Abstract
Intracellular Ca2+ mobilization is a useful readout to screen for agonists or antagonists of G-protein coupled receptors (GPCRs). Here, we describe methods to conduct high-throughput screening of stably or transiently transfected HTC4 cells expressing the individual S1P1–5 receptor subtypes. The cells are grown in 96-well plates and loaded with the cell permeable fluorescent Ca2+ indicator dye Fura-2-AM. Changes in intracellular Ca2+ levels in response to S1P or test compounds are detected using a FlexStation II scanning fluorometer with integrated fluidics transfer capabilities.
Keywords: Calcium assay, G-protein coupled receptor, Sphingosine-1-phosphate, Lysophospholipid, FlexStation, EDG receptor
1. Introduction
The 7-transmembrane, G-protein coupled receptor (GPCR) superfamily is the largest family of membrane proteins and a major focus of drug discovery (1). Upon activation, GPCRs undergo a conformational change that results in activation of associated heterotrimeric G-proteins at the cytoplasmic face of the cell membrane composed of Gα, Gβ, and Gγsubunits. Based on sequence homology, there are four major subclasses of Gα-proteins: Gαs, Gαi/o, Gαq/11, and Gα12/13 (2). GPCR activation results in the increase in GTPase activity and the subsequent dissociation of Gα from the Gβγ subunits, which then can interact with distinct effector proteins of several downstream signaling pathways. The Gαq/11 subclass of Gα proteins is able to activate phosphoinositol phospholipase Cβ can hydrolyze phosphatidylinositol-4,5-bisphosphate (PIP2) to generate diacylglycerol and inositol-1,4,5-trisphosphate (IP3). IP3 binds endoplasmic IP3-gated Ca2+ channels, causing the release of Ca2+ from intracellular stores (3). When ectopically expressed in cells, many GPCRs can stimulate Ca2+ mobilization. The resulting changes in cytosolic Ca2+ concentrations can provide a very sensitive and robust indicator of GPCR activation in functional assays that utilize Ca2+ sensitive dyes.
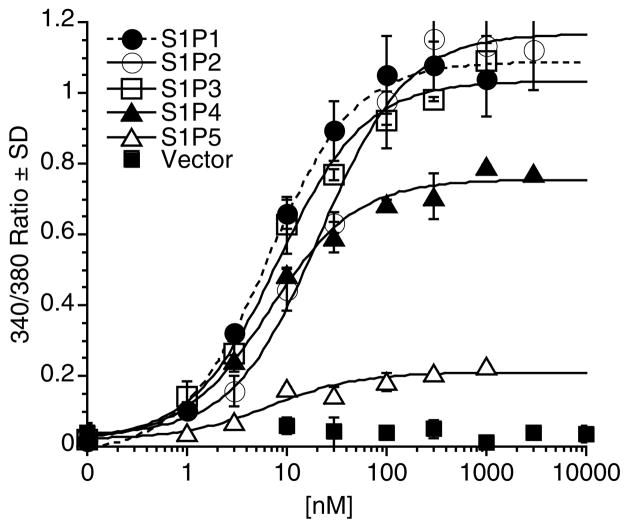
The lipid signaling molecule sphingosine-1-phosphate (S1P) functions in numerous physiological and pathophysiological conditions. Although several intracellular effects and targets have been reported (4–6), S1P exerts many of its biological effects by acting as a high affinity ligand for five highly homologous GPCRs, S1P1–5 (7, 8). The S1P receptor modulator FTY720 has been approved as frontline treatment for relapsing/remitting multiple sclerosis, establishing the S1P receptors as proven drug targets in human medicine. Our laboratory is interested in developing receptor subtype selective reagents and has extensive experience in screening compounds for activity on individual S1P receptor subtypes using Ca2+ mobilization assays. For most screening assays, we use cells stably expressing a receptor subtype; however, for mutagenesis studies we may perform Ca2+ mobilization assays using cells that have been transiently transfected with a receptor construct. The HTC4 rat hepatoma cell line lacks an endogenous Ca2+ mobilization response to S1P and thus is a suitable host cell line for measuring Ca2+ responses of either transiently or stably expressed S1P receptors (9) (Fig. 1).
Fig. 1.
S1P receptor activation by S1P. Intracellular Ca2+ mobilization in response to S1P was measured in HTC4 cells stably expressing individual S1P receptor subtypes (S1P1–4) or vector, or in HTC4 cells transiently transfected with S1P5 and Gαqz5. Samples were run in triplicate, and the mean ± SD was plotted.
