Abstract
Therapeutic agents that suppress apolipoprotein B (apoB) and microsomal triglyceride transfer protein (MTP) levels/activity are being developed in the clinic to benefit patients who are unable to reach target LDL-C levels with maximally tolerated lipid-lowering drugs. To compare and contrast the metabolic consequences of reducing these targets, murine-specific apoB or MTP antisense oligonucleotides (ASOs) were administered to chow-fed and high fat-fed C57BL/6 or to chow-fed and Western diet-fed LDLr−/− mice for periods ranging from 2 to 12 weeks, and detailed analyses of various factors affecting fatty acid metabolism were performed. Administration of these drugs significantly reduced target hepatic mRNA and protein, leading to similar reductions in hepatic VLDL/triglyceride secretion. MTP ASO treatment consistently led to increases in hepatic triglyceride accumulation and biomarkers of hepatotoxicity relative to apoB ASO due in part to enhanced expression of peroxisome proliferator activated receptor γ target genes and the inability to reduce hepatic fatty acid synthesis. Thus, although both drugs effectively lowered LDL-C levels in mice, the apoB ASO produced a more positive liver safety profile.
Keywords: microsomal triglyceride transfer protein, dyslipidemia, steatosis, lipoprotein metabolism, lipid droplets, familial hypercholesterolemia
Coronary heart disease is the leading cause of death in the United States (1). Although multiple risk factors associated with this disease process are being extensively investigated, it is well established that lowering plasma LDL cholesterol (LDL-C) reduces cardiovascular risk events (2). The most effective therapeutic agents for the treatment of elevated LDL-C levels and, ultimately, coronary heart disease are the statins (3). Although these drugs have proven to be potent and efficacious, a significant percentage of high-risk patients are unable to attain their ATP-III recommended LDL-C goals for several reasons, including ineffectiveness or intolerance to statins alone or in combination with other dyslipidemia agents (4, 5). These factors have led to a continued search for new drugs with unique mechanisms of action that lower plasma LDL-C levels.
Two proteins involved in the formation of apoB-containing lipoprotein particles that have recently received considerable attention as potential therapeutic targets are apolipoprotein B (apoB) and microsomal triglyceride transfer protein (MTP) (6–8). Both are essential for the assembly and secretion of the apoB-containing lipoproteins (i.e., VLDL) synthesized in the liver and chylomicrons produced by enterocytes of the small intestine. In the liver, apoB100 is the main structural protein component of VLDL and LDL particles.
Extensive biochemical studies indicate that apoB protein is primarily regulated at the post-transcriptional level (9). Initially, nascent apoB protein is transferred through the ER membrane and into the ER lumen. In the absence of sufficient lipid or functional MTP, apoB fails to be lipidated and undergoes retrograde translocation into the cytosol, where it is ubiquitinated and degraded. However, in the presence of sufficient lipid, apoB is lipidated in an MTP-dependent, two-step process, leading to the formation and secretion of a mature VLDL particle (10). VLDL then enters the circulation and is acted upon by lipolytic and lipid transfer enzymes that convert triglycerides TG-rich VLDL into cholesteryl ester (CE)-rich LDL particles. Therefore, reduction of apoB and/or MTP lowers LDL levels by decreasing the number of particles secreted into the circulation. In contrast, statins lower LDL-C principally by enhancement of LDL receptor-mediated clearance of LDL particles from the circulation (3).
Pharmacological suppression of hepatic apoB production via antisense oligonucleotide (ASO) treatment has also led to reductions in VLDL-C and LDL-C and the amelioration of atherosclerosis in murine preclinical models (11, 12). Furthermore, although a murine-specific apoB ASO reduced apoB production and secretion from the liver, hepatic steatosis and elevation of transaminases were not observed in these mice (11). In high fat-fed mice administered the apoB ASO, liver TG accumulation was shown to be reduced as a result of secondary, compensatory mechanisms that lead to reduced lipogenesis and increased fatty acid oxidation. Additionally, there were no effects on dietary fat absorption, chylomicron formation, or increases in intestinal TG accumulation due to limited ASO distribution to the small intestine.
Given the importance of MTP to VLDL production, we also wished to evaluate the effects of an inhibitor of MTP in C57BL/6 and LDLr−/− mice. However, experiments directly comparing the therapeutic reduction of apoB and MTP have not been possible because apoB is not targetable by small molecule approaches. Therefore, to perform such studies, we designed an ASO to murine MTP and directly compared its activity with that of an apoB antisense drug, ISIS 147764 (11), in preclinical pharmacology models.
MATERIALS AND METHODS
Antisense oligonucleotides
A series of uniform chimeric 20-mer phosphorothioate oligonucleotides containing 2’-O-methoxyethyl groups at positions 1–5 and 15–20 targeted to murine apoB, MTP, and a control ASO were synthesized and purified on an automated DNA synthesizer using phosphoramidite chemistry as previously described (13). The sequences evaluated were as follows: apoB ASO-ISIS 147764 (5′-GTCCCTGAAGATGTCAATGC-3′), MTP ASO-ISIS 144477 (5′-CCCAGCACCTGGTTTGCCGT-3′), and one of two control ASOs-ASO 1 (5′-AGCATAGTTAACGAGCTCCC-3′) or ASO 2 (5′-AGCATAGTTAACGAGCTCCC-3′), with underlining indicating 2’-O-methoxyethyl-modified bases.
Mice
Six week old male C57BL/6 or LDLr−/− (Jackson Laboratory) mice in cages (n = 3–5 per cage) on a 12-h light-dark cycle for the duration of the studies. All procedures and protocols were approved by an institutional animal care and use committee. C57BL/6 mice were fed chow or a high-fat diet (60% calories as fat; Research Diets D12492) (DIO model) for 3 weeks. After the diet acclimation period, mice were randomized based on TPC and nonHDL-C and then administered assigned ASOs in saline (5 mg/ml) by intraperitoneal injection at a dose of 25, 12.5, or 6.25 mg/kg twice weekly for 2 or 6 weeks. LDLr−/− mice were fed a standard chow diet or a Western diet (Harland Teklad Diet 88137) consisting of 42% of calories as fat and 0.15% cholesterol for 1 week. The mice were then bled, randomized based on TPC and nonHDL-C, and administered control ASO (50 mg/kg/week), apoB ASO (50 mg/kg/week), or MTP ASO (50 mg/kg/week) for periods ranging from 2 to 12 weeks while remaining on diet. For 12 week studies, mice received intraperitoneal injections of ASO once weekly for 6 weeks and then once every other week for the remainder of the study. Body weight was monitored throughout the study, with no significant differences between control and treated mice observed in any of the studies. At the end of treatment period, animals were fasted for 4 h and euthanized for blood and tissue analysis.
Plasma chemistry and lipoprotein cholesterol analysis
Plasma lipids and aminotransferase concentrations were quantified on an Olympus AU400e automated clinical chemistry analyzer (Melville, NY). HPLC analysis of LDLr−/− mice cholesterol distribution among VLDL-C, LDL-C, and HDL-C fractions was performed as described previously. Percent area under curve VLDL-C, LDL-C, and HDL-C was determined using Peakfit™ v 4.12 (Seasolve Software®, Framingham MA) and then multiplied by TPC to determine VLDL-C, LDL-C, and HDL-C, respectively.
Histology
Oil Red O (ORO) staining on sections of snap-frozen liver and intestinal samples was performed as described (14). For quantitation of lipid droplets, digital 10× (1,700 × 1,100 μm) images (four images per treatment group, with each image taken from a different mouse) of ORO-stained sections were analyzed with the ChromaColor™ software to identify all ORO-stained droplets. Those densitometric images were quantified using Image Pro Plus™ software, which sorted the droplets into one of four sizes: 5–50 μm2, 51–100 μm2, 101–1,000 μm2, or >1,000 μm2, with the largest size class considered to be an artifact of the staining procedure.
Western blotting
Liver homogenates were prepared and immunoblotted as described. GAPDH or tubulin was used as a protein loading control for all blots. For the quantitation of hepatic proteins, two samples from each treatment group were run on two separate blots (n = 4 samples per treatment). Western blots were visualized, and band density was analyzed using the LI-COR Odyssey™ imaging system. Fluorescence of the bands of interest were normalized to load controls and expressed as mean fold change relative to control ASO. For small intestine, a single blot was analyzed (n = 2 samples per treatment).
Tissue lipid composition
Liver TG, free cholesterol, and total cholesterol were extracted and analyzed as described previously (15). Intestinal lipid was quantitated after fasting the mice for 4–5 h. Mice were then euthanized, and the small intestine from the pyloric valve to the cecum was collected and divided into three equal pieces. The luminal contents were flushed with PBS from the proximal section, and each section was cut longitudinally with scissors and flattened, and the mucosal layer was scraped off with a glass slide. An aliquot of the cells was suspended in PBS. One-fifth of the suspension was brought to 1 N NaOH for protein determination by the Lowry method, and three-fifths of the lipid was extracted by the Bligh-Dyer method described previously. The extract was solubilized with 2% Triton ×100 in dH2O, and lipids were quantified using colorimetric assays as described for the liver lipid extraction.
