Abstract
Obese women have an increased risk to deliver large babies. However, the mechanisms underlying fetal overgrowth in these pregnancies are not well understood. Obese pregnant women typically have elevated circulating lipid levels. We tested the hypothesis that fatty acids stimulate placental amino acid transport, mediated via toll-like receptor 4 (TLR4) and mammalian target of rapamycin (mTOR) signaling pathways. Circulating NEFA levels and placental TLR4 expression were assessed in women with varying prepregnancy body mass index (BMI). The effects of oleic acid on system A and system L amino acid transport, and on the activation of the mTOR (4EBP1, S6K1, rpS6), TLR4 (IĸBɑ, JNK, p38 MAPK), and STAT3 signaling pathways were determined in cultured primary human trophoblast cells. Maternal circulating NEFAs (n = 33), but not placental TLR4 mRNA expression (n = 16), correlated positively with BMI (P < 0.05). Oleic acid increased trophoblast JNK and STAT3 phosphorylation (P < 0.05), whereas mTOR activity was unaffected. Furthermore, oleic acid doubled trophoblast system A activity (P < 0.05), without affecting system L activity. siRNA-mediated silencing of TLR4 expression prevented the stimulatory effect of oleic acid on system A activity. Our data suggest that maternal fatty acids can increase placental nutrient transport via TLR4, thereby potentially affecting fetal growth.
Keywords: nutrient transport, fatty acids, pregnancy, obesity
Maternal obesity affects almost 20% of all pregnancies in the United States (1), with obese women being more likely to deliver large babies (2). Fetal overgrowth is associated with increased risks of perinatal complications (3) and metabolic syndrome in childhood and later in life (4, 5). Moreover, obese women who were born large themselves have a higher probability of delivering large babies (6). Thus, fetal overgrowth has both short- and long-term adverse consequences, potentially also impacting future generations. The mechanisms underlying fetal overgrowth in maternal obesity are not well established. Previous studies have shown that placental nutrient transport is upregulated in pregnancies complicated by type-1 or gestational diabetes with fetal overgrowth (7, 8), raising the possibility that increased placental nutrient transport capacity contributes to increased fetal growth also in obese women.
Fatty acids are essential for fetal development. For example, they constitute part of cellular membranes, are precursors of bioactive molecules, and are important for proper development of the brain and retina (9). In addition, fatty acids may function as modulators of toll-like receptor 4 (TLR4) signaling activity (10, 11). Activation of TLR4 results in transcription of inflammatory related genes, mediated by numerous signaling pathways, including c-Jun N-terminal kinase (JNK), nuclear factor-ĸB (NFĸB), and p38 mitogen-activated protein kinase (MAPK). The TLR4 signaling network is complex. There are several adaptor proteins downstream of the TLR4 receptor, and depending on which adaptor proteins are recruited following receptor stimulation, different intracellular signaling pathways may be activated (12).
In human placenta, TLR4 is expressed in the maternal facing microvillous plasma membrane (MVM) of the syncytiotrophoblast (13, 14), the transporting epithelium, suggesting that placental TLR4 can be activated by factors in the maternal circulation. Recently it was reported that placental TLR4 mRNA expression is increased in obese ewes (15) and in Japanese macaques fed a high-fat diet (16). However, it is unknown whether maternal obesity affects TLR4 expression in human placenta. Furthermore, lipopolysaccharide (LPS), which activates TLR4 signaling (12), has been shown to increase system A amino acid transporter activity in rat liver (17), consistent with a link between TLR4 and amino acid transport.
The human placenta exhibits distinct alterations in its ability to transport nutrients in cases of altered fetal growth. Placenta from pregnancies complicated by fetal growth restriction have a reduced activity of the amino acid transporters system A and system L (18, 19), whereas these transporter activities are increased in diabetic pregnancies associated with fetal overgrowth (7, 8). The molecular mechanisms regulating placental amino acid transporters involve multiple signaling pathways (20). We have previously shown in cultured human primary trophoblast cells that the mammalian target of rapamycin (mTOR) signaling pathway is a positive regulator of system A and system L amino acid transporter activities (21). Furthermore, placental mTOR signaling is reduced in cases of fetal growth restriction (22). Data from our laboratory suggests that mTOR signaling is increased in response to maternal obesity (23, 24). Several factors modulate mTOR signaling, such as hormones and nutrients levels (25), and oleic acid has been reported to activate mTOR signaling (26). Activation of mTOR leads to phosphorylation of the downstream targets eukaryotic initiation factor 4E binding protein 1 (4EBP1), S6 kinase 1 (S6K1), and ribosomal protein S6 (rpS6) (25).
Cytokines and hormones represent another mechanism by which placental amino acid transport is regulated. For instance, interleukin 6 (IL-6), leptin, and tumor necrosis factor ɑ (TNF-ɑ) increases the activity of the system A amino acid transporter (27, 28). The effects of IL-6 and leptin, but not TNF-ɑ, are mediated through activation of signal transducer and activator of transcription 3 (STAT3) (27, 28). In a hepatocarcinoma cell line (HepG2), exposure to oleic acid increases phosphorylation of STAT3 (29) and activates mTOR signaling (26). Hence, oleic acid can activate two different signaling pathways, mTOR and STAT3, both known as positive regulators of placental amino acid transport.
We tested the hypothesis that circulating fatty acids and placental TLR4 expression are increased in women with high prepregnancy body mass index (BMI), and that fatty acids stimulate placental amino acid transport through TLR4, mTOR, and STAT3 signaling pathways. We investigated the effect of oleic acid on system A and system L amino acid transporter activity and activation of intracellular signaling pathways in cultured human primary trophoblast cells isolated from term placentas. We used oleic acid because it is one of the most predominant fatty acids in vivo and constitutes approximately 30% of maternal circulating nonesterified fatty acids (NEFAs) during pregnancy (30). Using RNA interference techniques, TLR4 expression was downregulated to test the hypothesis that oleic acid alters placental amino acid transport through interaction with this receptor.
MATERIALS AND METHODS
Subjects
Human term (>37 weeks gestation) placental tissue and/or maternal blood were collected from singleton pregnancies after informed consent (n = 57). Women with pregnancy complications (such as gestational diabetes and preeclampsia) or concurrent maternal diseases were excluded from the study. The protocols were approved by the Committee for Research Ethics at University of Gothenburg and the Institutional Review Board at University of Texas Health Science Center, San Antonio. Only nonlaboring women were included in the study of circulating NEFAs and placental TLR4 mRNA expression; their clinical characteristics are presented in Table 1 (n = 38).
TABLE 1.
