FSP27 is a lipid droplet (LD) associated protein that is transcriptionally regulated by peroxisome proliferator-activated receptor (PPAR)γ during differentiation in adipocytes (1–3). FSP27 was initially identified by its upregulation in TA1 cell adipogenesis and was shown to be highly expressed in brown and white adipose tissues (4–7). Its LD association and role in fat metabolism was discovered recently (1, 2, 8). Expression of FSP27 is associated with triglyceride (TG) accumulation in various cell types (1, 2), whereas its depletion in adipocytes causes increased lipolysis both in vivo and in vitro (1–3, 9, 10). White adipocytes of FSP27-KO mice have multilocular droplets and increased lipolysis, and these animals have higher mitochondrial oxidative metabolism (3, 11). This energy expenditure in turn protects the mice from diet-induced obesity and insulin resistance. In humans, FSP27 expression is positively associated with fat accumulation in adipocytes and insulin sensitivity (9, 10). Recent studies have demonstrated that FSP27 regulates LD morphology by playing a role in fusion of lipid droplets (12, 13).
Besides adipose tissue, FSP27 is also expressed in muscle and liver (14). The very first report on the regulation of FSP27 in liver was from Reddy’s laboratory in which Yu et al. elegantly showed that PPARγ overexpression in the liver of PPARα(−/−) mice induced FSP27 among other lipogenesis-related genes (7). Later on, it was confirmed that hepatic steatosis in leptin-deficient mice is promoted by FSP27 (15). In fact, a dramatic upregulation of FSP27 transcript occurs in the liver of ob/ob mice (8). The expression of FSP27 was markedly decreased in livers lacking the nuclear receptor PPARγ. It is now clearly established that FSP27 plays an important role in hepatic steatosis in mouse models by promoting PPARγ-mediated hepatic steatosis (7, 15). Forced expression of FSP27 in hepatocytes in vitro or in vivo leads to increased LD formation through increased triglyceride levels, whereas repressed FSP27 inhibits hepatic lipid accumulation in both db/db and high-fat diet-fed mice lacking mitogen-activated protein phosphatase-1 (MKP-1) (16).
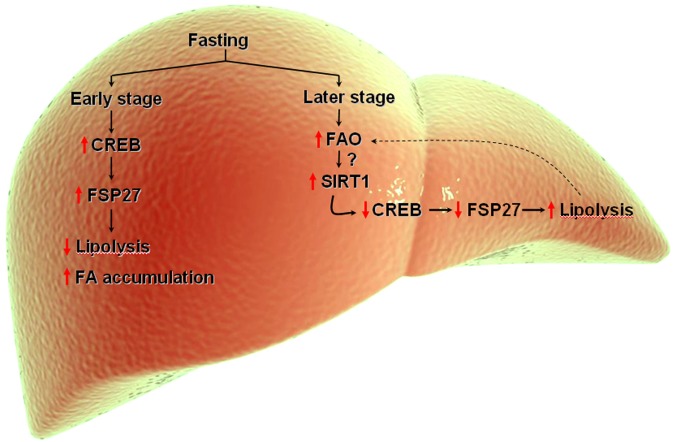
PPARγ-mediated nutritional regulation of FSP27 in white adipose tissue was recently suggested (17). PPARγ also regulates expression of FSP27 in hepatocytes (7, 15); however, the nutritional regulation of FSP27 in liver has not yet been studied. In this issue of the Journal of Lipid Research, Vila-Brau et al. (18) demonstrate that fasting regulates FSP27 in the liver of mice. They have shown that FSP27 expression in liver is regulated by fasting in a time-dependent manner. During the early stages of fasting, FSP27 expression increases, whereas it decreases at later stages. Consistent with the role of FSP27 in TG accumulation, its expression in liver would increase during early fasting to accommodate the storage of incoming fatty acids. Although not shown in the report by Vila-Brau et al. (18), it is conceivable that under the conditions used by the authors, hepatic TG levels are increased during early fasting. At a later stage of fasting, there is increased fatty acid oxidation (FAO); hence, a decrease in FSP27 expression will increase lipolysis to meet the requirement of fatty acids needed for oxidation. In fact, this whole process is consistent with the hypothesis that the FFAs coming from outside the tissue are not directly oxidized but first stored as triglycerides, which are then hydrolyzed to provide fuel to mitochondria.
Interestingly, Vila-Brau et al. found that PPARα, a master regulator of fasting in liver, is not responsible for inducing FSP27 expression (18). The authors followed a logical approach to show that FSP27 expression in the liver is regulated by cAMP-responsive element binding protein (CREB). Previously it has been shown that at the onset of fasting, hormonal changes produce an increase in both the level and activity of CREB-regulated transcript coactivator 2 (CRTC2), which induces the expression of hepatic gluconeogenic genes (19). Eighteen hours of fasting induces the activity of nutrient-sensing sirtuin 1 (SIRT1), which then deacetylates CRTC2 and promotes its downregulation (20). Overall, the studies support a model (Fig. 1) in which during early stages of fasting there is a CREB-dependent upregulation of FSP27 to accommodate the increased FA flux in the liver, whereas at a later stage, FAO would increase SIRT1 activity, perhaps by changes in NAD+/NADH, which might be responsible for decreased expression of FSP27 via suppression of CREB/CRTC2. This would cause increased lipolysis to support enhanced FAO. Indeed, FSP27 expression was increased when FAO was inhibited in liver cells, and FSP27 expression was induced to a higher level in the livers of Sirt1-LKO mice than in wild-type animals. However, the mechanism of FAO-induced SIRT1 activity remains to be determined. In addition, whether FFAs could directly regulate the expression of FSP27 in the liver remains an open question.
