Abstract
Angiopoietin-like 4 (ANGPTL4) is a regulator of LPL activity. In this study we examined whether different fatty acids have a differential effect on plasma ANGPTL4 levels during hyperinsulinemia in healthy lean males. In 10 healthy lean males, 3 hyperinsulinemic euglycemic clamps were performed during concomitant 6 h intravenous infusion of soybean oil (Intralipid® rich in PUFA), olive oil (Clinoleic® rich in MUFA) and control saline. In 10 other healthy lean males, 2 hyperinsulinemic clamps were performed during infusion of a mixed lipid emulsion containing a mixture of fish oil (FO), medium-chain triglycerides (MCTs), and long-chain triglycerides (LCTs) (FO/MCT/LCT; SMOFlipid®) or saline. FFA levels of approximately 0.5 mmol/l were reached during each lipid infusion. Plasma ANGPTL4 decreased during hyperinsulinemia by 32% (18–52%) from baseline. This insulin-mediated decrease in ANGPTL4 concentrations was partially reduced during concomitant infusion of olive oil and completely blunted during concomitant infusion of soybean oil and FO/MCT/LCT. The reduction in insulin sensitivity was similar between all lipid infusions. In accordance, incubation of rat hepatoma cells with the polyunsaturated fatty acid C22:6 increased ANGPTL4 expression by 70-fold, compared with 27-fold by the polyunsaturated fatty acid C18:2, and 15-fold by the monounsaturated fatty acid C18:1. These results suggest that ANGPTL4 is strongly regulated by fatty acids in humans, and is also dependent on the type of fatty acid.
Keywords: insulin sensitivity, fish oil, docosahexaenoic acid
Over the last decade, angiopoietin-like 4 (ANGPTL4) has emerged as a regulator of lipid metabolism (1). ANGPTL4 reduces clearance of VLDL and chylomicrons by inhibition of LPL activity and therefore increases plasma triglyceride (TG) concentration (2, 3). In addition, in transgenic mice ANGPTL 4 increases plasma FAs by stimulating white adipose tissue lipolysis (4).
In humans, ANGPTL4 is expressed ubiquitously, but predominantly in white adipose tissue and liver (5). In a cohort of 108 subjects, plasma ANGPTL4 positively correlated with fasting FFA and adipose tissue lipolysis (6). At present, it is known that plasma ANGPLTL4 levels increase during fasting, exercise, and chronic caloric restriction, and decrease during feeding and high insulin conditions (5). It is therefore hypothesized that during a negative energy balance or increased energy demand, ANGPTL4 is important for increasing lipid availability as energetic fuel. The above-mentioned conditions are characterized by high levels of plasma FFAs and it has been suggested that FFAs themselves are able to increase ANGPTL4 (5). This would serve to create a positive feedback loop with ongoing lipolysis in times of energy deficit. Indeed, expression of ANGPTL4 is directly stimulated by fatty acids in a number of tissues via activation of the peroxisome proliferator-activated receptors (PPARs) α, γ, and δ, which are the key transcriptional regulators of ANGPTL4 expression (5, 7–9). Insulin reduces circulating ANGPTL4 levels via mechanisms that are yet unknown. Recently, it was shown that an increase in plasma FFA by means of a lipid infusion during a hyperinsulinemic euglycemic clamp attenuates the insulin-mediated reduction of plasma ANGPTL4 (5). It remains unclear whether this blunted response is due to the FFA-induced insulin resistance or to the direct induction of ANGPTL4 by FFA.
We hypothesized that the reduction in insulin-mediated suppression of ANGPTL4 during concomitant infusion of a lipid emulsion is a direct effect of FFA on ANGPTL4 expression and not an indirect effect via induction of insulin resistance. Also, we hypothesized that a lipid emulsion rich in polyunsaturated fatty acids would have the most pronounced effect on ANGPTL4 because these fatty acids are known to be potent activators of peroxisome proliferator-activated receptors (PPARs). Therefore, we studied lean males during a hyperinsulinemic euglycemic clamp with concomitant infusion of different lipid emulsions or saline and assessed peripheral insulin sensitivity and measured ANGPTL4 levels. In addition, we measured ANGPTL4 expression in rat hepatoma cells after exposure to different fatty acids.
METHODS
Study participants
Twenty healthy lean males were included in this study. Participants were in good health, confirmed by medical history, physical examination, and routine blood chemistry. They had no family history of diabetes mellitus type-2 and had a normal glucose tolerance, assessed by an oral glucose tolerance test (10). Participants were nonsmokers, did not use any medication, and exercised at most two times a week. The protocol was approved by the Medical Ethical Committee of the Academic Medical Center in Amsterdam. All participants provided written informed consent before participation. Part of the data on insulin sensitivity and FFA levels during the hyperinsulinemic clamp in the group infused with olive oil and soybean oil has been published earlier (11).
Study design
Ten participants (group 1) underwent a hyperinsulinemic euglycemic clamp on three different occasions with concomitant infusion of either soybean oil 20% (Intralipid®, Fresensius Kabi Nederland B.V., Den Bosch, The Netherlands), olive oil 20% (Clinoleic®, Baxter, Clintec Benelux, Brussels, Belgium), or control saline 0.9%. Ten other participants (group 2) underwent a hyperinsulinemic euglycemic clamp during infusion of fish oil/medium-chain triglycerides/long-chain triglycerides (FO/MCTs/LCTs) (SMOFlipid®, Fresensius Kabi Nederland B.V., Den Bosch, The Netherlands) and control saline 0.9%. The order of the infusion of either the lipid emulsion or saline in both study groups was randomly assigned and each study occasion was scheduled at least 10 days apart.
