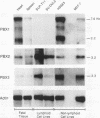
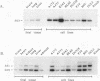
Abstract
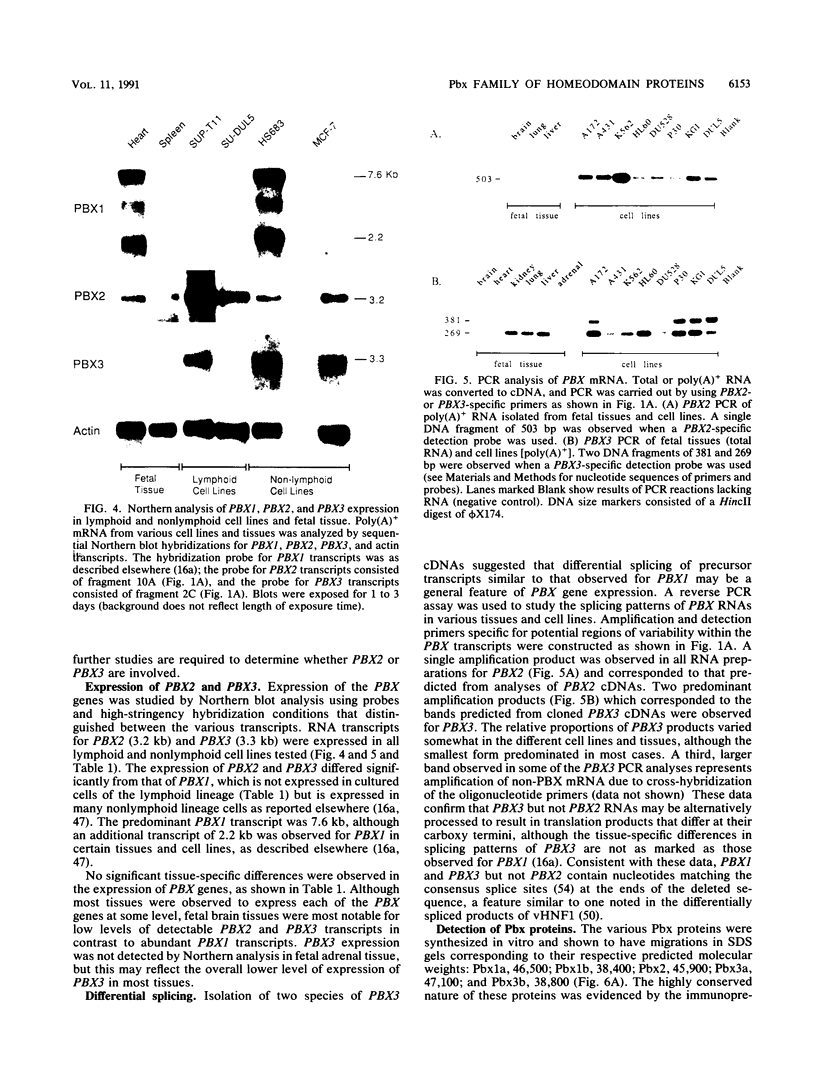
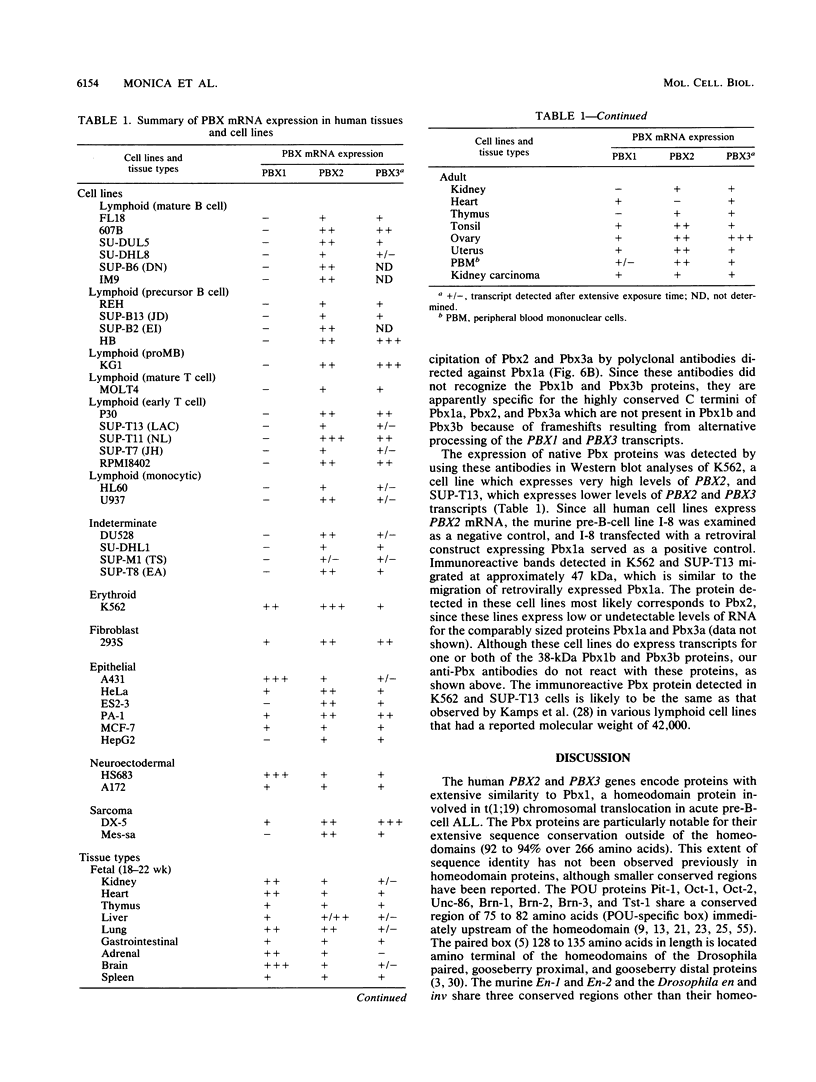
Two new homeobox genes, PBX2 and PBX3, were isolated on the basis of their extensive homology to PBX1, a novel human homeobox gene involved in t(1;19) translocation in acute pre-B-cell leukemias. The predicted Pbx2 and Pbx3 proteins are 92 and 94% identical to Pbx1 over a large region of 266 amino acids within and flanking their homeodomains, but all three proteins diverge significantly near their amino and carboxy termini. Chromosome in situ hybridizations demonstrated that the PBX genes are not clustered but map to separate chromosomal loci: PBX1, 1q23; PBX2, 3q22-23; PBX3, 9q33-34. Expression of PBX2 or PBX3 was not restricted to particular states of differentiation or development, as mRNA transcripts of these genes were detected in most fetal and adult tissues and all cell lines, unlike PBX1, which is not expressed in lymphoid cell lines. Similar to PBX1 RNA, PBX3 RNA is alternatively spliced to yield two translation products with different carboxy termini, a feature not observed for PBX2. Their extensive sequence similarity and widespread expression suggest a generalized, overlapping role for Pbx proteins in most cell types. Differences in their amino and carboxy termini may modulate their activities, mediated in part by differential splicing and, for PBX1, protein fusion following t(1;19) chromosomal translocation.
