Abstract
There is considerable disagreement regarding what constitutes a healthy diet. Ever since the influential work of Cannon and Richter, it was debated whether the ‘wisdom of the body’ will automatically direct us to the foods we need for healthy lives or whether we must carefully learn to eat the right foods, particularly in an environment of plenty. Although it is clear that strong mechanisms have evolved to prevent consumption of foods that have previously made us sick, it is less clear whether reciprocal mechanisms exist that reinforce the consumption of healthy diets. Here, we review recent progress in providing behavioural evidence for the regulation of intake and selection of proteins, carbohydrates and fats. We examine new developments in sensory physiology enabling recognition of macronutrients both pre- and post-ingestively. Finally, we propose a general model for central neural processing of nutrient-specific appetites. We suggest that the same basic neural circuitry responsible for the homoeostatic regulation of total energy intake is also used to control consumption of specific macro- and micronutrients. Similar to salt appetite, specific appetites for other micro- and macronutrients may be encoded by unique molecular changes in the hypothalamus. Gratification of such specific appetites is then accomplished by engaging the brain motivational system to assign the highest reward prediction to exteroceptive cues previously associated with consuming the missing ingredient. A better understanding of these nutrient-specific neural processes could help design drugs and behavioural strategies that promote healthier eating.
Keywords: Obesity, Diabetes, Macronutrient intake, Nutrient sensing, Neural control
Obesity and malnutrition negatively affect the lives of millions of people, and despite intensive research, no easy cures are in sight. Among the many factors contributing to these diseases, consumption of imbalanced and unhealthy diets is of central importance. Yet, there is considerable disagreement regarding what constitutes a healthy diet, both to prevent weight gain in healthy individuals and to promote weight loss in settings of obesity. Some believe that the ‘wisdom of the body’(1,2) will automatically direct us to the foods we need for healthy lives, while others are more sceptical and believe that we must carefully learn to eat the right foods to avoid succumbing to unhealthy diets. It is clear that strong mechanisms have evolved to prevent consumption of foods that have previously made us severely sick (conditioned taste aversion). Do reciprocal mechanisms exist to promote the consumption of healthy diets?
Nutrients can be classified as essential and non-essential. By definition, essential nutrients cannot be manufactured in the body and thus must be consumed in the diet to maintain health. Non-essential nutrients can be synthesised by the body, although often at considerable cost(3-5). It is thus plausible that mechanisms have evolved to actively seek essential, and possibly non-essential, nutrients(6-9). It is within this context of the selection and consumption of individual nutrients that the concept of macronutrient selection takes shape. All diets contain a mixture of the three macronutrients (protein, carbohydrate and fat), and each of the three macronutrients is either glorified or vilified in one diet fad or another. Yet, implicit within each of these arguments is the hypothesis that there exists a macronutrient composition that is ideal for health. These concepts raise the question of whether we actively regulate our consumption of the individual macronutrients, and if so, can we tap into this mechanism to specifically change our macronutrient preference. Imagine if we could take a pill that selectively reduces appetite for fatty foods.
To accept that intake of a macronutrient is regulated, several predictions should be met. First, intake of that macronutrient should be relatively stable under steady state conditions. Second, this intake should not depend on the menu, even if the menu consists of many different foods with different macronutrient compositions and other properties such as palatability, hydration level, viscosity and smoothness. Third, intake of the specific macronutrient should depend on the physiological need state. Fourth, intake of the macronutrient must be sensed to provide a negative feedback signal. This could be the macronutrient itself, one of its metabolites or a unique consequence of its metabolism, such as a signature profile of gut hormone release, acting either directly on the brain or via sensory neural pathways. Specific brain circuits would then use this feedback information to either enhance or reduce intake of the macronutrient in question by affecting both appetitive and satiety mechanisms. Collectively, these mechanisms should result in a selective, macronutrient-specific appetitive drive (motivation), which results in the correct selection from a menu, as demonstrated for Na intake(8,10,11). In other words, the subject has to ‘know’ exactly what it needs and to identify a source from a menu before ingesting large amounts of the other macronutrients. In human subjects, explicit knowledge about the composition and nutritional value of foods could be used to make the correct choice, yet such a solution is not available to animals, where implicit wanting would be the driving factor.
About 10 years ago, a multi-authored book was published asking the question whether the selection and consumption of the three macronutrients is regulated. Among the more than 30 chapters, both negative(12,13) and positive(14,15) evidence were presented. It was concluded that protein is quite strongly defended, while carbohydrate and fat intake are only weakly defended or not at all(16). The purpose of this review is to provide a brief appraisal of current concepts on macronutrient-specific appetites with emphasis on progress made over the last 10 years.
Behavioural evidence for the regulation of intake and selection of specific macronutrients
Proteins: strong evidence for defence of situation-specific target intake
Proteins are crucially important for growth and most of their building blocks, the amino acids, cannot easily be synthesised by the body. Therefore, intake of protein needs to be defended similarly to salt and vitamins. A number of studies have focused on the effect of variations in dietary protein quality and quantity on food intake. The consensus of this literature is that dietary protein can have a profound impact on food intake, via two similar but potentially separate mechanisms. The first mechanism is via absolute protein content, with high-protein diets tending to suppress food intake and moderately low-protein diets increasing food intake. For instance, it is well described that protein is the most satiating macronutrient on a per energy basis, and a large number of studies suggest that high-protein diets can decrease food intake and promote weight loss while maintaining lean mass(17,18). The second mechanism operates through protein quality: the amino acid profile. In particular, it seems clear that many species have the ability to rapidly detect and avoid diets that are severely imbalanced in their amino acid profile, and therefore unhealthy(19-21). This phenomenon has been demonstrated following the depletion of multiple amino acids and appears to represent a learned aversion(22,23). Gietzen and co-workers have demonstrated that this learned aversion is mediated by critical molecular events within the anterior piriform cortex (APC), in particular, the accumulation of uncharged tRNA and the resulting activation of the kinase GCN2 (general control non-depressible-2)(24,25). Thus, it is clear that variations in both dietary protein quantity and quality can have significant effects on food intake. It also seems likely that protein intake is regulated within general upper and lower limits to ensure a sufficient supply to support life(21).
Stronger evidence that protein selection is regulated comes from work testing whether protein selection is sensitive to changes in the need or demand for protein. These data collectively demonstrate that animals will increase their selection and consumption of protein when there is an increased need for protein, e.g., following a period of protein restriction, in growing animals, and during chronic injections of growth hormone, and that this regulation occurs independently from the regulation of energy intake(26).
Perhaps the most convincing data supporting the ability of an animal to navigate through ‘nutrient space’ derives from the Geometric Framework, developed by Steven Simpson and David Raubenheimer(27-29). Studies of macronutrient selection are impaired by the fact that an increase of one macronutrient must be offset by a decrease in a different macronutrient in order to maintain diets that are isoenergetic. The geometric approach addresses this and other concerns by using a geometric state–space model to quantify the intake of individual nutrients across a range of diets and choices. Consumption of any individual nutrient can be plotted relative to other components in the diet (e.g. protein v. carbohydrate or protein v. energy), with this analysis easily extended to the selection between multiple diets. The data suggest that species as diverse as insects, fish, rodents and pigs seek to consume a fixed amount of both protein and carbohydrate, and thus regulate intake around a specific protein:carbohydrate target(28,30-32). In addition, when faced with diets that do not allow an individual to simultaneously reach its protein and carbohydrate targets, evidence in insects and rodents indicates that protein intake is prioritised over carbohydrate intake. This effect has been termed ‘protein leveraging’, as small changes in protein content can induce profound changes in energy intake(28,29,32). From this perspective, the hyperphagia detected on a low-protein diet is due to the leveraging in an effort to consume a target amount of protein. The concept of protein leveraging has recently been extended to human subjects, with reductions in dietary protein leading to increases in energy intake, at least over the short term(33,34). Taken together, these data provide a compelling case for both the regulation of protein intake and a potential role for protein in the regulation of energy intake and obesity(29,35).