[6,6-2H2]glucose (Cambridge Isotopes, Andover, MA) was infused during the hyperinsulinemic clamp as a stable glucose isotope tracer to measure peripheral insulin sensitivity. Intralipid® is a 100% soybean based lipid emulsion mainly containing the polyunsaturated fatty acid C18:2 (linoleic acid). Clinoleic® is a 80% olive oil based lipid emulsion mainly containing the monounsaturated fatty acid C18:1 (oleic acid). SMOF lipid® contains a mixture of 30% soybean oil, 30% medium-chain TGs, 25% olive oil, and 15% fish oil, therefore containing the polyunsaturated eicosapentaenoic acid (EPA) (C20:3) and docosahexaenoic acid (DHA) (C22:6). The exact fatty acid compositions of the three different lipid emulsions are described in supplementary Table I.
Experimental protocol
Participants were admitted to the metabolic unit at 7.30 AM after an overnight fast. They were instructed not to exercise or drink alcohol, and to consume 250 g of carbohydrates per day during the 3 days prior to the study day. After weight and body composition measurement using bioelectrical impedance analysis (Maltron BF-906, Maltron International Ltd, Essex, England), a catheter was inserted into the dorsal vein of each hand. One catheter was used for infusion of insulin, glucose, and the stable glucose isotope. The other catheter was kept in a thermo-regulated (60°C) Plexiglas box for sampling of arterialized venous blood. To keep the sampling line patent, a slow infusion of NaCl 0.9% was used.
At t = 0 h (8:00 AM) a blood sample was drawn for determination of [6,6-2H2]glucose background enrichment and a primed continuous infusion of [6,6-2H2]glucose was started: prime 8.8 µmol/kg, continuous 0.11 µmol/kg·min. At t = 1 h (9:00 AM) blood samples were drawn for basal measurements of plasma glucose, FFA, ANGPTL4, and insulin. Thereafter the infusion of insulin (60 mU/m2·min−1) [Actrapid (100 IU/ml); Novo Nordisk Farma B.V. Alphen aan den Rijn, The Netherlands] was started. Plasma glucose concentration was measured every 10 min and a 20% glucose solution was infused at a variable rate to maintain euglycemia at 5 mmol/l. The 20% glucose solution was enriched with [6,6-2H2]glucose to approximate the values for enrichment reached in plasma and thereby minimizing changes in isotopic enrichment due to changes in the infusion rate of exogenous glucose. At the start of the clamp, a concomitant infusion of a lipid emulsion or saline was started for 6 h. The plasma FFA concentration was measured every 30 min and the lipid infusion rate of each lipid emulsion was adjusted to clamp the FFA levels at ∼0.5 mmol/l. The control saline infusion was kept at 100 ml/h.
At the end of the clamp, t = 7 h (3:00 PM), 5 blood samples were drawn at 5 min intervals for determination of glucose enrichments. Another blood sample was drawn for determination of insulin, FFA, and ANGPTL4.
Laboratory analyses
ANGPTL4 was measured by ELISA as described previously (1). Briefly, 96-well plates were coated with anti-human ANGPTL4 polyclonal goat IgG antibody (AF3485, R and D Systems) and incubated overnight at 4°C. Plates were washed extensively between each step. After blocking, 100 μl of 20-fold diluted human plasma was applied, followed by a 2 h incubation at room temperature. A standard curve was prepared using recombinant human ANGPTL4 (3485-AN, R and D Systems) at 0.3 to 2.1 ng/well. Next, 100 μl of diluted biotinylated anti-human ANGPTL4 polyclonal goat IgG antibody (BAF3485, R and D Systems) was added for 2 h, followed by addition of streptavidin-conjugated horseradish peroxidase for 20 min, and tetramethylbenzidine substrate reagent for 6 min. The reaction was stopped by addition of 50 μl of 10% H2SO4, and the absorbance was measured at 450 nm. The intra-assay coefficient of variation for the assay was determined at 7%.
Plasma glucose concentrations were measured with the glucose oxidase method using a Biosen C-line Plus glucose analyzer (EKF Diagnostics, Barleben/Magedeburg, Germany). Insulin was determined on an Immulite 2000 system (Diagnostic Products Corporation, Los Angeles, CA) with a chemiluminescent immunometric assay (intra-assay variation 3–6%; total-assay variation 4%; detection limit, 15 pmol/l). TGs were determined on a Hitachi-P (Roche Diagnostics GmbH, Mannheim, Germany), intra-assay variation 0.6–0.9% and inter-assay variation 1.6–2.0%.
Plasma FFA concentrations were measured with an enzymatic colorimetric method (NEFA-C test kit; Wako Chemicals GmbH, Neuss, Germany) with an intra-assay variation of 1%, inter-assay variation of 4–15%, and detection limit of 0.02 mmol/l. [6,6-2H2]glucose enrichment was measured with GC-MS as described in detail earlier (12, 13).