MTP activity assay
Aliquots of livers isolated from DIO mice treated with ASO for 6 weeks were snap frozen and sent to Chylos® Inc. (New York, NY), where MTP activity was quantitated as described previously (16).
Quantitation of TG and cholesterol absorption
DIO mice administered ASO for 6 weeks were fasted overnight and then received an intraperitoneal injection of poloxamer 407 at a dose of 1 mg/g body weight. One hour after injection, a baseline retro-orbital bleed was collected, and the mice were orally gavaged with 2.5 μCi of 3H triolein in 200 μl of olive oil. Animals were bled 90 and 180 min after gavage, and counts per minute (cpm) values were quantified from 20 μl of serum by liquid scintillation counting (LSC Instrument; Beckman). Cholesterol absorption in DIO mice treated with ASO for 6 weeks was quantitated using the dual fecal isotope method as described previously (17).
Hepatic ApoB and TG secretion
Hepatic and apoB secretion was carried out as previously described (18).
Analysis of hepatic fatty acid synthesis
Quantitation of hepatic fatty acid synthesis was carried out using the tritiated water method described previously (19).
Quantitative real-time PCR
Real-time PCR was performed as described (11). Sequences of the primer probe sets used are provided in supplementary Table I. All data were normalized to cyclophilin A mRNA levels and were expressed as percent of hepatic mRNA expression in control ASO-treated mice.
Statistical analysis
The majority of pharmacology experiments were performed in at least two independently performed studies with groups of 4 to 12 animals. All values are expressed as mean ± SEM. To determine statistical significance, one- and two-way ANOVA analysis with Tukey's post hoc test was carried out using GraphPad Prism 5™ software with statistical significance being set at p < 0.05.
RESULTS
Second-generation ASOs targeting murine apoB (ISIS 147764), whose pharmacology has been described in detail in two previous studies (11, 12), or MTP (ISIS 144477), which in primary mouse hepatocytes reduced MTP mRNA in a dose-dependent manner (supplementary Fig. 1), were used for all studies. To compare the pharmacological effects of apoB and MTP ASOs on safety, efficacy, and hepatic steatotic progression, three mouse models were used. Initially, ASOs were administered to chow-fed C57BL/6 mice twice weekly (12.5–50 mg/kg/week) for 2 or 6 weeks to assess their efficacy and tolerability. To further investigate the effects on liver TG accumulation between apoB and MTP ASO treatment, the two compounds were administered to DIO mice, a model of hepatic steatosis, for 2 or 6 weeks. Finally, because the severe familial hypercholesterolemia patient population is the primary indication for apoB and MTP drugs, male LDLr−/− mice were fed chow or Western diets and administered ASOs at 50 mg/kg/week for 6 weeks.
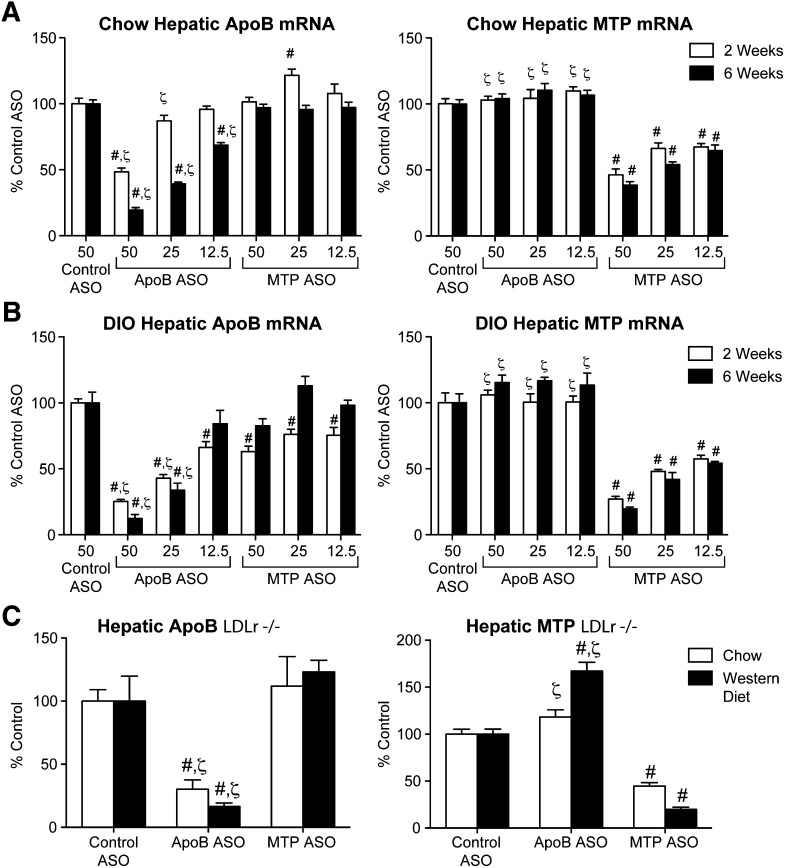
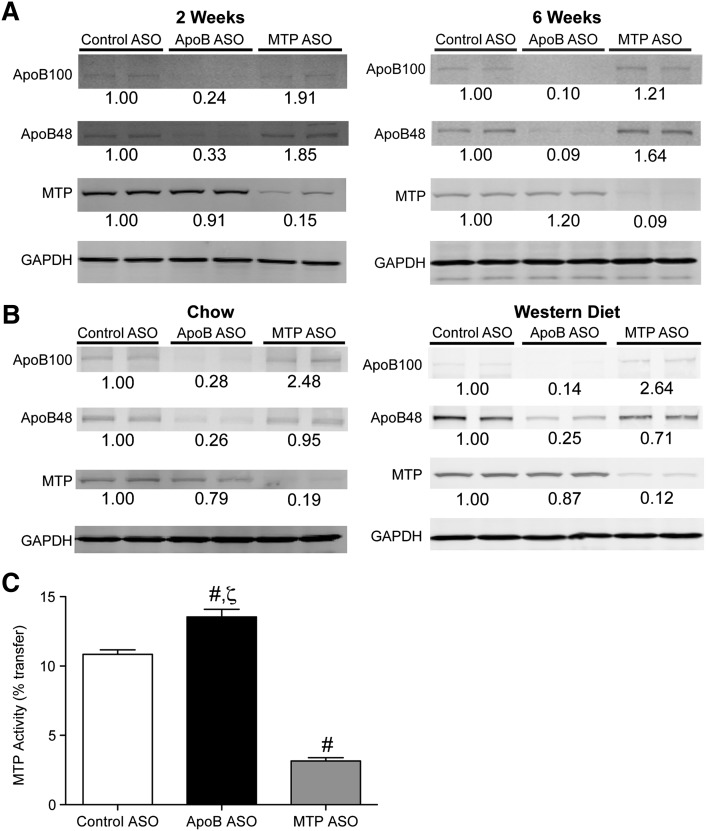
In all three mouse models, ASO administration led to significant, dose-dependent suppression of hepatic target mRNA expression, reaching 70% to 90% reduction when compared with control ASO-treated animals at the high dose after 6 weeks of treatment (Fig. 1). Consistent with the mRNA data, DIO and LDLr−/− mice treated with the apoB ASO-demonstrated reductions in hepatic target protein levels when compared with control ASO treatment (Fig. 2). The MTP ASO-treated DIO and LDLr−/− reductions in hepatic target protein were also consistent with the degree of hepatic MTP mRNA suppression. Furthermore, measurement of hepatic MTP activity in DIO mice after 6 weeks of MTP ASO treatment showed that the reduction in activity (−71%) was consistent with the hepatic MTP mRNA and total protein reductions (Fig. 2C). Administration of the apoB ASO significantly increased MTP activity by 25% when compared with control.
Fig. 1.
ApoB and MTP ASOs reduce liver target mRNA expression in three different mouse models. Chow-fed (n = 5–8 per group) (A) or DIO C57BL/6 mice (n = 4) (B) were administered ASO for 2 or 6 weeks. C: Chow-fed or Western diet-fed LDLr−/− mice were administered ASO for 6 weeks. Quantitative RT-PCR was carried out as described in Materials and Methods. Number on x axis indicates mg/kg/week dose. #Significantly different (P < 0.05) when compared with control ASO. ζSignificantly different when compared with MTP ASO treatment.
Fig. 2.
ApoB and MTP ASO administration reduced liver target protein expression in several murine models. A: DIO C57BL/6 mice were administered ASO (50 mg/kg/week) for 2 or 6 weeks. B: Chow-fed or Western diet-fed LDLr−/− were administered ASO for 6 weeks. Liver protein (50 μg) was immunoblotted with polyclonal Abs raised against apoB, MTP, or GAPDH (load control). Numbers below bands are mean density (n = 4) relative to control ASO-treated mice. C: Quantitation of hepatic MTP activity (n = 5 per group) in DIO mice administered ASO for 6 weeks. #Significantly different (p < 0.05) when compared with control ASO. ζSignificantly different when compared with MTP ASO treatment.