We have used transiently transfected HTC4 cells to measure Ca2+ mobilization responses mediated by several of the S1P receptor subtypes, and the responses are generally much less robust than the responses from the stable cell lines. When transiently expressing a S1P receptor subtype, we usually cotransfect a Gα protein of the Gαq/11 subclass, such as Gαq or Gα16, as these proteins can facilitate the coupling of many GPCRs to intracellular Ca2+ mobilization (10). However, some GPCRs normally couple to Gαi/o and will not utilize coexpressed Gαq/11 subclass proteins to elicit a measureable Ca2+ response. In that case, Ca2+ mobilization responses may be elicited if the receptors are coexpressed with a chimeric Gαq protein in which the last five carboxy terminus amino acids of Gαq are replaced with the corresponding Gαi/o sequence. The chimeric Gαq proteins (i.e., Gαqi5, Gαqo5, or Gαqz5) allow some GPCRs with Gαi-coupling specificity to stimulate Gαq-mediated Ca2+ mobilization (11–13). We have found that S1P1 can be coexpressed with either Gαqi5 or Gα16, and S1P2 with either Gα16 or Gαq to elicit a S1P-induced Ca2+ response. We have not tested cells that transiently expressed S1P3 or S1P4 receptors for Ca2+ responses. For cells that transiently express the S1P5 receptor, we tried several different Gα proteins and only coexpression of Gαqz5 was able to elicit a S1P-induced Ca2+ response. Even with the coexpressed Gα proteins, the transiently transfected cells generally show a much weaker response than the stable transfectants (i.e., compare the transiently transfected S1P5 response to the responses of the stable S1P1–4 cell lines to the transiently transfected S1P5 response shown in Fig. 1).
The FlexStation II scanning fluorometer with fluidics transfer capabilities can measure time-resolved changes in fluorescence and is suitable for performing intracellular Ca2+ mobilization assays in a 96-well format. When the fluorescent Ca2+ indicator dye Fura-2 binds to free Ca2+, the excitation peak of Fura-2 shifts to shorter wavelengths, but the 510-nm emission peak does not change (14). The dual excitation wavelength capability of the FlexStation II permits ratiometric measurements of Fura-2 peak emissions after excitations at 340 and 380 nm, and changes in the 340/380 ratio will reflect changes in intracellular-free Ca2+concentrations. The fluidics and ratiometric data acquisition capabilities of the FlexStation II permit time-resolved measurement of intracellular Ca2+ flux following the transfer of ligand or test compounds in GPCR functional assays. Here, we describe Ca2+ mobilization assays to detect S1P receptor activation in HTC4 cells stably or transiently expressing the individual S1P1–5 receptor subtypes. The cells are grown in 96-well plates and loaded with the cell permeable acetoxymethyl ester of Fura-2, Fura-2-AM, and changes in intracellular Ca2+ concentrations in response to S1P or test compounds are monitored using a FlexStation II scanning fluorometer.
2. Materials
2.1. Cells
2.1.1. Cell Culture
Cell growth media: Dulbecco’s modified Eagle medium supplemented with 10% (v/v) fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 2 mM glutamine.
HTC4 rat hepatoma cell line: The HTC4 cell line may be purchased for research purposes from the University of California, San Francisco, Cell Culture Facilities (product code CCLZR467). The cells are maintained in cell growth media and passaged 1:10 every 2–3 days, when they are ~70–80% confluent. For routine passaging, the cells are detached with Trypsin/EDTA; however, HyQTase cell detachment solution is used to detach the cells before replating onto assay plates.
2.1.2. Stable Cell Lines
The establishment and characterization of HTC4 cells stably transfected with S1P1–4 or pCDEF3 vector has been described previously (9, 14, 15). The cells were selected for stable expression in cell growth media supplemented with G418-sulfate (0.5 μg/ml) (see Note 1).
2.1.3. Reagents for Transient Transfections
Receptor expression plasmid, i.e., S1P5 subcloned in pCR3.1 expression plasmid.
Gα expression plasmid, i.e., Gαqz5 subcloned in pcDNA1 expression plasmid.
Effectene Transfection Reagent (Qiagen, #301425) includes Effectene, Enhancer, and Buffer EC solutions.
Opti-MEM I reduced serum medium (Invitrogen, #31985).
2.2. PLL-Coated Assay Plates
Coating the assay plates with PLL is necessary to ensure that the HTC4 cells will remain adherent to the plates throughout the assay. PLL-coated plates are also commercially available.