The fatty acid profile was measured at baseline. Therefore, 50 μl of plasma was transmethylated in 1 ml 3 M HCl by incubating for 4 h at 90°C in the presence of 10 nmol internal standard; the methyl ester of 18-methylnonadecanoic acid. After cooling, the aqueous layer was extracted in 2 ml hexane, and this extract was taken to dryness under nitrogen flow and resuspended in 80 μl of hexane. One microliter of this solution was injected into a Hewlett Packard GC 5890 equipped with an Agilent J and W HP-FFAP, 25 m, 0.20 mm, 0.33 µm GC Column and eluting fatty acid methylesters were detected by flame ionization detection. Fatty acid concentration was calculated using the known amount of internal standard and expressed as μM for plasma.
Calculations
Peripheral insulin sensitivity rate of disappearance (Rd) was calculated using the Steele equation for the nonsteady state (14, 15).
Statistics
Data are presented as median and range. Primary outcomes were the change in plasma ANGPTL4 concentration, expressed as the percentage of change from baseline concentration, and insulin sensitivity, expressed as glucose uptake (Rd) as the percentage from Rd during control saline. Differences between the FO/MCT/LCT in group 2 and soybean oil and olive oil in group 1 were each tested separately using the unpaired Mann-Whitney U test. Differences within each treatment group compared with control saline were tested using a nonparametric related Wilcoxon signed-rank test. Nonparametric analysis was chosen due to the relatively small number of participants. Correlations between baseline ANGPTL4 and fatty acid concentrations were calculated with the Spearmen correlation test. All statistical analyses were run on SPSS for Windows version 16.0 (SPSS, Chicago, IL). P < 0.05 was considered statistically significant.
In vitro study
Rat hepatoma FAO cells were grown in DMEM containing 10% (v/v) fetal bovine serum, 100 U/ml penicillin, and 100 ug/ml streptomycin. Cells were incubated with albumin only (control), albumin-bound oleic acid, linoleic acid, or docosahexaenoic acid (100 umol/l) for 48 h, followed by RNA isolation and quantitative RT-PCR. cDNA was prepared using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories BV, Veenendaal, The Netherlands). RT-PCR was carried out using platinum Taq polymerase (Invitrogen, Breda, The Netherlands) and SYBR Green using an iCycler PCR machine (Bio-Rad Laboratories BV). Melt curve analysis was performed to assure a single PCR product was formed. Rat ANGPTL4 cDNA was amplified using primers: 5′-GTTTGCAGACTCAGCTCAAGG-3′ and 5′-CCAAGAGGTCTATCTGGCTCTG-3′. ANGPTL4 expression data were normalized against the housekeeping genes 36B4.
We chose FAO cells because it is not possible to obtain robust induction of PPAR targets including ANGPTL4 in human hepatoma cell lines in response to fatty acids, probably because one of the essential mechanisms for fatty acid-dependent gene regulation is strongly down-regulated in these cell lines. Indeed, incubation of Huh-7 and HepG2 cell lines with fatty acids induced no ANGPTL4 expression (data not shown).
RESULTS
Anthropometric data are summarized in Table 1. Baseline characteristics of the participants did not differ between the two study groups. Weight and body fat percentage of each participant did not differ on the different study occasions.
TABLE 1.
Baseline characteristics
| Group 1 | Group 2 | P | |
| Age (years) | 23 (19–30) | 24 (20–25) | ns |
| Weight (kg) | 74.9 (70.3–91.4) | 74.9 (65.6–89.9) | ns |
| Body mass index (kg/m2) | 21.4 (19.6–25.7) | 22.2 (19.3–25.1) | ns |
| Body fat (%) | 14.0 (11.1–26.1) | 14.1 (9.8–24.4) | ns |
| Fasting glucose (mmol/l) | 4.7 (3.8–5.2) | 4.8 (4.2–5.2) | ns |
Data are expressed as median (range) N = 10 in each study group. ns, not significant.
Glucose metabolism
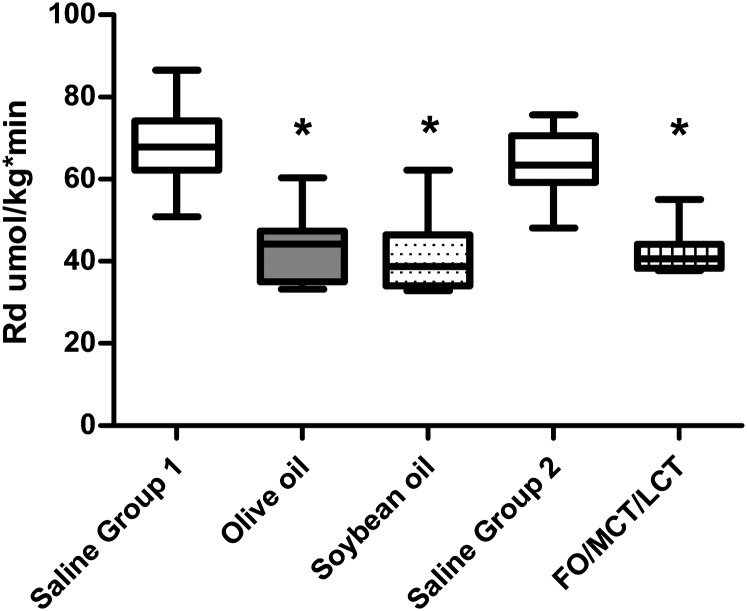
Infusion of all lipid emulsions decreased peripheral glucose uptake significantly compared with control saline (Fig. 1), as partially reported earlier (11). The decrement in peripheral insulin sensitivity expressed as a percentage of the control saline condition during FO/MCT/LCT infusion was 65.4% (50.7–87%), and not different compared with olive oil [56.6% (45.8–82.6%); P = 0.290] or compared with soybean oil [68% (44–84.0%); P = 0.940]. In group 1, the decrease in Rd was not different between olive oil and soybean oil (P = 0.455).
