Abstract
Leukotriene (LT)A4 and closely related allylic epoxides are pivotal intermediates in lipoxygenase (LOX) pathways to bioactive lipid mediators that include the leukotrienes, lipoxins, eoxins, resolvins, and protectins. Although the structure and stereochemistry of the 5-LOX product LTA4 is established through comparison to synthetic standards, this is the exception, and none of these highly unstable epoxides has been analyzed in detail from enzymatic synthesis. Understanding of the mechanistic basis of the cis or trans epoxide configuration is also limited. To address these issues, we developed methods involving biphasic reaction conditions for the LOX-catalyzed synthesis of LTA epoxides in quantities sufficient for NMR analysis. As proof of concept, human 15-LOX-1 was shown to convert 15S-hydroperoxy-eicosatetraenoic acid (15S-HPETE) to the LTA analog 14S,15S-trans-epoxy-eicosa-5Z,8Z,10E,12E-tetraenoate, confirming the proposed structure of eoxin A4. Using this methodology we then showed that recombinant Arabidopsis AtLOX1, an arachidonate 5-LOX, converts 5S-HPETE to the trans epoxide LTA4 and converts 5R-HPETE to the cis epoxide 5-epi-LTA4, establishing substrate chirality as a determinant of the cis or trans epoxide configuration. The results are reconciled with a mechanism based on a dual role of the LOX nonheme iron in LTA epoxide biosynthesis, providing a rational basis for understanding the stereochemistry of LTA epoxide intermediates in LOX-catalyzed transformations.
Keywords: 5-lipoxygenase, 15-lipoxygenase, hydroperoxy-eicosatetraenoic acid, nuclear magnetic resonance
The leukotrienes (LTs) are a family of lipid mediators derived from arachidonic acid and implicated in the pathogenesis of asthma and other inflammatory diseases (1). The biosynthetic pathway, unraveled three decades ago, is a model for related transformations to anti-inflammatory mediators including the lipoxins, eoxins, resolvins, protectins, and maresins (2–5). 5-Lipoxygenase (5-LOX) catalyzes the first two steps in leukotriene biosynthesis, namely the stereospecific oxygenation of arachidonic acid to 5S-hydroperoxy-eicosatetraenoic acid (5S-HPETE), and dehydration of the hydroperoxide to LTA4, the allylic 5,6-trans-epoxide from which the bioactive leukotrienes are derived (Fig. 1) (6–9). The importance of this pivotal intermediate is reflected in the extensive efforts directed toward perfecting the total synthesis of LTA4 and of closely related 5,6-epoxide isomers (as reviewed in Refs. 10–12).
Fig. 1.

The 5-LOX catalyzed pathway of LTA4 biosynthesis. 5-LOX oxygenates arachidonic acid to 5S-HPETE and then dehydrates the hydroperoxide to LTA4. Shown in gray, metabolism by LTA4 hydrolase produces the bioactive leukotriene LTB4.
LTA4 is highly unstable in physiological buffer at pH 7.4, with a half-life estimated as approximately 3 s at 25°C and 18 s at 4°C (13). Despite this instability, LTA4 was detected as an evanescent intermediate in short-term incubations of arachidonic acid with human leukocytes (6), and the radiolabeled product was isolated and, as the methyl ester derivative, shown to have similar chromatographic mobility and hydrolysis under acidic conditions as a synthetic standard (14). The stereochemistry has heretofore not been established directly on the biosynthetic product but was deduced from an understanding of the biosynthetic pathway and by study of the reactions of synthetic LTA4 and related isomers in transformations to the stable, bioactive leukotrienes LTB4 and LTC4 (15–17). Herein we report the isolation and purification of LTA epoxides as products of LOX enzymes in quantities sufficient for NMR analysis of the stereoconfigurations. The isolation method we developed here was successfully applied earlier to allene oxides (18) and to analysis of an LTA-related epoxide synthesized by a catalase-related hemoprotein (19), although not heretofore applied to lipoxygenase reactions. We identify substrate chirality as a determinant of the cis or trans configuration of the LTA epoxide and propose a model that predicts how these products are derived in the enzymatic transformation.