Fig. 1.
During early stages of fasting, FSP27 is upregulated in the liver by CREB to facilitate storage of fatty acids. At later stages of fasting, FAO is increased, which might upregulate SIRT1 activity that would inactivate and downregulate CREB, thus decreasing FSP27 expression and increasing lipolysis to release fatty acids to be fuel for FAO.
REFERENCES
- 1.Puri V., Konda S., Ranjit S., Aouadi M., Chawla A., Chouinard M., Chakladar A., Czech M. P. 2007. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J. Biol. Chem. 282: 34213–34218 [DOI] [PubMed] [Google Scholar]
- 2.Keller P., Petrie J. T., De Rose P., Gerin I., Wright W. S., Chiang S. H., Nielsen A. R., Fischer C. P., Pedersen B. K., MacDougald O. A. 2008. Fat-specific protein 27 regulates storage of triacylglycerol. J. Biol. Chem. 283: 14355–14365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., et al. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118: 2808–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Danesch U., Hoeck W., Ringold G. M. 1992. Cloning and transcriptional regulation of a novel adipocyte-specific gene, FSP27. CAAT-enhancer-binding protein (C/EBP) and C/EBP-like proteins interact with sequences required for differentiation-dependent expression. J. Biol. Chem. 267: 7185–7193 [PubMed] [Google Scholar]
- 5.Su A. I., Cooke M. P., Ching K. A., Hakak Y., Walker J. R., Wiltshire T., Orth A. P., Vega R. G., Sapinoso L. M., Moqrich A., et al. 2002. Large-scale analysis of the human and mouse transcriptomes. Proc. Natl. Acad. Sci. USA. 99: 4465–4470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams P. M., Chang D. J., Danesch U., Ringold G. M., Heller R. A. 1992. CCAAT/enhancer binding protein expression is rapidly extinguished in TA1 adipocyte cells treated with tumor necrosis factor. Mol. Endocrinol. 6: 1135–1141 [DOI] [PubMed] [Google Scholar]
- 7.Yu S., Matsusue K., Kashireddy P., Cao W. Q., Yeldandi V., Yeldandi A. V., Rao M. S., Gonzalez F. J., Reddy J. K. 2003. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 278: 498–505 [DOI] [PubMed] [Google Scholar]
- 8.Kim J. Y., Liu K., Zhou S., Tillison K., Wu Y., Smas C. M. 2008. Assessment of fat-specific protein 27 in the adipocyte lineage suggests a dual role for FSP27 in adipocyte metabolism and cell death. Am. J. Physiol. Endocrinol. Metab. 294: E654–E667 [DOI] [PubMed] [Google Scholar]
- 9.Rubio-Cabezas O., Puri V., Murano I., Saudek V., Semple R. K., Dash S., Hyden C. S., Bottomley W., Vigouroux C., Magre J., et al. 2009. Partial lipodystrophy and insulin resistant diabetes in a patient with a homozygous nonsense mutation in CIDEC. EMBO Mol. Med. 1: 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Puri V., Ranjit S., Konda S., Nicoloro S. M., Straubhaar J., Chawla A., Chouinard M., Lin C., Burkart A., Corvera S., et al. 2008. Cidea is associated with lipid droplets and insulin sensitivity in humans. Proc. Natl. Acad. Sci. USA. 105: 7833–7838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toh S. Y., Gong J., Du G., Li J. Z., Yang S., Ye J., Yao H., Zhang Y., Xue B., Li Q., et al. 2008. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS ONE. 3: e2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong J., Sun Z., Wu L., Xu W., Schieber N., Xu D., Shui G., Yang H., Parton R. G., Li P. 2011. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195: 953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jambunathan S., Yin J., Khan W., Tamori Y., Puri V. 2011. FSP27 Promotes Lipid Droplet Clustering and Then Fusion to Regulate Triglyceride Accumulation. PLoS ONE. 6: e28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puri V., Virbasius J. V., Guilherme A., Czech M. P. 2008. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol. (Oxf.). 192: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F. J. 2008. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 7: 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flach R. J., Qin H., Zhang L., Bennett A. M. 2011. Loss of mitogen-activated protein kinase phosphatase-1 protects from hepatic steatosis by repression of cell death-inducing DNA fragmentation factor A (DFFA)-like effector C (CIDEC)/fat-specific protein 27. J. Biol. Chem. 286: 22195–22202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karbowska J., Kochan Z. 2012. Intermittent fasting up-regulates Fsp27/Cidec gene expression in white adipose tissue. Nutrition. 28: 294–299 [DOI] [PubMed] [Google Scholar]
- 18.Vila-Brau A., De Sousa-Coelho A. L., Goncalves J. F., Haro D., Marrero P. F. Fs12p27/CIDEC is a CREB target gene induced during early fasting in liver and regulated by fatty acid oxidation rate. J. Lipid Res. 54: 592–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le Lay J., Tuteja G., White P., Dhir R., Ahima R., Kaestner K. H. 2009. CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 10: 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J., 3rd, et al. 2008. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 456: 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]