Fig. 1.
Peripheral glucose uptake (Rd) during intravenous saline or an intravenous lipid emulsion (Rd in umol/kg/min). *P < 0.01 compared with control saline (11). Boxplots represent median, 25th percentile, 75th percentile, minimum, and maximum, respectively.
ANGPTL4
Baseline ANGPTL4 concentrations were not different between or within groups (Table 2). Baseline ANGPTL4 concentrations did not correlate with FFA, total polyunsaturated fatty acids, or with the individual polyunsaturated fatty acids linoleic acid, EPA, and DHA or with TGs (data not shown).
TABLE 2.
Plasma concentrations
| Group 1 |
Group 2 |
||||
| Saline 1 | Olive Oil | Soybean Oil | Saline 2 | FO/MCT/LCT | |
| Insulin (pmol/l) | 560 (470–801) | 568 (303–861) | 588 (485–789] | 501 (397–641) | 538 (457–639) |
| FFA, t = 0 h (mmol/l) | 0.36 (0.21–0.71) | 0.39 (0.17–0.63) | 0.46 (0.13–0.60) | 0.50 (0.23–0.62) | 0.42 (0.21–0.56) |
| FFA, t = 6 h (mmol/l) | <0.02 | 0.46 (0.34–0.64)a | 0.49 (0.32–0.56)a | <0.02 | 0.51 (0.35–0.62)a |
| TGs, t = 0 h (mmol/l) | 0.91 (0.62–1.45) | 0.87 (0.33–1.42) | 0.87 (0.38–0.96) | 0.56 (0.35–1.35) | 0.61 (0.21–1.65) |
| ANGPTL4, t = 0 h (ng/ml) | 13.6 (5.5–21.2) | 11.6 (5.5–19.7) | 8.3 (2.3–40.6) | 10.4 (6.4–17.5) | 9.2 (5.7–14.2) |
| ANGPTL4, t = 6 h (% from t = 0 h) | 70.6 (48.4–81.9) | 76.5 (71.3–106.2)bc | 99.2 (87.5–183.2)a | 59.9 (52.3–78.3) | 103.1 (69.6–161.3)a |
Data are expressed as median (range) N = 10 in each study group.
P < 0.01 compared with saline.
P = 0.022 compared with saline.
P = 0.022 compared with soybean oil.
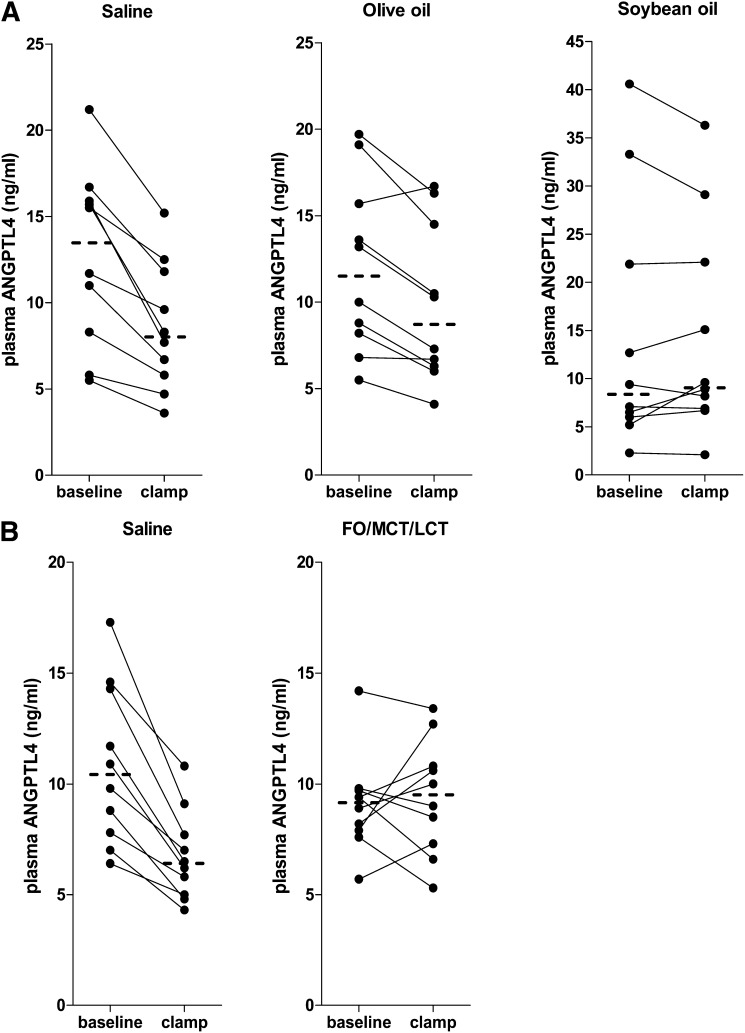
Hyperinsulinemia decreased plasma ANGPTL4 levels by 30% (18–52%) from baseline in the saline condition in group 1 and by 40% (22–48%) from baseline in the saline condition in group 2 (Fig. 2). During hyperinsulinemia with concomitant infusion of soybean oil and FO/MCT/LCT, the insulin-mediated reduction of ANGPTL4 levels was blunted (Fig. 2). The combined infusion of insulin and olive oil reduced ANGPTL4 levels by 24% (−28 to +6%) which was significantly less compared with saline (P = 0.022). This decrease in ANGPTL4 during infusion of insulin and olive oil was higher compared with soybean oil (P = 0.022) and tended to be higher compared with the FO/MCT/LCT condition (P = 0.075), meaning that soybean oil and FO/MCT/LCT were more potent in counteracting the insulin effects on ANGPTL4.