Carbohydrates: despite a strong basic attraction to sweets and learned avoidance if utilisation is chronically impaired, there is only weak defence of target intake
Glucose is the preferred nutrient for the brain and typically accounts for most of the energy requirements in human subjects. Furthermore, sugars are highly palatable and desired. Yet carbohydrate intake is not essential for survival per se, as energy and glucose can be derived from both fat and protein. However, a modest level of carbohydrate intake is necessary to avoid ketosis, and it seems likely that prolonged ketosis is at least undesirable. This may be the reason for animals to eat towards a carbohydrate target as determined in the geometric paradigm(28,31,32,36). However, protein takes priority over carbohydrate in this model.
Changes in food preferences when carbohydrate metabolism is impaired could also be interpreted as carbohydrate-specific regulation of intake. There is a large literature on food selection in diabetic rats(37-42), and the general consensus is that rats learn to avoid carbohydrates and instead prefer fat and protein, because of their inability to efficiently metabolise carbohydrates. Interestingly, in one report, induction of diabetes initially increased carbohydrate and protein intake, but after 3 weeks, rats switched to fat consumption(40). This suggests an initial attempt to overcome blocked glucose utilisation by increased carbohydrate intake, before they learn to circumvent glucose utilisation as the better strategy. This interpretation is consistent with the increased carbohydrate intake after systemic 2-deoxy-glucose-induced acute blockade of glucose utilisation(43) (but see(44) for different outcome).
There is also evidence that carbohydrate is not regulated. Although animals restricted for both energy and protein actively seek the missing nutrient, there is no evidence for carbohydrate-seeking in carbohydrate-restricted animals. Hamsters with restricted access to carbohydrate but provided with sufficient energy showed no preference for carbohydrate(45), in contrast to a strong preference for protein following protein restriction. A similar observation was made for hypothalamic neuropeptide-Y expression, which increased in response to both protein and energy restriction, but not following the isoenergetic restriction of carbohydrate(46). Taken together, the earlier data support the concept that carbohydrate is avoided if its utilisation is chronically impaired, but provide only limited support for the hypothesis that animals defend a specific intake of carbohydrate.
Fats: despite strong basic attraction to fatty foods, evidence for defence of target intake is lacking for fats in general, although it may exist for specific essential fatty acids
Fatty foods are very palatable and strongly preferred over dry foods with less creamy textures by human subjects and rodents. Most fatty acids are non-essential and thus can be synthesised by the body with the exception of α-linolenic and linoleic acids whose production requires desaturase enzymes that are lacking in many animal species including human subjects. Rats fed an n-3 fatty acid-deficient diet showed a robust preference to consume an n-3 fatty acid replete diet when given the choice over the n-3-deficient diet(47). Moreover, intake of the replete-diet progressively increased over 4 d of preference testing, indicating the contribution of post-ingestive learning. In another study, it was shown that mice with a genetic deletion of the fatty acid transporter CD36 were not able to detect and prefer another essential fatty acid, linoleic acid(48). These results provide evidence that rodents may possess a mechanism for regulating the intake of essential fatty acids just as they defend intake of thiamine.
In addition to essential fatty acids, there is some evidence for the regulation of total dietary lipids. For example, in free-living human subjects, it was found that fat intake on a given day was negatively correlated with fat intake 2 d later, suggesting some sort of delayed negative feedback regulation of fat intake(15). The existence of a fat-specific appetite was suggested in studies using Pavlovian conditioning by pairing separate conditioned stimuli to either fatty or sweet food rewards acting as unconditioned stimuli. Specifically, rats treated with intracerebroventricular agouti-related protein were observed to show, in the absence of food intake, enhanced appetitive responding towards stimuli that had been previously paired with fat and reduced responding towards stimuli previously paired with sucrose(49). However, studies using the geometric model have consistently found a lack of support for regulation of fat intake in a number of species(14). Therefore, with the exception of essential fatty acids, there is mixed evidence for physiological monitoring and precise regulation of fat intake.
When fats cannot be metabolised, the post-oral effects of ingested fat can become negative, thereby conditioning a reduction in fat intake. For example, a genetic mutation in Acads −/− mice renders them deficient in short-chain acyl-CoA dehydrogenase and therefore unable to oxidise SCFA. In a food choice situation, Acads-deficient mice shift consumption away from fat- and towards carbohydrate-containing diets, thus effectively preventing a reduction in total energies(50). This genetic model provides a tool for determining how signals from impaired SCFA oxidation are sensed and translated into feeding behaviour. The results from Acads −/− mice indicate that a deficiency in fatty acid oxidation can drive macronutrient selection and is similar to what has been reported in experimental diabetes where carbohydrate utilisation is impaired (see discussion earlier).
Several lines of evidence indicate that fat oxidation pathways are involved in the control of food intake, but whether or not these pathways regulate intake in a fat-specific manner is less clear. For example, the acute blockade of long-chain fatty acid oxidation potently stimulates food intake, and this response can be activated experimentally by using a variety of drugs that inhibit fatty acid oxidation including β-mercaptoacetate (MA). In a macronutrient choice paradigm, MA-treated rats increased their intake of protein and carbohydrate and decreased intake of fat(43). MA-treated rats did not eat more fat, even if fat was the only macronutrient source available, thus, MA increased only the intake of nutrients that could be metabolised.
None of the earlier studies suggest a macronutrient-specific appetite because blocking oxidation of SCFA (Acads-deficient mice) or long-chain fatty acids (pharmacological antagonists) does not produce a specific need for fat. Instead, the behavioural feeding response appears to be directed at: (1) locating the diet or nutrient that can provide sufficient energy; (2) avoiding the diet/nutrient that cannot provide sufficient energy or (3) exhibiting hyperphagia in response to the only diet/nutrient available when there is no choice. One exception appears to be that MA-treated rats fail to increase their intake of fat when it is the only macronutrient source available(43).
Exteroceptive and interoceptive cues for the detection of macronutrients
To ‘know’ which food source to select from, mechanisms must exist for detecting the necessary macronutrient before it is ingested. In the modern world, human subjects can rely on food labels and other explicit knowledge about foods, so that by just thinking about, or seeing foods(51), we can decide whether it is a good source for a given nutrient. Just as single-trial learning mechanisms have evolved to avoid toxic foods, learning helps select necessary and beneficial foods. Visual, olfactory, auditory and gustatory cues in the environment that are associated with specific foods become predictive for the beneficial post-ingestive consequences of eating this food through learning(52,53). The olfactory, and particularly the gustatory system, recognise certain nutrients through nutrient-specific receptor mechanisms, without the need for prior experience. Finally, once ingested, sensory mechanisms all along the alimentary canal and, after absorption, throughout the body, are used to encode the beneficial effects of their ingestion. Potential sites and mechanisms for the detection of the three macronutrients are discussed later.
Protein: there are excellent sensors for individual amino acids before and after ingestion, but quantitatively measuring protein intake is a challenging task
The umami (savory) flavour is often associated with protein-rich foods, and involves principally the detection of the amino acid glutamic acid or its salt, monosodium glutamate by members of the T1R taste receptor family, specifically the T1R1/T1R3 heterodimer(54,55), with additional involvement of the olfactory system. The complexity of this system should be noted in that: (1) umami is also represented by a rather diverse set of compounds(56), (2) the T1R1/T1R3 heterodimer appears to be capable of responding to amino acids other than glutamate(57) and (3) umami is also detected in the absence of T1R3(54). Thus, there is likely more than one receptor mediating umami taste, and individual amino acids besides monosodium glutamate represent unique tastes(58,59).