Full text
PDF
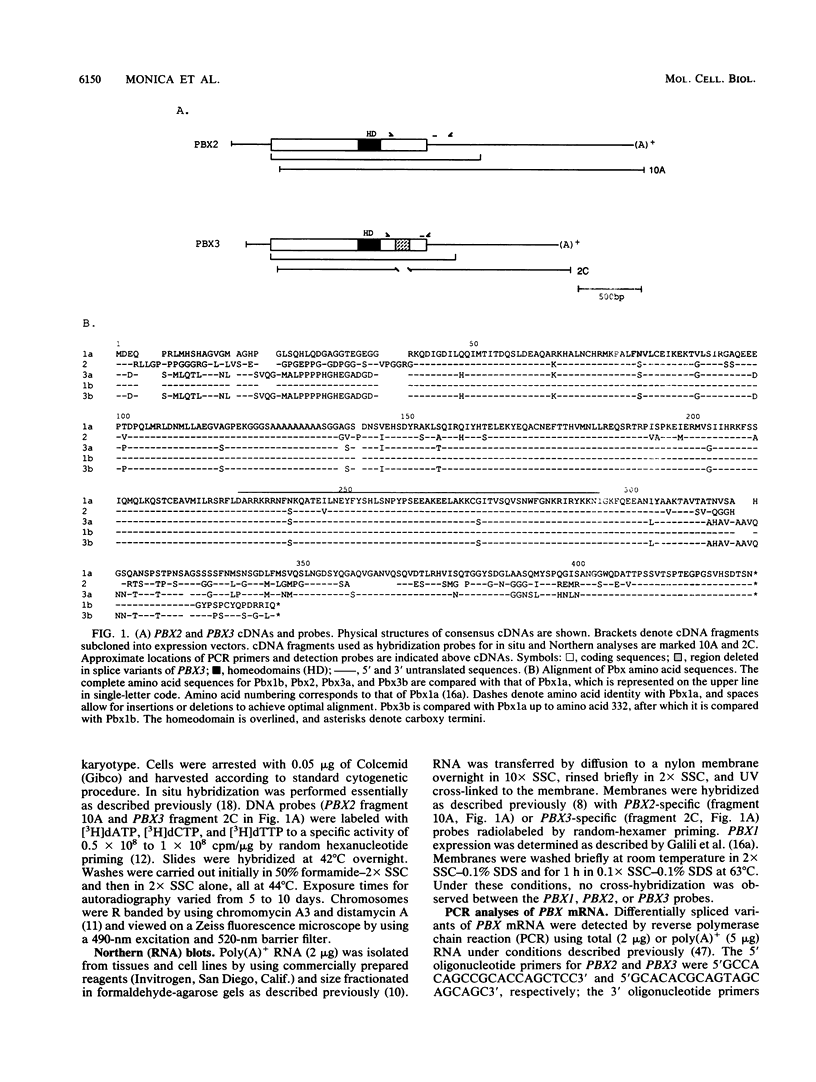

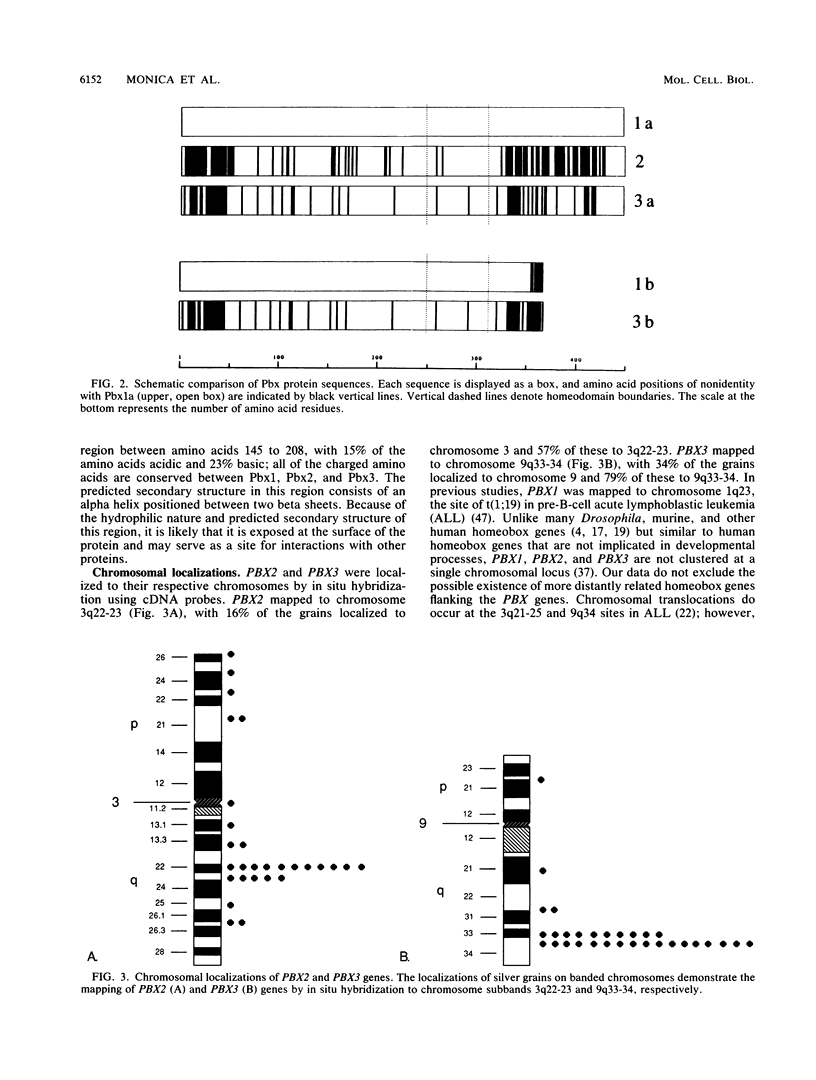



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. D., Lints T., Jenkins N. A., Copeland N. G., Strasser A., Harvey R. P., Adams J. M. Novel murine homeo box gene on chromosome 1 expressed in specific hematopoietic lineages and during embryogenesis. Genes Dev. 1991 Apr;5(4):509–520. doi: 10.1101/gad.5.4.509. [DOI] [PubMed] [Google Scholar]
- Barad M., Jack T., Chadwick R., McGinnis W. A novel, tissue-specific, Drosophila homeobox gene. EMBO J. 1988 Jul;7(7):2151–2161. doi: 10.1002/j.1460-2075.1988.tb03054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S., Bopp D., Burri M., Noll M. Structure of two genes at the gooseberry locus related to the paired gene and their spatial expression during Drosophila embryogenesis. Genes Dev. 1987 Dec;1(10):1247–1267. doi: 10.1101/gad.1.10.1247. [DOI] [PubMed] [Google Scholar]
- Boncinelli E., Somma R., Acampora D., Pannese M., D'Esposito M., Faiella A., Simeone A. Organization of human homeobox genes. Hum Reprod. 1988 Oct;3(7):880–886. doi: 10.1093/oxfordjournals.humrep.a136802. [DOI] [PubMed] [Google Scholar]
- Bopp D., Burri M., Baumgartner S., Frigerio G., Noll M. Conservation of a large protein domain in the segmentation gene paired and in functionally related genes of Drosophila. Cell. 1986 Dec 26;47(6):1033–1040. doi: 10.1016/0092-8674(86)90818-4. [DOI] [PubMed] [Google Scholar]
- Chen-Levy Z., Nourse J., Cleary M. L. The bcl-2 candidate proto-oncogene product is a 24-kilodalton integral-membrane protein highly expressed in lymphoid cell lines and lymphomas carrying the t(14;18) translocation. Mol Cell Biol. 1989 Feb;9(2):701–710. doi: 10.1128/mcb.9.2.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Meeker T. C., Levy S., Lee E., Trela M., Sklar J., Levy R. Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell. 1986 Jan 17;44(1):97–106. doi: 10.1016/0092-8674(86)90488-5. [DOI] [PubMed] [Google Scholar]
- Clerc R. G., Corcoran L. M., LeBowitz J. H., Baltimore D., Sharp P. A. The B-cell-specific Oct-2 protein contains POU box- and homeo box-type domains. Genes Dev. 1988 Dec;2(12A):1570–1581. doi: 10.1101/gad.2.12a.1570. [DOI] [PubMed] [Google Scholar]
- Derman E., Krauter K., Walling L., Weinberger C., Ray M., Darnell J. E., Jr Transcriptional control in the production of liver-specific mRNAs. Cell. 1981 Mar;23(3):731–739. doi: 10.1016/0092-8674(81)90436-0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Finney M., Ruvkun G., Horvitz H. R. The C. elegans cell lineage and differentiation gene unc-86 encodes a protein with a homeodomain and extended similarity to transcription factors. Cell. 1988 Dec 2;55(5):757–769. doi: 10.1016/0092-8674(88)90132-8. [DOI] [PubMed] [Google Scholar]