As expected, reduction of hepatic apoB and MTP led to significant reductions in TPC in the DIO mice and LDLr−/− mice fed chow or Western diet (Tables 1 and 2). In DIO mice (Table 1), the reductions in TPC were due to reductions in nonHDL-C and HDL-C, a plasma lipid profile that is similar to that observed in ApoB+/− or MTP+/− mice (20, 21). In addition, neither apoB nor MTP ASO had any effect on plasma TG, which was not surprising given the relatively low plasma TG levels of this model. However, apoB and MTP ASO treatment significantly decreased plasma TG in Western diet-fed LDLr−/− mice by −69% and −75%, respectively (Table 2). More detailed evaluation of plasma samples taken from apoB and MTP ASO-treated LDLr−/− mice indicated that the TPC and plasma TG reductions were primarily due to decreases in apoB-containing lipoproteins; however, there was a significant decrease (−30%) in HDL-C in the chow-fed LDLr−/− mice (Table 2). For VLDL, similar reductions were observed for apoB ASO (−58% for chow, −82% for Western diet) and MTP ASO (−71% for chow, −85% for Western diet) treatment. However, LDL-C was reduced to a greater extent by apoB ASO (−61% for chow, −73% for Western diet) versus MTP ASO (−40% for chow, −42% for Western diet) administration. Therefore, in DIO and LDLr−/− mouse models, 6 weeks of treatment with the apoB or MTP ASOs led to significant reductions in hepatic apoB or MTP protein that in turn led to decreased plasma TPC and, in a severely hyperlipidemic model, decreased plasma TG.
TABLE 1.
Plasma lipids, ALT, AST, and 3HB levels in DIO C57BL/6 mice administered ASOs for 2 or 6 weeks
| ALT | AST | TPC | Non-HDL-C | HDL-C | Plasma TG | Plasma 3HB | |
| U/L | U/L | mg/dl | mg/dl | mg/dl | mg/dl | mmol/l | |
| 2 week DIO C57BL/6 | |||||||
| Control ASO (50) | 33 ± 4 | 56 ± 4 | 169 ± 12 | 25 ± 2 | 137 ± 10 | 95 ± 2 | 274 ± 28 |
| ApoB ASO (50) | 71 ± 9 | 60 ± 4 | 92 ± 2ab | 12 ± 1ab | 79 ± 1ab | 73 ± 7 | 514 ± 42a |
| ApoB ASO (25) | 74 ± 24 | 101 ± 18 | 144 ± 9 | 20 ± 2 | 118 ± 7 | 93 ± 9 | 294 ± 66 |
| ApoB ASO (12.5) | 41 ± 1 | 53 ± 5 | 148 ± 6 | 22 ± 1 | 119 ± 4 | 85 ± 3 | 336 ± 27 |
| MTP ASO (50) | 243 ± 30ab | 172 ± 22ab | 143 ± 11 | 23 ± 2 | 114 ± 9 | 65 ± 4 | 484 ± 48a |
| MTP ASO (25) | 81 ± 9 | 89 ± 6 | 145 ± 4 | 21 ± 1 | 117 ± 4 | 79 ± 9 | 499 ± 72a |
| MTP ASO (12.5) | 45 ± 7 | 61 ± 3 | 176 ± 5 | 24 ± 1 | 140 ± 4 | 72 ± 2 | 437 ± 43a |
| 6 week DIO C57BL/6 | |||||||
| Control ASO (50) | 41 ± 5 | 76 ± 17 | 161 ± 20 | 31 ± 2 | 129 ± 18 | 90 ± 3 | 251 ± 32 |
| ApoB ASO (50) | 62 ± 26 | 59 ± 9 | 72 ± 1a | 12 ± 0.2ab | 64 ± 1a | 78 ± 11 | 355 ± 53 |
| ApoB ASO (25) | 50 ± 13 | 65 ± 9 | 91 ± 6a | 13 ± 1ab | 79 ± 6a | 94 ± 3 | 372 ± 89 |
| ApoB ASO (12.5) | 47 ± 18 | 51 ± 5 | 140 ± 1 | 26 ± 1 | 116 ± 1 | 95 ± 8 | 334 ± 32 |
| MTP ASO (50) | 349 ± 75ab | 184 ± 40ab | 114 ± 10a | 23 ± 2 | 95 ± 7 | 70 ± 2 | 580 ± 64a |
| MTP ASO (25) | 96 ± 25 | 76 ± 6 | 102 ± 8a | 22 ± 2a | 84 ± 7a | 80 ± 2 | 484 ± 53 |
| MTP ASO (12.5) | 89 ± 26 | 82 ± 17 | 126 ± 4 | 26 ± 2 | 105 ± 5 | 83 ± 4 | 383 ± 56 |
Values represent mean ± SEM (n = 4 per group). Number in parentheses indicates mg/kg/week dose.
Significantly different (P < 0.05) when compared with control ASO.
Significantly different when compared with MTP ASO-treated mice at the same dose.
TABLE 2.
Plasma lipids, ALT, and AST levels in chow-fed or Western diet-fed LDLr−/− mice administered ASOs for 6 weeks
| ALT | AST | TPC | VLDL-C | LDL-C | HDL-C | Plasma TG | |
| U/l | U/l | mg/dl | mg/dl | mg/dl | mg/dl | mg/dl | |
| 6 week chow-fed LDLr−/− mice | |||||||
| Control ASO (50) | 29 ± 1 | 66 ± 6 | 348 ± 24 | 17 ± 2.6 | 235 ± 15 | 97 ± 14 | 156 ± 11 |
| ApoB ASO (50) | 62 ± 12ab | 78 ± 10 | 167 ± 10a | 7 ± 1 | 92 ± 7a | 68 ± 6a | 82 ± 6a |
| MTP ASO (50) | 38 ± 7 | 76 ± 12 | 205 ± 10a | 5 ± 4 | 140 ± 8a | 60 ± 3 | 102 ± 6a |
| 6 week Western diet-fed LDLr−/−−/− mice | |||||||
| Control ASO (50) | 92 ± 23 | 139 ± 21 | 1546 ± 50 | 646 ± 42 | 787 ± 14 | 113 ± 9 | 585 ± 25 |
| ApoB ASO (50) | 149 ± 31b | 128 ± 10b | 407 ± 58ab | 114 ± 35a | 209 ± 36ab | 84 ± 10 | 182 ± 33a |
| MTP ASO (50) | 258 ± 46a | 207 ± 25a | 671 ± 81a | 98 ± 28a | 457 ± 57a | 116 ± 8 | 147 ± 25a |
Values represent mean ± SEM. For chow-fed LDLr−/− mice, n = 8–9 per group. For Western diet-fed LDLr−/− mice, n = 7–8 per group. Number in parentheses indicates mg/kg/week dose.
Significantly different (P < 0.05) when compared with control ASO.
Significantly different when compared with MTP ASO treatment.
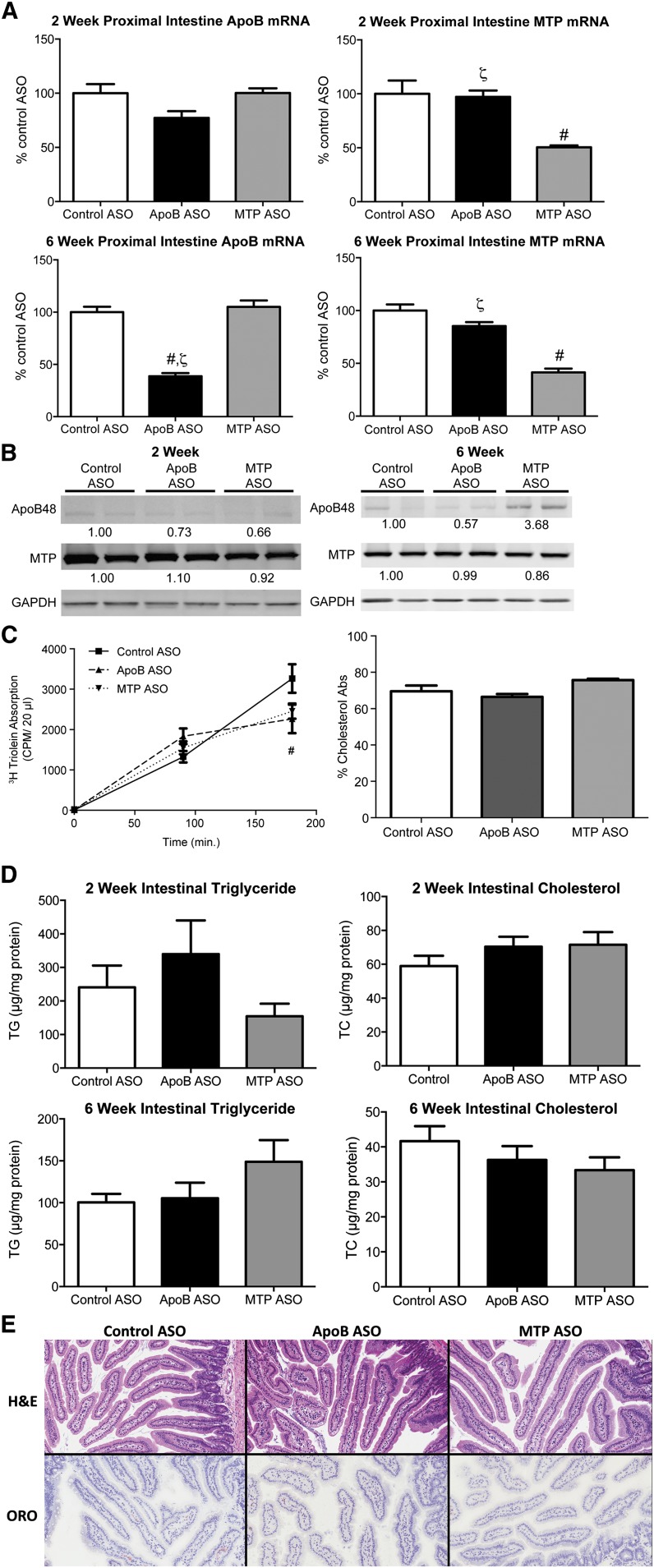
Suppression of intestinal expression of apoB or MTP can reduce lipid absorption and potentially lower plasma lipid levels by impairing intestinal secretion of apoB48-containing chylomicron particles. To determine if the amelioration of dyslipidemia observed with ASO treatment was due to alterations in intestinal metabolism, lipid absorption (Fig. 3) was quantified. Dietary food intake and animal body weights were unaffected by apoB or MTP ASO treatment; nor was there evidence of steatorrhea/loose stools (data not shown). Proximal intestinal apoB mRNA expression was unchanged after 2 weeks of apoB ASO treatment but was significantly reduced by 62% after 6 weeks (Fig. 3A). Intestinal apoB48 protein also appeared to be reduced, albeit more modestly than the apoB mRNA expression, with 6 weeks of ASO treatment (Fig. 3B). However, due to the weak and variable apoB48 protein bands, it is difficult to draw a distinct conclusion from the Western blot results. After 2 and 6 weeks of treatment, the MTP ASO led to significant reductions of −50% and −59% in proximal SI MTP mRNA expression, respectively. Unlike the apoB ASO, the MTP ASO had little to no effect on intestinal MTP protein expression and increased apoB48 protein expression. The reduced efficacy of the ASOs relative to liver was expected based on the observation in previous studies of limited distribution of ASO (22) to the intestine.