Protein selection is complicated by the fact that protein is more than a single substrate and because it is unclear whether animals are selecting for sensory cues from specific amino acids or crude protein (nitrogen). Infusing protein directly into the stomach or small intestine, thereby bypassing the oral cavity, is sufficient to condition learned flavour preferences similar to fat or carbohydrate(60-62). Because attempts to produce the same effects with infusion of glucose into the hepatic portal system have been less successful, it is thought that sensors at pre-absorptive sites, or linked to the absorptive process, can detect the arrival of all three macronutrients in the gut. The same sweet, umami, fat and bitter taste receptors found in the mouth are also expressed in select enterocytes throughout the small and large intestines where they can signal to primary afferent nerves or stimulate the release of gut hormones. In addition, after being absorbed into the bloodstream, macronutrients and their metabolites can generate hormonal signals by acting on the pancreas, liver and other organs, and can act directly on the brain. Collectively, these signalling mechanisms are thought to represent the post-ingestive consequences of a specific food or macronutrient which can be learned to be liked.
Learned associations between the post-ingestive effects of protein and flavours specific to that protein source may strongly influence protein intake and selection(63). For instance, DiBattista demonstrated that the strong protein preference demonstrated by protein-deprived hamsters was driven by the association of the high-protein diet with flavours associated with that diet(64). These data suggest that protein intake is not a specific, hard-wired appetite for protein, but instead a learned association between dietary cues and post-ingestive consequences.
Carbohydrates: while the human subjects gustatory system is largely blind to complex non-sweet carbohydrates there is abundant sensing of glucose, the major common currency of carbohydrates, in periphery and brain
The pre-ingestive detection of sugars is thought to involve both olfaction and taste(65,66). The T1R2/T1R3 heterodimer is responsible for mammalian sweet taste perception, and its location in the oral cavity mediates at least short-term preference for sugars(65,67). However, intragastric glucose infusions, bypassing the oral cavity, can also condition flavour preferences in rats(68). Post-oral sugar conditioning could depend on sweet taste receptors or, alternatively, the Na-GLUT, expressed in specialised gut epithelial cells that release specific gut hormones to communicate with the brain. For instance, sweet taste receptors are expressed on enteroendocrine cells within the gut, providing a potential mechanism for carbohydrate to induce changes in gut hormone secretion(69,70). Whether any of these identified or yet unidentified receptor mechanisms for the detection of simple carbohydrates in the gut contributes to the regulation of carbohydrate intake or balance remains an open question.
Fats: novel lipid sensors found in mouth and gut have not yet been characterised in the brain
Historically, the orosensory perception of fat has been attributed mainly to trigeminal(71) or olfactory(72,73) mechanisms. More recently, two lines of evidence for fat taste transduction mechanisms in taste receptor cells have been described: the delayed-rectifying K channel which is sensitive to PUFA(74), and fatty acid translocase (CD36) which is localised in lingual taste buds. In addition, the transient receptor potential type M5 was shown to be essential for fat taste in the mouse(75), suggesting involvement of G-protein-coupled receptors, possibly GPR40 and GPR120(76). CD36 knockout mice do not prefer a fatty acid emulsion as wild-type mice do, in two bottle 48 h preference tests(77,78) but do learn to prefer a flavoured solution paired with intragastric soyabean oil infusions(78), thus supporting a role of CD36 as a signalling protein for fat taste but not required for post-oral fat conditioning.
Based on systemic treatment with the fatty acid β-oxidation blocker mercaptoacetate, it has been proposed that mice may be able to detect fat via its oxidation products within taste receptor cells, independent of post-oral conditioning(79). Contrary to this idea is the finding that mice with genetically impaired SCFA oxidation respond normally to maize oil in 5-s lick tests, where post-ingestive learning is unlikely, but reduce responding in longer-term tests(50). However, such a mechanism needs to be verified by demonstrating a direct action of fatty acid oxidation inhibitors on taste receptor cell function.
Rats and mice do not discriminate between the oral effects of a nutritive or non-nutritive fat solution during brief presentations. However, rodents prefer a nutritive fat solution when post-oral consequences are allowed, e.g., when solutions are presented for a longer period of time to allow for post-oral consequences to occur(80), and during gastric conditioning when an oral flavour is paired with intragastric infusions of fat solutions (thus avoiding oral effects)(81,82). These data support the view that post-oral processes communicate the nutritional value of ingested fat solutions to the body. In gastric conditioning paradigms, fats that are high in PUFA and low in SCFA content are the most reinforcing(82), supporting the idea that oral as well as post-oral sensing helps to satisfy the evolutionary pressure to ingest sufficient amounts of the essential PUFA.
Post-oral effects of fat may involve processes in the stomach and gastrointestinal tract which could regulate the release of gut hormones (ghrelin, peptide YY and glucagon-like peptide-1), modulation of vagal afferents, absorption of nutrients into the circulation and subsequent communication with liver, pancreas or other peripheral tissues, which would then alter the release of hormones relevant to fat storage and glucose homoeostasis (e.g. leptin, insulin and glucagon). Individual nutrients affect incretin secretion(83). Enterocytes may sense TAG via fatty acid oxidation and influence eating through changes in intestinal vagal afferent activity(84).
Potential neural mechanisms integrating sensory inputs and leading to the expression of specific macronutrient appetite
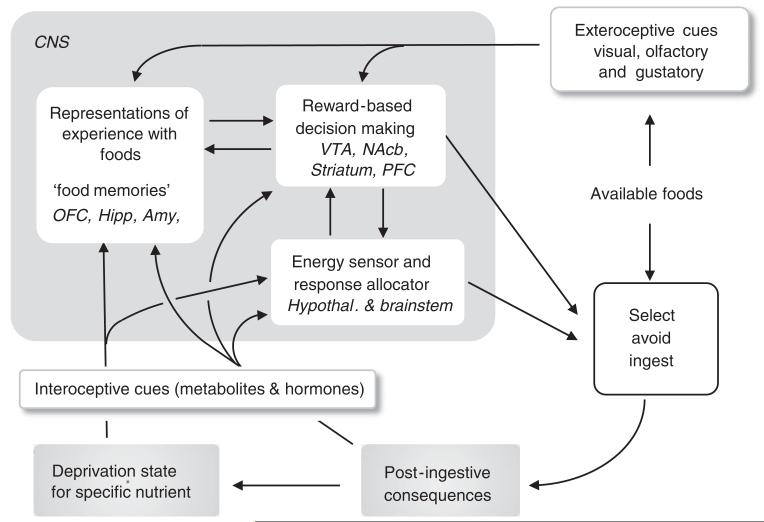
In the earlier two sections, we have reviewed the behavioural evidence for regulation, as well as the potential external and internal cues for the detection of specific macronutrients and their components. Here, we discuss the scarce knowledge about potential neural mechanisms that might be responsible for expression of the specific appetites and behavioural selection process. Accepting the neural circuitry for the homoeostatic control of energy balance as the prototype, we can distinguish at least two steps of neural processing. First, relevant sensory information is integrated by dedicated neural circuits generating a need state or hunger for energy. The major circuitry for this function is attributed to areas of the brainstem and hypothalamus (see(85) for a recent review). Neurons sensing the availability of all three energy providing macronutrients (see(86) for a recent review) ultimately determine activity of the agouti-related protein/neuropeptide-Y and melanocortin systems via the ancient fuel gauge AMP-regulated kinase. Activation of basomedial hypothalamic agouti-related protein neurons by low fuel availability is essential for the basic hunger drive to occur(87,88). In a second step, the motivational system residing in corticolimbic structures is engaged through the heightened incentive provided by the nutritional need state(89,90). This reward-based decision-making system takes both interoceptive and extroceptive sensory information into account and relies on earlier experience stored as ‘food memories’(52,53) (Fig. 1). As a result, attention is shifted from any other behaviour towards finding and eating food, and reward generated from gratification of the specific need provides the necessary reinforcement.