MATERIALS AND METHODS
Materials
Arachidonic acid and its methyl ester were purchased from NuChek Prep Inc. (Elysian, MN). Soybean LOX-1 (lipoxidase, type V) and α-tocopherol were purchased from Sigma (St. Louis, MO). 15S-HPETE was synthesized by reacting soybean LOX-1 with arachidonic acid followed by straight-phase HPLC (SP-HPLC) purification.
Synthesis and purification of enantiomeric HPETEs
Racemic hydroperoxides were prepared by vitamin E-controlled autoxidation (20). Arachidonic acid methyl ester (500 mg) was transferred to a 2 liter round-bottomed flask, mixed with 10% (w/w) α-tocopherol, and evaporated to dryness. The flask filled with oxygen, capped, and placed in an oven at 37°C. The oxygen was replenished daily. After 3 days, the lipid was dissolved in 10 ml of methylene chloride and stored at −30°C. The autoxidation sample was fractionated and partly purified using a 5 g silica Bond-Elut (Varian, Palo Alto, CA) with the solvents of hexane/ethyl acetate (three fractions of 10 ml of 95:5, followed by three of 10 ml of 90:10 and three of 10 ml of 85:5, v/v); the HPETE methyl esters were eluted in fractions 6 through 9. Racemic 5-HPETE and 15-HPETE methyl esters were then separated from other positional HPETE isomers by SP-HPLC using a semi-preparative Ultrasphere 10 μm silica column (25 × 1 cm) with a solvent system of hexane/isopropanol (100/1, by volume) run at a flow rate of 4 ml/min (Beckman) . The enantiomeric HPETE methyl esters were resolved using a semi-preparative, 25 × 1 cm Chiralpak AD column (Chiral Technologies Inc., West Chester, PA) with a solvent system of hexane/methanol (100:2, by volume) run at a flow rate of 4 ml/min (21).
To prepare the corresponding free acids, the purified HPETE methyl esters (1 mg) in 2 ml methanol/methylene chloride (10:1, by volume) were brought to room temperature, and an equal volume of 1 M KOH was added. The sample was mixed and kept at room temperature under an atmosphere of argon with occasional sonication in a water bath. After 20 min, the sample was acidified to pH 4.5 and extracted with an equal volume of methylene chloride. The organic phase was washed twice with water and then evaporated to dryness under a stream of nitrogen. The dried sample was redissolved in methanol and stored at −30°C. HPETEs were quantified based on the conjugated diene chromophore at 236/237 nm, ϵ = 25,000 (10 μg/ml = 0.75 AU).
Expression and purification of human 15-LOX-1 and Arabidopsis thaliana AtLOX1
The cDNA of human 15-LOX-1 was subcloned into the pET3a vector (with an N-terminal His6 tag), and the protein was expressed in BL21 cells. A typical preparation of a 100-ml culture was carried out as follows: 100 ml of 2XYT medium containing 100 μg/ml ampicillin was inoculated with a single colony of h15-LOX-1-His in BL21 cells and grown at 37°C at 250 rpm until OD600 reached 0.8. Isopropyl β-D-1-thiogalactopyranoside (0.5 mM) was then added to the culture, which was grown at 16°C, 220 rpm for 4 days. The cells were spun at 5,000 g for 20 min in a Beckman Avanti J-25I centrifuge, washed with 40 ml of 50 mM Tris (pH 7.9), pelleted again at 5,000 g for 20 min, and resuspended in 10 ml of 50 mM Tris (pH 8.0), 500 mM NaCl, 20% glycerol, and 100 μM PMSF. The spheroplasts were sonicated five times for 10 s using a model 50 Sonic Dismembrator (Fisher Scientific, Pittsburgh, PA) at a setting of 5. CHAPS detergent was added at a final concentration of 1% (w/w), and the sample was kept on ice for 20 min. The resulting membranes were spun at 5,000 g for 20 min at 4°C. h15-LOX-1 activity was present in the supernatant. The supernatant was loaded on a nickel-NTA column (0.5 ml bed volume; Qiagen, Gaithersburg, MD) equilibrated with 50 mM Tris buffer (pH 8.0), 500 mM NaCl. The column was then washed with the equilibration, buffer and the nonspecific bound proteins were eluted with 50 mM Tris buffer (pH 8.0), 500 mM NaCl, and 50 mM imidazole. The h15-LOX-1 was then eluted with 50 mM Tris buffer (pH 8.0), 500 mM NaCl, and 250 mM imidazole. Fractions of 0.5 ml were collected and assayed for the LOX activity. The positive fractions were dialyzed against 50 mM Tris buffer (pH 7.5) and 150 mM NaCl. The purity of the enzyme preparations was determined by SDS-PAGE and Coomassie Blue staining; the prominent band of h15-LOX-1 accounted for about 80% of the total protein.