Clinical characteristics of study subjects
| Characteristic | Normal BMI(BMI < 24.9) | High BMI(BMI ≥ 25.0) |
| N | 13 | 25 |
| BMI (kg/m2) | 22.0 ± 0.6 | 32.4 ± 1.4* |
| BMI range (min–max) | 18.4–24.4 | 25.0–54.3 |
| Maternal age (years) | 30.0 ± 1.6 | 27.2 ± 1.1 |
| Ethnicity (% Hispanic) | 69.2 | 72.0 |
| Parity (primiparous/multiparous) | 1/12 | 1/24 |
| Gestational age (weeks) | 39.2 ± 0.3 | 39.1 ± 0.2 |
| Birth weight (g) | 3,354 ± 80 | 3,420 ± 80 |
| Ponderal index (100 × BW / length3) | 2.50 ± 0.05 | 2.64 ± 0.05 |
| Placental weight (g) | 638 ± 34 | 730 ± 28* |
| Macrosomic (>4000 g) | 1 | 3 |
| Fetal gender (female/male) | 6/7 | 15/10 |
Data is presented as mean ± SEM. BMI based on prepregnancy weight or early pregnancy weight (<20 weeks gestation). *P < 0.05 normal BMI versus high BMI, t-test. BW, birth weight.
Measurement of plasma NEFAs
Fasting maternal blood was collected prior to Cesarean section. NEFA concentrations were determined with an enzymatic colorimetric assay (Wako Diagnostics, Richmond, VA).
Isolation and maintenance of trophoblast cells
Placental villous cytotrophoblasts were isolated as previously described (31, 32) by DNase/trypsin digestion and purified by separation on a Percoll gradient (n = 19 placentas). This protocol has been widely used and validated, and results in the isolation of cells that are expressing cytokeratin 7, a syncytial marker, that do not express vimentin, and that undergo biochemical differentiation as measured by hCG release (21, 27, 32, 33). The cells were plated on 6-well plates at a density of 1.5 × 106 per well (for amino acid uptake experiments and protein expression analysis) or 3.5 × 106 per well (for amino acid uptake after siRNA transfection). The cells were cultured for a total of 90 h at 37°C in 95% air, 5% CO2 atmosphere. The cell culture media (10% FBS, 45% high glucose DMEM, 45% Ham's F-12, supplemented with antibiotics) was changed daily. Sixty-six hours after plating, to allow for syncytialization, 400 µM oleic acid (dissolved in 10% BSA; Sigma-Aldrich, St. Louis, MO) was added to the cell culture media. Similar fatty acid concentrations have previously been used by us (31) and others (34). Although high for a single fatty acid, the concentration is within the physiological range (∼350–600 µM) of total NEFA concentration in maternal circulation during late pregnancy (30). Control cells were treated with an equal amount of the appropriate stock buffer (10% fatty acid free BSA in PBS). The cells were incubated for 24 h before measurement of amino acid uptake or protein expression analysis.
RNA interference (siRNA)
Transfection of primary trophoblast cell cultures was carried out as previously described (27, 35). Twenty hours after plating, the trophoblast cells were transfected with cell culture media containing 0.1 µM TLR4 siRNA (Validated MISSION siRNA, SASI_Hs01_00122250, NM_138554; Sigma-Aldrich) and 0.3% Dharmafect transfection reagent (Thermo Scientific, Rockford, IL) for 24 h. Mock transfected trophoblast cells were given an equal amount of Dharmafect transfection reagent. After 66 h (total culture time), trophoblast cells were treated with either control media or 400 µM oleic acid, and incubated for a further 24 h before measuring system A and system L activities. We have previously shown that transfecting trophoblast cells with noncoding siRNA does not affect system A activity or interfere with cellular differentiation (27).
Characterization and viability of isolated cells
As an indicator of biochemical differentiation, release of human chorionic gonadotropin (hCG) into the cell culture media was assessed with a commercial ELISA (β-hCG ELISA; DRG Instruments, Marburg, Germany) at 18 and 66 h after plating the cells. To detect contamination from cells of mesenchymal origin in the cell cultures, expression of vimentin (ab20346; Abcam, Cambridge, UK) was measured by Western blot as previously described (32). Lactate dehydrogenase (LDH) release into the cell culture media was measured by an in vitro assay (Sigma-Aldrich). Cell culture media from trophoblast cells lysed by sonication were used as a positive control for the LDH assay.
Harvesting cells
After 90 h culture, trophoblast cells were harvested for Western blot analysis by adding buffer D (250 mM sucrose, 50 mM Hepes/Tris, pH 7.0, at room temperature) and collected with a rubber spatula. Phosphatase inhibitor cocktail 1 and 2 and protease inhibitor cocktail (Sigma-Aldrich) were added to the cell lysates, and protein concentrations were determined by the method of Bradford (Bio-Rad, Hercules, CA). Cell lysates were stored at –80°C until further analysis.
Western blotting
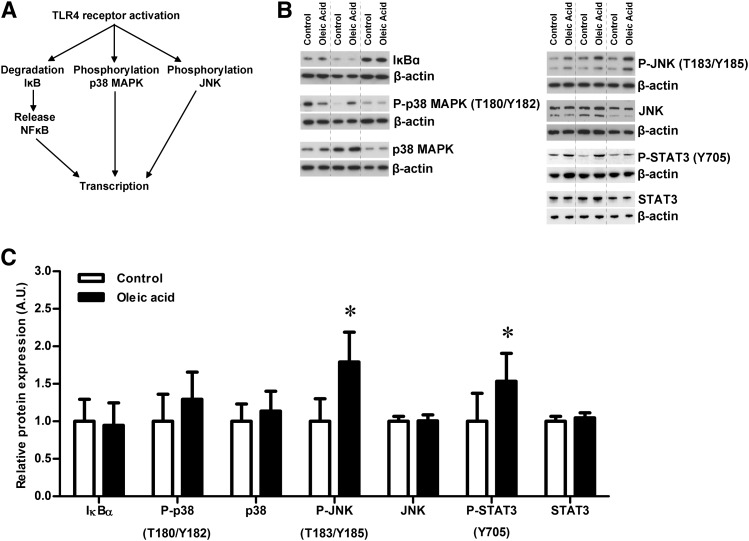
Western blots were performed on NuPAGE precast 4–12% gels (Invitrogen, Carlsbad, CA) or SDS-PAGE as previously described (31, 32). After electrophoresis and transfer, the membranes were blocked in 5% (phosphorylated STAT3 was blocked in 2.5%) nonfat milk in TBS with 0.1% tween (TBS-T) for 1 h. Primary antibodies were purchased from Cell Signaling Technology (Davers, MA): 4EBP1 (#9452), phosphorylated 4EBP1 (#9459; T37/46), phosphorylated 4EBP1 (#9455; T70), IĸBɑ (#4812), JNK (#9252), phosphorylated JNK (#4668; T183/Y185), p38 MAPK (#9212), phosphorylated p38 MAPK (#4511; T180/Y182), rpS6 (#2217), phosphorylated rpS6 (#4858; S235/236), S6K1 (#9202), phosphorylated S6K1 (#9205; T389), STAT3 (#9139), and phosphorylated STAT3 (#9145; Y705). Schematic representation of the TLR4 and mTOR signaling pathways are presented in Figs. 3A and 4A. The antibodies were diluted in TBS-T containing 3–5% BSA, and primary antibody incubations were carried out overnight at 4°C. After washing, membranes were incubated with the appropriate peroxidase-labeled IgG antibody for 1 h at room temperature. Immunolabeling was detected using SuperSignal Dura West or enhanced chemilumescent detection solution (Thermo Scientific). All membranes were stripped (2% SDS, 62.5 mM Tris-HCl, 100 mM β-mercaptoethanol; 35 min at 60°C), and thereafter reprobed with an antibody targeting β-actin (A2228; Sigma-Aldrich). The relative density of bands was measured by densitometry using ImageJ software (National Institutes of Health), and the expression of the target protein was normalized to the density of the β-actin band. The mean value of controls was arbitrarily assigned a value of 1.0 for comparisons between groups.
