Abstract
The National Marrow Donor Program, in partnership with the American Society for Blood and Marrow Transplantation, sponsored and organized a series of symposia to identify complex issues affecting the delivery of hematopoietic cell transplantation (HCT) and to collaboratively develop options for solutions. “Hematopoietic Cell Transplantation in 2020: A System Capacity Initiative” used a deliberative process model to engage professional organizations, experts, transplant centers, and stakeholders in a national collaborative effort. Year 2 efforts emphasized data analysis and identification of innovative ideas to increase HCT system efficiency, address future capacity requirements, and ensure adequate reimbursement for HCT programs to meet the projected need for HCT. This report highlights the deliberations and recommendations of Year 2 and the associated symposium held in September 2011.
Keywords: Allogeneic transplant, Care delivery model, Reimbursement, Healthcare disparities, Workforce
INTRODUCTION
To better understand the status of existing personnel and center infrastructure needed to support hematopoietic cell transplantation (HCT) in the United States, and to address system capacity challenges that would impede the future growth of HCT, the National Marrow Donor Program (NMDP) has sponsored and organized a series of multiyear symposia to identify complex issues affecting the delivery of HCT and collaboratively develop options for solutions. “Hematopoietic Cell Transplantation in 2020: A System Capacity Initiative” (SCI), organized by the NMDP in 2009 in partnership with the American Society for Blood and Marrow Transplantation (ASBMT), uses a deliberative process model to engage professional organizations, experts, transplant centers, and stakeholders in a national collaborative effort [1]. The program, now in its third year, has identified innovative ideas to increase efficiency using current capacity, address future capacity requirements, and ensure adequate reimbursement of HCT programs to meet the current and projected need for HCT (Figure 1). Year 1 focused on needs assessments and identification of factors that would have the greatest impact on future capacity. Year 2 emphasized data analysis and the development of pilot projects to address capacity issues. Year 3 will focus on establishing metrics for program evaluation, implementing pilot demonstration projects, and disseminating findings. Deliberations and recommendations from year 1 have been published previously [2]. This report highlights the deliberations and recommendations of year 2 and the associated symposium held in September 2011. More details on the program are available online at http://www.marrow.org/SCI.
Figure 1.
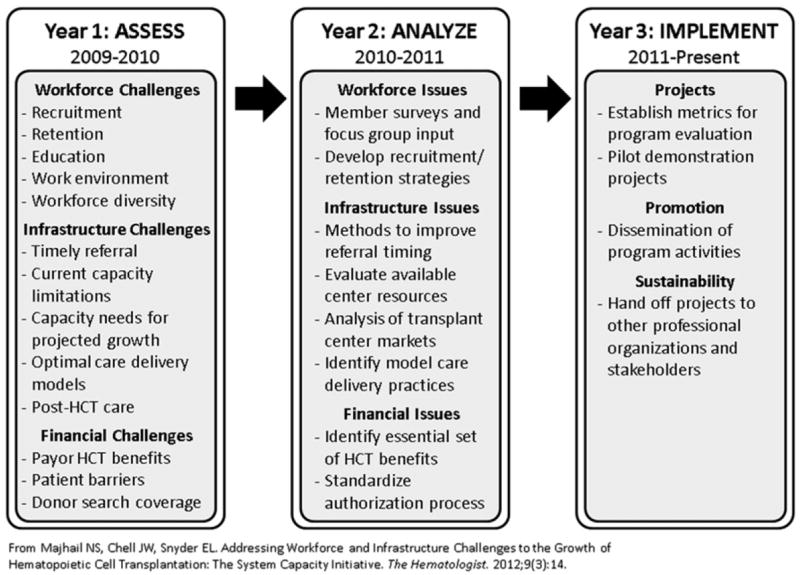
HCT in 2020: System Capacity Initiative. (Reprinted with permission. [1])
DELIBERATIVE PROCESS
Pre-Symposium Year 2
Between October 2010 and September 2011, the SCI Steering Committee, its Chair, and NMDP lead staff served to coordinate the expanded activities of the Working Groups (WGs). In year 2, the Steering Committee acknowledged the need for a Pharmacy Workforce WG. As a result of the year 1 deliberations, the Steering Committee also determined that the aims of the Facilities and Capacity and Care Delivery Model WGs overlapped, and thus combined the 2 WGs for year 2. The resulting 7 year 2 WGs were Physician Workforce, Advanced Practice Professionals (APPs) Workforce, Nursing Workforce, Pharmacy Workforce, Diversity and Health Care Disparities, Facility Capacity and Care Delivery Model, and Financial. Each WG comprised 13-20 key professionals, representatives of academic organizations, thought leaders, and stakeholders. The 7 WGs conducted their work via monthly teleconferences over the year. The goal for year 2 was to analyze data from year 1 needs assessments and collect additional data required to facilitate informed planning and implementation of recommended initiatives.