Fig. 3.
Intestinal effects of ApoB and MTP ASOs in DIO mice. A: Proximal intestine mRNA expression of targeted genes in 50 mg/kg/week control ASO-, apoB ASO-, and MTP ASO-treated (n = 5 per group) DIO animals were determined by quantitative PCR as described in Materials and Methods. B: One hundred micrograms of protein isolated from the proximal intestine was immunoblotted with polyclonal Abs raised against apoB, MTP, or GAPDH (load control). Numbers below bands are mean density (n = 2) relative to control ASO-treated mice. C: Quantitation of 3H-triolein absorption in DIO mice administered ASO for 6 weeks was carried out as described in Materials and Methods. Cholesterol absorption was carried out in DIO mice administered ASO for 6 weeks using the dual fecal isotope method. D: Proximal intestine triglyceride and total cholesterol was quantitated as described in Materials and Methods. E: Images of ORO-stained snap frozen proximal intestinal sections from mice treated with ASO for 6 weeks. ORO-stained sections were counterstained with hematoxylin. Bars represent mean; error bars represent SEM. #Significantly different (p < 0.05) when compared with control ASO. ζSignificantly different (p < 0.05) when compared with MTP ASO-treated mice at same dosage.
To determine if the reduction of intestinal apoB and MTP led to physiologic effects on lipid and sterol absorption, we measured TG and cholesterol absorption in DIO mice treated with ASOs for 6 weeks (Fig. 3C). Poloxamer 407-treated mice were administered a tritiated triolein gavage, and the appearance of radioactivity was quantitated over a 3 h period. ApoB ASO-treated animals had a modest, but significantly reduced, secretion of tritiated triolein into the plasma (−30%) at the 3 h time point when compared with control ASO. The MTP ASO also showed a nonsignificant tendency (−25%) to reduce tritiated triolein when compared with the control ASO. Neither compound demonstrated a significant effect on cholesterol absorption as measured by the dual fecal isotope method. Proximal intestine histology and lipid content were also analyzed. Consistent with minimal effects on total lipid absorption, there were no significant changes in TG or total cholesterol concentrations when quantitated and compared with the control ASO-treated mice (Fig. 3D); nor were there observable increases in neutral lipid accumulation detected using H&E and ORO staining methods (Fig. 3E).
In two previous publications (11, 12), no reductions were observed in intestinal apoB48 protein levels; nor were there any effects on lipid absorption observed after extended administration of the apoB ASO in the DIO and LDLr−/− mice used in those experiments. However, in this most recent study, a modest but significant reduction in apoB48 levels was noted. Nonetheless, the apparent reduction in apoB48 protein levels did not produce increased intestinal lipid deposition or reduced cholesterol/TG absorption. This variation in the protein modulation may be attributed to differences in mouse progeny, dietary constituents, experimental protocols, or test reagents in studies performed over the last decade.
After demonstration that the apoB and MTP ASO were sufficiently efficacious, we evaluated the safety profile of the two ASOs using a number of parameters (Tables 1–3). Liver TG and CE concentrations were evaluated in chow-fed C57BL/6, DIO, and LDLr−/− mice. In chow-fed C57BL/6 mice, hepatic TG levels (Table 3) were only increased by the MTP ASO at 6 weeks. Liver CE concentrations, however, were significantly increased in apoB ASO-treated animals after 2 weeks and in apoB and MTP ASO-treated animals after 6 weeks. Hepatic free cholesterol concentrations were similar between all groups at both time points (data not shown). Despite these differences in liver TG accumulation, administration of apoB or MTP ASO to chow-fed mice did not lead to changes in body weight progression or hepatic transaminases (data not shown), indicating that apoB and MTP ASO were well tolerated in the absence of a dietary challenge.
TABLE 3.
Effect of ApoB and MTP ASO administration on liver neutral lipid accumulation in chow-fed and dietary-challenged C57BL/6 and LDLr−/− mice
| 2 Week Chow | 6 Week Chow | 2 Week DIO | 6 Week DIO | 6 Week Chow LDLr−/− | 6 Week WD LDLr | |
| mg/g tissue | mg/g tissue | mg/g tissue | mg/g tissue | mg/g tissue | mg/g tissue | |
| Liver triglyceride | ||||||
| Control ASO (50) | 6.9 ± 1.6 | 11.4 ± 1.1 | 10.2 ± 1.6 | 15.1 ± 5.1 | 14.9 ± 1.3 | 45.3 ± 10.4 |
| ApoB ASO (50) | 11.9 ± 1.0 | 15.2 ± 1.3b | 33.9 ± 3.4a | 35.3 ± 7.9b | 28.6 ± 2.5a | 108.3 ± 11.5ab |
| MTP ASO (50) | 12.3 ± 2.1 | 23.4 ± 3.6a | 33.9 ± 4.9a | 110.7 ± 17.7a | 31.3 ± 2.5a | 175.2 ± 27.1a |
| Liver cholesteryl ester | ||||||
| Control ASO (50) | 3.7 ± 0.2 | 3.6 ± 0.3 | 1.2 ± 0.4 | 2.6 ± 0.7 | 1.7 ± 0.4 | 8.1 ± 1.5 |
| ApoB ASO (50) | 5.3 ± 0.4a | 4.5 ± 0.1ab | 3.5 ± 0.7a | 11.9 ± 2.8a | 5.5 ± 0.4ab | 31.1 ± 2.6a |
| MTP ASO (50) | 4.4 ± 0.2 | 5.3 ± 0.2a | 4.1 ± 0.5a | 10.5 ± 0.6a | 3.6 ± 0.5a | 27 ± 3.5a |
Values represent mean ± SEM. For chow, n = 5 per group; for DIO mice, n = 5 per group. For chow-fed LDLr−/− mice, n = 8–9 per group; for Western diet-fed LDLr−/− mice, n = 11–13 per group. Number in parentheses indicates mg/kg/week dose.
Significantly different (P < 0.05) when compared with control ASO.
Significantly different (P < 0.05) when compared with MTP ASO treatment.
A markedly different safety profile was observed in the face of a dietary challenge. Two weeks of high-dose (50 mg/kg/week) apoB or MTP ASO treatment in DIO mice led to a significant 3.3-fold elevation in liver TG when compared with controls (Table 3). However, after 6 weeks of apoB ASO administration, hepatic TG concentrations were relatively unchanged from those levels observed after 2 weeks. In contrast, 6 weeks treatment with the MTP ASO resulted in a further 3.2-fold increase in liver TG relative to the 2 week treatment values. Similar to chow-fed mice, DIO mice treated with MTP and apoB ASO showed significant increases in hepatic CE after both 2 and 6 weeks. As in the chow-fed animals, liver free cholesterol was similar in all groups (data not shown). Analysis of hepatic transaminases revealed that, unlike observations made in chow-fed mice, 2 week high-dose MTP ASO treatment significantly increased alanine aminotransferase (ALT) and aspartate aminotransferase (AST) by 7.4-fold and 3.1-fold, respectively, when compared with control. Similarly, 6 week high-dose MTP ASO treatment in DIO mice significantly increased plasma ALT and plasma AST by 8.5-fold and 2.4-fold, respectively, when compared with control (Table 1). In contrast, 2 week and 6 week high-dose apoB ASO treatment led to a nonsignificant increase in ALT levels of 2.1- and 1.5-fold, respectively, with no change in AST. Finally, DIO mice dosed at 25 and 12.5 mg/kg/week with the MTP ASO or apoB ASO demonstrated no significant changes in plasma transaminase levels. In chow-fed LDLr−/− mice, only apoB ASO led to significant increases (2.1-fold) in ALT concentrations when compared with controls. As with DIO mice, Western diet-fed LDLr−/− mice treated with the MTP ASO displayed significant increases in plasma ALT and AST concentrations of 2.8-fold and 1.4-fold, respectively, when compared with control (Table 2), whereas apoB ASO treatment tended to increase plasma ALTs by 1.6-fold. Unlike chow and DIO mice, chow-fed LDLr−/− mice dosed with the apoB or MTP ASO showed similar increases in liver TG. In Western diet-fed LDLr−/− mice, apoB ASO significantly increased hepatic TG 2.4-fold, whereas MTP ASO showed a 3.9-fold increase in liver TG that was significantly greater than the apoB ASO and control ASO treatments (Table 3). As with the chow and DIO mice, liver CE was significantly increased to a similar extent with apoB ASO and MTP ASO treatment, and hepatic free cholesterol concentrations did not change with either treatment (data not shown).