Fig. 3.
Oleic acid increases phosphorylation of JNK and STAT3 in trophoblast cells. (A) Schematic figure for activation of TLR4 signaling and effect on analyzed downstream target proteins. (B) Western blots of trophoblast cells incubated for 24 h with 400 µM oleic acid or control medium. Western blot for expression and/or phosphorylation (P) of IĸBɑ, p38 MAPK, JNK, and STAT3 in control and oleic acid exposed trophoblast cells from three different placentas. (C) While expression of IĸBɑ, p38 MAPK, total JNK, and total STAT3 was unaffected by the oleic acid exposure, the levels of phosphorylated JNK and STAT3 were significantly increased. Protein expression was normalized to the density of the β-actin band, and the mean of the controls was assigned a value of 1.0 AU. *P < 0.05 oleic acid versus control (paired t-test), n = 6.
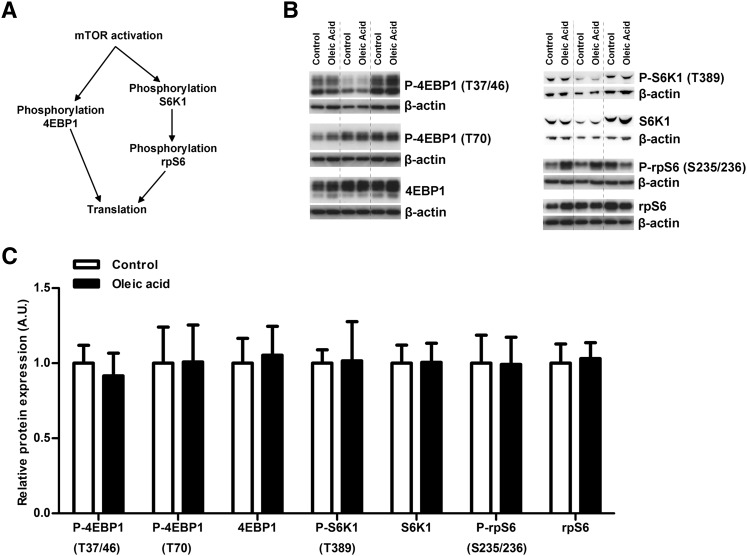
Fig. 4.
Oleic acid does not affect trophoblast mTOR signaling. (A) Schematic figure for activation of mTOR signaling and effect on analyzed downstream target proteins. (B) Western blots for expression and phosphorylation (P) of 4EBP1, S6K1, and rpS6 in trophoblast cells incubated for 24 h with 400 µM oleic acid or control medium. Western blot showing control and oleic acid exposed trophoblast cells from three different placentas. (C) There was no significant alteration in either expression or phosphorylation of these downstream targets in the mTOR signaling pathway. Protein expression was normalized to the density of the β-actin band, and the mean of the controls was assigned a value of 1.0 AU, n = 6.
Quantification of TLR4 mRNA expression
We used RNA STAT-60 (Tel-Test Inc., Friendswood, TX) according to the manufacturer's instructions to isolate RNA from cultured trophoblast cells. For determination of TLR4 expression in placentas from women with varying BMI, tissue samples were collected within 30 min of delivery. After removing decidua and chorionic plate, several small randomly collected villous tissue pieces were rinsed in ice-cold physiological saline and placed in RNAlater (Qiagen, Valencia, CA) at 4°C. After 24 h, the RNAlater was removed and the samples were stored at −80°C until further processing. RNA was isolated with RNeasy mini kit (Qiagen). Concentration and quality of the purified RNA samples were determined on a Nanodrop (Thermo Scientific, Wilmington, DE). In cultured trophoblast cells, TLR4 expression was measured with QuantiTect primer assay and QuantiFast SYBR Green PCR master mix (Qiagen) on a LightCycler (Roche Diagnostics, Mannheim, Germany). In placentas from normal and high BMI women, TLR4 expression was measured with SYBR Green master mix (Applied Biosystems, Carlsbad, CA) on a StepOnePlus real-time PCR system (Applied Biosystems). Primer design: forward, 5′-TTTGGACAGTTTCCCACATTGA-3′ reverse, 5′-AAGCATTCCCACCTTTGTTGG-3′. Succinate dehydrogenase complex, subunit A (SDHA) and TATA box binding protein (TBP) were selected as housekeeping genes, with primers designed according to a previously published report (21). All samples were analyzed in at least duplicates. The amplified transcripts were quantified using the relative standard curve method and normalized using the geometric mean of SDHA and TBP. The mean value of control or normal BMI women were arbitrarily assigned a value of 1.0 for comparisons between groups.
Measurement of amino acid uptake
Measuring activity of system A and system L amino acid transporters was performed as previously reported (21). System A transporter activity was measured as sodium-dependent 14C-methylaminoisobutyric acid (MeAIB) uptake, and system L transporter activity by 2-amino-2norbornanecarboxylic acid (BCH)-inhibitable uptake of 3H-leucine. Cells were incubated in buffer with sodium (135 mM NaCl, 5 mM KCl, 1.8 mM CaCl2, 1 mM MgCl2, 10 mM Hepes, 5.6 mM glucose; pH 7.4), or without sodium (135 mM choline chloride replaced NaCl). The sodium-free buffer also contained 1 mM BCH. Uptakes were stopped after 8 min by rapidly washing the cells three times with ice-cold, isotope- and sodium-free buffer. After washing, cells were lysed with distilled water to release the isotopes. The water, with released isotopes, was removed and mixed with scintillation fluid, and radioactivity was counted in a β-counter. Protein content of lysed cells was measured. Transporter-mediated uptake was calculated by subtracting the nonmediated uptake (sodium-free/BCH buffer) from the total uptake (sodium containing buffer). Uptakes were expressed as picomoles of amino acid uptake per milligram of protein per minute.
Data presentation and statistics
The results are presented as mean ± SEM. Statistical analysis was carried out with GraphPad Prism 5 (version 5.04; GraphPad Software, La Jolla, CA). Differences between two groups were evaluated statistically by t-test, and for multiple groups by repeated measures ANOVA followed by Tukey's post hoc test. Correlation between maternal BMI and circulating NEFAs was evaluated by Pearson's correlation coefficient. A P-value less than 0.05 was considered significant.