Tissue culture microplates: 96-well, black-walled, clearbottomed plates (Costar, #3603).
PLL solution: Add 50 ml sterile, autoclaved H2O to one 5-mg bottle of poly-L-lysine hydrobromide (Sigma, #P6282-5MG). Warm the solution in 37°C water bath and vortex to dissolve.
Add 50 μl/well of PLL solution to the 96-well assay plates using a multichannel pipette. Tap the plates to ensure that the wells are completely covered with solution and incubate at room temperature for 1 h.
Remove the solution by aspirating the wells or inverting the plates vigorously. Rinse with 200 μl/well of autoclaved H2O and allow the plates to dry in a tissue culture hood with the air blower on. The dried plates may be stored at room temperature for several months (see Note 2).
2.3. Reagents and Solutions for the Assay
HyQTase cell detachment solution (Thermo Scientific, #SV30030.01).
Krebs buffer: 120 mM NaCl, 5 mM KCl, 0.62 mM MgSO4, 1.8 mM CaCl2, 10 mM HEPES, 6 mM, glucose, pH 7.4. Weigh 7.01 g NaCl, 0.37 g KCl, 0.153 g MgSO4·7 H2O, 0.265 g CaCl2·2H2O, 2.6 g HEPES, and 1.08 g glucose, and dissolve in 900 ml H2O. Adjust the pH to 7.4 with NaOH and bring the volume to 1 L with H2O. Filter sterilize and store at 4°C.
Fura-2-AM (1 μg/μl in DMSO): Dissolve one tube (50 μg) of Fura-2-AM (Invitrogen, #F1221) in 50 μl DMSO. Warm the tube to room temperature and dissolve the solution by pipetting up and down. The Fura-2-AM solution should be reconstituted shortly before use. Once reconstituted, the Fura-2-AM solution should be protected from light. The solution may be stored at −20°C for 1 month.
10% Pluronic in DMSO: Dissolve 200 mg pluronic F-127 (Invitrogen, #P6867) in 2 ml DMSO. To dissolve the Pluronic F-127, incubate the solution in a 37°C water bath for 5–10 min and vortex. Store the solution at room temperature. If the solution appears cloudy, incubate in a 37°C water bath before use.
BSA/PBS: Charcoal-stripped BSA, 1 mM in PBS. Commercial grade BSA often contains S1P contamination. Incubation with activated charcoal removes lipid contaminants. Dissolve 1.67 g fatty acid free BSA (fraction V, Sigma, #A6003) in 25 ml PBS (calcium and magnesium free) in a 50-ml conical tube. Add 2.5 g activated charcoal (Sigma, #C-9157) and rotate at 4°C overnight. The next day, filter the solution through a 0.45-μM syringe filter and then a 0.22-μM syringe filter. Aliquot and store at −20°C.
S1P—dried aliquots: S1P (Avanti Polar Lipids, #860492P) is dissolved in methanol to a final concentration of 10 mM. 500 nmol (50 μl) aliquots are dispensed into glass vials and dried under argon gas. The dried aliquots are stored at −80°C and are stable up to 1 year.
S1P—1 mM stock solution complexed with BSA: Each 500 nmol dry S1P aliquot is reconstituted with 500 μl of BSA/PBS to make the 1 mM S1P stock solution These may be stored at 20°C for several months (see Note 3).
Test compounds: Stock solutions of test compounds are prepared similarly to the S1P stock solutions. Generally, test compounds may be dissolved or diluted in DMSO or BSA/PBS to make 10 mM (in DMSO) or 1 mM (in BSA/PBS) stock solutions.
2.4. Equipment and Supplies for the Assay
FlexStation II scanning fluorometer (Molecular Devices).
SoftMax Pro software (Molecular Devices).
FlexStation black pipette tips (Molecular Devices, #9000-0911).
Compound plates: 96-well, U bottom plates (Falcon, #353077).
PLL-coated assay plates (see Subheading 2.2).
50 ml Reagent reservoir (Corning, #4870).
Multichannel pipetter, 30–300 μl.
Repeat pipetter.
3. Methods
3.1. Transient Expression of S1P5
For this protocol, HTC4 cells are cotransfected with S1P5 and Gαqz5 using Effectene Transfection Reagent. We used a 3:1 ratio of S1P5: Gαqz5 in our transfections in order to optimize the signal to background ratio. This protocol can be adapted for HTC4 cells transiently expressing either S1P1 or S1P2 and a suitable Gα protein (see Note 4).
