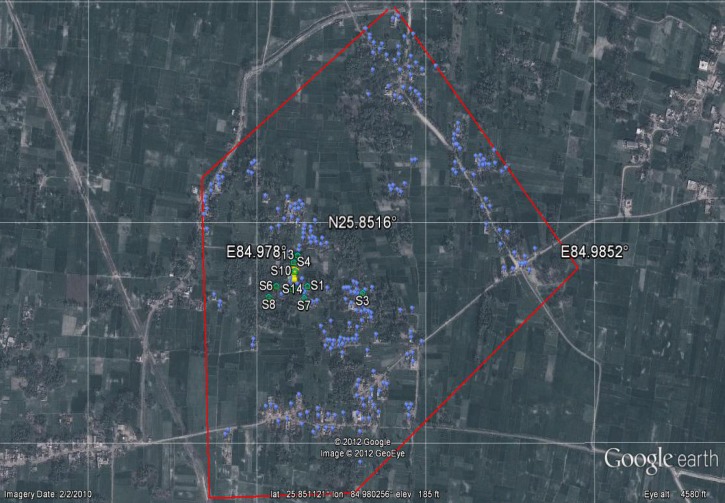
The GPS coordinates taken of households in Rasulpur and Sutihaar, including those households with an active case of VL from 2009 to 2011, indicate that there is a clustering of cases within villages. The most likely cluster in Rasulpur contains 16 of 27 cases with a relative risk (RR) of 11.89 (P < 0.001) within a radius of 100 m. In Sutihaar, the most likely cluster contained 17 of 18 cases, RR = 138.34 (P < 0.001), and a radius of 54 m. The outlying case was 102.3 m from the nearest cluster in Sutihaar. Figures 3 and 4 show images of the two villages with the active case households mapped.
Figure 3.
Google Earth image of households and VL cases from 2009 to 2011 in Rasulpur. The red line around the village is the study area perimeter. Yellow houses (R1–23) represent case households, blue balloons represent non-case households, and the yellow pushpin marks the center of the case cluster (16/27 cases, RR = 11.89, P < 0.001).
Figure 4.
Google Earth image of households and VL cases from 2009 to 2011 in Sutihaar. The red line around the village is the study area perimeter. Green houses (S1–14) represent case households, blue balloons represent non-case households, and the yellow pushpin represents the center point of the cluster radius (17/18 cases, RR = 138.34, P < 0.001).
Rasulpur.
There were 27 cases of VL in Rasulpur village from 2009 to 2011. In the census survey conducted by Genesis Laboratories, Inc. in 2010 (unpublished data), of 1,752 people in the village, the prevalence of the disease was 1.54%. Of 27 cases in Rasulpur, 25.93% were female, and 74.07% were male. Males were significantly more likely to contract VL than females (OR = 2.809, P = 0.026, CI for the odds ratio = 1.178–6.699) (Tables 1 and 2). In the youngest age range (0–10 years), 25.9% were infected; 11- to 20-year-old individuals had 22.2% of the total cases, and finally, 51.9% of the cases were in the oldest age range of 21 years and over (Tables 1 and 2). The median was 21 years for all cases, whereas the median for the whole of Rasulpur village was 25 years.
Table 1.
Factors analyzed as potential risk factors of VL in Rasulpur computed from sleeping conditions surveys (N = 135)
| Factor | Cases | No. (%) | Non-cases | No. (%) | OR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Male/female | 20/7 | 20 (74.1)/7 (25.9) | 532/523 | 532 (50.4)/523 (49.6) | 2.8 (1.2–6.7) | 0.026* |
| Male/female ages 0–10 years | 4/3 | 7 (25.9) | 86/120 | 206 (18.7) | 0.5 (0.1–2.50 | 0.673 |
| Male/female ages 11–20 years | 6/0 | 6 (22.2) | 144/129 | 273 (24.7) | 0.0 (0.0–†) | 0.060 |
| Male/female ages 21+ years | 10/4 | 14 (51.9) | 336/289 | 625 (56.6) | 0.5 (0.1–1.5) | 0.298 |
Significantly increases the chances of contracting VL.