Year 2 Symposium
All WGs convened in September 2011 for a 1-1/2-day symposium in Minneapolis, Minnesota. The WGs presented progress reports and prioritized initiatives, then elicited feedback through audience polling and round table discussions. Before the symposium, the WGs analyzed initiatives to determine priorities based on (1) benefits to increasing system capacity; (2) resources needed, costs, and organizations to engage; (3) potential challenges to implementation; and (4) metrics to determine effectiveness of the initiative. Additional prioritization occurred via audience polling at the symposium, with a specific focus on rating each initiative for potential impact, role of the NMDP in implementation, and estimated time required to complete the initiative. Round table discussion notes were qualitatively analyzed for saturation of themes across the workforce WGs. Common themes regarding the challenges faced by the HCT workforce were summarized (Table 1). As follow-up to the year 1 symposium, a special presentation on ethical issues inherent in HCT addressed challenges specific to the work environment, compassion fatigue, and burnout experienced by the HCT workforce.
Table 1.
HCT Workforce Challenges: Common Themes among System Capacity Initiative Workforce Working Groups
Recruitment
|
Retention
|
Diversity
|
PRIORITIZED INITIATIVES BY WORKING GROUP
Here we describe the initiatives for each WG that received the highest priority ratings by symposium attendees (Figure 2).
Figure 2.
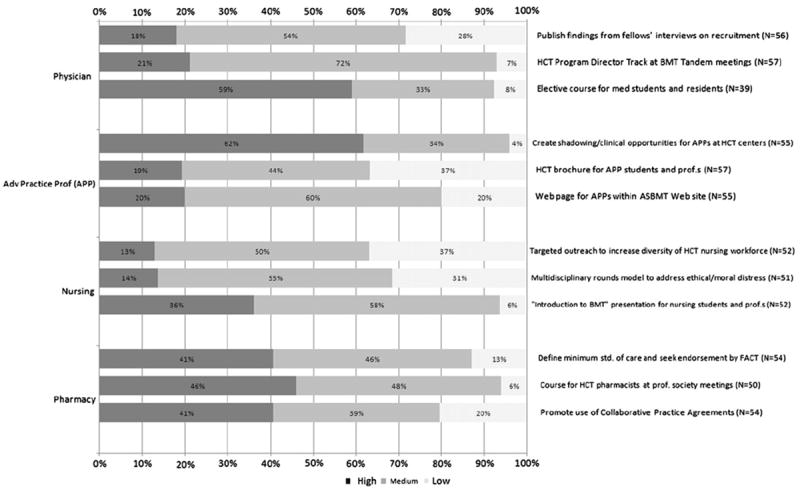
System Capacity Initiative workforce recommendations: impact on capacity.
Physician Workforce
-
Develop and implement elective course for fourth-year medical school students and first/second-year residents.
Findings from group interviews of fellows conducted during year 1 underscored the importance of early exposure to HCT in physicians’ career paths. The course will provide earlier exposure to HCT as a career and is intended to positively influence career paths. Key components of the rotation will expose the learner to outpatient HCT care, survivorship issues, decision making processes, the intellectual stimulation provided by an HCT career, the science related to HCT, and mentorship opportunities.
-
Develop and implement an HCT Program Directors’ track at BMT Tandem Meetings.
This forum would provide a resource for education on and discussion of issues related to fellow training in HCT. An inaugural meeting was organized as part of the 2012 BMT Tandem Meetings.
-
Disseminate results of group interviews on workforce, recruitment, and retention conducted with Fellowship Program Directors and fellows.
The group interviews and needs assessment surveys were very informative. Key findings show that earlier exposure to HCT and mentoring are critical, work/life balance is an important consideration for a career in HCT, and training/curriculum needs improvement and standardization. Results from the Transplant Provider and Center Factors Survey, currently in the field, will direct initiatives to address these findings. Wider dissemination of results will inform the broader HCT community of barriers to physician recruitment and retention.