Fig. 1.
Schematic flow diagram showing possible neural processing of external and internal food cues leading to nutrient-specific appetites. Representations of experience with a particular food (food memories) take into account (a) exteroceptive cues including taste, available before ingestion of significant amounts, (b) post-ingestive consequences elicited by ingesting the food (digestion, absorption and metabolism), and (c) the prevailing deprivation state for the particular nutrient at the time of replenishment. The hypothalamic energy sensor may be involved in generating a general hunger signal (incentive), and the cortico-limbic, reward-based decision-making circuitry may confer the behavioural specificity for the selection process. Amy, amygdala; Hipp, hippocampal complex; NAcb, nucleus accumbens; OFC, orbitofrontal cortex; PFC, prefrontral cortex; VTA, ventral tegmental area.
We suggest that this same basic two-step neural processing model is responsible for the homoeostatic-like regulation of individual macro- and micronutrients (Fig. 1). The difference is that instead of satisfying a general energy deficit, the system satisfies nutrient-specific appetites. For example, it has been shown that similar to food seeking in general(89), sodium appetite depends on the mesolimbic dopamine system(91). An intriguing possibility is that lateral hypothalamic orexin neurons provide the connection between steps one and two of the model(9). Orexin neurons connect the hypothalamus, where the specific need state is generated, with the mesolimbic dopamine system, which confers selectivity of behavioural action. In a seminal paper, Liedtke et al.(9) have recently demonstrated the molecular changes occurring in hypothalamic orexin neurons of Na depleted mice. They went on to show that pharmacological prevention of these changes greatly and selectively reduced Na appetite, without affecting thirst and hunger(9), suggesting that distinct molecular signatures of specific need states may be generated within hypothalamic orexin neurons. It is well known that orexin neurons are stimulated by hypoglycaemia(92) and fasting(93), and activation of orexin neurons is associated with conditioned reward seeking for foods and drugs(94). Furthermore, local administration of orexin to the ventral tegmental area, the home of mesolimbic dopamine neurons, reinstates extinct reward seeking for foods and drugs(94,95). Thus, this path-way could account for the expression of the ‘wisdom of the body’, by reinforcing only the behavioural actions that contribute to general well-being.
Protein: individual amino acid sensing in the piriform cortex and metabolism-coupled protein effects in the hypothalamus – search for the missing link
The most straight-forward mechanism for protein detection would be a direct effect of amino acids on brain areas regulating food intake(96), as both dietary and circulating amino acids clearly have access to the brain(97,98). As described previously, exposure to a diet that is devoid of an essential amino acid induces a rapid, learned aversion that requires critical signalling events within the APC(24,25). Lesions of the APC block the aversive response to an imbalanced diet, as does replacement of the missing amino acid locally within the APC(24,99-101), thus indicating the APC as a direct detector of essential amino acids. Subsequent studies indicate that a build-up of uncharged tRNA and the activation of the general control non-depressible-2 kinase are the key cellular mechanism involved. Indeed, general control non-depressible-2-deficient mice fail to exhibit the aversive response to a diet devoid of essential amino acids(99). These studies provide a clear neuroanatomical and cellular model for the avoidance of imbalanced and thus unhealthy diets, but it is unclear whether this mechanism contributes more generally to the selection of protein.
In addition, there is strong evidence that the branched-chain amino acid (BCAA) leucine acts locally within the hypothalamus to suppress food intake(102-105), at least in part via activation of mammalian target of rapamycin/S6 kinase 1, inhibition of AMP-regulated kinase signalling(103,105,106), and via activation of BCAA metabolism(102,107). Thus the hypothalamus is clearly capable of responding to amino acids, suggesting that BCAA may provide a unique circulating signal of dietary protein content(105,108). However, anorectic leucine effects are primarily observed when it is added in excess, and whether circulating amino acids provide a specific signal of protein status remains unclear. Mice with defects in BCAA metabolism and resulting increases in circulating BCAA show normal protein intake, even when allowed to self-select between high- and low-protein diets supplemented with or without BCAA(107). Thus, despite the clear anorexigenic effects of pharmacological leucine doses on food intake, its role as a physiological protein signal is unclear.
The intriguing possibility that orexin and other lateral hypothalamic neurons might provide a link between the specific protein-deficient state and the motivational system has not been explored. However, both the APC(109) and the medial hypothalamic sites responding to leucine, project directly to the lateral hypothalamus, including orexin neurons(110). Thus, protein-deficiency may engage similar neural pathways as Na deficiency(9).
Carbohydrate: hypothalamic glucose sensing may generate need state and drive specific appetite by engaging with mesolimbic dopamine system
As discussed earlier, the presence of carbohydrates in the diet and in the circulation are is detected by at least two distinct mechanisms, sweet taste and the metabolic effects of glucose and other simple sugars. Glucose-sensing neurons, through a mechanism involving GLUT2 and glucokinase, are either excited or inhibited by surrounding glucose (see(86) for a recent review). If brain glucose-sensing would be critically involved in the regulation or defence of carbohydrate intake, one would expect that loss-of-function manipulation would selectively stimulate carbohydrate seeking. Unfortunately, in the majority of studies that demonstrate increased food intake induced by impairment of brain glucosensing such as insulin or 2-deoxy-D-glucose(111) administration and genetic deletion of glucokinase(112), only total food intake, but not macronutrient selection, was measured. In only one experiment, systemic administration of 2-deoxy-D-glucose induced a selective hunger for carbohydrate(43), but it is not clear whether impaired brain glucosensing was responsible. However, third ventricular administration of insulin which, in contrast to 2-deoxy-glucose, enhances glucosensing and decreases total food intake(113), selectively reduced fat, not carbohydrate, intake in a three-choice paradigm(114). Thus, although brain glucosensing would be in an ideal position to modulate carbohydrate intake selectively, there is no direct evidence for such a mechanism.
As glucose on the tongue and carbohydrates in the gut can powerfully stimulate the mesolimbic dopamine system and condition flavour preferences(115-117), it will be interesting to look for a role of hypothalamic orexin neurons as potential mediators.
Fat: are fat-specific neuropeptides pharmacological artefacts or do they serve regulation of fat intake?
The possibility that essential fatty acids are detected directly in the brain, similar to amino acids(23), is suggested by the observation that consumption of an n-3 fatty acid deficient diet was accompanied by a decline in forebrain n-3 fatty acid content(47); however, this possibility needs to be validated by targeted repletion of the missing fatty acid in specific brain sites. In analogy to Na depletion, it would be particularly interesting to look for molecular changes in orexin and other hypothalamic neurons possibly encoding the specific need state.
Initially, there was excitement about the possibility that specific neurotransmitters and peptides drive selective intake of macronutrients, e.g. that the neuropeptides galanin and orexin stimulate lipid intake, and that norepinephrine via the α2-receptor as well as neuropeptide-Y stimulate carbohydrate intake(118,119). It would be interesting to test the effects of these neuropeptides with the geometric model to determine whether the observed preferences were mainly due to the specific sensory properties of the macronutrient samples rather than a defended target intake. It is clear that certain neuropeptides and transmitters change their expression levels with diets enriched with certain macronutrients. In particular, high-fat diets via elevated circulating TAG levels increase expression of galanin, enkephalin and dynorphin in paraventricular neurons, as well as orexin in lateral hypothalamic neurons. However, rather than providing negative feedback to curb further fat intake as would be expected in a regulated system, these mechanisms are apparently working in a positive feedback fashion to further enhance high-fat intake(120,121).