95% CI cannot be calculated because of a zero in the data.
Table 2.
Factors analyzed as potential risk factors of VL in Rasulpur computed from census surveys (N = 239)
| Factor | Case households | Non-case households | OR (95% CI) | P value |
|---|---|---|---|---|
| Sleeping on a bed | 22 (22/22) | 100 (100/100) | –* | –* |
| Thatch and/or mud as housing construction material | 19 (19/22) | 49 (49/100) | 6.6 (1.8–23.7) | 0.003† |
| Ownership of zero livestock | 1 (1/22) | 30‡ (30/236) | 0.3 (0.0–2.5) | 0.433 |
| Ownership of 1–3 livestock vs. zero | 17 (17/22) | 84‡ (84/236) | 6.1 (0.8–47.6) | 0.103 |
| Ownership of 4+ livestock vs. zero | 4 (4/22) | 122‡ (122/236) | 1.0 (0.1–9.1) | 0.578 |
| Sleeping outside | 22 (22/22) | 98 (98/100) | 0.0 (0.0–§) | 0.796 |
| Ownership of (a) bovid(s) | 19 (19/22) | 160‡ (160/239) | 0.3 (0.1–1.1) | 0.102 |
OR could not be computed, because there were zero case households not sleeping on a bed and zero non-case households not sleeping on a bed.
Significantly increases the chances of contracting VL.
Calculated from Genesis Laboratories, Inc. Rasulpur village census survey in 2010.
95% CI cannot be calculated because of a zero in the data.
Construction material varied from houses built only with brick to houses constructed only of thatch. Some of the brick houses were covered with cement, whereas others were plastered with mud. Additionally, some houses were constructed using any combination of the above materials. Thatch, brick, cement, tin, and tile were all used as roofing materials singly and combined. In Rasulpur, 40.7% of households used thatch as a construction material, and 59.3% of households did not use thatch. When evaluating thatch and mud as potential risk factors, the OR for case households in Rasulpur using thatch and/or mud compared with all other materials was OR = 6.592 (P = 0.003, CI for OR = 1.834–23.690). The thatch and/or mud significantly increased the likelihood of a case compared with the non-case households (Tables 1 and 2).
The quantity and type of livestock per case household were also examined (Tables 1 and 2). Households with active VL cases owned from 0 to 15 livestock, which included goats, cattle, buffalo, and oxen. There were 7.4% of the houses with no livestock, 77.8% of the households with one to three animals, and 6.8% of the households with four or more livestock. ORs were calculated using information from the Rasulpur census survey done by Genesis Laboratories, Inc. in 2010, and it was found that having zero livestock did not significantly increase the chance of developing VL (OR = 0.327, P = 0.433, CI for the OR = 0.0424–2.521). Analysis for one to three livestock owned and four or more livestock owned per household did not indicate that livestock had any effect on the chance of having a case (OR for one to three livestock versus zero livestock = 6.071, P = 0.103, CI for the OR = 0.774–47.609; OR for four or more livestock versus zero livestock = 0.984, P = 0.578, CI for the OR = 0.106–9.124). Additionally, bovid ownership was not protective (OR = 0.320, P = 0.102, CI for the OR = 0.0919–1.113).
Of 27 cases of VL from January of 2009 to July of 2011, symptom onset was highest in the April through June quarter, averaging 4.3 cases/quarter (Figure 4). July through September had the second highest average number of new cases, with 3.0 cases/quarter, followed by January through March (1.7 cases/quarter) and finally, October through December (1.5 cases/quarter). Of all the people diagnosed with VL in Rasulpur, 70.4% contracted the disease one time, 11.1% had been diagnosed two times, and 7.4% reported recurrence of the disease four separate times. Three people did not answer this question. The median number of days until patients sought treatment in Rasulpur was 30.0 (IQR = 30.0, Q1 = 30, Q3 = 60); 70.4% of the cases received treatment at a government hospital, whereas 25.9% chose to go to a private institution. One individual reported having received treatment from both a government hospital and a private hospital.
Sutihaar.