- Fletcher C., Heintz N., Roeder R. G. Purification and characterization of OTF-1, a transcription factor regulating cell cycle expression of a human histone H2b gene. Cell. 1987 Dec 4;51(5):773–781. doi: 10.1016/0092-8674(87)90100-0. [DOI] [PubMed] [Google Scholar]
- Frain M., Swart G., Monaci P., Nicosia A., Stämpfli S., Frank R., Cortese R. The liver-specific transcription factor LF-B1 contains a highly diverged homeobox DNA binding domain. Cell. 1989 Oct 6;59(1):145–157. doi: 10.1016/0092-8674(89)90877-5. [DOI] [PubMed] [Google Scholar]
- Frigerio G., Burri M., Bopp D., Baumgartner S., Noll M. Structure of the segmentation gene paired and the Drosophila PRD gene set as part of a gene network. Cell. 1986 Dec 5;47(5):735–746. doi: 10.1016/0092-8674(86)90516-7. [DOI] [PubMed] [Google Scholar]
- Gehring W. J., Hiromi Y. Homeotic genes and the homeobox. Annu Rev Genet. 1986;20:147–173. doi: 10.1146/annurev.ge.20.120186.001051. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Hart C. P., Fainsod A., Ruddle F. H. Sequence analysis of the murine Hox-2.2, -2.3, and -2.4 homeo boxes: evolutionary and structural comparisons. Genomics. 1987 Oct;1(2):182–195. doi: 10.1016/0888-7543(87)90011-5. [DOI] [PubMed] [Google Scholar]
- Hatano M., Roberts C. W., Minden M., Crist W. M., Korsmeyer S. J. Deregulation of a homeobox gene, HOX11, by the t(10;14) in T cell leukemia. Science. 1991 Jul 5;253(5015):79–82. doi: 10.1126/science.1676542. [DOI] [PubMed] [Google Scholar]
- He X., Treacy M. N., Simmons D. M., Ingraham H. A., Swanson L. W., Rosenfeld M. G. Expression of a large family of POU-domain regulatory genes in mammalian brain development. Nature. 1989 Jul 6;340(6228):35–41. doi: 10.1038/340035a0. [DOI] [PubMed] [Google Scholar]
- Herr W., Sturm R. A., Clerc R. G., Corcoran L. M., Baltimore D., Sharp P. A., Ingraham H. A., Rosenfeld M. G., Finney M., Ruvkun G. The POU domain: a large conserved region in the mammalian pit-1, oct-1, oct-2, and Caenorhabditis elegans unc-86 gene products. Genes Dev. 1988 Dec;2(12A):1513–1516. doi: 10.1101/gad.2.12a.1513. [DOI] [PubMed] [Google Scholar]
- Hunger S. P., Galili N., Carroll A. J., Crist W. M., Link M. P., Cleary M. L. The t(1;19)(q23;p13) results in consistent fusion of E2A and PBX1 coding sequences in acute lymphoblastic leukemias. Blood. 1991 Feb 15;77(4):687–693. [PubMed] [Google Scholar]
- Ingraham H. A., Chen R. P., Mangalam H. J., Elsholtz H. P., Flynn S. E., Lin C. R., Simmons D. M., Swanson L., Rosenfeld M. G. A tissue-specific transcription factor containing a homeodomain specifies a pituitary phenotype. Cell. 1988 Nov 4;55(3):519–529. doi: 10.1016/0092-8674(88)90038-4. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Herrup K., Auerbach B. A., Davis C. A., Rossant J. Subtle cerebellar phenotype in mice homozygous for a targeted deletion of the En-2 homeobox. Science. 1991 Mar 8;251(4998):1239–1243. doi: 10.1126/science.1672471. [DOI] [PubMed] [Google Scholar]