Fig. 2.
Plasma ANGPTL4 concentrations at baseline and during a 6 h hyperinsulinemic clamp with concomitant infusion of either: saline, olive oil and soybean oil (A) or control saline and FO/MCT/LCT (B). N = 10 in each study group. Dashed lines indicate median concentrations at t = 0 h and t = 6 h.
Plasma FFA
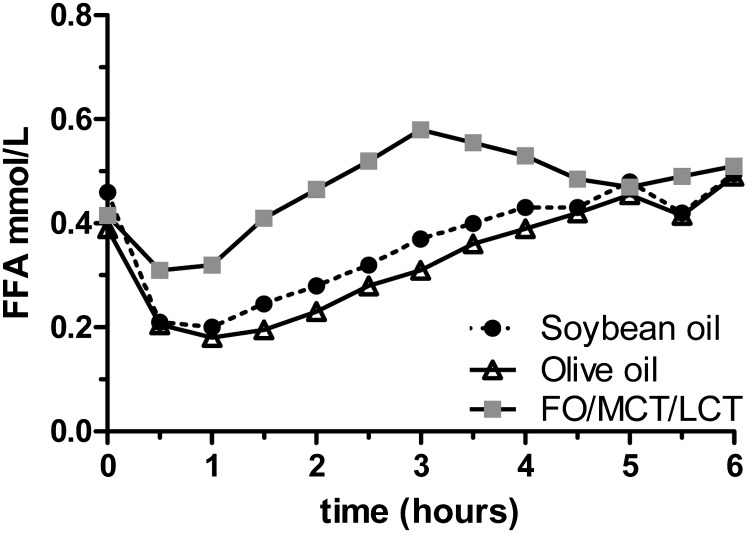
As expected, plasma FFA levels were completely suppressed during hyperinsulinemia in the saline condition. FFA levels during infusion of olive oil and soybean oil were comparable. FFA levels during the infusion of FO/MCT/LCT were higher for the first 4 h, but similar thereafter (Fig. 3).
Fig. 3.
Plasma FFA concentrations during the 6 h hyperinsulinemic euglycemic clamp with concomitant infusion of soybean oil, olive oil or FO/MCT/LCT. Data are expressed as median. N = 10 per group.
The total amount of lipid emulsion infused was not different between soybean oil [681 ml (344–1271 ml)] and olive oil [735 ml (554–1055 ml)], but was significantly lower during FO/MCT/LCT infusion [364 ml (305–421 ml)].
ANGPTL4 expression in rat hepatoma cells
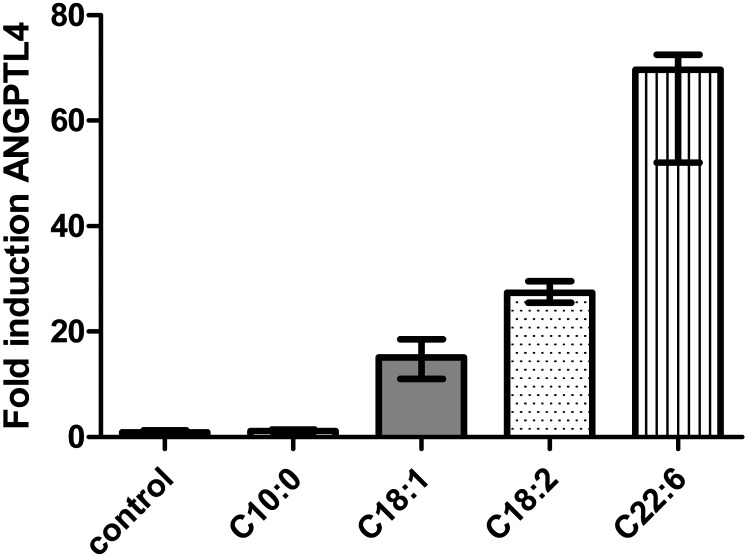
The direct effect of the different fatty acids on ANGPTL4 expression in rat hepatoma cells is shown in Fig. 4. The polyunsaturated fatty acid C22:6 increased ANGPTL4 expression by 70-fold, the polyunsaturated fatty acid C18:2 by 27-fold, and the monounsaturated fatty acid C18:1 by 15-fold. Incubation with the medium-chain fatty acid C10:0 did not increase ANGPTL4 expression.
Fig. 4.
Expression of ANGPTL4 in rat hepatoma cells after incubation during 48 h with albumin, C:10, C18:1, C18:2, and C22:6.