Sutihaar had 18 VL cases between January of 2009 and July of 2011; of those cases, 66.7% were females, and 33.3% were males. The youngest group, 0–10 years, had 61.1% of the cases, ages 11–20 years had 11.1% of the cases, and 27.8% of the cases were individuals over the age of 21 years, with the median = 8.5 years. Housing material included brick, thatch, cement, mud, and different combinations thereof. As for roofing materials, tile, thatch, and brick/cement were used. An evaluation of all housing materials and roofing materials indicated that 66.7% of the houses with cases of VL had thatch in their building materials, whereas 33.3% of the houses did not include thatch in their building materials.
In Sutihaar, livestock number and type ranged from zero to seven animals per household, which included goats, buffalo, and cattle, and one household had both goats and buffalo. Of 18 VL cases, 38.9% of the households owned no livestock at all, 55.6% of the households owned between one and three animals, and 5.6% owned four or more livestock.
Average new case development in Sutihaar peaked between April and June (Figure 2), with three cases per quarter per year. The quarter with the second highest number of cases of VL from 2009 to July of 2011 was October through December, with 2.5 cases/quarter experiencing symptom onset. During the quarters of January through March and July through September, there was only an average of 1.0 case per quarter per year and 0.5 cases per quarter, respectively. Of 18 cases in Sutihaar, only one individual reported having been diagnosed more than one time before contracting the disease for a second time in 2009–2010. The median time until treatment was sought for all cases in Sutihaar was 30.0 days (IQR = 0, Q1 = 30, Q3 = 30); 50% of the individuals sought treatment at a government hospital, whereas the other 50.0% attended a private institution.
Sleeping conditions surveys.
There were a total of 135 sleeping conditions surveys conducted, with 122 questionnaires answered in Rasulpur and 13 questionnaires answered in Sutihaar. The household surveys accounted for a total of 1,217 people, with 1,121 people in Rasulpur and 96 people in Sutihaar. From 135 households surveyed, Rasulpur reported 22 VL cases, and Sutihaar reported 13 VL cases (Table 3). There were 5.2% of households with members who do not sleep outside: zero members not sleeping outside in Rasulpur (Tables 1 and 2) and five members not sleeping outside in Sutihaar. There were only 2.2% of the households, all from houses in Sutihaar, where all family members reported not sleeping on a bed. However, 18 people in Rasulpur and 24 people in Sutihaar do not use a bed (1.6% and 25%, respectively). There were 19 men and 22 women (one response with sex unanswered) not sleeping on a bed: 8 men and 10 women in Rasulpur and 11 men and 12 women in Sutihaar.
Table 3.
Sleeping conditions surveys for household information and individuals (three VL case households reported not sleeping on a bed)
| Total | Rasulpur | Sutihaar | |
|---|---|---|---|
| Number of households surveyed | 135 | 122 | 13 |
| Number of VL case households surveyed | 35 | 22 | 13 |
| Number of households with members not sleeping outside | 7 | 2 | 5 |
| Number of households not using a bed | 3 | 0 | 3 |
| Number of people surveyed | 1,217 | 1,121 | 96 |
| Number of people not using a bed | 42 (3.5%) | 18 (1.6%) | 24 (25%) |
| Number and sex of people not using a bed | 19 M/22 F/1 unknown | 8 M/10 F | 11 M/12 F/1 unknown |
F = female; M = male.
Of 135 sleeping conditions surveys, 51.1% of households reported the use of wooden beds, 47.4% reported the use of cots made of jute, and 0.7% reported the use of a plastic wire bed (Table 4). The VL cases surveyed reported 34.3% of households sleeping on wooden beds and 42.9% sleeping on jute cots (Table 4). There were 8.6% of the case households that reported not sleeping on a bed of any kind (Table 4). However, it should be noted that not all households reported having every member of the house sleeping on a bed; some houses had only a few members on beds, whereas others had only a couple, typically children, sleeping on the ground, with a total of 3.5% of the people from all surveys not using a bed.
Table 4.