Advanced Practice Professionals Workforce
-
Develop a Web page for advanced practice professionals (APPs) within the ASBMT Web site.
The goal of this initiative is to centralize HCT resources for APPs and others interested in the profession. This Web site will increase visibility of job postings, education resources, student rotations, and networking opportunities. It will serve as a nationally recognized resource for transplant center staff (eg, HCT program directors, physicians, administrators) and help build collaboration and maintain effective relationships between HCT APPs and physicians.
-
Create and disseminate a brochure for APPs that promotes career options in HCT.
The APP WG has devoted a significant amount of time to developing recommendations for the Optimal Work Model, specifically defining the role of APPs within the HCT care team. The Optimal Work Model brochure is intended to assist established and student APPs considering entering the field, as well as APPs currently practicing in HCT or other disciplines, in learning more about how to best use APPs in HCT. Highlights from the brochure include the following important topics: description of APPs’ contribution to the HCT care team, work hours and schedules, multi-disciplinary support staff, patient care models, salary and compensation, and professional development and nonclinical APP support.
-
Engage HCT programs to increase the number of shadowing and clinical rotation opportunities for APPs.
The WG assessed the APP student education initiative, HCT 101 lecture, piloted by the WG in year 2. The pilot was designed to promote recruitment of APP students and APPs practicing in non-HCT fields to a career in HCT (n = 49). Preliminary data showed that 43% of the students were not previously familiar with the HCT profession, and that after the lecture, 37% were “likely or very likely to pursue a career in HCT.” The long-term vision is to make educational opportunities such as this available through the ASBMT APP Web site.
Nursing Workforce
-
Develop the “Introduction to Blood and Marrow Transplantation (BMT)” presentation.
This initiative focused on outreach to senior students in accredited nursing programs. The presentation and learning objectives highlight HCT as a career option for nursing students as well as nurses not currently practicing within the HCT specialty.
-
Develop a program to address ethical/moral distress.
Year 1 survey results identified moral and ethical distress and compassion fatigue as major issues among nurses. Professional caregivers experience anxiety, frustration, and pressure related to their work and often have no outlets to discuss these experiences or difficult patient care communication issues with their healthcare professional peers [3]. One approach to addressing these issues is implementing multidisciplinary rounds (eg, Schwartz Center rounds, ethics rounds) to provide an opportunity to discuss ethical concerns or share feelings about cases relating to local/national issues.
An exploratory session on ethical case studies was held during the year 2 symposium. A multidisciplinary subgroup was tasked with developing recommendations for discussing moral and ethical issues related to specific points of care in the HCT continuum, from preparation for HCT to survivorship.
-
Increase the diversity of the HCT nursing workforce.
The WG plans to make outreach to culturally diverse nursing associations and conferences (eg, International Cultural Cancer Conference, National Coalition of Ethnic and Minority Nurse Associations, National Black Nurses Association) to expose nurses from diverse communities to HCT as a career path option. Strategies to successfully engage these nurses include disseminating culturally appropriate information on how a HCT nursing workforce interested in and qualified to serve diverse and underserved populations can increase access to transplant.
Pharmacy Workforce
-
Provide training opportunities for pharmacists.
The WG recommended development of training courses for HCT pharmacists to be offered at professional meetings and/or online. These courses would highlight career opportunities within HCT and aim to expand the knowledge base and improve the skill set of HCT pharmacists. TheWG will create a full-day HCT course for new pharmacists, intermediate pharmacists, and resident/seasoned pharmacists.
-
Define a minimum standard of pharmaceutical care.
The level of pharmaceutical care provided for patients at transplant centers across the United States (both inpatient and outpatient) varies widely. A defined minimum standard of pharmaceutical care, endorsed by accreditation organizations (eg, Foundation for the Accreditation of Cellular Therapy), would provide a staffing benchmark and improve consistency of care nationally. Centers would be required to adhere to this benchmark to obtain and maintain accreditation.
-
Develop and use collaborative practice agreements (CPAs).