DISCUSSION
This study demonstrates the differential effects of different lipid emulsions on circulating ANGPTL4 levels in lean males during hyperinsulinemia. It is known that insulin reduces ANGPTL4 levels (1, 16, 17). This finding was confirmed in our subjects with a significant decrease in circulating ANGPTL4 during the hyperinsulinemic euglycemic clamps. We now show for the first time that the insulin-mediated decrease in ANGPTL4 levels is blunted by concomitant infusion of a lipid emulsion containing a high percentage of polyunsaturated fatty acids or a lipid emulsion containing, among other FFAs, the omega-3 polyunsaturated fatty acids EPA and DHA. The insulin-mediated decrease in ANGPTL4 was much less attenuated upon infusion of olive oil, containing the highest percentage of the monounsaturated fatty acid C18:1.
Because reduction in peripheral insulin sensitivity was not different between all lipid emulsions, these results suggest a direct and differential effect of different lipid emulsions on plasma ANGPTL4 depending on the specific fatty acid composition of the lipid emulsion.
These findings are in line with the in vitro experiments, showing the lowest expression of ANGPTL4 after incubation with C18:1, and highest induction by DHA. SMOFlipid® also contains medium-chain fatty acids. The in vitro experiment in rat hepatoma cells showed no effect of C10:0 on ANGPTL4 expression. This can be explained by the fact that ANGPTL4 expression is under control of PPARs, and medium-chain fatty acids do not activate PPARs. Therefore, the blunted insulin-induced reduction in plasma ANGPTL4 during infusion of FO/MCT/LCT emulsion could not be explained by the presence of medium-chain fatty acids, and is probably related to the potent effect of PUFAs, especially DHA. ANGPTL4 expression is under control of the PPAR family (7–9). Because polyunsaturated fatty acids are known to be potent activators of PPARs, it seems likely that the blunted insulin-mediated decrements during infusion of soybean oil and FO/MCT/LCT is related to transcriptional stimulation of ANGPTL4 via activated PPARs. Depending on the type of tissue involved, the effect of fatty acids on ANGPTL4 mRNA can be mediated by different PPAR isotypes (18). Recently it was shown that PPARδ, but not PPARα, serves as a plasma FFA sensor in the liver (19). Krey et al. (20) showed that the affinity of PPARδ for linoleic acid is higher than for oleic acid, and that 8(S)-HETE (an arachidonic acid metabolite), linolenic, and linoleic acids are the most potent natural ligands for PPARδ. This suggests that differences in the observed ANGPTL4 concentrations may be related to the specific effect of the different fatty acids used on PPAR activation. In addition, metabolites of the polyunsaturated fatty acids might have been responsible for part of the observed effect on ANGPTL4. Our in vitro data indicate that the differences in plasma ANGPTL4 concentrations could be explained by a more potent effect of polyunsaturated fatty acids on expression and production of ANGPTL4 by hepatocytes. In humans, plasma ANGPTL4 seems to be predominantly derived from the liver, but is also secreted by other tissues (5, 21). Incubation of human myotubes for 20 h with palmitate, stearate, palmitoleate, oleate, linoleate, or a combination of palmitate and linoleate all increased ANGPTL4 expression to a similar extent (6). This suggests that in muscle as compared to liver, ANGPTL4 induction depends neither on the chain length nor on the presence of double bonds of fatty acids, but rather, represented a general long-chain FA effect (6).
To date, the role of ANGPTL4 has not been completely elucidated. Previous studies have shown that ANGPTL4 inhibits LPL activity by converting active LPL dimers into inactive LPL monomers and accordingly reduces plasma TG clearance (1). In line, ANGPTL4 overexpression in mice markedly increases plasma TG levels (4, 22).
Although experimental animal studies support the role of ANGPTL4 in the regulation of LPL activity and plasma TG levels, its effect in humans is less clear. In the postprandial state, suppression of ANGPTL4 by insulin may be important for clearance and storage of TG-rich lipoproteins, whereas in the fasting state, ANGPTL4 ensures fuel supply by stimulating adipose tissue lipolysis and reducing TG clearance (1).
From a study of diabetic rats, it has been suggested that the fatty acid-induced stimulation of ANGPTL4 might serve to protect tissues from FFA-induced cellular toxicity via a reduction in hydrolysis of TGs (23). Plasma ANGPTL4 in humans has been correlated with adiposity and fasting FFA, but a significant correlation between plasma TG levels and ANGPTL 4 has not been established (24). In line with this, we did not find a significant correlation between fasting TGs, total FFAs, and ANGPTL4. In addition, baseline ANGPTL4 levels did not correlate with fasting total PUFA or linoleic acid, EPA, and DHA concentrations. Population-based sequencing of ANGPTL4 however uncovered variations that are associated with lower plasma TGs (25).
In the current study, we combined hyperinsulinemia with high levels of circulating FFA, a condition characteristic for obese insulin-resistant subjects. Recently it has been shown that in young healthy subjects insulin reduced ANGPTL4 expression in adipose tissue by 70%, while this effect was absent in insulin-resistant diabetic subjects and older control subjects. ANGPTL4 expression in adipose tissue in that study was linked to insulin resistance and body mass index, and it was therefore suggested to contribute to the hypertriglyceridemia present in insulin resistant individuals (26). An association between adipose tissue ANGPTL4 expression and LPL activity was not found (26). Therefore, the role of ANGPTL4 in insulin resistance and hypertriglyceridemia in human subjects remains to be elucidated.