Sleeping conditions surveys for number and type of beds used (three VL case households reported not sleeping on a bed)
| Wood | Jute | Plastic wire | |
|---|---|---|---|
| Total number of households surveyed using beds | 69 | 64 | 1 |
| VL cases surveyed | 12 | 15 |
Housing construction materials were reported on the questionnaires. The use of thatch, mud, a combination of thatch and mud, and all other materials was listed. Of the surveys with no cases (N = 100), 32% of all houses used thatch as a building material, 13% of all houses used mud, 4% of all houses used both mud and thatch together, and 51% of all houses used a material other than thatch or mud. For the surveys of only the VL case households, 39% used thatch, 17% used mud, 8% used thatch and mud, and 36% used other materials.
Of 135 total questionnaires collected, 134 households reported using some type of cover while sleeping, whereas one survey failed to answer this particular question. Women all reported using their clothing as a cover, such as a saree or salwar (133/133 and 26/133, respectively), and men reported wearing clothing; 77 men reported using a towel, 39 men reported using a dhoti, and 83 men reported using a lungi. (Dhoti and lungi are both traditional wraps worn by men that can be made into a short wrap above the knee or worn long covering to around the ankles.) When children's covers were reported, they were always listed as wearing their clothes to bed. Forty percent of the VL case households reported using towels as covers for the men and sarees as covers for the women. All women from the case households used sarees for sleeping covers, and in 65.7% of the cases, men reported using a towel, lungi, or dhoti, whereas 2.9% of the case households answered that the men sleep in their clothes.
Conclusions
India is one of three countries that accounts for an estimated 300,000 of 500,000 cases of VL (kala-azar) occurring annually.4 The current study assessed associated factors in VL cases contracted from January of 2009 to July of 2011 in two villages of the Saran district of Bihar, India. These data indicated that there were more cases of the disease in males over the age of 21 years, whereas females between the ages of 0 and 10 years were most frequently infected with VL (Figure 1). Similar to the work by Ranjan and others,6 those individuals 21 years and older accounted for the highest percentage of cases (42%), whereas the current study also had 40% of the total cases in children ages 0–10 years. Additionally, 58% (N = 26) of the current study's total cases of VL were male, which has also been seen in previous studies in India and is thought to relate to a higher incidence of exposure as a result of clothing and habits,23,24 with men in Rasulpur significantly more likely to contract the disease than women (Tables 1 and 2). From the sleeping conditions surveys, it was found that men most frequently reported sleeping in a towel or traditional cloth wraps called lungi or dhoti. This result supports findings by Kumar and others23 indicating that the towel, lungi, and dhoti all leave the men's upper bodies exposed, whereas sarees and salwars cover at least a portion of the women's torso and arms. Studies in both Bangladesh and Nepal show similar results, with a higher incidence of cases in men. When evaluated for different age groups, those individuals between ages 15 and 40 years followed by ages 5–14 years contracted the disease most frequently in Nepal, whereas children 3–14 years showed the highest incidence in Bangladesh followed by individuals between 15 and 45 years.5,25
Of 45 cases in this study, the annual quarter with the highest symptom onset was April to June followed by July to September and October to December. This finding is in contrast to the findings in the work by Bern and others5 in Bangladesh; July to September showed the highest case incidence, and October to December had the second most reported cases followed closely by the April to June quarter. Leishmaniasis is said to have a typical incubation period of 2–6 months. Sand fly numbers in Bihar increase in November before the population dies out during the colder winter months between mid-December and early February. Thus, it is possible that patients contracted the disease during the second sand fly peak in November or as the numbers began to increase after the winter months in March, and they did not develop symptoms until sometime between April and June. However, as the work by Bern and others5 points out, the incubation period can be extremely variable, with symptoms appearing anywhere from 10 days after infection to over 2 years in some cases, and thus, seasonal correlations are difficult.
From 45 cases in Sutihaar and Rasulpur, the strongest correlating factor was the presence of another case in the home or proximity to another case. Of 18 cases in Sutihaar, 17 cases were clustered in an area with a radius of 54 m, and in Rasulpur, there was a case cluster including 16 of 27 cases within a radius of 100 m. The study shows that the proximity to another case is a significant risk factor in both villages. Bern and others5 also found a strong association between rate of infection and proximity to another case, and they suggest that this association may be a result of genetics affecting the progression of L. donovani in members of effected households as well as the limited range of sand fly dispersal.5 Additionally, members of the same household may be affected similarly by nutritional influences; thus, households suffering from malnutrition are likely to have individuals with lowered immune systems and therefore, be at higher risk of developing diseases, which Bern and others22 found in Bangladesh with individuals with a lower meat intake.