A more likely mechanism that could be responsible for a homoeostatic-like negative feedback regulation of fat intake may use the same general system outlined earlier (Fig. 1). Low-fat oxidation detected in the periphery(84) and/or directly in the brain(122) may activate hypothalamic energy sensor neurons via AMP-regulated kinase phos-phorylation. This would in turn lead to activation of the agouti-related protein system and possibly fat-specific molecular changes in orexin and other lateral hypothalamic neurons as demonstrated for Na deficiency(9). In support of such molecular changes, our preliminary observations suggest that mice with genetic deletion of the ability to oxidise SCFA show elevated hypothalamic AMP-regulated kinase expression on high-fat diet compared with wild-type mice (BK Richards, unpublished results).
Conclusions and unanswered questions
It is now commonly accepted that the consumption of energy and essential nutrients such as Na, vitamins and certain amino- and fatty acids is at least controlled and defended, if not regulated, in a homeostatic-like manner, is now commonly accepted. A much weaker case for such ‘wisdom of the body’ can be made for the non-essential macronutrients, carbohydrate, fat and protein, although a review of the recent literature provides some new insights encouraging continued inquiry of this question. We propose that the same basic neural circuitry responsible for the homoeostatic-regulation of total energy intake when energy expenditure is held constant is also used to control consumption of specific macro- and micronutrients. Thus, in addition to a general appetitive drive, specific appetites are likely encoded by unique molecular changes in the hypothalamus(9) that occur via largely unknown changes in interoceptive signalling. Gratification of such specific appetites is then accomplished by engaging the brain motivational system. The cortico-limbic circuitry assigns the highest reward prediction to exteroceptive cues previously associated with consuming the missing ingredient. Thus, we would argue that the depletion of a given nutrient results in an increased rewarding value (incentive salience) of that nutrient. For example, protein-deprived animals would find protein particularly rewarding, much more than any other nutrient, and more than a protein-replete animal. The increased incentive salience would result in a heightened reinforcement of cues associated with that food source, particularly in the deplete state, such that the depleted animal specifically seeks food sources that provide the needed nutrient, and particularly those sources that previously met this need. It will be interesting to test this model under conditions of relative protein-, carbohydrate-, or fat-deficiencies. In particular, it will be interesting to (1) search for possible nutrient-specific molecular changes within hypothalamic circuits, (2) characterise the sensitivity and selectivity of the midbrain dopamine system in response to nutrient-specific cues and (3) identify the nature and location of the relevant ‘food memories’. The potential ‘reward’ of such research could be big, as it could lead to the development of drugs that make us want to eat less fatty foods, or help design behavioural strategies that promote healthier eating.
Acknowledgements
We thank Katie Bailey for editorial assistance. Supported by National Institutes of Health Grants DK047348 and DK071082 (H.R.B.), DK081563 (C.D.M.), DK053113 (B.K.R.), RR02195 and DK092587 (H.M.). None of the authors declares a conflict of interest. All authors contributed equally to the writing of this review paper.
Abbreviations
- APC
anterior piriform cortex
- BCAA
branched-chain amino acid
- MA
mercaptoacetate
References
- 1.Cannon W. The Wisdom of the Body. Norton; New York: 1939. [Google Scholar]
- 2.Richter C. Total self-regulatory functions of animals and human beings. Harvey Lect Ser. 1942–1943;38:63–103. [Google Scholar]
- 3.Livesey G. The energy equivalents of ATP and the energy values of food proteins and fats. Br J Nutr. 1984;51:15–28. doi: 10.1079/bjn19840005. [DOI] [PubMed] [Google Scholar]
- 4.Veldhorst MA, Westerterp-Plantenga MS, Westerterp KR. Gluconeogenesis and energy expenditure after a high-protein, carbohydrate-free diet. Am J Clin Nutr. 2009;90:519–526. doi: 10.3945/ajcn.2009.27834. [DOI] [PubMed] [Google Scholar]
- 5.Kalhan SC, Kilic I. Carbohydrate as nutrient in the infant and child: range of acceptable intake. Eur J Clin Nutr. 1999;53(Suppl. 1):S94–100. doi: 10.1038/sj.ejcn.1600749. [DOI] [PubMed] [Google Scholar]
- 6.Rozin P. Adaptive food sampling patterns in vitamin deficient rats. J Comp Physiol Psychol. 1969;69:126–132. doi: 10.1037/h0027940. [DOI] [PubMed] [Google Scholar]
- 7.Fromentin G, Gietzen DW, Nicolaidis S. Aversion-preference patterns in amino acid- or protein-deficient rats: a comparison with previously reported responses to thiamin-deficient diets. Br J Nutr. 1997;77:299–314. doi: 10.1079/bjn19970031. [DOI] [PubMed] [Google Scholar]
- 8.Tindell AJ, Smith KS, Berridge KC, et al. Dynamic computation of incentive salience: “wanting” what was never “liked”. J Neurosci. 2009;29:12220–12228. doi: 10.1523/JNEUROSCI.2499-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liedtke WB, McKinley MJ, Walker LL, et al. Relation of addiction genes to hypothalamic gene changes sub-serving genesis and gratification of a classic instinct, sodium appetite. Proc Natl Acad Sci USA. 2011;108:12509–12514. doi: 10.1073/pnas.1109199108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berridge KC, Flynn FW, Schulkin J, et al. Sodium depletion enhances salt palatability in rats. Behav Neurosci. 1984;98:652–660. doi: 10.1037//0735-7044.98.4.652. [DOI] [PubMed] [Google Scholar]
- 11.Tindell AJ, Smith KS, Pecina S, et al. Ventral pallidum firing codes hedonic reward: when a bad taste turns good. J Neurophysiol. 2006;96:2399–2409. doi: 10.1152/jn.00576.2006. [DOI] [PubMed] [Google Scholar]
- 12.Galef BGJ. Is There a specific appetite for Protein? In: Berthoud HR, Seeley RJ, editors. Neural and Metabolic Control of Macronutrient Intake. CRC Press; Boca Raton, FL: 2000. pp. 19–28. [Google Scholar]
- 13.Friedman M. Too many choices? Acritical essay on macronutrient selection. In: Berthoud HaS RJ, editor. Neural and Metabolic Control of Macronutrient Intake. CRC Press; Boca Raton, FL: 2000. pp. 11–28. [Google Scholar]
- 14.Simpson SJ, Raubenheimer D. Geometric models of macronutrient selection. In: Berthoud HR, Seeley RJ, editors. Neural and Metabolic Control of Macronutrient Intake. CRC Press; Boca Raton, FL: 2000. pp. 29–42. [Google Scholar]
- 15.de Castro JM. Macronutrient selection in free-feeding humans: evidence for long-term regulation. In: Berthoud HR, Seeley RJ, editors. Neural and Metabolic Control of Macronutrient Intake. CRC Press; Boca Raton, FL: 2000. pp. 43–59. [Google Scholar]
- 16.Berthoud HR, Seeley RJ. Neural and Metabolic Control of Macronutrient Intake. CRC Press; Boca Raton, FL: 2000. [Google Scholar]
- 17.Westerterp-Plantenga MS, Nieuwenhuizen A, Tome D, et al. Dietary protein, weight loss, and weight maintenance. Annu Rev Nutr. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. [DOI] [PubMed] [Google Scholar]