Our study has some limitations. First, we aimed at a similar exposure to FFA during infusion of all three lipid emulsions, because the FFA-induced reduction in insulin sensitivity is dose dependent (27). Although the absolute FFA concentrations during the last 1.5 h of the clamp were not different, the rise in FFA concentration during infusion of FO/MCT/LCT was much steeper and the total infusion rate to reach similar levels of FFA was lower. Despite the lower infusion rate of FO/MCT/LCT, in addition to its lower soybean oil content, the potency to attenuate the insulin effect on ANGPTL4 was similar to pure soybean oil. This can be explained by the finding that DHA induced ANGPTL4 expression most potently in rat hepatoma cells. However, we cannot exclude that the higher level of plasma FFA during the first several hours of the clamp had an independent effect on our results. Second, we only measured ANGPTL4 concentrations during insulin infusion. It would be interesting to see whether lipid infusion without coinfusion of insulin also induces changes in plasma ANGPTL4. Finally, the design of our study does not allow any conclusions to be drawn on how plasma lipids interfere with ANGPTL4 levels during hyperinsulinemia in humans.
In conclusion, the insulin-induced suppression of ANGPTL4 is abolished during infusion of a lipid emulsion high in polyunsaturated fatty acids and is reduced during infusion of a lipid emulsion high in monounsaturated fatty acids. Omega-3 fatty acids seem to have the highest potency to induce ANGPTL4. It remains to be studied whether higher plasma ANGPTL4 concentrations during both hyperinsulinemia and elevated circulating FFA explain the postprandial hypertriglyceridemia in obesity and insulin resistance.
Supplementary Material
Footnotes
Abbreviations:
- ANGPTL4
- angiopoietin-like 4
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- FO
- fish oil
- LCT
- long-chain triglyceride
- MCT
- medium-chain triglyceride
- PPAR
- peroxisome proliferator-activated receptor
- Rd
- rate of disappearance
- TG
- triglyceride
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of two tables and four figures.
REFERENCES
- 1.Kersten S. 2005. Regulation of lipid metabolism via angiopoietin-like proteins. Biochem. Soc. Trans. 33: 1059–1062 [DOI] [PubMed] [Google Scholar]
- 2.Yoshida K., Shimizugawa T., Ono M., Furukawa H. 2002. Angiopoietin-like protein 4 is a potent hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein lipase. J. Lipid Res. 43: 1770–1772 [DOI] [PubMed] [Google Scholar]
- 3.Xu A., Lam M. C., Chan K. W., Wang Y., Zhang J., Hoo R. L., Xu J. Y., Chen B., Chow W. S., Tso A. W., et al. 2005. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc. Natl. Acad. Sci. USA. 102: 6086–6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mandard S., Zandbergen F., van Straten E., Wahli W., Kuipers F., Muller M., Kersten S. 2006. The fasting-induced adipose factor/angiopoietin-like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J. Biol. Chem. 281: 934–944 [DOI] [PubMed] [Google Scholar]
- 5.Kersten S., Lichtenstein L., Steenbergen E., Mudde K., Hendriks H. F., Hesselink M. K., Schrauwen P., Muller M. 2009. Caloric restriction and exercise increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler. Thromb. Vasc. Biol. 29: 969–974 [DOI] [PubMed] [Google Scholar]
- 6.Staiger H., Haas C., Machann J., Werner R., Weisser M., Schick F., Machicao F., Stefan N., Fritsche A., Haring H. U. 2009. Muscle-derived angiopoietin-like protein 4 is induced by fatty acids via peroxisome proliferator-activated receptor (PPAR)-delta and is of metabolic relevance in humans. Diabetes. 58: 579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgiadi A., Lichtenstein L., Degenhardt T., Boekschoten M. V., van Bilsen M., Desvergne B., Muller M., Kersten S. 2010. Induction of cardiac Angptl4 by dietary fatty acids is mediated by peroxisome proliferator-activated receptor beta/delta and protects against fatty acid-induced oxidative stress. Circ. Res. 106: 1712–1721 [DOI] [PubMed] [Google Scholar]
- 8.Kersten S., Mandard S., Tan N. S., Escher P., Metzger D., Chambon P., Gonzalez F. J., Desvergne B., Wahli W. 2000. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferator-activated receptor target gene. J. Biol. Chem. 275: 28488–28493 [DOI] [PubMed] [Google Scholar]
- 9.Yoon J. C., Chickering T. W., Rosen E. D., Dussault B., Qin Y., Soukas A., Friedman J. M., Holmes W. E., Spiegelman B. M. 2000. Peroxisome proliferator-activated receptor gamma target gene encoding a novel angiopoietin-related protein associated with adipose differentiation. Mol. Cell. Biol. 20: 5343–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. 2003. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 26 (Suppl. 1): S5–S20.