The intent of CPAs is to authorize the pharmacist to work in a collaborative fashion with the physician. CPAs set forth guidelines for collaboration between the physician and pharmacist. Through this initiative, the WG will (1) identify transplant centers that currently use CPA; (2) arrange for presentations at the upcoming ASBMT/Center for International Blood and Marrow Transplant Research (CIBMTR) BMT Tandem Meetings to provide sample agreements, instructions on how to implement CPAs, and best practices; (3) publish evaluation outcomes from transplant centers that successfully use CPA; (4) encourage transplant centers to develop goals of pharmaceutical therapy and a CPA; (5) formally recommend that CPAs be evidence-based and include provisions for management of acute exacerbations of disease; and (6) pilot the development and use of CPAs.
Finance
The Financial WG acknowledged that health insurance coverage and benefits for transplantation remain highly variable and often lack components important to the patient undergoing HCT. Initiatives for the Financial WG included the following:
-
Disseminate WG products and resources to facilitate care of transplant recipients.
Year 1 efforts focused on (1) development of a set of model health insurance benefits that should be available to all patients undergoing HCT, including coverage for the actual transplantation benefit, search and procurement benefits, travel, lodging, clinical trial coverage, and prescription medications, and (2) generation of a standard authorization form that transplant centers can submit to all payors to request approval for search and transplantation on behalf of patients (accessible online at http://marrow.org/HD/Payor/Transplant_Reimbursement_Resource_Center.aspx). In year 2, strategies were activated to distribute these materials to key audiences, such as small health plans, self-funded accounts, reinsurers, professional payor societies, benefit consultants, healthcare professionals, transplant networks, and HCT program administrators.
-
Assist transplant centers by clarifying and expanding medical billing codes for HCT-associated procedures.
It was acknowledged that available codes for transplantation are ambiguous and not comprehensive. A series of recommendations were identified to promote consistency in coding HCT-associated procedures (eg, search and procurement of a marrowproduct) and to clarify existing codes, such as rewording the Current Procedural Terminology code definition for donor leukocyte infusion. The WG determined that it was critical to proactively review International Statistical Classification of Diseases and Related Health Problems, 10th Revision procedural codes and request appropriate new codes as the HCT and cellular therapy fields evolve.
-
Continue to support the development of standard coverage indications for HCT through the Health Resources and Services Administration Advisory Council on Blood and Stem Cell Transplantation.
This effort was recognized as falling outside the primary scope of effort of the Financial WG; however, multiple members of the WG were involved in this Health Resources and Services Administration effort. The standard coverage indications for HCT initiative were deemed critical and central to the HCT field, and provide payors with important evidence-based guidelines justifying ongoing support for HCT patients.
Facility Capacity and Care Delivery Model
The following prioritized initiatives focused on assessing market potential and reducing late referral for HCT through increased engagement with referring physicians:
-
Assess transplant center market potential.
The NMDP will continue to calculate transplantation demand and unmet transplantation needs (market potential) within every US market using Surveillance Epidemiology and End Results and CIBMTR data. Thiessen polygon boundaries were constructed that grouped transplant centers into market areas serving common patient populations: adult versus pediatric and malignant versus nonmalignant diseases. An example of the resulting market areas and associated unmet needs is shown in Figure 3. These market potential assessments improve understanding of individual market barriers and will assist transplant centers in planning to meet the demand for HCT within their market. The WG will develop strategies to disseminate the data to individual transplant centers.
-
Improve referral timing, rates, and process effectiveness.
The WG will continue to advise the NMDP Medical Education staff on their initiatives to (1) increase engagement with US physicians who refer patients for transplantation; (2) identify transplant centers that have the highest unmet needs of transplant recipients by increasing local education outreach to referring physicians; (3) implement a new continuing medical education series focusing on increasing awareness of the appropriate application of transplantation, patient eligibility, and the importance of timely planning; and (4) develop and assess a resource based on published posttransplantation care consensus guidelines [4] for referring physicians.
The WG plans to explore the possibility of partnering with transplant centers and other organizations (eg, Foundation for the Accreditation of Cellular Therapy) to address the need for posttransplantation care plans. This initiative will strengthen transplant centers’ relationships with physicians providing posttransplantation care. The WG will continue to characterize model care delivery practices through NMDP network transplant center site visits. Model practices will guide efforts to better reach market potential for network transplant centers.
Figure 3.