RESULTS
Maternal BMI, plasma fatty acids, and placental TLR4 expression
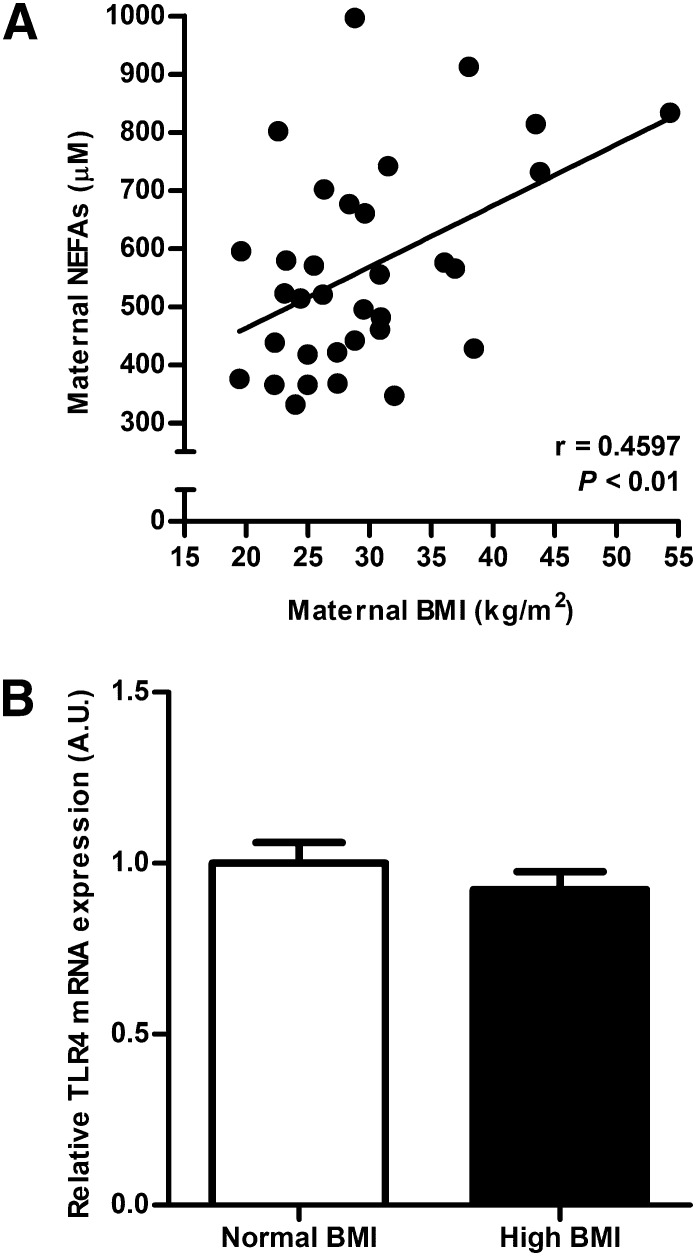
Maternal prepregnancy/early pregnancy BMI correlated positively with fasting plasma NEFAs at term (P < 0.01, n = 33; Fig. 1A). The placental mRNA expression of TLR4 was not influenced by maternal BMI because the expression level did not differ between normal BMI (n = 8) and high BMI (n = 8) groups (Fig. 1B).
Fig. 1.
Effect of maternal obesity on plasma fatty acids and placental TLR4 expression. (A) Maternal plasma NEFAs at term are increased in women with high prepregnancy/early pregnancy BMI. Pearson correlation, r = 0.4597, P < 0.01, n = 33. (B) mRNA expression of TLR4 in human placenta collected from women with normal BMI (18.4–24.4 kg/m2) or high prepregnancy/early pregnancy BMI (27.4–43.8 kg/m2). The expression level of TLR4 was not affected by maternal BMI. The mean value for the normal BMI group was assigned a value of 1.0 AU, n = 8/group.
Characterization of cultured trophoblast cells
Increased levels of hCG were detected at 66 h after plating compared with 18 h (36 ± 8 versus 1,497 ± 377 mIU/mg protein/hour; P < 0.05, n = 6), confirming that trophoblast cells differentiate into syncytium in culture. After 90 h, no expression of vimentin was detected in the cultures of isolated trophoblast cells (data not shown). The release of LDH into the cell culture media was similar between control (0.029 ± 0.009 optical density (OD) units; n = 3) and oleic acid-treated cells (0.028 ± 0.006 OD; n = 3). As a positive control, trophoblast cells subjected to sonication released almost a 6-fold larger amount of LDH (0.164 ± 0.088 OD; n = 3) compared with controls or oleic acid-treated cells.
System A activity is increased by oleic acid
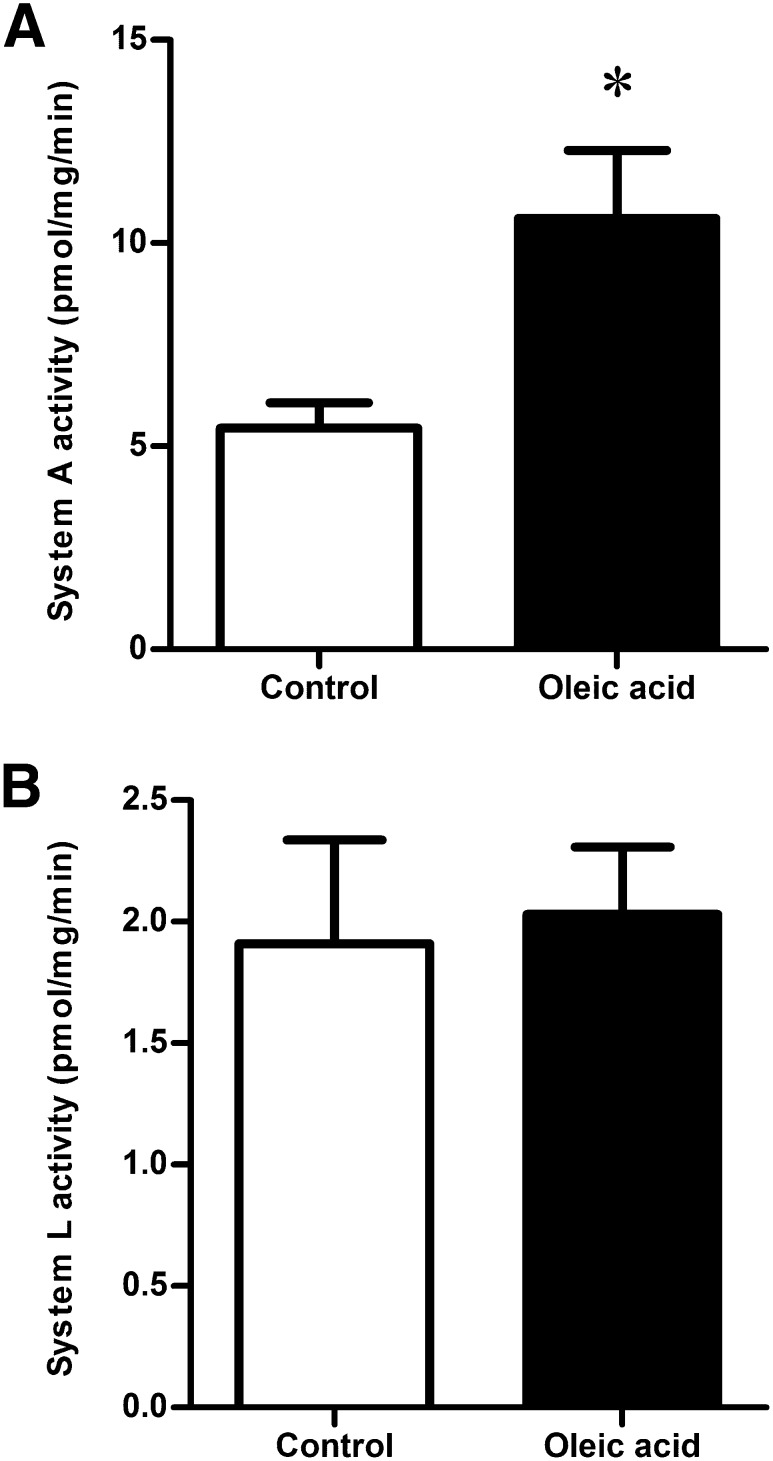
Activity of the system A amino acid transporter was measured by sodium-dependent uptake of 14C-MeAIB and was increased in trophoblast cells exposed to oleic acid for 24 h (+94%, P < 0.05, n = 6; Fig. 2A). In contrast, system L amino acid transporter activity, assessed by BCH-inhibitable uptake of 3H-leucine, was not altered by oleic acid treatment (n = 6; Fig. 2B).
