Abstract
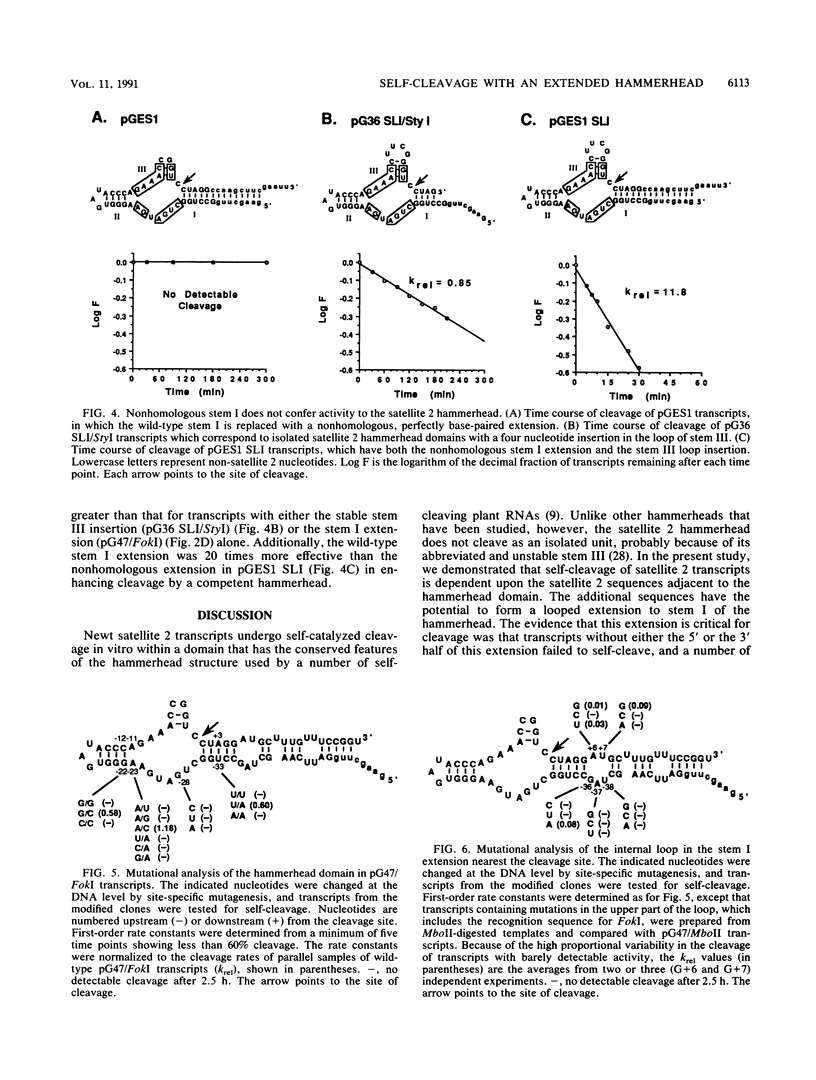
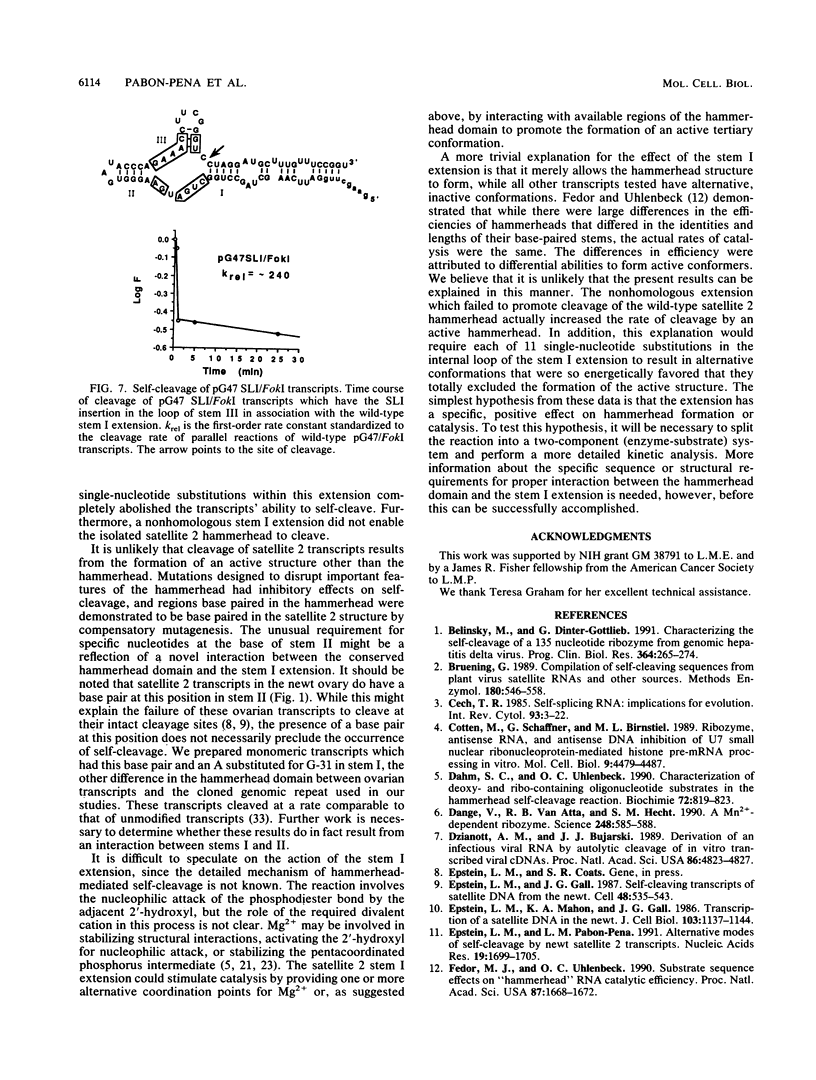
Synthetic transcripts of satellite 2 DNA from newts undergo self-catalyzed, site-specific cleavage in vitro. Cleavage occurs within a domain that is similar to the hammerhead domain used by a number of self-cleaving, infectious plant RNAs. The newt hammerhead has a potentially unstable structure due to a stem composed of two base pairs and a 2-nucleotide loop, and unlike other hammerheads that have been studied, it cannot cleave as an isolated unit. Here we show that cleavage by a single newt hammerhead requires additional satellite 2 sequences flanking both ends of the hammerhead domain. We also present a structural model of a truncated satellite 2 transcript which is capable of cleavage. The structure includes an internally looped extension to one of the conserved stems of the hammerhead. By in vitro mutagenesis, the identities of each of the five nucleotides composing one of the internal loops were shown to be critical for cleavage. Additional evidence that the extension stimulates self-cleavage in a manner other than by simply stabilizing the hammerhead is presented.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Belinsky M., Dinter-Gottlieb G. Characterizing the self-cleavage of a 135 nucleotide ribozyme from genomic hepatitis delta virus. Prog Clin Biol Res. 1991;364:265–274. [PubMed] [Google Scholar]
- Bruening G. Compilation of self-cleaving sequences from plant virus satellite RNAs and other sources. Methods Enzymol. 1989;180:546–558. doi: 10.1016/0076-6879(89)80123-5. [DOI] [PubMed] [Google Scholar]
- Cech T. R. Self-splicing RNA: implications for evolution. Int Rev Cytol. 1985;93:3–22. doi: 10.1016/s0074-7696(08)61370-4. [DOI] [PubMed] [Google Scholar]
- Cotten M., Schaffner G., Birnstiel M. L. Ribozyme, antisense RNA, and antisense DNA inhibition of U7 small nuclear ribonucleoprotein-mediated histone pre-mRNA processing in vitro. Mol Cell Biol. 1989 Oct;9(10):4479–4487. doi: 10.1128/mcb.9.10.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahm S. C., Uhlenbeck O. C. Characterization of deoxy- and ribo-containing oligonucleotide substrates in the hammerhead self-cleavage reaction. Biochimie. 1990 Nov;72(11):819–823. doi: 10.1016/0300-9084(90)90191-i. [DOI] [PubMed] [Google Scholar]
- Dange V., Van Atta R. B., Hecht S. M. A Mn2(+)-dependent ribozyme. Science. 1990 May 4;248(4955):585–588. doi: 10.1126/science.2185542. [DOI] [PubMed] [Google Scholar]