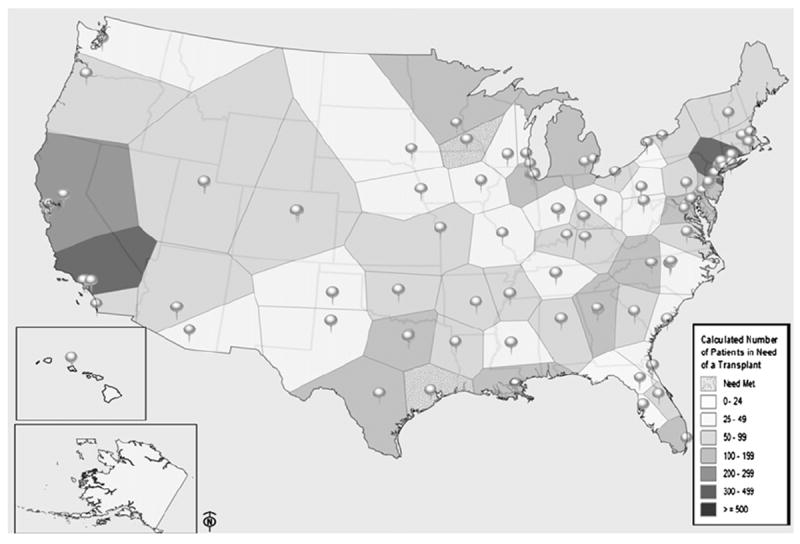
Unmet need for allogeneic HCT in US transplant center market areas. The figure shows adult patients (age 20-54, all diagnoses) within each market area who potentially needed HCT and did not receive one. Each market area is defined by a Thiessen polygon, in which boundaries are defined by the perpendicular bisectors of the lines connecting the location of adjacent transplant centers.
Diversity and Health Care Disparities Advisory Group
In collaboration with other WGs, this WG ensured that issues related to diversity and healthcare disparities were considered in year 2 deliberations. Specific recommendations of this WG include the following:
-
Analysis of US allogeneic HCT markets for minority and underserved populations.
This initiative will build on the Facilities Capacity and Care Delivery Model WG market assessment project to map out underserved allogeneic HCT markets in the United States and will inform policy on patient and transplant center characteristics within those markets that may impede access to HCT.
-
Identify funding for short-term education programs to increase HCT workforce diversity.
Needs assessment survey findings suggested that early exposure to the field of HCT plays a crucial role in the decision making process for medical and other health professional students. The creation of short-term health professional education programs before, for example, medical and nursing school presents an opportunity to mentor high-potential, diverse students earlier in their academic careers.
-
Provide expertise on diversity- and health disparity–focused activities.
The WG will continue to provide expertise on SCI activities that may potentially improve access to HCT among previously underrepresented groups. For example, the WG supports the efforts of the Nursing Workforce WG to recruit nurses from diverse communities to the HCT field by providing culturally appropriate language and identifying community-specific needs for outreach efforts. In addition, the WG will continue to assess the impact of factors that contribute to issues of diversity and healthcare disparities (eg, socioeconomic status) through the Diversity Metrics Project. This project, implemented in 2011, aims to describe and report on key diversity and healthcare disparity measures within transplant and donor recruitment. The continuation of this project through year 3 will direct refinements in data collection methods, analysis, and program recommendations to key stakeholder audiences.
IMPACT
The data collection and analysis from year 2 will be used to inform implementation strategies and activities in year 3. To ensure appropriate stewardship of the SCI issues going forward, strategic activities will be aimed at securing partner and stakeholder engagement, determining the role of partnerships within each initiative, resolving barriers to implementation, and evaluating progress of the priority initiatives adopted. Areas for future HCT-related health services and health policy research identified during the year 2 deliberations include analyzing HCT market potential, evaluating the effectiveness of BMT education and outreach to students/fellows, and establishing transplant center benchmarks for structure, staffing, and care delivery models. To implement this research agenda, focusing on its translation to practice and care delivery, it will be critical to sustain existing collaborations as well as create new partnerships with HCT-focused organizations, healthcare professionals, and academic institutions.
Acknowledgments
We thank the NMDP Board of Directors, the Patient Services/CIBMTR Health Services Research program, other NMDP staff, and professional societies that helped with this effort. We also acknowledge the participation of the Steering Committee and Working Group members listed in Appendix 1.