- 18.Potier M, Darcel N, Tome D. Protein, amino acids and the control of food intake. Curr Opin Clin Nutr Metab Care. 2009;12:54–58. doi: 10.1097/MCO.0b013e32831b9e01. [DOI] [PubMed] [Google Scholar]
- 19.Sanahuja JC, Harper AE. Effect of dietary amino acid pattern on plasma amino acid pattern and food intake. Am J Physiol. 1963;204:686–690. doi: 10.1152/ajplegacy.1963.204.4.686. [DOI] [PubMed] [Google Scholar]
- 20.Leung PM, Rogers QR, Harper AE. Effect of amino acid imbalance on dietary choice in the rat. J Nutr. 1968;95:483–492. doi: 10.1093/jn/95.3.483. [DOI] [PubMed] [Google Scholar]
- 21.Harper AE, Peters JC. Protein intake, brain amino acid and serotonin concentrations and protein self-selection. J Nutr. 1989;119:677–689. doi: 10.1093/jn/119.5.677. [DOI] [PubMed] [Google Scholar]
- 22.Koehnle TJ, Russell MC, Gietzen DW. Rats rapidly reject diets deficient in essential amino acids. J Nutr. 2003;133:2331–2335. doi: 10.1093/jn/133.7.2331. [DOI] [PubMed] [Google Scholar]
- 23.Gietzen DW, Hao S, Anthony TG. Mechanisms of food intake repression in indispensable amino acid deficiency. Annu Rev Nutr. 2007;27:63–78. doi: 10.1146/annurev.nutr.27.061406.093726. [DOI] [PubMed] [Google Scholar]
- 24.Hao S, Sharp JW, Ross-Inta CM, et al. Uncharged tRNA and sensing of amino acid deficiency in mammalian piriform cortex. Science. 2005;307:1776–1778. doi: 10.1126/science.1104882. [DOI] [PubMed] [Google Scholar]
- 25.Rudell JB, Rechs AJ, Kelman TJ, et al. The anterior piriform cortex is sufficient for detecting depletion of an indispensable amino acid, showing independent cortical sensory function. J Neurosci. 2011;31:1583–1590. doi: 10.1523/JNEUROSCI.4934-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musten B, Peace D, Anderson GH. Food intake regulation in the weanling rat: self-selection of protein and energy. J Nutr. 1974;104:563–572. doi: 10.1093/jn/104.5.563. [DOI] [PubMed] [Google Scholar]
- 27.Cheng K, Simpson SJ, Raubenheimer D. A geometry of regulatory scaling. Am Nat. 2008;172:681–693. doi: 10.1086/591686. [DOI] [PubMed] [Google Scholar]
- 28.Simpson SJ, Raubenheimer D. Geometric analysis of macronutrient selection in the rat. Appetite. 1997;28:201–213. doi: 10.1006/appe.1996.0077. [DOI] [PubMed] [Google Scholar]
- 29.Simpson SJ, Raubenheimer D. Obesity: the protein leverage hypothesis. Obes Rev. 2005;6:133–142. doi: 10.1111/j.1467-789X.2005.00178.x. [DOI] [PubMed] [Google Scholar]
- 30.Kyriazakis I, Emmans GC. Diet selection in pigs: dietary choices made by growing pigs following a period of underfeeding with protein. Anim Prod. 1991;52:337–346. [Google Scholar]
- 31.Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr Res Rev. 1997;10:151–179. doi: 10.1079/NRR19970009. [DOI] [PubMed] [Google Scholar]
- 32.Sorensen A, Mayntz D, Raubenheimer D, et al. Protein-leverage in mice: the geometry of macronutrient balancing and consequences for fat deposition. Obesity (Silver Spring) 2008;16:566–571. doi: 10.1038/oby.2007.58. [DOI] [PubMed] [Google Scholar]
- 33.Simpson SJ, Batley R, Raubenheimer D. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite. 2003;41:123–140. doi: 10.1016/s0195-6663(03)00049-7. [DOI] [PubMed] [Google Scholar]
- 34.Gosby AK, Conigrave AD, Lau NS, et al. Testing protein leverage in lean humans: a randomised controlled experimental study. PLoS ONE. 2011;6:e25929. doi: 10.1371/journal.pone.0025929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brooks RC, Simpson SJ, Raubenheimer D. The price of protein: combining evolutionary and economic analysis to understand excessive energy consumption. Obes Rev. 2010;11:887–894. doi: 10.1111/j.1467-789X.2010.00733.x. [DOI] [PubMed] [Google Scholar]
- 36.Mayntz D, Raubenheimer D, Salomon M, et al. Nutrient-specific foraging in invertebrate predators. Science. 2005;307:111–113. doi: 10.1126/science.1105493. [DOI] [PubMed] [Google Scholar]
- 37.Bartness TJ, Rowland NE. Diet selection and metabolic fuels in three models of diabetes mellitus. Physiol Behav. 1983;31:539–545. doi: 10.1016/0031-9384(83)90079-3. [DOI] [PubMed] [Google Scholar]
- 38.Epstein LH, Salvy SJ, Carr KA, et al. Food reinforcement, delay discounting and obesity. Physiol Behav. 2010;100:438–445. doi: 10.1016/j.physbeh.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bellush LL, Rowland NE. Dietary self-selection in diabetic rats: an overview. Brain Res Bull. 1986;17:653–661. doi: 10.1016/0361-9230(86)90197-8. [DOI] [PubMed] [Google Scholar]
- 40.Kanarek RB, Ho L. Patterns of nutrient selection in rats with streptozotocin-induced diabetes. Physiol Behav. 1984;32:639–645. doi: 10.1016/0031-9384(84)90319-6. [DOI] [PubMed] [Google Scholar]
- 41.Tepper BJ, Kanarek RB. Selection of protein and fat by diabetic rats following separate dilution of the dietary sources. Physiol Behav. 1989;45:49–61. doi: 10.1016/0031-9384(89)90165-0. [DOI] [PubMed] [Google Scholar]
- 42.Tordoff MG, Tepper BJ, Friedman MI. Food flavor preferences produced by drinking glucose and oil in normal and diabetic rats: evidence for conditioning based on fuel oxidation. Physiol Behav. 1987;41:481–487. doi: 10.1016/0031-9384(87)90084-9. [DOI] [PubMed] [Google Scholar]
- 43.Singer LK, York DA, Bray GA. Macronutrient selection following 2-deoxy-D-glucose and mercaptoacetate administration in rats. Physiol Behav. 1998;65:115–121. doi: 10.1016/s0031-9384(98)00141-3. [DOI] [PubMed] [Google Scholar]
- 44.Ritter S, Ritter JB, Cromer L. 2-Deoxy-D-glucose and mercaptoacetate induce different patterns of macronutrient ingestion. Physiol Behav. 1999;66:709–715. doi: 10.1016/s0031-9384(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 45.DiBattista D. Effects of time-restricted access to protein and to carbohydrate in adult mice and rats. Physiol Behav. 1991;49:263–269. doi: 10.1016/0031-9384(91)90042-m. [DOI] [PubMed] [Google Scholar]
- 46.White BD, He B, Dean RG, et al. Low protein diets increase neuropeptide Y gene expression in the basomedial hypothalamus of rats. J Nutr. 1994;124:1152–1160. doi: 10.1093/jn/124.8.1152. [DOI] [PubMed] [Google Scholar]
- 47.Dunlap S, Heinrichs SC. Neuronal depletion of omega-3 fatty acids induces flax seed dietary self-selection in the rat. Brain Res. 2009;1250:113–119. doi: 10.1016/j.brainres.2008.10.072. [DOI] [PubMed] [Google Scholar]
- 48.Gaillard D, Laugerette F, Darcel N, et al. The gustatory pathway is involved in CD36-mediated orosensory perception of long-chain fatty acids in the mouse. FASEB J. 2008;22:1458–1468. doi: 10.1096/fj.07-8415com. [DOI] [PubMed] [Google Scholar]
- 49.Tracy AL, Clegg DJ, Johnson JD, et al. The melanocortin antagonist AgRP (83–132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav. 2008;89:263–271. doi: 10.1016/j.pbb.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith Richards BK, Belton BN, York B, et al. Mice bearing Acads mutation display altered postingestive but not 5-s orosensory response to dietary fat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R311–R319. doi: 10.1152/ajpregu.00488.2003. [DOI] [PubMed] [Google Scholar]