The cDNA of Arabidopsis thaliana AtLOX1 was subcloned into the pET14b vector (with an N-terminal His6 tag). The protein was expressed in Escherichia coli BL21 (DE3) cells and purified by nickel affinity chromatography according to a previously published protocol (22).
Biphasic reaction conditions for preparation of LTA epoxides
Enzyme reactions were performed at 0°C, with the HPETE substrate initially in hexane (5 ml, bubbled for 30 min before use with argon to decrease the O2 concentration, and containing ∼200 μM HPETE) layered over the recombinant LOX enzyme (1–2 mg; ∼20 nmol) in 400 μl of Tris buffer (pH 7.5 for h15-LOX-1 and pH 6.0 for AtLOX1). The reaction was initiated by vigorous vortex mixing of the two phases. After 1.5 min, the hexane phase was collected and scanned from 200 to 350 nm in ultraviolet (UV) light by using a Perkin-Elmer Lambda-35 spectrophotometer. The hexane phase was evaporated to about 2 ml under a stream of nitrogen, treated with ethanol (20 μl) and ethereal diazomethane for 10 s at 0°C, rapidly blown to dryness, and kept in hexane at −80°C until further analysis.
HPLC analyses
Aliquots of the methylated hexane phase were analyzed by RP-HPLC using a Waters Symmetry column (25 × 0.46 cm) using a solvent of MeOH/20 mM triethylamine (pH 8.0) (90/10 by volume) at a flow rate of 1 ml/min, with on-line UV detection (1100 series diode array detector; Agilent) (19). Further purification was achieved by SP-HPLC using a Beckmann Ultrasphere 5 μm silica column (25 × 0.46 cm) using a solvent of hexane/triethylamine (100/0.5) run at 1 ml/min.
NMR analysis
1H NMR and 1H,1H COSY NMR spectra were recorded on a Bruker AV-III 600 MHz spectrometer at 283 K. The parts/million values are reported relative to residual nondeuterated solvent (δ = 7.16 ppm for C6H6). Typically, 1,024 scans were acquired for a 1-D spectrum on ∼20 μg of LTA epoxide.
GC-MS analysis of 5-oxo-ETE
The methoxime derivative was prepared by treatment with methoxylamine hydrochloride (10 mg/ml in pyridine) at room temperature for 16 h. The trimethylsilyl ester derivative was then prepared using bis(trimethylsilyl)-trifluoroacetamide/pyridine (12 μl, 5:1, v/v) for 2 h at room temperature. Analysis of the trimethylsilyl ester methoxime derivative was carried out in the positive ion electron impact mode (70 eV) using a Thermo Finnigan Trace DSQ ion trap GC-MS with the Xcalibur data system. Samples were injected at 150°C, and after 1 min the temperature was programmed to 300°C at 20°C/min. The spectrum was recorded by repetitive scanning over the range of 50–500 m/z.