Financial disclosure: Funding for this conference was made possible (in part) by grant 1R13HL110705-01 from the National Institutes of Health, Yale School of Medicine, Yale University and the National Marrow Donor Program. The views expressed in written conference materials or publications and by speakers and moderators do not necessarily reflect the official policies of the Department of Health and Human Services, nor does the mention of trade names, commercial practices, or organizations imply endorsement by the US Government.
APPENDIX 1. STEERING COMMITTEE AND WORKING GROUP MEMBERS
STEERING COMMITTEE
Chair: Edward L. Snyder, MD, Yale University/Yale–New Haven Hospital; Past Chair, NMDP Board of Directors
Robert Baitty, U.S. Department of Health and Human Services, Health Resources and Services Administration, Division of Transplantation
Michael Boo, JD, NMDP
Arthur Bracey, MD, St Luke’s Episcopal Hospital
Linda Burns, MD, University of Minnesota Medical Center, Fairview
Jeffrey Chell, MD, NMDP, CIBMTR
Dennis Confer, MD, NMDP, CIBMTR
Thomas L. Joseph, MPS, CAE, ASBMT
Helen Leather, BPharm, University of Florida
Navneet S. Majhail, MD, MS, NMDP, CIBMTR
Richard T. Maziarz, MD, Knight Cancer Institute, Oregon Health & Science University
Elizabeth Murphy, EdD, RN, NMDP
Joyce Neumann, RN, MSN, AOCN, University of Texas M.D. Anderson Cancer Center
J. Douglas Rizzo, MD, MS, Medical College of Wisconsin
Barry A. Schatz, Cardinal Bernardin Cancer Center, Loyola University Medical Center
Shelley Tims Grant, US Department of Health and Human Services, Health Resources and Services Administration, Division of Transplantation
Deborah S. Yolin Raley, PA-C, Dana-Farber Cancer Institute
Lead Staff
Stacy Stickney Ferguson, MSW, LICSW, NMDP
Joan Jarosh, NMDP
WORKING GROUP MEMBERS
Physician Workforce Working Group
Chair: Linda Burns, MD, University of Minnesota Medical Center, Fairview
Vice Chair: Claudio Anasetti, MD, H. Lee Moffitt Cancer Center and Research Institute, ASBMT
Nancy Bunin, MD, Children’s Hospital of Philadelphia
Christopher Flowers, MD, The Emory Clinic
James Gajewski, MD, Oregon Health & Science University
Thomas L. Joseph, MPS, CAE, ASBMT
Mark Litzow, MD, Mayo Clinic Rochester
Navneet S. Majhail, MD, MS, NMDP, CIBMTR
Edgar L. Milford, MD, Brigham and Women’s Hospital
Jeffrey Schriber, MD, Banner Good Samaritan Medical Center
George Selby, MD, University of Oklahoma Medical Center and the Children’s Hospital
Shalini Shenoy, MD, St Louis Children’s Hospital
Ann Woolfrey, MD, Seattle Cancer Care Alliance
Lead Staff
Darlene Haven, NMDP
Kerri Heinen, MT, CHTC, NMDP Tia Houseman, NMDP
Willis Navarro, MD, NMDP
Advanced Practice Professionals Workforce Working Group
Chair: Deborah Yolin Raley, PA-C, Dana-Farber Cancer Institute
Vice Chair: Erin Medoff, APRN, Yale University/Yale–New Haven Hospital
Gilbert Ciocci, NSN, FNP-BC, Duke University Medical Center
Theresa Donohue, PA-C, Hematology Branch, National Heart, Lung and Blood Institute, National Institutes of Health
Julia Gourde, RN, CNP, Mayo Clinic Rochester
Amy Joyce, MSN, ANP, AOCN, Dana-Farber Cancer Institute
Shelly Mentzer, MMS, PA-C, Fred Hutchinson Cancer Research Center
Eneida Nemecek, MD, Doernbecher Children’s Hospital; Oregon Health & Science University
Stephany Rodriguez, RN, MS, NP, University of California San Francisco
Nancy Shreve, NP, MS, FNP, Children’s Mercy Hospital
Susan Slater, MN, FNP-BC, Oregon Health & Science University
Kristen Sterling, APRN-BC, Medical College of Georgia