- 51.Toepel U, Knebel JF, Hudry J, et al. The brain tracks the energetic value in food images. Neuroimage. 2009;44:967–974. doi: 10.1016/j.neuroimage.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Davidson TL, Morell JR, Benoit SC. Memory and macronutrient regulation. In: Berthoud HR, Seeley RJ, editors. Neural and Metabolic Control of Macronutrient Intake. CRC Press; Boca Raton, FL: 2000. pp. 203–217. [Google Scholar]
- 53.Benoit SC, Davis JF, Davidson TL. Learned and cognitive controls of food intake. Brain Res. 2010;1350:71–76. doi: 10.1016/j.brainres.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Damak S, Rong M, Yasumatsu K, et al. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science. 2003;301:850–853. doi: 10.1126/science.1087155. [DOI] [PubMed] [Google Scholar]
- 55.Palmer RK. The pharmacology and signaling of bitter, sweet, and umami taste sensing. Mol Interv. 2007;7:87–98. doi: 10.1124/mi.7.2.9. [DOI] [PubMed] [Google Scholar]
- 56.Delay ER, Eddy MC, Eschle BK. Behavioral studies of umami: tales told by mice and rats. Ann NY Acad Sci. 2009;1170:41–45. doi: 10.1111/j.1749-6632.2009.03933.x. [DOI] [PubMed] [Google Scholar]
- 57.Nelson G, Chandrashekar J, Hoon MA, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 58.Chaudhari N, Pereira E, Roper SD. Taste receptors for umami: the case for multiple receptors. Am J Clin Nutr. 2009;90:738S–742S. doi: 10.3945/ajcn.2009.27462H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Maruyama Y, Pereira E, Margolskee RF, et al. Umami responses in mouse taste cells indicate more than one receptor. J Neurosci. 2006;26:2227–2234. doi: 10.1523/JNEUROSCI.4329-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Perez C, Ackroff K, Sclafani A. Carbohydrate- and protein-conditioned flavor preferences: effects of nutrient preloads. Physiol Behav. 1996;59:467–474. doi: 10.1016/0031-9384(95)02085-3. [DOI] [PubMed] [Google Scholar]
- 61.Perez C, Lucas F, Sclafani A. Carbohydrate, fat, and protein condition similar flavor preferences in rats using an oral-delay procedure. Physiol Behav. 1995;57:549–554. doi: 10.1016/0031-9384(94)00366-d. [DOI] [PubMed] [Google Scholar]
- 62.Sclafani A. Psychobiology of food preferences. Int J Obes Relat Metab Disord. 2001;25(Suppl. 5):S13–S16. doi: 10.1038/sj.ijo.0801905. [DOI] [PubMed] [Google Scholar]
- 63.Miller MG, Teates JF. Acquisition of dietary self-selection in rats with normal and impaired oral sensation. Physiol Behav. 1985;34:401–408. doi: 10.1016/0031-9384(85)90203-3. [DOI] [PubMed] [Google Scholar]
- 64.DiBattista D, Mercier S. Role of learning in the selection of dietary protein in the golden hamster (Mesocricetus auratus) Behav Neurosci. 1999;113:574–586. doi: 10.1037//0735-7044.113.3.574. [DOI] [PubMed] [Google Scholar]
- 65.Zukerman S, Touzani K, Margolskee RF, et al. Role of olfaction in the conditioned sucrose preference of sweet-ageusic T1R3 knockout mice. Chem Senses. 2009;34:685–694. doi: 10.1093/chemse/bjp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rhinehart-Doty JA, Schumm J, Smith JC, et al. A non-taste cue of sucrose in short-term taste tests in rats. Chem Senses. 1994;19:425–431. doi: 10.1093/chemse/19.5.425. [DOI] [PubMed] [Google Scholar]
- 67.Nelson G, Hoon MA, Chandrashekar J, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 68.Sclafani A, Glass DS, Margolskee RF, et al. Gut T1R3 sweet taste receptors do not mediate sucrose-conditioned flavor preferences in mice. Am J Physiol Regul Integr Comp Physiol. 2010;299:R1643–R1650. doi: 10.1152/ajpregu.00495.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Steinert RE, Gerspach AC, Gutmann H, et al. The functional involvement of gut-expressed sweet taste receptors in glucose-stimulated secretion of glucagon-like peptide-1 (GLP-1) and peptide YY (PYY) Clin Nutr. 2011;30:524–532. doi: 10.1016/j.clnu.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 70.Kokrashvili Z, Mosinger B, Margolskee RF. Taste signaling elements expressed in gut enteroendocrine cells regulate nutrient-responsive secretion of gut hormones. Am J Clin Nutr. 2009;90:822S–825S. doi: 10.3945/ajcn.2009.27462T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laugerette F, Gaillard D, Passilly-Degrace P, et al. Do we taste fat? Biochimie. 2007;89:265–269. doi: 10.1016/j.biochi.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 72.Kinney NE, Antill RW. Role of olfaction in the formation of preference for high-fat foods in mice. Physiol Behav. 1996;59:475–478. doi: 10.1016/0031-9384(95)02086-1. [DOI] [PubMed] [Google Scholar]
- 73.Ramirez I. Role of olfaction in starch and oil preference. Am J Physiol. 1993;265:R1404–R1409. doi: 10.1152/ajpregu.1993.265.6.R1404. [DOI] [PubMed] [Google Scholar]
- 74.Gilbertson TA, Fontenot DT, Liu L, et al. Fatty acid modulation of K + channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 75.Liu P, Shah BP, Croasdell S, et al. Transient receptor potential channel type M5 is essential for fat taste. J Neurosci. 2011;31:8634–8642. doi: 10.1523/JNEUROSCI.6273-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cartoni C, Yasumatsu K, Ohkuri T, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–8382. doi: 10.1523/JNEUROSCI.0496-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Laugerette F, Passilly-Degrace P, Patris B, et al. CD36 involvement in orosensory detection of dietary lipids, spontaneous fat preference, and digestive secretions. J Clin Invest. 2005;115:3177–3184. doi: 10.1172/JCI25299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sclafani A, Ackroff K, Abumrad NA. CD36 gene deletion reduces fat preference and intake but not post-oral fat conditioning in mice. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1823–R1832. doi: 10.1152/ajpregu.00211.2007. [DOI] [PubMed] [Google Scholar]
- 79.Matsumura S, Saitou K, Miyaki T, et al. Mercaptoacetate inhibition of fatty acid beta-oxidation attenuates the oral acceptance of fat in BALB/c mice. Am J Physiol Regul Integr Comp Physiol. 2008;295:R82–R91. doi: 10.1152/ajpregu.00060.2008. [DOI] [PubMed] [Google Scholar]
- 80.Ackroff K, Vigorito M, Sclafani A. Fat appetite in rats: the response of infant and adult rats to nutritive and non-nutritive oil emulsions. Appetite. 1990;15:171–188. doi: 10.1016/0195-6663(90)90018-4. [DOI] [PubMed] [Google Scholar]
- 81.Lucas F, Ackroff K, Sclafani A. Dietary fatinduced hyperphagia in rats as a function of fat type and physical form. Physiol Behav. 1989;45:937–946. doi: 10.1016/0031-9384(89)90218-7. [DOI] [PubMed] [Google Scholar]
- 82.Ackroff K, Lucas F, Sclafani A. Flavor preference conditioning as a function of fat source. Physiol Behav. 2005;85:448–460. doi: 10.1016/j.physbeh.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 83.Wu T, Rayner CK, Jones K, et al. Dietary effects on incretin hormone secretion. Vitam Horm. 2010;84:81–110. doi: 10.1016/B978-0-12-381517-0.00003-5. [DOI] [PubMed] [Google Scholar]
- 84.Langhans W, Leitner C, Arnold M. Dietary fat sensing via fatty acid oxidation in enterocytes: possible role in the control of eating. Am J Physiol Regul Integr Comp Physiol. 2011;300:R554–R565. doi: 10.1152/ajpregu.00610.2010. [DOI] [PubMed] [Google Scholar]