Conversely, Poché and others26 discovered that sand flies are not limited by short flight ranges and were found as high as 18.5 m in the tops of palm trees in Bihar, India. Genetics certainly play a role in family member's immune systems and their likelihood to succumb to the disease as well as how individuals respond to treatment. Additionally, asymptomatic individuals can still act as a reservoir for parasite transmission. Topno and others27 found that 13% of neighbors of VL cases were seropositive for leishmaniasis without showing symptoms.15,28 In South Asia, it has been reported that living close to a previous case of VL strongly increases the risk of contracting the disease as well as acquiring a subclinical infection.22 However, given the results and the clustering of many cases both related and unrelated, it is possible that the grouping of cases is a result of pockets of parasite activity and not the sand fly vectors.
VL is a disease predominately of the poor, and there is some evidence that housing materials, poor sanitary conditions, and malnutrition increase susceptibility,16,17,22 although Kumar and others23 found no connection between income and VL in Varanasi, India. Housing materials, such as thatch and mud used as a plaster, have been said to provide resting and breeding sites for sand flies, thus increasing the risk of VL infection. Significantly increased VL risk in association with thatch and/or mud housing material was seen in this study in the village of Rasulpur, and with additional samples in Sutihaar, a similar trend would likely be seen. These results are in keeping with the work by Ranjan and others,6 which found that education and type of walls within houses were both risk factors associated with contracting VL in India.
Bern and others22 found ownership of livestock to be protective for those individuals living in areas of VL in Nepal, specifically those individuals owning bovids. In the current study, however, bovids as well as all livestock combined were not found to be protective. However, the current study may have overestimated or underestimated some of the risk factors as a result of the small sample size and the evaluation of a fairly homogenous high-risk population. Northern Bihar state in India is a highly endemic area for VL, and the only current governmental control method is spraying of DDT. Although DDT was highly effective in reducing and nearly eliminating VL in India during the 1950s malaria eradication program,10,11 there are recent concerns of pesticide resistance among disease transmitting sand flies as a result of inconsistent spraying efforts. Additionally, India may face problems with increased spread of the disease to more urban areas as a result of migration as well as the possibility of coinfections, making the disease even more lethal.12 Because of the high incidence of VL in India and the difficulty of its control, it is essential that risks associated with contracting the disease be determined to aid in proper management strategies. As the current study indicated, the highly clustered nature of the active cases of VL may assist in targeted control efforts and the eventual eradication of the disease from the area.
ACKNOWLEDGMENTS
This project was funded by Bill and Melinda Gates Foundation Grant No. 51112. The authors would also like to thank Mukesh Roy, Sanjay Singh, and Kate Ingenloff for their help with the project as well as Kevin Aldrich, Alyssa Brayshaw, Bryn Lawrence, an old friend and Robyn Raban for assistance with editing. Much thanks to an anonymous reviewer for suggestions that helped to improve the paper immensely.
Footnotes
Authors' addresses: Diana Perry, Kandice Dixon, Rajesh Garlapati, Alex Gendernalik, David Poché, and Richard Poché, Genesis Laboratories, Inc. NA, Wellington, CO, E-mails: diana@genesislabs.com, kandice@genesislabs.com, raj@genesislabs.com, alex@genesislabs.com, david@genesislabs.com, and richard@genesislabs.com.