Fig. 2.
Effect of oleic acid on amino acid transporter activity. System A amino acid transporter activity (A) was doubled after 24 h incubation with 400 µM oleic acid, while system L amino acid transporter activity (B) was unaffected. *P < 0.05 oleic acid versus control (paired t-test), n = 6.
Oleic acid increases phosphorylation of JNK and STAT3
In trophoblast cells incubated with oleic acid, phosphorylation of JNK (T183/Y185) and STAT3 (Y705) was significantly increased compared with control (P < 0.05, n = 6; Fig. 3), while the levels of total JNK and STAT3 remained stable. There was no difference in expression levels of IĸBɑ (NFĸB inhibitor) or p38 MAPK (T180/Y182) between control and oleic acid-treated cells (n = 6; Fig. 3). Similarly, there was no alteration in activity of the mTOR signaling pathway after exposure to oleic acid as measured by expression and phosphorylation of 4EBP1, S6K1, and rpS6 (n = 6; Fig. 4).
Silencing TLR4 prevents oleic acid stimulation of system A activity
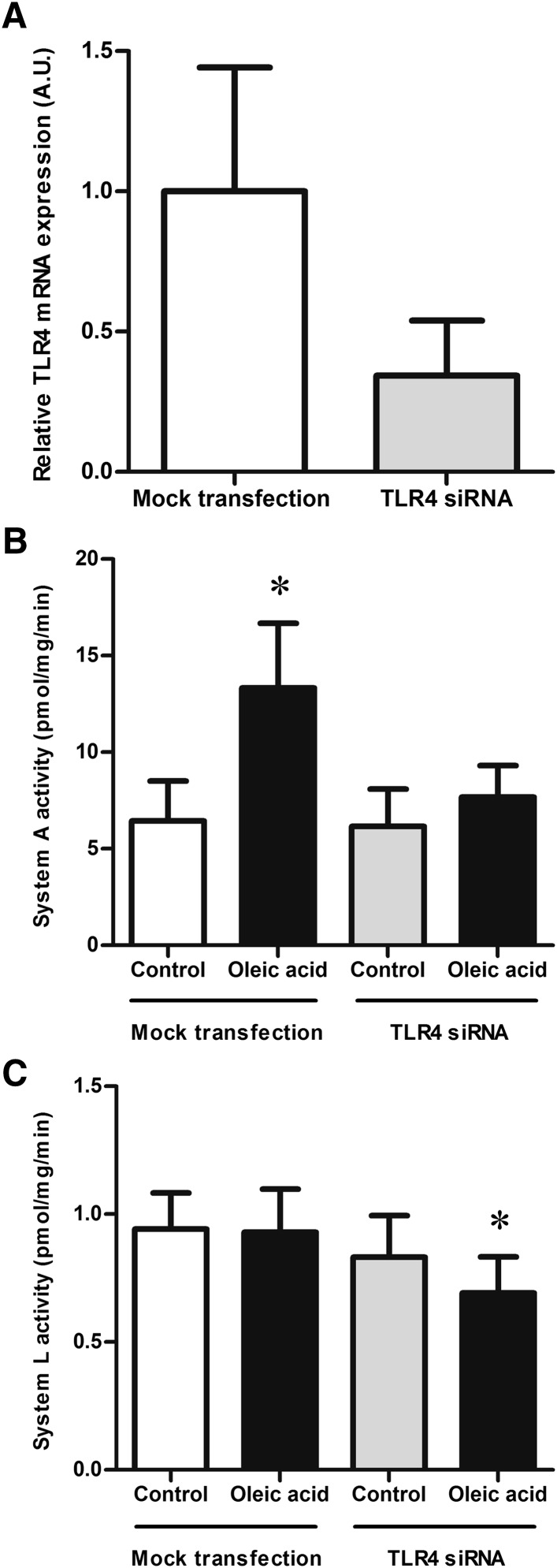
Transfecting trophoblast cells with siRNA targeting TLR4 decreased the mRNA levels of TLR4 to approximately one third of control (n = 3; Fig. 5A). Treatment with transfection reagent alone (between 20 and 44 h after plating) did not change the response of trophoblast cells to oleic acid. Exposure to oleic acid doubled the system A activity (P < 0.05, n = 7; Fig. 5B), while the activity of the system L transporter remained unchanged (n = 7; Fig. 5C). Reducing TLR4 expression did not affect basal system A activity (n = 7; Fig. 5B). However, silencing TLR4 prevented the stimulation of system A activity by oleic acid (n = 7; Fig. 5B). Basal system L activity was not affected by silencing TLR4. However, silencing TLR4 and exposing the cells to oleic acid resulted in a small but significant decrease in the system L activity compared with controls (both mock-transfected and TLR4-silenced control cells) as well as mock-transfected oleic acid-treated trophoblast cells (P < 0.05, n = 7; Fig. 5C).
Fig. 5.
Amino acid transporter activity after TLR4 silencing. (A) Transfecting trophoblast cells with siRNA targeting TLR4 after 20 h in culture reduced TLR4 mRNA expression to approximately one third of controls at 90 h in culture. Relative TLR4 mRNA expression was measured by quantitative real-time PCR and normalized against the housekeeping genes SDHA and TBP. The mean of the controls was assigned a value of 1.0 AU, n = 3. B, C: Mock transfection with the transfection reagent Dharmafect alone did not alter the response to oleic acid of either system A or system L amino acid transporters. System A activity (B) was increased after a 24 h incubation with oleic acid, while system L activity (C) was unaffected. In trophoblast cells transfected with siRNA targeting TLR4, oleic acid did not increase the system A activity (B), but reduced the system L activity (C). *P < 0.05 compared with all other groups; repeated measures ANOVA followed by Tukey's post hoc test, n = 7.