- 85.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 86.Levin BE, Magnan C, Dunn-Meynell A, et al. Metabolic sensing and the brain: who, what, where, and how? Endocrinology. 2011;152:2552–2557. doi: 10.1210/en.2011-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Luquet S, Perez FA, Hnasko TS, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 88.Krashes MJ, Koda S, Ye C, et al. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Berridge KC. Food reward: brain substrates of wanting and liking. Neurosci Biobehav Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- 91.Lucas LR, Pompei P, McEwen BS. Correlates of deoxycorticosterone-induced salt appetite behavior and basal ganglia neurochemistry. Ann NY Acad Sci. 1999;897:423–428. doi: 10.1111/j.1749-6632.1999.tb07912.x. [DOI] [PubMed] [Google Scholar]
- 92.Cai XJ, Evans ML, Lister CA, et al. Hypoglycemia activates orexin neurons and selectively increases hypothalamic orexin-B levels: responses inhibited by feeding and possibly mediated by the nucleus of the solitary tract. Diabetes. 2001;50:105–112. doi: 10.2337/diabetes.50.1.105. [DOI] [PubMed] [Google Scholar]
- 93.Diano S, Horvath B, Urbanski HF, et al. Fasting activates the nonhuman primate hypocretin (orexin) system and its postsynaptic targets. Endocrinology. 2003;144:3774–3778. doi: 10.1210/en.2003-0274. [DOI] [PubMed] [Google Scholar]
- 94.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 95.Cason AM, Smith RJ, Tahsili-Fahadan P, et al. Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav. 2010;100:419–428. doi: 10.1016/j.physbeh.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mellinkoff SM, Frankland M, Boyle D, et al. Relationship between serum amino acid concentration and fluctuations in appetite. J Appl Physiol. 1956;8:535–538. doi: 10.1152/jappl.1956.8.5.535. [DOI] [PubMed] [Google Scholar]
- 97.Choi YH, Chang N, Fletcher PJ, et al. Dietary protein content affects the profiles of extracellular amino acids in the medial preoptic area of freely moving rats. Life Sci. 2000;66:1105–1118. doi: 10.1016/s0024-3205(00)00414-8. [DOI] [PubMed] [Google Scholar]
- 98.Hawkins RA, O’Kane RL, Simpson IA, et al. Structure of the blood-brain barrier and its role in the transport of amino acids. J Nutr. 2006;136:218S–226S. doi: 10.1093/jn/136.1.218S. [DOI] [PubMed] [Google Scholar]
- 99.Maurin AC, Jousse C, Averous J, et al. The GCN2 kinase biases feeding behavior to maintain amino acid homeostasis in omnivores. Cell Metab. 2005;1:273–277. doi: 10.1016/j.cmet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Russell MC, Koehnle TJ, Barrett JA, et al. The rapid anorectic response to a threonine imbalanced diet is decreased by injection of threonine into the anterior piriform cortex of rats. Nutr Neurosci. 2003;6:247–251. doi: 10.1080/1028415031000151567. [DOI] [PubMed] [Google Scholar]
- 101.Leung PM, Rogers QR. Importance of prepyriform cortex in food-intake response of rats to amino acids. Am J Physiol. 1971;221:929–935. doi: 10.1152/ajplegacy.1971.221.3.929. [DOI] [PubMed] [Google Scholar]
- 102.Blouet C, Jo YH, Li X, et al. Mediobasal hypothalamic leucine sensing regulates food intake through activation of a hypothalamus-brainstem circuit. J Neurosci. 2009;29:8302–8311. doi: 10.1523/JNEUROSCI.1668-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cota D, Proulx K, Smith KA, et al. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 104.Morrison CD, Xi X, White CL, et al. Amino acids inhibit Agrp gene expression via an mTOR-dependent mechanism. Am J Physiol Endocrinol Metab. 2007;293:E165–E171. doi: 10.1152/ajpendo.00675.2006.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ropelle ER, Pauli JR, Fernandes MF, et al. A central role for neuronal AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) in high-protein diet-induced weight loss. Diabetes. 2008;57:594–605. doi: 10.2337/db07-0573. [DOI] [PubMed] [Google Scholar]
- 106.Morrison CD, White CL, Wang Z, et al. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Purpera MN, Shen L, Taghavi M, et al. Impaired branched chain amino acid metabolism alters feeding behavior and increases orexigenic neuropeptide expression in the hypothalamus. J Endocrinol. 2012;212:85–94. doi: 10.1530/JOE-11-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Newgard CB, An J, Bain JR, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9:311–326. doi: 10.1016/j.cmet.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Blevins JE, Truong BG, Gietzen DW. NMDA receptor function within the anterior piriform cortex and lateral hypothalamus in rats on the control of intake of amino acid-deficient diets. Brain Res. 2004;1019:124–133. doi: 10.1016/j.brainres.2004.05.089. [DOI] [PubMed] [Google Scholar]
- 110.Elias CF, Saper CB, Maratos-Flier E, et al. Chemically defined projections linking the mediobasal hypothalamus and the lateral hypothalamic area. J Comp Neurol. 1998;402:442–459. [PubMed] [Google Scholar]
- 111.Berthoud HR, Mogenson GJ. Ingestive behavior after intracerebral and intracerebroventricular infusions of glucose and 2-deoxy-D-glucose. Am J Physiol. 1997;233:R127–R133. doi: 10.1152/ajpregu.1977.233.3.R127. [DOI] [PubMed] [Google Scholar]
- 112.Bady I, Marty N, Dallaporta M, et al. Evidence from glut2-null mice that glucose is a critical physiological regulator of feeding. Diabetes. 2006;55:988–995. doi: 10.2337/diabetes.55.04.06.db05-1386. [DOI] [PubMed] [Google Scholar]
- 113.Woods SC, Lotter EC, McKay LD, et al. Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- 114.Chavez M, Riedy CA, Van Dijk G, et al. Central insulin and macronutrient intake in the rat. Am J Physiol. 1996;271:R727–R731. doi: 10.1152/ajpregu.1996.271.3.R727. [DOI] [PubMed] [Google Scholar]
- 115.Lenoir M, Serre F, Cantin L, et al. Intense sweetness surpasses cocaine reward. PLoS ONE. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hajnal A, Smith GP, Norgren R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am J Physiol Regul Integr Comp Physiol. 2004;286:R31–R37. doi: 10.1152/ajpregu.00282.2003. [DOI] [PubMed] [Google Scholar]
- 117.Sclafani A, Touzani K, Bodnar RJ. Dopamine and learned food preferences. Physiol Behav. 2011;104:64–68. doi: 10.1016/j.physbeh.2011.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tempel DL, Leibowitz KJ, Leibowitz SF. Effects of PVN galanin on macronutrient selection. Peptides. 1988;9:309–314. doi: 10.1016/0196-9781(88)90265-3. [DOI] [PubMed] [Google Scholar]
- 119.Clegg DJ, Air EL, Woods SC, et al. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology. 2002;143:2995–3000. doi: 10.1210/endo.143.8.8977. [DOI] [PubMed] [Google Scholar]
- 120.Wortley KE, Chang GQ, Davydova Z, et al. Peptides that regulate food intake: orexin gene expression is increased during states of hypertriglyceridemia. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1454–R1465. doi: 10.1152/ajpregu.00286.2002. [DOI] [PubMed] [Google Scholar]
- 121.Chang GQ, Karatayev O, Ahsan R, et al. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: role of circulating triglycerides. Am J Physiol Endocrinol Metab. 2007;292:E561–E570. doi: 10.1152/ajpendo.00087.2006. [DOI] [PubMed] [Google Scholar]
- 122.Obici S, Feng Z, Morgan K, et al. Central administration of oleic acid inhibits glucose production and food intake. Diabetes. 2002;51:271–275. doi: 10.2337/diabetes.51.2.271. [DOI] [PubMed] [Google Scholar]