References
- 1.Desjeux P. The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg. 2001;95:239–243. doi: 10.1016/s0035-9203(01)90223-8. [DOI] [PubMed] [Google Scholar]
- 2.Poché D, Garlapati R, Ingenloff K, Remmers J, Poché R. Bionomics of phlebotomine sand flies from three villages in Bihar, India. J Vector Ecol. 2010;36:1–12. doi: 10.1111/j.1948-7134.2011.00119.x. [DOI] [PubMed] [Google Scholar]
- 3.Bora D. Epidemiology of visceral leishmaniasis in India. Natl Med J India. 1999;12:62–68. [PubMed] [Google Scholar]
- 4.Desjeux P. Leishmaniasis: current situation and new perspectives. CIMID. 2004;27:305–318. doi: 10.1016/j.cimid.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Bern C, Hightower AW, Chowdhury R, Ali M, Amann J, Wagatsuma Y, Haque R, Kurkjian K, Vaz LE, Begum M, Akter T, Cetre-Sossah CB, Ahluwalia IB, Dotson E, Secor WE, Breiman RF, Maguire J. Risk factors for kala-azar in Bangladesh. Emerg Infect Dis. 2005;11:655–662. doi: 10.3201/eid1105.040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranjan A, Sur D, Singh VP, Siddigue NA, Manna B, Lal CS, Sinha PK, Kishore K, Bhattacharya SK. Risk factors for Indian kala-azar. Am J Trop Med Hyg. 2005;73:74–78. [PubMed] [Google Scholar]
- 7.Singh SP, Reddy CSD, Rabindra NM, Sundar S. Knowledge, attitude, and practices related to kala-azar in rural areas of Bihar State, India. Am J Trop Med Hyg. 2006;75:505–508. [PubMed] [Google Scholar]
- 8.Dey A, Sharma M, Singh S. First case of indigenous visceral leishmaniasis from central India. Am J Trop Med Hyg. 2007;77:95–98. [PubMed] [Google Scholar]
- 9.Choudhury N, Saxena NBL. Visceral leishmaniasis in India—a brief review. J Comm Dis. 1987;19:332–340. [PubMed] [Google Scholar]
- 10.Kishore K, Kumar V, Kesari S, Dinesh DS, Kumar AJ, Das P, Bhattacharya SK. Vector control in leishmaniasis. Indian J Med Res. 2006;123:467–472. [PubMed] [Google Scholar]
- 11.Killick-Kendrick R. The biology and control of phlebotomine sand flies. Clin Dermatol. 1999;17:279–289. doi: 10.1016/s0738-081x(99)00046-2. [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO) Urbanization: an increasing risk factor for leishmaniasis. Wkly Epidemiol Rec. 2002;77:365–372. [PubMed] [Google Scholar]
- 13.Khanal B, Picado A, Bhattara NR, Van Der Auwera G, Das ML, Ostyn B, Davies CR, Boelaert M, Dujardin J, Rijal S. Spatial analysis of Leishmania donovani exposure in humans and domestic animals in a recent kala azar focus in Nepal. Parasitology. 2010;137:1597–1603. doi: 10.1017/S0031182010000521. [DOI] [PubMed] [Google Scholar]
- 14.Desjeux P. Leishmaniasis. Public health aspects and control. Clin Dermatol. 1996;14:417–423. doi: 10.1016/0738-081x(96)00057-0. [DOI] [PubMed] [Google Scholar]
- 15.Claborn DM. The biology and control of leishmaniasis vectors. J Glob Infect Dis. 2010;2:127–134. doi: 10.4103/0974-777X.62866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cerf BJ, Jones TC, Badaro R, Sampaio D, Teixeira R, Johnson WD. Malnutrition as a risk factor for severe visceral leishmaniasis. J Infect Dis. 1987;156:1030–1033. doi: 10.1093/infdis/156.6.1030. [DOI] [PubMed] [Google Scholar]
- 17.Thakur CP. Socio-economics of visceral leishmaniasis in Bihar (India) Trans R Soc Trop Med Hyg. 2000;94:156–157. doi: 10.1016/s0035-9203(00)90255-4. [DOI] [PubMed] [Google Scholar]
- 18.Rijal S, Uranw S, Chappuis F, Picado A, Khanal B, Paudel IS, Andersen EW, Meheus F, Ostyn B, Dasl ML, Davies C, Boelaert M. Epidemiology of Leishmania donovani infection inhigh-transmission foci in Nepal. Trop Med Int Health. 2010;15:21–28. doi: 10.1111/j.1365-3156.2010.02518.x. [DOI] [PubMed] [Google Scholar]