RESULTS
Method development, preparation, and analysis of 14,15-LTA4
To prepare and isolate LTA epoxides, we used a biphasic reaction system, kept at 0°C, with the HPETE substrate dissolved in hexane and a highly active LOX enzyme in aqueous buffer. Upon vigorous mixing, the more polar hydroperoxide partitions into the aqueous phase and reacts with enzyme, and the less polar epoxide product instantly back-extracts into the hexane and is thereby protected from hydrolysis. For the initial development and validation of the method, we required an enzyme capable of LTA-type epoxide synthesis and with the highest possible catalytic activity. Our preparation of recombinant human 15-LOX-1 proved suitable; it converts its primary product, 15S-HPETE, to several derivatives, including the LTA4 analog 14,15-LTA4 (23).
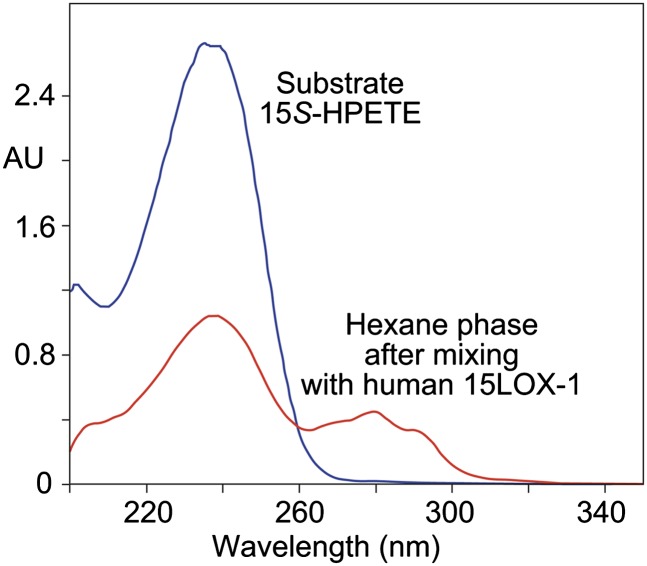
In optimizing the conditions for the reaction of 15S-HPETE with 15-LOX-1, we used a 12.5-fold excess of hexane over the aqueous phase (pH held at 7.5) and a mixing time of 90 s at 0°C. Comparison of the UV spectrum of the hexane phase before and after vortex mixing provided quick feedback on the extent of reaction. In the example shown in Fig. 2, a new conjugated triene chromophore typical of an LTA-type epoxide (λmax 280 nm) is evident after mixing. The substrate concentration has diminished markedly and is only partially replaced by the conjugated triene. Hydrolysis of the epoxide produces 8,15- and 14,15-dihydroxy derivatives that do not extract into hexane, especially at the elevated pH of 7.5, and the same applies to any dioxygenation products or epoxyalcohol derivatives (23). Analysis of the aqueous phase from these biphasic reactions revealed the presence of dihydro(pero)xy derivatives (prominently with all-trans conjugated trienes, λmax 269 nm, typical of epoxide hydrolysis products, but including dihydroperoxides) as well as a mixture of more polar derivatives with trans-trans conjugated dienes (not identified, but possibly epoxyalcohols formed from conjugated triene-containing dihydroperoxides). Overall, about 60% of the starting 15S-HPETE was consumed, with an estimated recovery of LTA-type epoxide under the above reaction conditions of 10–15% (taking into account the molar absorbance of the conjugated trienes is about 1.5–2 times the value for a conjugated diene) (24, 25), with the balance of products being accounted for mainly by the nonextractable, more polar derivatives.
Fig. 2.
UV analysis of LTA epoxide formation under biphasic reaction conditions. The UV spectrum of 15S-HPETE substrate (40 μg/ml) in 5 ml hexane was recorded before the reaction and after vortex mixing for 90 s at 0–4°C with 1.5 mg 15-LOX-1 enzyme in 400 μl 0.1 M Tris (pH 7.5).