HTC4 cells (1.5 × 106) are plated in 6-cm dishes in 5 ml of cell growth media and incubated at 37°C in a 5% CO2 cell incubator overnight.
After 24 h, the cell growth media is replaced with 4 ml of Opti-MEM serum-free media (see Note 5).
In a 1.5-ml microcentrifuge tube, combine 150 μl EC buffer, 0.75 μg S1P5 expression plasmid, 0.25 μg Gαqz5 expression plasmid, and 8 μl Enhancer. Vortex for 1 s. Incubate at room temperature for 2–5 min.
Add 25 μl Effectene to the tube, vortex 10 s, and incubate at room temperature for 5–10 min.
Add 1 ml Opti-MEM to the tube, pipette up and down two times gently, and apply the complexes dropwise to the cells in the 6-cm dish. Swirl the dish gently to distribute the transfection complexes evenly.
Incubate the cells with the transfection complexes at 37°C in a 5% CO2 cell incubator overnight (~16 h).
The next morning, detach the cells with HyQTase cell detachment solution, and pellet by centrifugation at 200 × g for 5 min. Resuspend the cells in cell growth media at a concentration of 2.5 × 105/ml.
Dispense 100 μl/well into a PLL-coated 96-well using a repeating pipetter (25,000 cells/well). Incubate the cells for 24 h in a 5% CO2 incubator at 37°C (see Note 6).
3.2. Stably Transfected Cells
HTC4 cells stably expressing a S1P receptor subtype that are in log phase growth are detached with HyQTase cell detachment solution, pelleted by centrifugation at 200 × g for 5 min, and resuspended in cell growth media at a concentration of 2–3.5 × 105/ml. Cells are replated in PLL-coated 96-well microplates in a volume of 100 μl (20,000–35,000 cells/well), and cultured overnight in a 5% CO2 incubator at 37°C (see Note 7).
3.3. Running the Assay
The Krebs buffer used to serum starve, load the cells, and run the assay is prewarmed to 37°C in a water bath.
24 h after plating, the transiently or stably transfected cells into the PLL-coated assay plates, remove the cell growth media by inverting the plate vigorously without dislodging the adherent cells (see Note 8). Add 100 μl/well of prewarmed Krebs buffer. Incubate the cells for 2–3 h at 37°C in 5% CO2 incubator (see Note 9).
Make the Fura-2-AM/Pluronic loading solution immediately before applying it to cells: Combine 25 μl of Fura-2-AM (1 μg/μl in DMSO) with 25 μl of 10% Pluronic F-127 solution in a 50-ml tube. Pipette up and down to mix. Add 5.5 ml of prewarmed Krebs buffer and mix.
Turn on the FlexStation II instrument before loading the cells, and set the temperature setpoint to allow the reading chamber temperature to stabilize to 37°C.
Remove the Krebs buffer from the cells by inverting the plate vigorously and add 50 μl/well of the Fura-2-AM/Pluronic loading solution. Incubate the cells at 37°C for 30 min to allow uptake of the Fura-2-AM.
Prepare the test compounds for screening in Krebs buffer, and if necessary make up any serial dilutions for dose–response curves. Apply them to the compound plate (270 μl/well) (see Note 10).
Put the compound plate in the compound drawer of the FlexStation II instrument, and load tips in the tip drawer.
Remove the Fura-2-AM/Pluronic solution from the assay plate by inverting vigorously, and add 100 μl/well of pre-warmed Krebs buffer. Place the assay plate in the reading chamber of the FlexStation II, allow the plate to stabilize for 5 min, and start the assay.
3.4. FlexStation II Instrument Settings
A method should be set up in advance using the SoftMax Pro software. The parameters we used are shown in Table 1. While the plate is equilibriating in the FlexStation II, the method can be adjusted for the tips and compound plate transfers.
Table 1.