DISCUSSION
The novel finding in this study is that oleic acid stimulates system A amino acid transport in primary human trophoblast cells mediated by TLR4. This is, to the best of our knowledge, the first evidence that fatty acids stimulate amino acid transport in any tissue. The unaffected system L transporter activity together with the unchanged LDH release after oleic acid exposure indicates specific stimulation of system A amino acid transport rather than a general effect on, for example, cell membrane permeability. As maternal fatty acid levels progressively rise during pregnancy (36), this activation of system A amino acid transport by fatty acids may function as a natural stimulus to increase placental amino acid uptake in all pregnancies. However, the additionally elevated circulating fatty acid levels of obese pregnant women may further activate placental amino acid transporting systems, contributing to increased birth weights in these pregnancies.
Placental system A amino acid transport activity is stimulated by a variety of signaling molecules, including adipokines such as globular adiponectin (33), IL-6 (27), and leptin (28). Activation of trophoblast system A activity by globular adiponectin and IL-6 involves an increase in the protein expression of the system A isoform SNAT2 (sodium-dependent neutral amino acid transporter) (27, 33). In cultured trophoblast cells, silencing STAT3 expression prevented stimulation of system A activity by globular adiponectin and IL-6 (27, 33) demonstrating that regulation of trophoblast amino acid uptake by these adipokines is mediated through STAT3. In addition, STAT3 may also be involved in the increased system A activity in response to leptin (28). Collectively, these data suggest that STAT3 signaling is an important regulator of placental system A amino acid transporter activity.
In several cell types, amino acid deprivation results in upregulation of system A amino acid transporter activity (37–40), including the trophoblast-like human choriocarcinoma cell line BeWo (38, 40). Amino acid deprivation increases SNAT2 expression in BeWo trophoblast-like cells (38, 40). In cell types other than trophoblast cells, activation of JNK signaling has been implicated as an important component in the adaptive response to amino acid deprivation (37, 39). Whether JNK is important for the upregulation of system A activity in trophoblast cells is currently unknown.
We demonstrated that oleic acid activates both JNK and STAT3. These findings are consistent with the possibility that JNK and/or STAT3 signaling constitutes a link between elevated NEFAs and increased placental amino acid uptake. How activation of these signaling pathways may mediate oleic acid-stimulated increase of system A activity remains to be established. JNK and STAT3 signaling may cooperate in regulating placental system A activity since these two pathways are known to interact (41–43).
It is well established that mTOR functions as a nutrient sensor, and we have previously demonstrated that this signaling pathway is a positive regulator of placental amino acid transport (21, 22). Because oleic acid has been reported to activate mTOR signaling in HepG2 cells (29), mTOR is a potential mediator of oleic acid-stimulated amino acid transport. However, oleic acid-stimulated trophoblast system A activity without a concurrent increase in phosphorylation of 4EBP1, S6K1, or rpS6 (all well-established readouts of mTOR activity) indicates that system A activation after oleic acid treatment is independent of the mTOR signaling pathway. Taken together our results suggest that there are at least two separate mechanisms regulating system A amino acid transporter activity in trophoblast cells, mTOR-dependent as well as mTOR-independent pathways.
To investigate a possible causal link between oleic acid and system A activity, the expression of TLR4 was silenced with siRNA. We have previously demonstrated that transfecting trophoblast cells with noncoding siRNA does not affect system A transporter activity (27). Similarly, in this study, reducing the expression of TLR4 did not alter the basal amino acid transport activity. Furthermore, transfection reagent alone did not affect their response to oleic acid. However, the effect of oleic acid on trophoblast system A amino acid transport was abolished by silencing of TLR4. In TLR4-deficient cells, oleic acid was unable to stimulate system A activity, suggesting that this receptor is critical in mediating the effect of oleic acid on trophoblast amino acid uptake. In general agreement with our findings, LPS stimulation (which also initiates TLR4 signaling) increases activity of hepatic system A but not system L amino acid transport (17). As with the system A transporter, basal activity of the system L transporter was not affected by reducing TLR4 expression. This finding suggests that TLR4 is not critical for maintaining basal trophoblast system A or system L amino acid transporter activity. Unexpectedly, reducing the TLR4 expression in the trophoblast cells combined with oleic acid exposure caused a small but significant reduction (∼20%) in the system L amino acid transport activity. These results suggest that oleic acid modulates trophoblast system L activity in the absence of TLR4; however, the underlying mechanism remains to be established. Oleic acid activates multiple signaling pathways, including the peroxisome proliferator-activated receptors (PPAR), which are important for placental function (44, 45). It is therefore possible that any of these alternative signaling pathways may mediate the decrease in system L activity in TLR4-silenced cells in response to oleic acid.
The placentas used for isolation of trophoblast cells were from women of unknown prepregnancy BMI; presumably placentas from women with a variety of BMIs are included. Hence, the trophoblast cells will almost certainly have been exposed to diverse in vivo conditions, potentially resulting in differences sustained throughout culture, which may contribute to some variability in our results. However, the cultured trophoblast cells capacity to respond to oleic acid through TLR4 and stimulate amino acid transport is most likely not compromised since maternal BMI does not change placental TLR4 expression.
In the human placenta, TLR4 is localized to the MVM of the syncytiotrophoblast (13, 14), where it can be readily affected by circulating maternal fatty acids. However, whether fatty acids bind directly to the TLR4 receptor or interact with the receptor through another mechanism remains unknown (46, 47). The data in this report suggest that oleic acid in the maternal circulation modifies placental function by affecting TLR4 signaling. The current study has been focused on oleic acid, a monounsaturated fatty acid, which constitutes almost one third of maternal NEFAs during late pregnancy (30). How fatty acid species other than oleic acid might affect amino acid transport remains to be established. However, in other tissues, it has been shown that different fatty acid species modulate TLR4 signaling differently, with saturated fatty acids activating and polyunsaturated fatty acids inhibiting TLR4 signaling (10, 11). TLR4 was required for oleic acid to stimulate amino acid transport, and oleic acid activated JNK and STAT3. We therefore speculate that saturated fatty acids increase whereas polyunsaturated fatty acids may reduce placental system A amino acid transporter activity.
Placental TLR4 gene expression has been reported to be upregulated in animal models of maternal obesity (15), and it is increased by maternal high-fat diet (16) and inflammation (48). In contrast, human placenta appears to respond to maternal obesity differently, because placental expression of TLR4 was not influenced by maternal BMI. Nonetheless we demonstrate that intact TLR4 expression is necessary for oleic acid to stimulate system A activity. It is possible that trophoblast TLR4 signaling represents a link between high dietary fat intake/obesity and placental function, which subsequently affects fetal growth. Other required molecular components in the signaling cascade linking oleic acid and increased system A activity remain to be determined.
In conclusion, our data suggest that elevated maternal levels of oleic acid activates trophoblast JNK and STAT3 signaling as well as increase system A amino acid transport activity. Furthermore, TLR4 appears to be a critical component for the stimulatory effect of oleic acid on system A activity because reducing TLR4 expression prevents the increase in amino acid transport after oleic acid exposure. However, intact TLR4 expression is not required for maintaining basal system A or system L amino acid transporter activity.