- Dzianott A. M., Bujarski J. J. Derivation of an infectious viral RNA by autolytic cleavage of in vitro transcribed viral cDNAs. Proc Natl Acad Sci U S A. 1989 Jul;86(13):4823–4827. doi: 10.1073/pnas.86.13.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Gall J. G. Self-cleaving transcripts of satellite DNA from the newt. Cell. 1987 Feb 13;48(3):535–543. doi: 10.1016/0092-8674(87)90204-2. [DOI] [PubMed] [Google Scholar]
- Epstein L. M., Mahon K. A., Gall J. G. Transcription of a satellite DNA in the newt. J Cell Biol. 1986 Oct;103(4):1137–1144. doi: 10.1083/jcb.103.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Pabón-Peña L. M. Alternative modes of self-cleavage by newt satellite 2 transcripts. Nucleic Acids Res. 1991 Apr 11;19(7):1699–1705. doi: 10.1093/nar/19.7.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedor M. J., Uhlenbeck O. C. Substrate sequence effects on "hammerhead" RNA catalytic efficiency. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1668–1672. doi: 10.1073/pnas.87.5.1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein P. A., Buzayan J. M., Bruening G. Two sequences participating in the autolytic processing of satellite tobacco ringspot virus complementary RNA. Gene. 1989 Oct 15;82(1):53–61. doi: 10.1016/0378-1119(89)90029-2. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Davies C., Sheldon C. C., Jeffries A. C., Symons R. H. Self-cleaving viroid and newt RNAs may only be active as dimers. Nature. 1988 Jul 21;334(6179):265–267. doi: 10.1038/334265a0. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of plus and minus RNAs of a virusoid and a structural model for the active sites. Cell. 1987 Apr 24;49(2):211–220. doi: 10.1016/0092-8674(87)90562-9. [DOI] [PubMed] [Google Scholar]
- Forster A. C., Symons R. H. Self-cleavage of virusoid RNA is performed by the proposed 55-nucleotide active site. Cell. 1987 Jul 3;50(1):9–16. doi: 10.1016/0092-8674(87)90657-x. [DOI] [PubMed] [Google Scholar]
- Hampel A., Tritz R., Hicks M., Cruz P. 'Hairpin' catalytic RNA model: evidence for helices and sequence requirement for substrate RNA. Nucleic Acids Res. 1990 Jan 25;18(2):299–304. doi: 10.1093/nar/18.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haseloff J., Gerlach W. L. Simple RNA enzymes with new and highly specific endoribonuclease activities. Nature. 1988 Aug 18;334(6183):585–591. doi: 10.1038/334585a0. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Kuo M. Y., Sharmeen L., Dinter-Gottlieb G., Taylor J. Characterization of self-cleaving RNA sequences on the genome and antigenome of human hepatitis delta virus. J Virol. 1988 Dec;62(12):4439–4444. doi: 10.1128/jvi.62.12.4439-4444.