SoftMax Pro software settings
| Settings options | Data reduction | |||
|---|---|---|---|---|
| Fluorescence | Wavelength combination: !LM1/!LM2 | |||
| Bottom read | Kinetic reduction: peak | |||
|
|
||||
| Ex | Em | Cutoff* | Smoothing: moving average | |
|
|
||||
| Lm1 | 340 | 510 | 495 | (Number of points) × 3
|
| Lm2 | 380 | 510 | 495 | Limits |
|
|
||||
| Time: 70 s | Min RFU: 0 | |||
| Interval: 4.22 s | Max RFU: 35,000 | |||
| Reads: 17 | Lag time: 0 | |||
|
|
||||
| PMT: high | End time: 70
|
|||
| Reads/well: 8 | Baseline options: | |||
|
|
||||
| Compound transfer | Zero baseline: 1 point | |||
| T1: 130, 2, 50 @ 16 | ||||
|
|
||||
| Automix: Off | ||||
| Calibrate: On | ||||
| AutoRead: Off | ||||
Acknowledgments
We thank Dr. Bruce Conklin (University of California, San Francisco) for generously providing chimeric G-protein expression plasmids and Dr. Edward Goetzl (University of California, San Francisco) for the stable S1P receptor cell lines. This work was supported by NIH grant CA-092160.
Footnotes
The HTC4 cell line was originally chosen as a host cell line for S1P receptors after extensive screening for cell lines with low endogenous responses to S1P (9). Other cell lines have been engineered to stably express S1P receptors (16–18) and in some cases may be commercially available. The Ca2+ mobilization assay conditions may differ substantially for different cell lines; for instance, CHO cells require probenecid in the loading and assay buffers to inhibit dye extrusion.
We usually coat at least 10–12 plates at a time, and for convenience change the solutions by inverting the plates vigorously (to remove as much liquid as possible) over a pan in a tissue culture hood. The plates may be kept sterile throughout the coating process. Alternatively the plates may be sterilized afterwards by exposing the surfaces to be sterilized to a gemicidal ultraviolet lamp in a tissue culture hood for 20–30 min.
To dissolve dried S1P, we add the appropriate solvent (either methanol or BSA/PBS) and then sonicate for a few minutes in a sonication bath, with intermittent vortexing. The S1P stock solutions always appear somewhat cloudy; however, we find this does not affect the potency of the S1P provided we vortex the stock solution immediately before taking an aliquot.
The transiently transfected S1P1 and S1P2 receptor activation assays differ from the S1P5 assay described here in a few aspects. The S1P1 and S1P2 constructs have a FLAG-tag sequence (DYKDDDDK) fused to the N terminus of the receptor sequence and are subcloned in pCDNA3.1 plasmid. For S1P1, a 1:1 ratio of receptor to Gαqi5 or Gα16 is used for transfection, and for S1P2, a 1:1 ratio of receptor to Gαq or Gα16 is used. 40,000 cells/well are replated into the PLL-coated assay plate, and the serum deprivation time is 3.5–4 h. The assays are otherwise similar to the S1P5 receptor activation assay.
In our experience, it is critical to use Opti-MEM rather than normal cell growth media during the transfection.
Whereas the stable cells generally give a very robust signal, the timing of the transient transfection and replating of the cells is critical in order to obtain a robust signal. For the assays utilizing the transiently transfected cells, the cells are plated on Day 1 in the evening, and transfection complexes are applied to the cells 24 h later (Day 2—evening). 16 h after transfection, the cells are replated (Day 3—morning), and serum deprivation of the cells in preparation for the Ca2+ mobilization assays is started 24 h later (Day 4—morning).
For all assays (both transient transfections and stable cell lines), cells are plated so as to be 75–90% confluent at the time of the assay. We find the number of cells to plate differs for HTC4 cells that express the different receptor subtypes. For the stably transfected cells expressing the individual S1P receptor subtypes, we plate: S1P1—20,000 cells/well, S1P2—25,000 cells/well, S1P3—35,000 cells/well, S1P4—30,000 cells/well, vector—30,000 cells/well.
Inverting the plate vigorously over a suitable waste receptacle is an efficient method to remove the media. The cells do not need to be kept sterile at this point, but care must be taken to not lose too many adherent cells during changing the media. Loss of cells during the assay can be monitored by checking the cells under a microscope periodically.
Serum contains many growth factors including S1P and LPA; therefore, incubating the cells in the absence of serum is critical for a robust ligand response. The incubation in Krebs buffer serves to deprive the cells of serum. Depending on the receptor, the optimal serum deprivation time may range from 2 to 5 h.
We set up the compound concentrations in the compound plate at 3× the final concentration, so that 50 μl of compound can be dispensed into assay plate wells that contain cells in 100 μl of buffer, to achieve the final 1× compound concentration. We dispense 270 μl of 3× compound into each well of the U bottom 96-well compound plate so that 50 μl volumes can be dispensed to either triplicate or quadruplicate wells of the assay plate (~50 μl/well of compound plate is dead-volume).