Mary Thoma, PA-C, Mayo Clinic Rochester
Cindy Treviño, ANP-BC, AOCN, University of Texas M.D. Anderson Cancer Center
Michael Wilson, PA-C, H. Lee Moffitt Cancer Center & Research Institute
Lead Staff
Megan Cooper, CHTC, NMDP
Jason Dehn, MPH, CHTC, NMDP
Joan Jarosh, NMDP
Nursing Workforce Working Group
Chair: Joyce Neumann, RN, MSN, AOCN, University of Texas M.D. Anderson Cancer Center
Vice Chair: Kim Schmit-Pokorny, RN, MSN, OCN, University of Nebraska Medical Center
Dennis Confer, MD, NMDP, CIBMTR
Lourine Davis, RN, BSN, OCN, University of Texas M.D. Anderson Cancer Center
Rosemary C. Ford, RN, BSN, OCN, Seattle Cancer Care Alliance
Mutsuko Holiman, RN, BSN, Massachusetts General Hospital
Stephanie Jardine, BSN, RN, Oncology Nursing Society
Martha Lassiter, MSN, OCN, Duke University Medical Center
Elizabeth Murphy, EdD, RN, NMDP
Elaine Z. Stenstrup, MSN, RN, ACNS-BC, AOCNS, University of Minnesota Medical Center, Fairview
Terry Sylvanus, RN, MSN, ACNS-BC, AOCN, H. Lee Moffitt Cancer Center and Research Institute
D. Kathryn Tierney, RN, PhD, Stanford University Medical Center
Lead Staff
Alexandra De Kesel Lofthus, MNM, CHTC, NMDP
Ellen Denzen, MS, NMDP
Lynn Pepple, NMDP
Pharmacy Workforce Working Group
Chair: Helen Leather, BPharm, University of Florida; Principal HLL Communications, LLC
Vice Chair: Laura Wiggins, PharmD, Shands Hospital at the University of Florida
Joseph Bubalo, PharmD, BCPS, BCOP, Oregon Health & Science University
Ashley Morris Engemann, PharmD, BCOP, Duke University Medical Center
Christopher Fausel, PharmD, BCPS, BCOP, Indiana University Simon Cancer Center
Alison M. Gulbis, PharmD, BCOP, University of Texas M.D. Anderson Cancer Center
Cindy Ippoliti, PharmD, Weill Cornell Medical Center
Tippu Khan, PharmD, BCOP, University of North Carolina Hospitals
Scott Lanum, PharmD, BCOP, University of Washington School of Pharmacy, Fred Hutchinson Cancer Center
Julie Merten, PharmD, BCPS, BCOP, Mayo Clinic Rochester
Jamie Shapiro, PharmD, BCOP, H. Lee Moffitt Cancer Center and Research Institute
Sepideh Shayani, PharmD, City of Hope Medical Center
Connie Sizemore, PharmD, Northside Hospital
Tracey Walsh-Chocolaad, PharmD, BCOP, Oncology Pharmacy Consultant
Casey Williams, PharmD, BCOP, University of Kansas Medical Center
Lead Staff
Lyndsey Aspaas, CHTC, NMDP
Susie Burke, NMDP
Pam Robinett, NMDP
Facility Capacity and Care Delivery Model Working Group
Chair: Jeffrey Chell, MD, NMDP, CIBMTR
Vice Chair: Michael Lill, MD, Cedars-Sinai Medical Center
Robert Baitty, US Department of Health and Human Services, Health Resources and Services Administration, Division of Transplantation
A. John Barrett, MD, FRCP, National Institutes of Health, National Heart, Lung, and Blood Institute, Hematology Branch
Michael Boo, JD, NMDP
Katie Bruce, PharmD, Monroe Carell Jr Children’s Hospital at Vanderbilt
Colleen Chapleau, Iowa Marrow Donor Program, University of Iowa Hospitals and Clinics
Theresa Franco, RN, MSN, Cancer Care Service Line; Nebraska Medical Center
Corina Gonzalez, MD, Georgetown University Hospital
Norm Hubbard, MBA, Seattle Cancer Care Alliance
Dennis Irwin, MD, OptumHealth Care Solutions
Shirley Johnson, RN, MS, MBA, City of Hope
Michael Jhin, NMDP Board of Directors
Marion Kalbacker, MSW, LCSW, Duke University Medical Center; Association of Pediatric Oncology Social Work
Linda Kelley, PhD, Connell O’Reilly Cell Manipulation Core Facility, Dana-Farber Cancer Institute
Kimberley Lower, CMOM, Florida Center for Cellular Therapy