- 19.Singh SP, Hasker E, Picado A, Gidwani K, Malaviya P, Singh RP, Boelaert M, Sundar S. Risk factors for visceral leishmaniasis in India; further evidence on the role of domestic animals. Trop Med Int Health. 2010;15:29–35. doi: 10.1111/j.1365-3156.2010.02515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boelaert M, Meheus F, Sanchez A, Singh SP, Vanlerberghe V, Picado A, Meessen B, Sundar S. The poorest of the poor: a poverty appraisal of households affected by visceral leishmaniasis in Bihar, India. Trop Med Int Health. 2009;14:639–644. doi: 10.1111/j.1365-3156.2009.02279.x. [DOI] [PubMed] [Google Scholar]
- 21.Harhay MO, Olliaro PL, Vaillant M, Chappuis F, Lima MA, Ritmeijer K, Costa CH, Costa DL, Rijal S, Sundar S, Balasegaram M. Who is a typical patient with visceral leishmaniasis? Characterizing the demographic and nutritional profile of patients in Brazil, East Africa, and South Asia. Am J Trop Med Hyg. 2011;84:543–550. doi: 10.4269/ajtmh.2011.10-0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bern C, Courtenay O, Alvar J. Of cattle, sand flies and men: a systematic review of risk factor analysis for South Asian visceral leishmaniasis and implications for elimination. PLoS Negl Trop Dis. 2010;4:1–9. doi: 10.1371/journal.pntd.0000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar R, Kumar P, Chowdhary RK, Pai K, Kumar K, Pandey HP, Singh VP, Sundar S. Kala-azar epidemic in Varanasi district, India. Bull World Health Organ. 1999;77:371–374. [PMC free article] [PubMed] [Google Scholar]
- 24.Rai R, Sehgal P. Kala-azar in Varanasi (U.P.): preliminary observations. J Commun Dis. 1990;22:120–123. [PubMed] [Google Scholar]
- 25.Bern C, Joshi AB, Jha SN, Das ML, Hightower A, Thakur GD, Bista MB. Factors associated with visceral leishmaniasis in Nepal: bed-net use is strongly protective. Am J Trop Med. 2000;63:184–188. doi: 10.4269/ajtmh.2000.63.184. [DOI] [PubMed] [Google Scholar]
- 26.Poché R, Garlapati R, Elnaiem D, Perry D, Poché D. The role of Palmyra palm trees (Borassus flabellifer) and sand fly distribution in northeastern India. J Vector Ecol. 2012;37:148–153. doi: 10.1111/j.1948-7134.2012.00211.x. [DOI] [PubMed] [Google Scholar]
- 27.Topno RK, Das V, Ranjan A, Pandey K, Singh D, Kumar N, Siddiqui NA, Singh VP, Kesari S, Kumar N, Bimal S, Kumar AJ, Meena C, Kumar R, Das P. Asymptomatic infection with visceral leishmaniasis in a disease-endemic area in Bihar, India. Am J Trop Med Hyg. 2010;83:502–506. doi: 10.4269/ajtmh.2010.09-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ibrahim ME, Lambson B, Yousie AO, Deifalla NS, Alnaiem DA, Ismail A, Yousif H, Ghalib HW, Khalil EAG, Kadaro A, Barker DC, El Hassan AM. Kala-azar in a high transmission focus: an ethnic and geographic dimension. Am J Trop Med Hyg. 1999;61:941–944. doi: 10.4269/ajtmh.1999.61.941. [DOI] [PubMed] [Google Scholar]
- 29.Sundar S, Rosenkaimer F, Murray HW. Successful treatment of refactory visceral leishmaniasis in India using antimony plus interferon-γ. J Infect Dis. 1994;170:659–662. doi: 10.1093/infdis/170.3.659. [DOI] [PubMed] [Google Scholar]
- 30.Kulldorff M. Information Management Services, Inc. SaTScanTM v9.0: Software for the Spatial and Space-Time Scan Statistics. 2009. http://www.satscan.org/ Available at. Accessed October 1, 2012. [Google Scholar]