Therefore, upon receiving a dietary challenge, DIO and LDLr−/− mice treated with the MTP ASO developed more extensive hepatic steatosis and liver injury, as evidenced by elevated transaminases, when compared with apoB ASO treatment. Using these models, we attempted to identify the factors that would lead to such differences in the development of steatosis observed between the two targets.
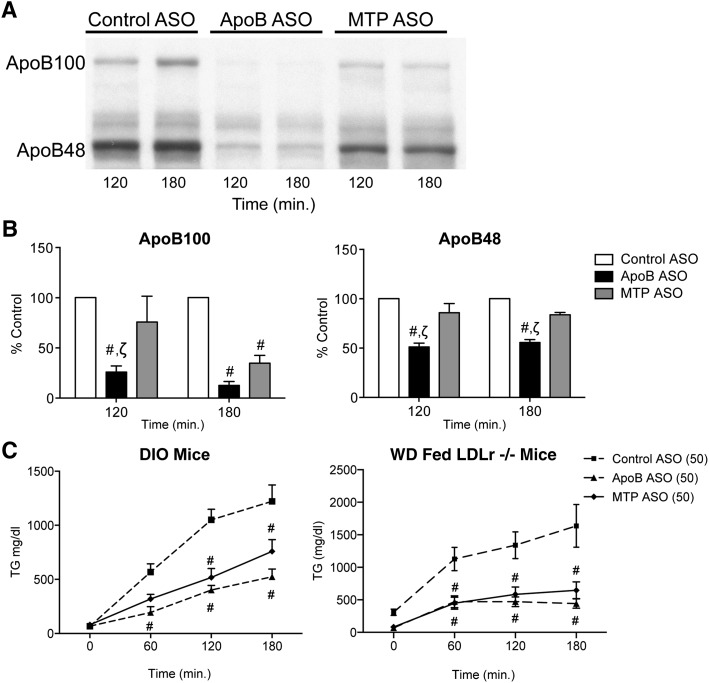
To determine if the discrepancies in hepatic TG accumulation between apoB and MTP ASO treatment were due to differences in VLDL secretion, apoB and TG accumulation was quantified in ASO-treated mice administered a Triton WR1339/35S methionine bolus (Fig. 4). Our data showed that apoB and MTP ASO administration significantly reduced TG secretion to a similar extent in DIO and Western diet-fed LDLr−/− mice when compared with control. However, quantitation of 35S apoB protein secretion in DIO mice demonstrated that, relative to MTP, the apoB ASO was a more potent inhibitor of apoB48 secretion at the 120 min and at the 180 min time points. The relative insensitivity of apoB48 to MTP suppression has been well documented in vitro and in vivo (23–25). Therefore, the differences in TG accumulation between apoB ASO and MTP ASO-treated mice were not due to differences in reduction of hepatic TG secretion.
Fig. 4.
Hepatic ApoB and TG secretion in ApoB-treated or MTP ASO-treated (50 mg/kg/week) DIO mice. Fasted DIO mice (n = 4 oer treatment group) were injected with a 35S Met/Triton WR1339 bolus. Animals were bled at the 60, 120, and 180 min time points, and apoB-100, apoB-48, and TG levels were analyzed. A: TG levels at each time point were determined by colorimetric assay in control ASO (square with dashed line), apoB ASO (diamond with solid line), and MTP ASO-treated mice (triangle with dashed line) (left panel) and Western diet-fed LDLr−/− mice (right panel). B: Plasma (5 μl) from the 120 and 180 min time points were analyzed by SDS-PAGE. The gels were dried down and exposed to film. C: Films were scanned and densitometry on the apoB100, and apoB48 bands were assessed for control ASO-treated (gray bars), apoB ASO-treated (black bars), and MTP ASO-treated (hatched bars) mice. Bars represent mean; error bars represent SEM. #P < 0.05 when compared with control ASO. ζSignificantly different (P < 0.05) when compared with MTP ASO treatment.
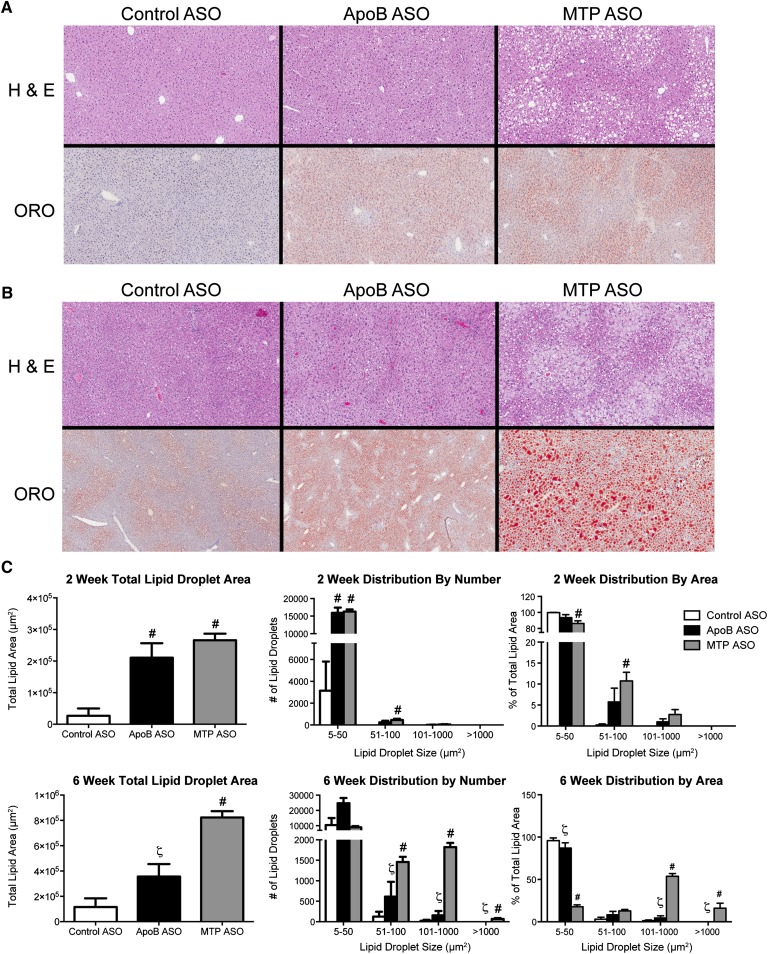
Densitometric analysis of ORO-stained histological sections (Fig. 5A, B) was used to examine treatment differences in lipid droplet density and size distribution after 2 and 6 weeks of treatment. At 2 weeks (Fig. 5C), the total lipid droplet area (i.e., the ORO-stained area) of apoB and MTP ASO-treated livers showed similar significant elevations when compared with the control ASO animals, consistent with the liver TG concentrations obtained from tissue extractions (Table 3). Further analysis of lipid droplet size distribution demonstrated that a majority of the lipid droplets in all three ASO treatments were of small diameter (5–50 μm2). However, with MTP ASO treatment there was a significant increase in the number of larger lipid droplets and in the percentage of total lipid area of larger lipid droplets (51–100 μm2) when compared with controls (Fig. 5C).
Fig. 5.
Effects of ApoB and MTP ASO (50 mg/kg/week) on liver lipid droplets in DIO mice. A: Representative images of H&E- and ORO-stained liver sections from DIO mice treated for 2 weeks with control ASO (50 mg/kg/week), apoB ASO (50 mg/kg/week), or MTP ASO (50 mg/kg/week). B: Representative images of H&E- and ORO-stained liver sections from DIO mice treated for 6 weeks with control ASO (50 mg/kg/week), apoB ASO (50 mg/kg/week), or MTP ASO (50 mg/kg/week). ORO-stained sections were counterstained with hematoxylin. C: Quantitation of lipid droplet number and size distribution in DIO mice administered ASO for 2 or 6 weeks. ORO-stained liver sections from ASO-treated DIO mice (n = 4 per group) were analyzed as described in Materials and Methods. Bars represent mean; error bars represent SEM. #P < 0.05 when compared with control ASO. ζSignificantly different (P < 0.05) when compared with MTP ASO treatment.