Miriam Markowitz, MSc, Children’s National Medical Center
Carolyn Messner, DSW, MSW, LCSW-R, BCD, Association of Oncology Social Work, CancerCare
Robert Negrin, MD, Stanford Hospital and Clinics
Leslie Parran, RN, MS, University of Minnesota Medical Center, Fairview, ASBMT Steering Committee
Barry Schatz, Loyola University Medical Center
Pat Stiff, MD, Loyola University Medical Center
William Vaughan, MD, University of Alabama-Birmingham
Kent Walters, MBA, CMPE, University of Texas M.D. Anderson Cancer Center
Daniel Weisdorf, MD, University of Minnesota Medical Center, Fairview, CIBMTR
Yvonne Ybarra, South Texas Blood and Tissue Center
Lead Staff
Emilie Clancy, NMDP
Rachelle Plonsky, NMDP
Andrea Selleck, NMDP
Financial Working Group
Chair: Richard T. Maziarz, MD, Knight Cancer Institute, Oregon Health & Science University
Vice Chair: Richard Champlin, MD, University of Texas M.D. Anderson Cancer Center
Bonnie Anderson, FLMI, HIA, MHP, FAHM, LifeTrac Network
Peggy Appel, MHA, Northwest Marrow Transplant Program
Michael Boo, JD, NMDP
Ruth Brentari, National Transplant Services, Kaiser Permanente
Allan J. Chernov, MD, Health Care Quality and Policy, Blue Cross and Blue Shield of Texas
Mary Foote, Aetna
Krishna Komanduri, MD, University of Miami Sylvester Cancer Center
Navneet S. Majhail, MD, MS, NMDP, CIBMTR
Patricia Martin, RN, BSN, Anthem WellPoint
Philip J. McCarthy Jr, MD, Roswell Park Cancer Institute
Christopher P. Pricco, OptumHealth, Complex Medical Conditions
Michael Rabin, MBA, MPA, City of Hope National Medical Center
Deborah Rodriguez, Cigna LifeSOURCE Transplant Network, Cigna
Debbie Stenhjem, Seattle Cancer Care Alliance
Lead Staff
Stephanie Farnia, MPH, NMDP
Debbie Jacobson, NMDP
Joan Jarosh, NMDP
Aaron Schnell, NMDP
Diversity and Health Care Disparities Advisory Group
Chair: Arthur Bracey, MD, St Luke’s Episcopal Hospital
Deborah A. Abroal, Mayor’s Office of Special Projects and Community Events, New York City
Theresa M. Boyd, MD, Life South Community Blood Centers
Rex L. Crawley, PhD, Robert Morris University
Airam da Silva, MPH, Icla da Silva Foundation
Michael Jones, MBA, NMDP
Naynesh Kamani, MD, Children’s National Medical Center
Robert D. Lorentz, PhD, Be The Match Foundation
Eneida Nemecek, MD, Doernbecher Children’s Hospital, Oregon Health & Science University
Shelley Tims Grant, US Department of Health and Human Services, Health Resources and Services Administration, Division of Transplantation, Healthcare Systems Bureau
Randal K. Wada, MD, Cancer Research Center of Hawaii
Lead Staff
Martha E. Burton Santibáñez, MPA, NMDP
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Majhail NS, Chell JW, Snyder EL. Addressing workforce and infrastructure challenges to the growth of hematopoietic cell transplantation: the System Capacity Initiative. Hematologist. 2012;9:14. [Google Scholar]
- 2.Majhail NS, Murphy EA, Denzen EM, et al. The National Marrow Donor Program’s Symposium on Hematopoietic Cell Transplantation in 2020: a health care resource and infrastructure assessment. Biol Blood Marrow Transplant. 2012;18:172–182. doi: 10.1016/j.bbmt.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz Center for Compassionate Healthcare. Schwartz Center rounds. [July 31, 2012]; Available from: http://www.theschwartzcenter.org/ViewPage.aspx?pageId=20.
- 4.Majhail NS, Rizzo JD, Lee SJ, et al. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2012;18:348–371. doi: 10.1016/j.bbmt.2011.12.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