After preparing the methyl esters of the hexane extract by brief reaction with diazomethane at 0°C, the remaining 15S-HPETE and its products were analyzed on RP-HPLC and SP-HPLC using conditions suitable for LTA-type epoxides (13, 26). Fig. 3 illustrates RP-HPLC analysis with UV detection at 270 nm. The unreacted 15S-HPETE (at 7.2 min retention time, the largest peak on the chromatogram but weakly detected at 270 nm) is immediately followed by a minor keto derivative (at 7.6 min retention time, a conjugated dienone, λmax 281 nm) and then by a well-resolved peak of the putative LTA-type epoxide (at 9.7 min, a conjugated triene, λmax 280 nm). This product was collected from SP-HPLC runs with a solvent of hexane containing 0.1% triethylamine. The pooled aliquots of LTA epoxide methyl ester (∼20 μg) were analyzed by 1H-NMR in d6-benzene (Fig. 4). The 2-D COSY spectrum (described below) helps confirm the proton assignments. The expanded regions for the olefinic protons (5.0–6.5 ppm) and the epoxide protons (2.6–3.1 ppm) illustrate the splitting of individual signals and associated coupling constants from which the stereochemistry can be derived. These provide the configuration of the double bonds, in particular identifying the conjugated triene as 8Z,10E,12E, and on the epoxide protons the 2 Hz coupling between H14 and H15 identifies the epoxide configuration as trans. Based on these analyses and with the reasonable assumption that the original 15S configuration is retained, the structure of the epoxide product can be defined as 14S,15S-trans-epoxy-eicosa-5Z,8Z,10E,12E-tetraenoate. This confirms the structure originally proposed for this intermediate (23, 27), now named eoxin A4, precursor of the proinflammatory eoxins of human eosinophils in asthma (3, 28).
Fig. 3.
RP-HPLC analysis of the reaction of 15-LOX-1 with 15S-HPETE. RP-HPLC analysis used a Waters Symmetry C18 column (0.46 × 25 cm), a flow rate of 1 ml/min, and a solvent system of methanol/20 mM triethylamine (pH 8.0) (90/10, by volume) with UV detection at 270 nm.
Fig. 4.
NMR analysis of 14,15-LTA4. Spectra were recorded in d6-benzene at 283 K using a Bruker 600 MHz spectrometer. The two-dimensional H,H-COSY spectrum is shown below; shown above is an expanded view of the olefinic protons (5.0–6.5 ppm) and epoxide protons (2.6–3.1 ppm).
Leukotriene epoxide formation from enantiomeric HPETE substrates
To investigate the significance of substrate chirality to LTA epoxide formation, initially we examined the reaction of 15-LOX-1 with the mirror image substrate, 15R-HPETE. The analyses showed, however, that the major product by far is the keto derivative 15-oxo-eicosatetraenoic acid (data not shown). Only traces of a mixture of very minor products exhibiting an LTA-related UV chromophore were detectable.
With the requirement that the LOX enzyme used in these preparations should have very high catalytic activity (which our available preparations of mammalian 5-LOX did not), we switched to the use of recombinant Arabidopsis AtLOX1 as the arachidonate 5-LOX (22). This enzyme is a homolog of the potato 5-LOX (22) that is capable of LTA epoxide synthesis and has been used as a model for the study of 5-LOX-catalyzed leukotriene synthesis in earlier studies (7, 29, 30). Although the native activity of these plant enzymes is as a linoleic acid 9S-lipoxygenase, they form 5S-HPETE as a prominent product from arachidonic acid and can further convert this to LTA4 (22, 29, 30). Indeed, after vortex mixing of AtLOX1 with 5S-HPETE at 0°C, UV spectroscopy of the hexane phase showed a decrease in substrate and appearance of a new chromophore with λmax at 280 nm, characteristic of LTA4 epoxide (Fig. 5A). After methyl esterification with diazomethane, the product was analyzed by RP-HPLC (Fig. 5B). The complete structure was then established by 1H-NMR (see supplementary data). Most importantly, this confirmed the 5,6-trans configuration of the epoxide (J5,6 = 2 Hz) (31) (Fig. 5C).