Acknowledgments
The authors are grateful to patients and staff at the Sahlgrenska University Hospital (SUH) in Gothenburg and the University Hospital (UH) in San Antonio for making collection of placental tissue possible. In addition, the authors are indebted to S. Johansson (SUH) and E. Miller (UH) who were responsible for tissue collection.
Footnotes
Abbreviations:
- 4EBP1
- eukaryotic initiation factor 4E binding protein 1
- AU
- arbitrary unit
- BCH
- 2-amino-2norbornanecarboxylic acid
- BMI
- body mass index
- hCG
- human chorionic gonadotropin
- IL
- interleukin
- JNK
- c-Jun N-terminal kinase
- LDH
- lactate dehydrogenase
- MAPK
- mitogen-activated protein kinase
- MeAIB
- methylaminoisobutyric acid
- mTOR
- mammalian target of rapamycin
- MVM
- microvillous plasma membrane
- NFκB
- nuclear factor-κB
- OD
- optical density unit
- rpS6
- ribosomal protein S6
- S6K1
- S6 kinase 1
- SDHA
- succinate dehydrogenase complex subunit A
- siRNA
- small interfering RNA
- SNAT
- sodium dependent neutral amino acid transporter
- STAT
- signal transducer and activator of transcription
- TBP
- TATA box binding protein
- TLR
- toll-like receptor
- TNF
- tumor necrosis factor
This work was supported by National Institutes of Health Grant R01-DK-089989, the Swedish Research Council, the Swedish Society of Endocrinology, the Swedish federal government under the LUA/ALF agreement, the Novo Nordisk Foundation, and the Wilhelm and Martina Lundgren Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Chu S. Y., Kim S. Y., Bish C. L. 2009. Prepregnancy obesity prevalence in the United States, 2004–2005. Matern. Child Health J. 13: 614–620 [DOI] [PubMed] [Google Scholar]
- 2.Athukorala C., Rumbold A. R., Willson K. J., Crowther C. A. 2010. The risk of adverse pregnancy outcomes in women who are overweight or obese. BMC Pregnancy Childbirth. 10: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X., Decker A., Platt R. W., Kramer M. S. 2008. How big is too big? The perinatal consequences of fetal macrosomia. Am. J. Obstet. Gynecol. 198: 517.e1–6. [DOI] [PubMed] [Google Scholar]
- 4.Boney C. M., Verma A., Tucker R., Vohr B. R. 2005. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 115: e290–e296 [DOI] [PubMed] [Google Scholar]
- 5.Chiavaroli V., Giannini C., D'Adamo E., de Giorgis T., Chiarelli F., Mohn A. 2009. Insulin resistance and oxidative stress in children born small and large for gestational age. Pediatrics. 124: 695–702 [DOI] [PubMed] [Google Scholar]
- 6.Cnattingius S., Villamor E., Lagerros Y. T., Wikstrom A. K., Granath F. 2011. High birth weight and obesity-a vicious circle across generations. Int. J. Obes. (Lond.) 36: 1320–1324. [DOI] [PubMed] [Google Scholar]
- 7.Jansson T., Ekstrand Y., Bjorn C., Wennergren M., Powell T. L. 2002. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 51: 2214–2219 [DOI] [PubMed] [Google Scholar]
- 8.Jansson T., Wennergren M., Powell T. L. 1999. Placental glucose transport and GLUT 1 expression in insulin-dependent diabetes. Am. J. Obstet. Gynecol. 180: 163–168 [DOI] [PubMed] [Google Scholar]
- 9.Haggarty P. 2010. Fatty acid supply to the human fetus. Annu. Rev. Nutr. 30: 237–255 [DOI] [PubMed] [Google Scholar]
- 10.Wong S. W., Kwon M. J., Choi A. M., Kim H. P., Nakahira K., Hwang D. H. 2009. Fatty acids modulate Toll-like receptor 4 activation through regulation of receptor dimerization and recruitment into lipid rafts in a reactive oxygen species-dependent manner. J. Biol. Chem. 284: 27384–27392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang S., Rutkowsky J. M., Snodgrass R. G., Ono-Moore K. D., Schneider D. A., Newman J. W., Adams S. H., Hwang D. H. 2012. Saturated fatty acids activate TLR-mediated pro-inflammatory signaling pathways. J. Lipid Res. 53: 2002–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Y. C., Yeh W. C., Ohashi P. S. 2008. LPS/TLR4 signal transduction pathway. Cytokine. 42: 145–151 [DOI] [PubMed] [Google Scholar]
- 13.Beijar E. C., Mallard C., Powell T. L. 2006. Expression and subcellular localization of TLR-4 in term and first trimester human placenta. Placenta. 27: 322–326 [DOI] [PubMed] [Google Scholar]
- 14.Holmlund U., Cebers G., Dahlfors A. R., Sandstedt B., Bremme K., Ekstrom E. S., Scheynius A. 2002. Expression and regulation of the pattern recognition receptors Toll-like receptor-2 and Toll-like receptor-4 in the human placenta. Immunology. 107: 145–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu M. J., Du M., Nathanielsz P. W., Ford S. P. 2010. Maternal obesity up-regulates inflammatory signaling pathways and enhances cytokine expression in the mid-gestation sheep placenta. Placenta. 31: 387–391 [DOI] [PubMed] [Google Scholar]
- 16.Frias A. E., Morgan T. K., Evans A. E., Rasanen J., Oh K. Y., Thornburg K. L., Grove K. L. 2011. Maternal high-fat diet disturbs uteroplacental hemodynamics and increases the frequency of stillbirth in a nonhuman primate model of excess nutrition. Endocrinology. 152: 2456–2464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue Y., Bode B. P., Abcouwer S., Souba W. W. 1995. Attenuation of the endotoxin-stimulated increase in hepatic amino acid transport with a glucocorticoid receptor antagonist. J. Surg. Res. 58: 693–701 [DOI] [PubMed] [Google Scholar]
- 18.Jansson T., Scholtbach V., Powell T. L. 1998. Placental transport of leucine and lysine is reduced in intrauterine growth restriction. Pediatr. Res. 44: 532–537 [DOI] [PubMed] [Google Scholar]
- 19.Jansson T., Wennergren M., Illsley N. P. 1993. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J. Clin. Endocrinol. Metab. 77: 1554–1562 [DOI] [PubMed] [Google Scholar]
- 20.Lager S., Powell T. L. 2012. Regulation of nutrient transport across the placenta. J. Pregnancy. 2012: article ID 179827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roos S., Kanai Y., Prasad P. D., Powell T. L., Jansson T. 2009. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am. J. Physiol. Cell Physiol. 296: C142–C150 [DOI] [PubMed] [Google Scholar]
- 22.Roos S., Jansson N., Palmberg I., Saljo K., Powell T. L., Jansson T. 2007. Mammalian target of rapamycin in the human placenta regulates leucine transport and is down-regulated in restricted fetal growth. J. Physiol. 582: 449–459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaccioli F., Jansson T., Powell T. 2011. Activation of placental mammalian target of rapamycin signaling in obese women. Placenta. 32: A134 [Google Scholar]