Analysis of livers from animals treated for 6 weeks revealed a more pronounced histological phenotype (Fig. 5B). In silico quantitation (Fig. 5C) showed that, much like liver TG concentrations, MTP ASO had significantly more total lipid area when compared with apoB and control, whereas apoB ASO had a total lipid area that was intermediate between the control and MTP ASO. Much like the 2 week time point, apoB ASO treatment tended to increase the number of lipid droplets and the percentage of total lipid area in the 5 to 50 μm2 and the 51 to 100 μm2 size classes. The MTP ASO showed a significantly different lipid droplet size distribution, with a majority of the lipid droplets and lipid droplet area found in the larger lipid droplets (101–1,000 μm2 and >1,000 μm2).
The formation and maintenance of lipid droplets relies upon a number of important transcription factors, including peroxisome proliferator activated receptor γ (PPARγ) and protein cofactors. Previous studies have demonstrated that PPARγ can increase lipid droplet size via increased expression of fat-specific protein 27 (FSP27) (26).
Based on microarray data and studies described previously (11, 12), we had identified PPARγ as being regulated by apoB ASO. Quantitation of hepatic mRNA (Table 4) revealed that, although there were no significant differences compared with control ASO, 2 weeks of apoB ASO treatment led to significant decreases in hepatic mRNA expression of PPARγ and PPARγ-regulated genes FSP27 and CD36 as well as the adipocyte-specific marker adipsin relative to the MTP ASO. Furthermore, apoB and MTP ASO treatment significantly reduced hepatic SCD-1 mRNA expression, a key enzyme in the lipogenic pathway (27). After 6 weeks of apoB ASO treatment, DIO mice again showed significant reductions in hepatic PPARγ mRNA when compared with MTP ASO-treated mice (Table 5). In contrast, the hepatic expression of PPARγ and its target genes CD36 and FSP27, as well as the adipocyte-specific marker adipsin, which is more highly expressed with PPARγ overexpression (28), were significantly greater in MTP ASO-treated animals.
TABLE 4.
Hepatic mRNA expression of a subset of key metabolic genes after 2 weeks of 50 mg/kg/week ASO treatment in DIO mice
| Week 2 | Control ASO | ApoB ASO | MTP ASO |
| Fatty acid metabolism | |||
| SREBP1-c | 100 ± 12 | 36 ± 6a | 40 ± 9a |
| ACC1 | 100 ± 17 | 57 ± 14 | 92 ± 18 |
| SCD-1 | 100 ± 31 | 13 ± 4a | 14 ± 3a |
| AMPKα2 | 100 ± 12 | 103 ± 12 | 82 ± 7 |
| PPARγ-related genes | |||
| PPARγ | 100 ± 7 | 62 ± 7b | 128 ± 27 |
| FSP27 | 100 ± 21 | 95 ± 14b | 178 ± 26 |
| CD36 | 100 ± 12 | 84 ± 8b | 165 ± 27 |
| Adipsin | 100 ± 23 | 43 ± 17b | 204 ± 48 |
Values represent mean ± SEM (n = 5 per group).
Significantly different (P < 0.05) when compared with control ASO.
Significantly different (P < 0.05) when compared with MTP ASO treatment.
TABLE 5.
Hepatic mRNA expression of a subset of key metabolic genes after 6 weeks of 50 mg/kg/week ASO treatment in DIO mice
| Week 6 | Control ASO | ApoB ASO | MTP ASO |
| Lipogenic pathway | |||
| SREBP1-c | 100 ± 9 | 116 ± 13b | 70 ± 6 |
| ACC1 | 100 ± 8 | 41 ± 4a | 65 ± 11a |
| SCD-1 | 100 ± 22 | 3 ± 1a | 14 ± 4a |
| AMPKα2 | 100 ± 10 | 166 ± 13ab | 101 ± 8 |
| PPARγ-related genes | |||
| PPARγ | 100 ± 8 | 44 ± 5ab | 162 ± 9a |
| FSP27 | 100 ± 14 | 64 ± 8b | 231 ± 34a |
| CD36 | 100 ± 9 | 75 ± 3b | 275 ± 21a |
| Adipsin | 100 ± 22 | 33 ± 17.b | 389 ± 86a |
Values represent mean ± SEM (n = 4 per group).
Significantly different (P < 0.05) when compared with control ASO.
Significantly different (P < 0.05) when compared with MTP ASO treatment.
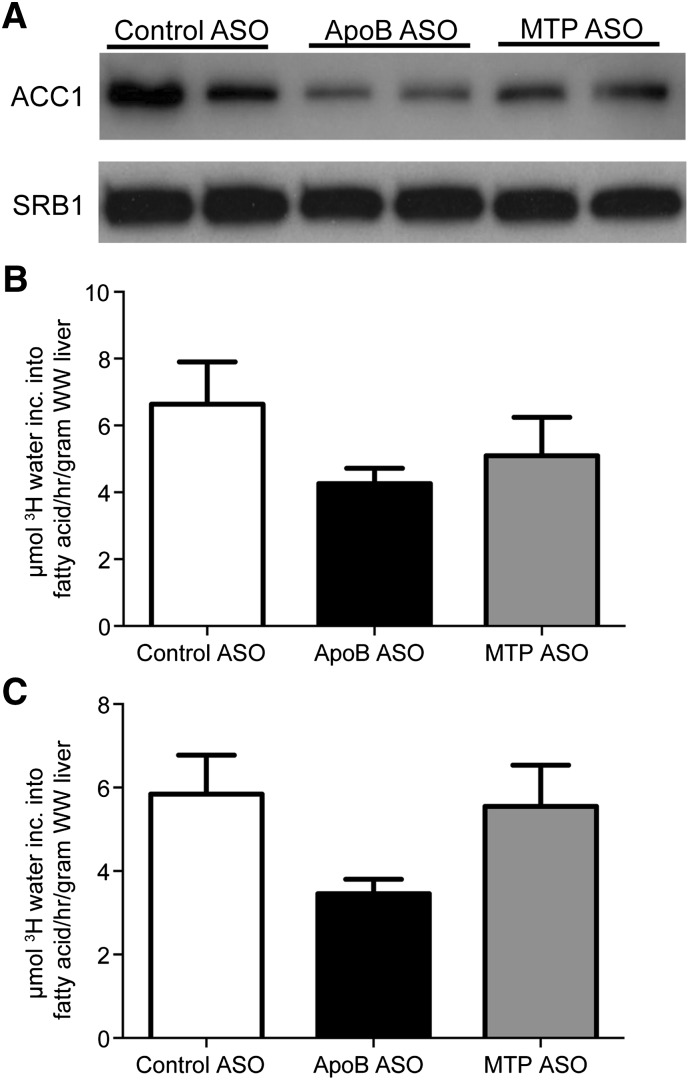
Because increased hepatic de novo lipogenesis can contribute to liver TG accumulation, we studied this synthetic process by evaluating transcriptional factors and fatty acid synthesis in mice treated with ASO for 6 or 12 weeks. The differences in liver TG concentrations after 12 weeks of treatment were similar to shorter-duration studies (4.3 mg/g liver wet weight [WW] with control ASO vs. 20.3 mg/g liver WW with apoB ASO vs. 81.7 mg/g liver WW with MTP ASO). DIO mice administered the highest dose of apoB or MTP ASO showed similar reductions in acetyl-coA carboxylase 1 (ACC1) mRNA expression (Table 5), a key enzyme involved in fatty acid synthesis. Furthermore, after 6 weeks of ASO treatment, hepatic AMPKα2 mRNA significantly increased by 66% when compared with control. Hepatic ACC1 protein expression was also reduced by both drug treatments, whereas apoB ASO-treated animals showed more pronounced protein reduction relative to MTP ASO treatment (Fig. 6) after 6 weeks of ASO treatment. When hepatic lipogenesis was quantified after 6 and 12 weeks of treatment, this process tended to be reduced at both time points with the apoB ASO, but these changes did not achieve statistical significance (P = 0.28 and P = 0.14, respectively). MTP ASO treatment tended to suppress lipogenesis at 6 weeks (P = 0.58) but had no effect after 12 weeks (P = 0.96). Therefore, the differences in TG accumulation between apoB and MTP ASO treatment appear to be due in part to 1) the inability of MTP ASO-treated animals after long-term treatment to suppress lipogenesis, 2) the development of larger lipid droplets with the MTP ASO, and 3) reduced PPARγ pathway mRNA expression produced by apoB ASO relative to the MTP ASO.
Fig. 6.
Effects of ApoB and MTP ASOs on fatty acid synthesis in DIO mice. A: Hepatic expression of ACC1 protein in DIO mice administered 50 mg/kg/week of ASO for 6 weeks. B: Hepatic fatty acid synthesis in DIO mice (n = 6–12 per group) administered 50 mg/kg/week of ASO for 6 weeks. C: Hepatic fatty acid synthesis in DIO mice (n = 5/group) administered 50 mg/kg/week of ASO for 12 weeks. Fatty acid synthesis was measured using the tritiated water method described in Materials and Methods. Bars represent mean; error bars represent SEM.