- 11.Brands M., Hoeks J., Sauerwein H. P., Ackermans A. T., Ouwens M., Lammers N. M., van der Plas M. N., Schrauwen P., Groen A. K., Serlie M. J. 2011. Short-term increase of plasma free fatty acids does not interfere with intrinsic mitochondrial function in healthy young men. Metabolism. 60: 1398–1405 [DOI] [PubMed] [Google Scholar]
- 12.Ackermans M. T., Ruiter A. F., Endert E. 1998. Determination of glycerol concentrations and glycerol isotopic enrichments in human plasma by gas chromatography/mass spectrometry. Anal. Biochem. 258: 80–86 [DOI] [PubMed] [Google Scholar]
- 13.Ackermans M. T., Pereira Arias A. M., Bisschop P. H., Endert E., Sauerwein H. P., Romijn J. A. 2001. The quantification of gluconeogenesis in healthy men by (2)H2O and [2-(13)C]glycerol yields different results: rates of gluconeogenesis in healthy men measured with (2)H2O are higher than those measured with [2-(13)C]glycerol. J. Clin. Endocrinol. Metab. 86: 2220–2226 [DOI] [PubMed] [Google Scholar]
- 14.Finegood D. T., Bergman R. N., Vranic M. 1987. Estimation of endogenous glucose production during hyperinsulinemic-euglycemic glucose clamps. Comparison of unlabeled and labeled exogenous glucose infusates. Diabetes. 36: 914–924 [DOI] [PubMed] [Google Scholar]
- 15.Steele R. 1959. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann. N. Y. Acad. Sci. 82: 420–430 [DOI] [PubMed] [Google Scholar]
- 16.Mattijssen F., Kersten S. 2012. Regulation of triglyceride metabolism by angiopoietin-like proteins. Biochim. Biophys. Acta. 1821: 782–789 [DOI] [PubMed] [Google Scholar]
- 17.van Raalte D. H., Brands M., Serlie M. J., Mudde K., Stienstra R., Sauerwein H. P., Kersten S., Diamant M. 2012. Angiopoietin-like protein 4 is differentially regulated by glucocorticoids and insulin in vitro and in vivo in healthy humans. Exp. Clin. Endocrinol. Diabetes. 120: 598–603 [DOI] [PubMed] [Google Scholar]
- 18.Lichtenstein L., Kersten S. 2010. Modulation of plasma TG lipolysis by angiopoietin-like proteins and GPIHBP1. Biochim. Biophys. Acta. 1801: 415–420 [DOI] [PubMed] [Google Scholar]
- 19.Sanderson L. M., Degenhardt T., Koppen A., Kalkhoven E., Desvergne B., Muller M., Kersten S. 2009. Peroxisome proliferator-activated receptor beta/delta (PPARbeta/delta) but not PPARalpha serves as a plasma free fatty acid sensor in liver. Mol. Cell. Biol. 29: 6257–6267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krey G., Braissant O., L'Horset F., Kalkhoven E., Perroud M., Parker M. G., Wahli W. 1997. Fatty acids, eicosanoids, and hypolipidemic agents identified as ligands of peroxisome proliferator-activated receptors by coactivator-dependent receptor ligand assay. Mol. Endocrinol. 11: 779–791 [DOI] [PubMed] [Google Scholar]
- 21.Mandard S., Zandbergen F., Tan N. S., Escher P., Patsouris D., Koenig W., Kleemann R., Bakker A., Veenman F., Wahli W., et al. 2004. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J. Biol. Chem. 279: 34411–34420 [DOI] [PubMed] [Google Scholar]
- 22.Köster A., Chao Y. B., Mosior M., Ford A., Gonzalez-DeWhitt P. A., Hale J. E., Li D., Qiu Y., Fraser C. C., Yang D. D., et al. 2005. Transgenic angiopoietin-like (angptl)4 overexpression and targeted disruption of angptl4 and angptl3: regulation of triglyceride metabolism. Endocrinology. 146: 4943–4950 [DOI] [PubMed] [Google Scholar]
- 23.Wang Y., Puthanveetil P., Wang F., Kim M. S., Abrahani A., Rodrigues B. 2011. Severity of diabetes governs vascular lipoprotein lipase by affecting enzyme dimerization and disassembly. Diabetes. 60: 2041–2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staiger H., Machicao F., Werner R., Guirguis A., Weisser M., Stefan N., Fritsche A., Haring H. U. 2008. Genetic variation within the ANGPTL4 gene is not associated with metabolic traits in white subjects at an increased risk for type 2 diabetes mellitus. Metabolism. 57: 637–643 [DOI] [PubMed] [Google Scholar]
- 25.Romeo S., Pennacchio L. A., Fu Y., Boerwinkle E., Tybjaerg-Hansen A., Hobbs H. H., Cohen J. C. 2007. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat. Genet. 39: 513–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruge T., Sukonina V., Kroupa O., Makoveichuk E., Lundgren M., Svensson M. K., Olivecrona G., Eriksson J. W. 2012. Effects of hyperinsulinemia on lipoprotein lipase, angiopoietin-like protein 4, and glycosylphosphatidylinositol-anchored high-density lipoprotein binding protein 1 in subjects with and without type 2 diabetes mellitus. Metabolism. 61: 652–660 [DOI] [PubMed] [Google Scholar]
- 27.Belfort R., Mandarino L., Kashyap S., Wirfel K., Pratipanawatr T., Berria R., DeFronzo R. A., Cusi K. 2005. Dose-response effect of elevated plasma free fatty acid on insulin signaling. Diabetes. 54: 1640–1648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.