- 24.Jansson N., Rosario F. J., Gaccioli F., Lager S., Jones H. N., Roos S., Jansson T., Powell T. L. 2013. Activation of placental mTOR signaling and amino acid transporters in obese women giving birth to large babies. J. Clin. Endocrinol. Metab. 98: 105–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos S., Powell T. L., Jansson T. 2009. Placental mTOR links maternal nutrient availability to fetal growth. Biochem. Soc. Trans. 37: 295–298 [DOI] [PubMed] [Google Scholar]
- 26.Arous C., Naimi M., Van Obberghen E. 2011. Oleate-mediated activation of phospholipase D and mammalian target of rapamycin (mTOR) regulates proliferation and rapamycin sensitivity of hepatocarcinoma cells. Diabetologia. 54: 954–964 [DOI] [PubMed] [Google Scholar]
- 27.Jones H. N., Jansson T., Powell T. L. 2009. IL-6 stimulates system A amino acid transporter activity in trophoblast cells through STAT3 and increased expression of SNAT2. Am. J. Physiol. Cell Physiol. 297: C1228–C1235 [DOI] [PubMed] [Google Scholar]
- 28.von Versen-Höynck F., Rajakumar A., Parrott M. S., Powers R. W. 2009. Leptin affects system A amino acid transport activity in the human placenta: evidence for STAT3 dependent mechanisms. Placenta. 30: 361–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim K., Kim H. Y., Son E. J., Heo J., Cheong J. 2009. Oleic acid inhibits hepatic insulin signaling through deregulation of STAT3 activation and C/EBPalpha expression. Cell. Signal. 21: 1269–1276 [DOI] [PubMed] [Google Scholar]
- 30.Villa P. M., Laivuori H., Kajantie E., Kaaja R. 2009. Free fatty acid profiles in preeclampsia. Prostaglandins Leukot. Essent. Fatty Acids. 81: 17–21 [DOI] [PubMed] [Google Scholar]
- 31.Magnusson-Olsson A. L., Lager S., Jacobsson B., Jansson T., Powell T. L. 2007. Effect of maternal triglycerides and free fatty acids on placental LPL in cultured primary trophoblast cells and in a case of maternal LPL deficiency. Am. J. Physiol. Endocrinol. Metab. 293: E24–E30 [DOI] [PubMed] [Google Scholar]
- 32.Lager S., Jansson N., Olsson A. L., Wennergren M., Jansson T., Powell T. L. 2011. Effect of IL-6 and TNF-alpha on fatty acid uptake in cultured human primary trophoblast cells. Placenta. 32: 121–127 [DOI] [PubMed] [Google Scholar]
- 33.Jones H. N., Jansson T., Powell T. L. 2010. Full-length adiponectin attenuates insulin signaling and inhibits insulin-stimulated amino acid transport in human primary trophoblast cells. Diabetes. 59: 1161–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathmaperuma A. N., Mana P., Cheung S. N., Kugathas K., Josiah A., Koina M. E., Broomfield A., Delghingaro-Augusto V., Ellwood D. A., Dahlstrom J. E., et al. 2010. Fatty acids alter glycerolipid metabolism and induce lipid droplet formation, syncytialisation and cytokine production in human trophoblasts with minimal glucose effect or interaction. Placenta. 31: 230–239 [DOI] [PubMed] [Google Scholar]
- 35.Forbes K., Desforges M., Garside R., Aplin J. D., Westwood M. 2009. Methods for siRNA-mediated reduction of mRNA and protein expression in human placental explants, isolated primary cells and cell lines. Placenta. 30: 124–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couch S. C., Philipson E. H., Bendel R. B., Pujda L. M., Milvae R. A., Lammi-Keefe C. J. 1998. Elevated lipoprotein lipids and gestational hormones in women with diet-treated gestational diabetes mellitus compared to healthy pregnant controls. J. Diabetes Complications. 12: 1–9 [DOI] [PubMed] [Google Scholar]
- 37.Hyde R., Cwiklinski E. L., MacAulay K., Taylor P. M., Hundal H. S. 2007. Distinct sensor pathways in the hierarchical control of SNAT2, a putative amino acid transceptor, by amino acid availability. J. Biol. Chem. 282: 19788–19798 [DOI] [PubMed] [Google Scholar]
- 38.Jones H. N., Ashworth C. J., Page K. R., McArdle H. J. 2006. Expression and adaptive regulation of amino acid transport system A in a placental cell line under amino acid restriction. Reproduction. 131: 951–960 [DOI] [PubMed] [Google Scholar]
- 39.López-Fontanals M., Rodriguez-Mulero S., Casado F. J., Derijard B., Pastor-Anglada M. 2003. The osmoregulatory and the amino acid-regulated responses of system A are mediated by different signal transduction pathways. J. Gen. Physiol. 122: 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Novak D., Quiggle F., Haafiz A. 2006. Impact of forskolin and amino acid depletion upon System A activity and SNAT expression in BeWo cells. Biochimie. 88: 39–44 [DOI] [PubMed] [Google Scholar]
- 41.Zhang X., Wrzeszczynska M. H., Horvath C. M., Darnell J. E., Jr 1999. Interacting regions in Stat3 and c-Jun that participate in cooperative transcriptional activation. Mol. Cell. Biol. 19: 7138–7146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim C. P., Cao X. 1999. Serine phosphorylation and negative regulation of Stat3 by JNK. J. Biol. Chem. 274: 31055–31061 [DOI] [PubMed] [Google Scholar]
- 43.Miyazaki T., Bub J. D., Iwamoto Y. 2008. c-Jun NH(2)-terminal kinase mediates leptin-stimulated androgen-independent prostate cancer cell proliferation via signal transducer and activator of transcription 3 and Akt. Biochim. Biophys. Acta. 1782: 593–604 [DOI] [PubMed] [Google Scholar]
- 44.Fournier T., Tsatsaris V., Handschuh K., Evain-Brion D. 2007. PPARs and the placenta. Placenta. 28: 65–76 [DOI] [PubMed] [Google Scholar]
- 45.Schaiff W. T., Barak Y., Sadovsky Y. 2006. The pleiotropic function of PPAR gamma in the placenta. Mol. Cell. Endocrinol. 249: 10–15 [DOI] [PubMed] [Google Scholar]
- 46.Schaeffler A., Gross P., Buettner R., Bollheimer C., Buechler C., Neumeier M., Kopp A., Schoelmerich J., Falk W. 2009. Fatty acid-induced induction of Toll-like receptor-4/nuclear factor-kappaB pathway in adipocytes links nutritional signalling with innate immunity. Immunology. 126: 233–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fessler M. B., Rudel L. L., Brown J. M. 2009. Toll-like receptor signaling links dietary fatty acids to the metabolic syndrome. Curr. Opin. Lipidol. 20: 379–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arce R. M., Barros S. P., Wacker B., Peters B., Moss K., Offenbacher S. 2009. Increased TLR4 expression in murine placentas after oral infection with periodontal pathogens. Placenta. 30: 156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]