Another potential contributing factor to hepatic steatosis is reduced fatty acid oxidation. An accepted in vivo biomarker for fatty acid oxidation is β-hydroxybutyrate (3HB) (29). In DIO mice treated for 2 weeks, plasma 3HB levels were significantly increased with apoB and MTP ASO treatment (Table 1), indicating that these animals were compensating for elevated levels of hepatic TG levels by increasing fatty acid oxidation. However, after 6 weeks of treatment, 3HB levels remained elevated only with MTP ASO treatment, possibly because apoB ASO-treated mice no longer had elevated liver TG concentrations.
DISCUSSION
Given that antisense drugs share similar pharmacokinetic properties and distribute predominantly in the liver rather than intestine, we performed head-to-head comparisons with target-specific antisense drugs to evaluate the metabolic consequences of hepatic apoB and MTP suppression. Our studies reveal that the ASO-mediated modulation of apoB or MTP mRNA resulted in similar reductions in their respective hepatic mRNA levels, with comparable concurrent suppression in hepatic TG secretion and serum lipid levels. Despite equivalent impairment of VLDL secretion, MTP reduction produced persistent increased hepatic TG accumulation that resulted in increased ALT elevations relative to the apoB ASO.
In earlier publications from our laboratory (11, 12), we suggested that antisense suppression of apoB does not result in hepatic steatosis due to secondary compensatory changes in the expression of multiple enzymes involved in lipogenic and β-oxidative pathways in the liver. Data presented here confirm and extend those observations. When liver fat was quantified in DIO mice administered the apoB ASO, there was an early, transient increase in hepatic TG levels observed after 2 weeks of treatment, but by 6 weeks, liver TG returned to levels present in control animals (Table 3). A number of experiments using transcriptional profiling and metabolomic analyses were performed to determine the physiological sequelae of apoB reduction. Those analyses revealed that apoB ASO treatment down-regulated several key lipogenic genes, including SCD-1, ACC1, and PPARγ. Consistent with these findings, hepatic fatty acid synthesis, as determined by tritiated water analytical methods, demonstrated that de novo lipogenesis was decreased in vivo (Fig. 6).
In addition to decreased fatty acid synthesis, another adaptive response to increased liver TG is to enhance oxidation of fatty acid stores (30). Extensive studies have shown that AMP-activated protein kinase α (AMPKα) is a key energy sensor whose expression has been correlated with increased fatty acid oxidation and decreased lipogenesis (30, 31). As described previously and shown herein, AMPKα mRNA levels were increased after apoB ASO treatment (Table 5). An established method to detect hepatic β oxidation is to evaluate 3HB, or ketone levels, in vivo (32, 33). Using that technique, it appeared that 3HB was increased in apoB ASO-treated DIO mice after 2 weeks of treatment (Table 1), when liver TG levels were elevated in comparison to controls (Table 3), but after 6 weeks, when hepatic TG levels were similar to controls, 3HB levels returned to those observed in control ASO-treated mice. This was in contrast to MTP ASO-treated animals, which had elevated plasma TG and 3HB levels and at the highest administered dose and increased transaminase levels at both the 2 week and 6 week time points. Sustained fatty acid oxidation has been shown to increase reactive oxygen species, which, through many well documented pathways, including the peroxidation of polyunsaturated fatty acids, leads to enhanced oxidative stress, thus potentially contributing to the development of liver injury (30).
In MTP ASO-treated mice, many lipogenic genes were also down-regulated, with similar reductions as observed in apoB ASO-treated mice such as SCD-1, whereas less robust down-regulation was demonstrated for ACC1. Finally, AMPKα levels were unaffected by MTP ASO treatment. Regardless of these reductions in lipogenic genes, de novo fatty acid synthesis, as determined using tritiated water, was not significantly reduced in MTP-treated mice (Fig. 6), perhaps due to more modest effects on ACC1 mRNA and protein. ACC1 is a key enzyme that catalyzes the synthesis of malonyl CoA and plays a pivotal role in de novo lipogenesis and mitochondrial fatty acid oxidation (34). These observations suggest that blocking VLDL secretion with an MTP or apoB ASO may trigger common metabolic pathways to compensate for increased hepatic TG deposition, including down-regulation of lipogenesis and increased β-oxidation. These adaptive processes have also been observed in mice with a targeted apoB38.9 mutation (35) and in hepatocytes isolated from humans with familial hypobetalipoproteinemia (36).
Another potential contributor to the consistent hepatic steatosis observed in MTP mice but not in apoB ASO-treated mice relates to differential effects upon PPARγ-regulated genes that are thought to be involved in lipid droplet formation and homeostasis. We observed key differences between apoB and MTP ASO treatment in lipid droplet size (Fig. 5) and the expression of PPARγ and FSP27 genes (Tables 4 and 5) previously implicated in lipid droplet metabolism. Others have shown that FSP27, a lipid droplet associated protein, is integral to the PPARγ-dependent development of hepatic steatosis in mice (37). In vitro evidence indicates that FSP27 overexpression results in TG accumulation and increased lipid droplet size in AML12 cells, primary hepatocytes (37), and COS cells due in part to decreases in fatty acid oxidation and TG turnover. Furthermore, recent in vivo experiments demonstrated that overexpression of FSP27 in liver-specific PPARγ−/− mice partially restored hepatic TG accumulation (37). Recent evidence indicates that FSP27 facilitates lipid droplet fusion and expansion (38, 39), which in turn reduces lipolytic activity by limiting lipolytic enzymes (i.e., HSL and ATGL) access to substrate. This reduces TG turnover from lipid droplets, which causes accumulation of lipid within the droplet. In the MTP ASO-treated animals, hepatic FSP27 mRNA expression and lipid droplet size were increased relative to controls and apoB ASO-treated animals. It is possible that the elevated FSP27 expression in MTP ASO-treated animals is shunting the TG normally exported in VLDL particles away from the fatty acid oxidation pathway and into lipid droplets where the TG cannot be mobilized. Therefore, differential effects on both fatty acid synthesis and lipid droplet morphology may help explain the difference in liver TG levels observed between high dose apoB and MTP ASO treatment.
In summary, although apoB and MTP play central roles in hepatic lipoprotein secretion, pharmacological suppression of these proteins leads to distinct differences in metabolic sequelae. Although there were similar reductions in hepatic TG secretion after 6 weeks of ASO treatment, MTP ASO-treated mice accumulated significantly more hepatic TG than apoB ASO-treated mice. Our data suggest that this is likely due to the complex, multiphasic interplay of factors including decreased lipogenesis and changes in lipid droplet metabolism, as exemplified by reductions in hepatic lipid droplet size and FSP27 mRNA expression with apoB ASO treatment relative to MTP ASO treatment. Although we believe that the factors elucidated in this manuscript contribute to the phenotypes observed in mice after apoB and MTP ASO treatment, other metabolic processes may occur. Further work is necessary to define the precise signaling mechanisms and potential alternate pathways that differentiate the effects of long-term reductions in apoB and MTP in liver metabolism and lipid homeostasis.
Supplementary Material
Acknowledgments
The authors thank Drs. Stanley T. Crooke, Brett Monia, Michael McCaleb, Brenda Baker, Walter Singleton, and Richard Geary for helpful discussions and review of the manuscript and Yuhong Jiang for technical assistance in Oil Red O staining of liver and intestine.
Footnotes
Abbreviations:
- ACC1
- acetyl-coA carboxylase 1
- ALT
- alanine aminotransferase
- ASO
- antisense oligonucleotide
- AST
- aspartate aminotransferase
- CE
- cholesteryl ester
- FSP27
- fat-specific protein 27
- 3HB
- β-hydroxybutyrate
- MTP
- microsomal triglyceride transfer protein
- PPARγ
- peroxisome proliferator activated receptor γ
- TG
- triglyceride
- WW
- wet weight
All studies were funded by Isis Pharmaceuticals.