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei H. Y., Kaaret T. W., Bruice T. C. A computational approach to the mechanism of self-cleavage of hammerhead RNA. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9727–9731. doi: 10.1073/pnas.86.24.9727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odai O., Hiroaki H., Sakata T., Tanaka T., Uesugi S. The role of a conserved guanosine residue in the hammerhead-type RNA enzyme. FEBS Lett. 1990 Jul 2;267(1):150–152. doi: 10.1016/0014-5793(90)80311-6. [DOI] [PubMed] [Google Scholar]
- Perreault J. P., Labuda D., Usman N., Yang J. H., Cedergren R. Relationship between 2'-hydroxyls and magnesium binding in the hammerhead RNA domain: a model for ribozyme catalysis. Biochemistry. 1991 Apr 23;30(16):4020–4025. doi: 10.1021/bi00230a029. [DOI] [PubMed] [Google Scholar]
- Ruffner D. E., Stormo G. D., Uhlenbeck O. C. Sequence requirements of the hammerhead RNA self-cleavage reaction. Biochemistry. 1990 Nov 27;29(47):10695–10702. doi: 10.1021/bi00499a018. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sarver N., Cantin E. M., Chang P. S., Zaia J. A., Ladne P. A., Stephens D. A., Rossi J. J. Ribozymes as potential anti-HIV-1 therapeutic agents. Science. 1990 Mar 9;247(4947):1222–1225. doi: 10.1126/science.2107573. [DOI] [PubMed] [Google Scholar]
- Saville B. J., Collins R. A. A site-specific self-cleavage reaction performed by a novel RNA in Neurospora mitochondria. Cell. 1990 May 18;61(4):685–696. doi: 10.1016/0092-8674(90)90480-3. [DOI] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. Mutagenesis analysis of a self-cleaving RNA. Nucleic Acids Res. 1989 Jul 25;17(14):5679–5685. doi: 10.1093/nar/17.14.5679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon C. C., Symons R. H. RNA stem stability in the formation of a self-cleaving hammerhead structure. Nucleic Acids Res. 1989 Jul 25;17(14):5665–5677. doi: 10.1093/nar/17.14.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons R. H. Self-cleavage of RNA in the replication of small pathogens of plants and animals. Trends Biochem Sci. 1989 Nov;14(11):445–450. doi: 10.1016/0968-0004(89)90103-5. [DOI] [PubMed] [Google Scholar]
- Uhlenbeck O. C. A small catalytic oligoribonucleotide. Nature. 1987 Aug 13;328(6131):596–600. doi: 10.1038/328596a0. [DOI] [PubMed] [Google Scholar]
- Watson N., Gurevitz M., Ford J., Apirion D. Self cleavage of a precursor RNA from bacteriophage T4. J Mol Biol. 1984 Jan 25;172(3):301–323. doi: 10.1016/s0022-2836(84)80028-5. [DOI] [PubMed] [Google Scholar]
- Zuker M., Stiegler P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981 Jan 10;9(1):133–148. doi: 10.1093/nar/9.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]