- Joyner A. L., Martin G. R. En-1 and En-2, two mouse genes with sequence homology to the Drosophila engrailed gene: expression during embryogenesis. Genes Dev. 1987 Mar;1(1):29–38. doi: 10.1101/gad.1.1.29. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Look A. T., Baltimore D. The human t(1;19) translocation in pre-B ALL produces multiple nuclear E2A-Pbx1 fusion proteins with differing transforming potentials. Genes Dev. 1991 Mar;5(3):358–368. doi: 10.1101/gad.5.3.358. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Murre C., Sun X. H., Baltimore D. A new homeobox gene contributes the DNA binding domain of the t(1;19) translocation protein in pre-B ALL. Cell. 1990 Feb 23;60(4):547–555. doi: 10.1016/0092-8674(90)90658-2. [DOI] [PubMed] [Google Scholar]
- Kissinger C. R., Liu B. S., Martin-Blanco E., Kornberg T. B., Pabo C. O. Crystal structure of an engrailed homeodomain-DNA complex at 2.8 A resolution: a framework for understanding homeodomain-DNA interactions. Cell. 1990 Nov 2;63(3):579–590. doi: 10.1016/0092-8674(90)90453-l. [DOI] [PubMed] [Google Scholar]
- Kohler S., Galili N., Sklar J. L., Donlon T. A., Blume K. G., Cleary M. L. Expression of bcr-abl fusion transcripts following bone marrow transplantation for Philadelphia chromosome-positive leukemia. Leukemia. 1990 Aug;4(8):541–547. [PubMed] [Google Scholar]
- Kongsuwan K., Allen J., Adams J. M. Expression of Hox-2.4 homeobox gene directed by proviral insertion in a myeloid leukemia. Nucleic Acids Res. 1989 Mar 11;17(5):1881–1892. doi: 10.1093/nar/17.5.1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld K., Saint R. B., Beachy P. A., Harte P. J., Peattie D. A., Hogness D. S. Structure and expression of a family of Ultrabithorax mRNAs generated by alternative splicing and polyadenylation in Drosophila. Genes Dev. 1989 Feb;3(2):243–258. doi: 10.1101/gad.3.2.243. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristie T. M., Sharp P. A. Interactions of the Oct-1 POU subdomains with specific DNA sequences and with the HSV alpha-trans-activator protein. Genes Dev. 1990 Dec;4(12B):2383–2396. doi: 10.1101/gad.4.12b.2383. [DOI] [PubMed] [Google Scholar]
- Lonai P., Orr-Urtreger A. Homeogenes in mammalian development and the evolution of the cranium and central nervous system. FASEB J. 1990 Mar;4(5):1436–1443. doi: 10.1096/fasebj.4.5.1968407. [DOI] [PubMed] [Google Scholar]
- Macdonald P. M., Ingham P., Struhl G. Isolation, structure, and expression of even-skipped: a second pair-rule gene of Drosophila containing a homeo box. Cell. 1986 Dec 5;47(5):721–734. doi: 10.1016/0092-8674(86)90515-5. [DOI] [PubMed] [Google Scholar]