REFERENCES
- 1.Heron M. 2007. Deaths: leading causes for 2004. Natl. Vital Stat. Rep. 56: 1–95 [PubMed] [Google Scholar]
- 2.LaRosa J. C. 2007. Low-density lipoprotein cholesterol reduction: the end is more important than the means. Am. J. Cardiol. 100: 240–242 [DOI] [PubMed] [Google Scholar]
- 3.Baigent C., Keech A., Kearney P. M., Blackwell L., Buck G., Pollicino C., Kirby A., Sourjina T., Peto R., Collins R., et al. 2005. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 366: 1267–1278 [DOI] [PubMed] [Google Scholar]
- 4.Armitage J. 2007. The safety of statins in clinical practice. Lancet. 370: 1781–1790 [DOI] [PubMed] [Google Scholar]
- 5.Stein E. 2002. The lower the better? Reviewing the evidence for more aggressive cholesterol reduction and goal attainment. Atheroscler. Suppl. 2: 19–23 [DOI] [PubMed] [Google Scholar]
- 6.Lilly S. M., Rader D. J. 2007. New targets and emerging therapies for reducing LDL cholesterol. Curr. Opin. Lipidol. 18: 650–655 [DOI] [PubMed] [Google Scholar]
- 7.Burnett J. R., Huff M. W. 2006. Cholesterol absorption inhibitors as a therapeutic option for hypercholesterolaemia. Expert Opin. Investig. Drugs. 15: 1337–1351 [DOI] [PubMed] [Google Scholar]
- 8.Akdim F., Stroes E. S., Kastelein J. J. 2007. Antisense apolipoprotein B therapy: where do we stand? Curr. Opin. Lipidol. 18: 397–400 [DOI] [PubMed] [Google Scholar]
- 9.Davis R. A. 1999. Cell and molecular biology of the assembly and secretion of apolipoprotein B-containing lipoproteins by the liver. Biochim. Biophys. Acta. 1440: 1–31 [DOI] [PubMed] [Google Scholar]
- 10.Shelness G. S., Sellers J. A. 2001. Very-low-density lipoprotein assembly and secretion. Curr. Opin. Lipidol. 12: 151–157 [DOI] [PubMed] [Google Scholar]
- 11.Crooke R. M., Graham M. J., Lemonidis K. M., Whipple C. P., Koo S., Perera R. J. 2005. An apolipoprotein B antisense oligonucleotide lowers LDL cholesterol in hyperlipidemic mice without causing hepatic steatosis. J. Lipid Res. 46: 872–884 [DOI] [PubMed] [Google Scholar]
- 12.Mullick A. E., Fu W., Graham M. J., Lee R. G., Witchell D., Bell T. A., Whipple C. P., Crooke R. M. 2011. Antisense oligonucleotide reduction of apoB-ameliorated atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 52: 885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koller E., Vincent T. M., Chappell A., De S., Manoharan M., Bennett C. F. 2011. Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res. 39: 4795–4807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sheehan D. C., Hrapchak B. B. 1980. Theory and practice of histotechnology. CV Mosby, St Louis, MO. [Google Scholar]
- 15.Lee R. G., Kelley K. L., Sawyer J. K., Farese R. V., Jr, Parks J. S., Rudel L. L. 2004. Plasma cholesteryl esters provided by lecithin:cholesterol acyltransferase and acyl-coenzyme a:cholesterol acyltransferase 2 have opposite atherosclerotic potential. Circ. Res. 95: 998–1004 [DOI] [PubMed] [Google Scholar]
- 16.Rava P., Athar H., Johnson C., Hussain M. M. 2005. Transfer of cholesteryl esters and phospholipids as well as net deposition by microsomal triglyceride transfer protein. J. Lipid Res. 46: 1779–1785 [DOI] [PubMed] [Google Scholar]
- 17.Wang D. Q., Carey M. C. 2003. Measurement of intestinal cholesterol absorption by plasma and fecal dual-isotope ratio, mass balance, and lymph fistula methods in the mouse: an analysis of direct versus indirect methodologies. J. Lipid Res. 44: 1042–1059 [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y. L., Hernandez-Ono A., Siri P., Weisberg S., Conlon D., Graham M. J., Crooke R. M., Huang L. S., Ginsberg H. N. 2006. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J. Biol. Chem. 281: 37603–37615 [DOI] [PubMed] [Google Scholar]
- 19.Shimano H., Horton J. D., Shimomura I., Hammer R. E., Brown M. S., Goldstein J. L. 1997. Isoform 1c of sterol regulatory element binding protein is less active than isoform 1a in livers of transgenic mice and in cultured cells. J. Clin. Invest. 99: 846–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Farese R. V., Jr, Ruland S. L., Flynn L. M., Stokowski R. P., Young S. G. 1995. Knockout of the mouse apolipoprotein B gene results in embryonic lethality in homozygotes and protection against diet-induced hypercholesterolemia in heterozygotes. Proc. Natl. Acad. Sci. USA. 92: 1774–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leung G. K., Veniant M. M., Kim S. K., Zlot C. H., Raabe M., Bjorkegren J., Neese R. A., Hellerstein M. K., Young S. G. 2000. A deficiency of microsomal triglyceride transfer protein reduces apolipoprotein B secretion. J. Biol. Chem. 275: 7515–7520 [DOI] [PubMed] [Google Scholar]
- 22.Yu R. Z., Kim T. W., Hong A., Watanabe T. A., Gaus H. J., Geary R. S. 2007. Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab. Dispos. 35: 460–468 [DOI] [PubMed] [Google Scholar]
- 23.Haghpassand M., Wilder D., Moberly J. B. 1996. Inhibition of apolipoprotein B and triglyceride secretion in human hepatoma cells (HepG2). J. Lipid Res. 37: 1468–1480 [PubMed] [Google Scholar]
- 24.Nicodeme E., Benoist F., McLeod R., Yao Z., Scott J., Shoulders C. C., Grand-Perret T. 1999. Identification of domains in apolipoprotein B100 that confer a high requirement for the microsomal triglyceride transfer protein. J. Biol. Chem. 274: 1986–1993 [DOI] [PubMed] [Google Scholar]
- 25.Raabe M., Veniant M. M., Sullivan M. A., Zlot C. H., Bjorkegren J., Nielsen L. B., Wong J. S., Hamilton R. L., Young S. G. 1999. Analysis of the role of microsomal triglyceride transfer protein in the liver of tissue-specific knockout mice. J. Clin. Invest. 103: 1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nishino N., Tamori Y., Tateya S., Kawaguchi T., Shibakusa T., Mizunoya W., Inoue K., Kitazawa R., Kitazawa S., Matsuki Y., et al. 2008. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118: 2808–2821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyazaki M., Flowers M. T., Sampath H., Chu K., Otzelberger C., Liu X., Ntambi J. M. 2007. Hepatic stearoyl-CoA desaturase-1 deficiency protects mice from carbohydrate-induced adiposity and hepatic steatosis. Cell Metab. 6: 484–496 [DOI] [PubMed] [Google Scholar]
- 28.Yu S., Matsusue K., Kashireddy P., Cao W. Q., Yeldandi V., Yeldandi A. V., Rao M. S., Gonzalez F. J., Reddy J. K. 2003. Adipocyte-specific gene expression and adipogenic steatosis in the mouse liver due to peroxisome proliferator-activated receptor gamma1 (PPARgamma1) overexpression. J. Biol. Chem. 278: 498–505 [DOI] [PubMed] [Google Scholar]
- 29.Hodson L., Frayn K. N. 2011. Hepatic fatty acid partitioning. Curr. Opin. Lipidol. 22: 216–224 [DOI] [PubMed] [Google Scholar]
- 30.Browning J. D., Horton J. D. 2004. Molecular mediators of hepatic steatosis and liver injury. J. Clin. Invest. 114: 147–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hardie D. G., Pan D. A. 2002. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem. Soc. Trans. 30: 1064–1070 [DOI] [PubMed] [Google Scholar]
- 32.Tremblay-Mercier J., Tessier D., Plourde M., Fortier M., Lorrain D., Cunnane S. C. 2010. Bezafibrate mildly stimulates ketogenesis and fatty acid metabolism in hypertriglyceridemic subjects. J. Pharmacol. Exp. Ther. 334: 341–346 [DOI] [PubMed] [Google Scholar]
- 33.Glien M., Haschke G., Schroeter K., Pfenninger A., Zoller G., Keil S., Muller M., Herling A. W., Schmoll D. 2011. Stimulation of fat oxidation, but no sustained reduction of hepatic lipids by prolonged pharmacological inhibition of acetyl CoA carboxylase. Horm. Metab. Res. 43: 601–606 [DOI] [PubMed] [Google Scholar]
- 34.Savage D. B., Choi C. S., Samuel V. T., Liu Z. X., Zhang D., Wang A., Zhang X. M., Cline G. W., Yu X. X., Geisler J. G., et al. 2006. Reversal of diet-induced hepatic steatosis and hepatic insulin resistance by antisense oligonucleotide inhibitors of acetyl-CoA carboxylases 1 and 2. J. Clin. Invest. 116: 817–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin X., Schonfeld G., Yue P., Chen Z. 2002. Hepatic fatty acid synthesis is suppressed in mice with fatty livers due to targeted apolipoprotein B38.9 mutation. Arterioscler. Thromb. Vasc. Biol. 22: 476–482 [DOI] [PubMed] [Google Scholar]
- 36.Zhong S., Magnolo A. L., Sundaram M., Zhou H., Yao E. F., Di Leo E., Loria P., Wang S., Bamji-Mirza M., Wang L., et al. 2010. Nonsynonymous mutations within APOB in human familial hypobetalipoproteinemia: evidence for feedback inhibition of lipogenesis and postendoplasmic reticulum degradation of apolipoprotein B. J. Biol. Chem. 285: 6453–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matsusue K., Kusakabe T., Noguchi T., Takiguchi S., Suzuki T., Yamano S., Gonzalez F. J. 2008. Hepatic steatosis in leptin-deficient mice is promoted by the PPARgamma target gene Fsp27. Cell Metab. 7: 302–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gong J., Sun Z., Wu L., Xu W., Schieber N., Xu D., Shui G., Yang H., Parton R. G., Li P. 2011. Fsp27 promotes lipid droplet growth by lipid exchange and transfer at lipid droplet contact sites. J. Cell Biol. 195: 953–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jambunathan S., Yin J., Khan W., Tamori Y., Puri V. 2011. FSP27 promotes lipid droplet clustering and then fusion to regulate triglyceride accumulation. PLoS ONE. 6: e28614. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.