References
- 1.Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 2.Offermanns S, Simon MI. Genetic analysis of mammalian G-protein signalling. Oncogene. 1998;17:1375–1381. doi: 10.1038/sj.onc.1202173. [DOI] [PubMed] [Google Scholar]
- 3.Singer WD, Brown HA, Sternweis PC. Regulation of eukaryotic phosphatidylinositol-specific phospholipase C and phospholipase D. Annu Rev Biochem. 1997;66:475–509. doi: 10.1146/annurev.biochem.66.1.475. [DOI] [PubMed] [Google Scholar]
- 4.Kohno M, Momoi M, Oo ML, Paik JH, Lee YM, Venkataraman K, Ai Y, Ristimaki AP, Fyrst H, Sano H, Rosenberg D, Saba JD, Proia RL, Hla T. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol Cell Biol. 2006;26:7211–7223. doi: 10.1128/MCB.02341-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strub GM, Maceyka M, Hait NC, Milstien S, Spiegel S. Extracellular and intracellular actions of sphingosine-1-phosphate. Adv Exp Med Biol. 2010;688:141–155. doi: 10.1007/978-1-4419-6741-1_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hla T, Brinkmann V. Sphingosine 1-phosphate (S1P): Physiology and the effects of S1P receptor modulation. Neurology. 2011;76:S3–S8. doi: 10.1212/WNL.0b013e31820d5ec1. [DOI] [PubMed] [Google Scholar]
- 8.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: mechanistic insights from gene deletion studies and reverse pharmacology. Pharmacol Ther. 2007;115:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 9.An S, Bleu T, Zheng Y. Transduction of intracellular calcium signals through G protein-mediated activation of phospholipase C by recombinant sphingosine 1-phosphate receptors. Mol Pharmacol. 1999;55:787–794. [PubMed] [Google Scholar]
- 10.Offermanns S, Simon MI. G alpha 15 and G alpha 16 couple a wide variety of receptors to phospholipase C. J Biol Chem. 1995;270:15175–15180. doi: 10.1074/jbc.270.25.15175. [DOI] [PubMed] [Google Scholar]
- 11.Coward P, Chan SD, Wada HG, Humphries GM, Conklin BR. Chimeric G proteins allow a high-throughput signaling assay of Gi-coupled receptors. Anal Biochem. 1999;270:242–248. doi: 10.1006/abio.1999.4061. [DOI] [PubMed] [Google Scholar]
- 12.Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 13.Conklin BR, Herzmark P, Ishida S, Voyno-Yasenetskaya TA, Sun Y, Farfel Z, Bourne HR. Carboxyl-terminal mutations of Gq alpha and Gs alpha that alter the fidelity of receptor activation. Mol Pharmacol. 1996;50:885–890. [PubMed] [Google Scholar]
- 14.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 15.Graler MH, Goetzl EJ. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 2004;18:551–553. doi: 10.1096/fj.03-0910fje. [DOI] [PubMed] [Google Scholar]
- 16.Koide Y, Uemoto K, Hasegawa T, Sada T, Murakami A, Takasugi H, Sakurai A, Mochizuki N, Takahashi A, Nishida A. Pharmacophore-based design of sphingosine 1-phosphate-3 receptor antagonists that include a 3,4-dialkoxybenzophenone scaffold. J Med Chem. 2007;50:442–454. doi: 10.1021/jm060834d. [DOI] [PubMed] [Google Scholar]
- 17.Murakami A, Takasugi H, Ohnuma S, Koide Y, Sakurai A, Takeda S, Hasegawa T, Sasamori J, Konno T, Hayashi K, Watanabe Y, Mori K, Sato Y, Takahashi A, Mochizuki N, Takakura N. Sphingosine 1-phosphate (S1P) regulates vascular contraction via S1P3 receptor: investigation based on a new S1P3 receptor antagonist. Mol Pharmacol. 2010;77:704–713. doi: 10.1124/mol.109.061481. [DOI] [PubMed] [Google Scholar]
- 18.Yamazaki Y, Kon J, Sato K, Tomura H, Sato M, Yoneya T, Okazaki H, Okajima F, Ohta H. Edg-6 as a putative sphingosine 1- phosphate receptor coupling to Ca(2+) signaling pathway. Biochem Biophys Res Commun. 2000;268:583–589. doi: 10.1006/bbrc.2000.2162. [DOI] [PubMed] [Google Scholar]