- Mavilio F., Simeone A., Giampaolo A., Faiella A., Zappavigna V., Acampora D., Poiana G., Russo G., Peschle C., Boncinelli E. Differential and stage-related expression in embryonic tissues of a new human homoeobox gene. Nature. 1986 Dec 18;324(6098):664–668. doi: 10.1038/324664a0. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Garber R. L., Wirz J., Kuroiwa A., Gehring W. J. A homologous protein-coding sequence in Drosophila homeotic genes and its conservation in other metazoans. Cell. 1984 Jun;37(2):403–408. doi: 10.1016/0092-8674(84)90370-2. [DOI] [PubMed] [Google Scholar]
- McGinnis W., Levine M. S., Hafen E., Kuroiwa A., Gehring W. J. A conserved DNA sequence in homoeotic genes of the Drosophila Antennapedia and bithorax complexes. 1984 Mar 29-Apr 4Nature. 308(5958):428–433. doi: 10.1038/308428a0. [DOI] [PubMed] [Google Scholar]
- Mermod N., O'Neill E. A., Kelly T. J., Tjian R. The proline-rich transcriptional activator of CTF/NF-I is distinct from the replication and DNA binding domain. Cell. 1989 Aug 25;58(4):741–753. doi: 10.1016/0092-8674(89)90108-6. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Monica K., Chen-Levy Z., Cleary M. L. Small G proteins are expressed ubiquitously in lymphoid cells and do not correspond to Bcl-2. Nature. 1990 Jul 12;346(6280):189–191. doi: 10.1038/346189a0. [DOI] [PubMed] [Google Scholar]
- Mul Y. M., Verrijzer C. P., van der Vliet P. C. Transcription factors NFI and NFIII/oct-1 function independently, employing different mechanisms to enhance adenovirus DNA replication. J Virol. 1990 Nov;64(11):5510–5518. doi: 10.1128/jvi.64.11.5510-5518.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Monaci P., Tomei L., De Francesco R., Nuzzo M., Stunnenberg H., Cortese R. A myosin-like dimerization helix and an extra-large homeodomain are essential elements of the tripartite DNA binding structure of LFB1. Cell. 1990 Jun 29;61(7):1225–1236. doi: 10.1016/0092-8674(90)90687-a. [DOI] [PubMed] [Google Scholar]
- Nourse J., Mellentin J. D., Galili N., Wilkinson J., Stanbridge E., Smith S. D., Cleary M. L. Chromosomal translocation t(1;19) results in synthesis of a homeobox fusion mRNA that codes for a potential chimeric transcription factor. Cell. 1990 Feb 23;60(4):535–545. doi: 10.1016/0092-8674(90)90657-z. [DOI] [PubMed] [Google Scholar]
- O'Neill E. A., Fletcher C., Burrow C. R., Heintz N., Roeder R. G., Kelly T. J. Transcription factor OTF-1 is functionally identical to the DNA replication factor NF-III. Science. 1988 Sep 2;241(4870):1210–1213. doi: 10.1126/science.3413485. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Kauvar L. M., Drees B., Kornberg T. The engrailed locus of Drosophila: structural analysis of an embryonic transcript. Cell. 1985 Jan;40(1):37–43. doi: 10.1016/0092-8674(85)90306-x. [DOI] [PubMed] [Google Scholar]
- Rey-Campos J., Chouard T., Yaniv M., Cereghini S. vHNF1 is a homeoprotein that activates transcription and forms heterodimers with HNF1. EMBO J. 1991 Jun;10(6):1445–1457. doi: 10.1002/j.1460-2075.1991.tb07665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro C., Mermod N., Andrews P. C., Tjian R. A family of human CCAAT-box-binding proteins active in transcription and DNA replication: cloning and expression of multiple cDNAs. Nature. 1988 Jul 21;334(6179):218–224. doi: 10.1038/334218a0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Tamkun J. W., Hartzell G. W., 3rd The structure and function of the homeodomain. Biochim Biophys Acta. 1989 Jul 28;989(1):25–48. doi: 10.1016/0304-419x(89)90033-4. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Weiner A. J. Structural relationships among genes that control development: sequence homology between the Antennapedia, Ultrabithorax, and fushi tarazu loci of Drosophila. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4115–4119. doi: 10.1073/pnas.81.13.4115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm R. A., Das G., Herr W. The ubiquitous octamer-binding protein Oct-1 contains a POU domain with a homeo box subdomain. Genes Dev. 1988 Dec;2(12A):1582–1599. doi: 10.1101/gad.2.12a.1582. [DOI] [PubMed] [Google Scholar]
- Verrijzer C. P., Kal A. J., Van der Vliet P. C. The DNA binding domain (POU domain) of transcription factor oct-1 suffices for stimulation of DNA replication. EMBO J. 1990 Jun;9(6):1883–1888. doi: 10.1002/j.1460-2075.1990.tb08314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wharton K. A., Yedvobnick B., Finnerty V. G., Artavanis-Tsakonas S. opa: a novel family of transcribed repeats shared by the Notch locus and other developmentally regulated loci in D. melanogaster. Cell. 1985 Jan;40(1):55–62. doi: 10.1016/0092-8674(85)90308-3. [DOI] [PubMed] [Google Scholar]
- Wright C. V., Cho K. W., Oliver G., De Robertis E. M. Vertebrate homeodomain proteins: families of region-specific transcription factors. Trends Biochem Sci. 1989 Feb;14(2):52–56. doi: 10.1016/0968-0004(89)90043-1. [DOI] [PubMed] [Google Scholar]
- Zamrod Z., Stumph W. E. U4B snRNA gene enhancer activity requires functional octamer and SPH motifs. Nucleic Acids Res. 1990 Dec 25;18(24):7323–7330. doi: 10.1093/nar/18.24.7323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D. X., Yen T. S. The ubiquitous transcription factor Oct-1 and the liver-specific factor HNF-1 are both required to activate transcription of a hepatitis B virus promoter. Mol Cell Biol. 1991 Mar;11(3):1353–1359. doi: 10.1128/mcb.11.3.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]