Fig. 5.
Product analyses from reactions of 5S- and 5R-HPETE with 5-LOX. A: UV spectrum of 5S-HPETE in hexane before and after vortex mixing with 5-LOX enzyme (AtLOX1). B: Reversed-phase HPLC analyses of the product methyl esters from 5S-HPETE. Column: Waters Symmetry C18, 25 × 0.46 cm; solvent, methanol/20 mM triethylamine (pH 8.0) (90/10, by volume); flow rate, 1 ml/min, UV detection at 270 nm. There was no sign on our HPLC chromatograms of a conjugated triene-containing keto derivative, a reported nonenzymatic rearrangement product of LTA4 epoxide (46). C: Partial 1H-NMR spectrum (2.45–3.25 ppm) of the trans-LTA epoxide product from 5S-HPETE. D–F: The UV (D), HPLC (E), and NMR (F) analyses of 5R-HPETE reaction with 5-LOX.
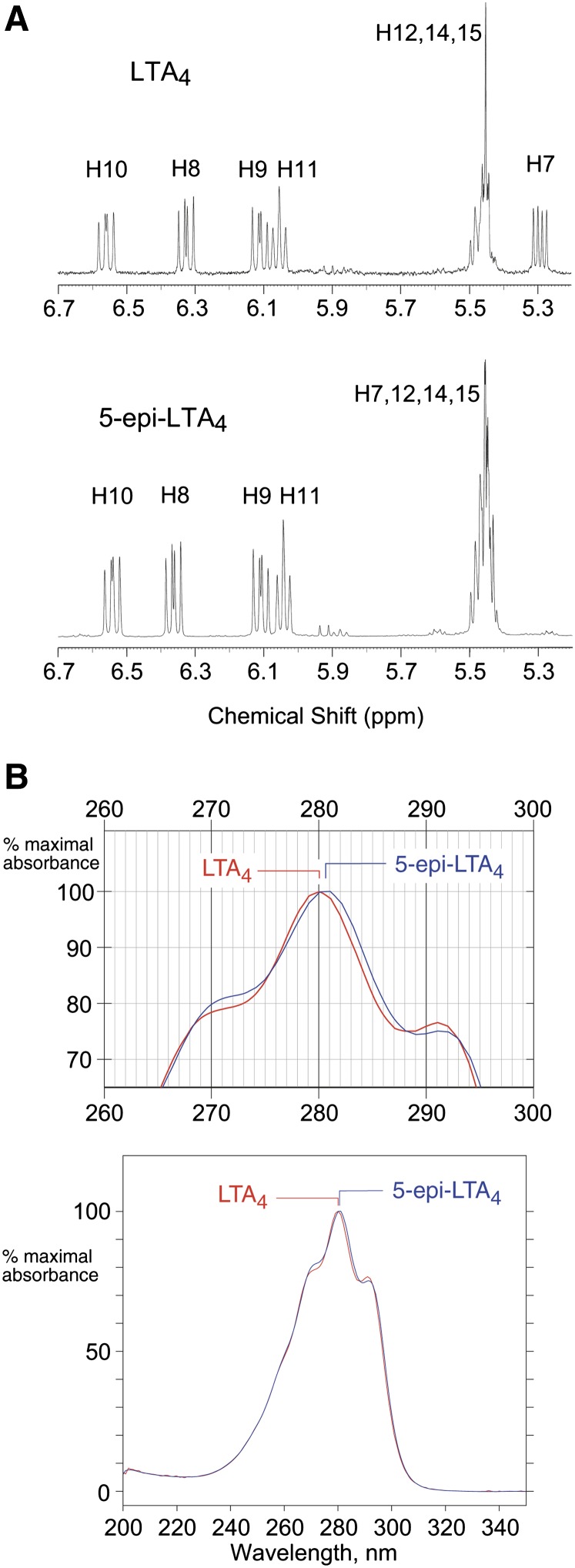
Incubation of the 5R enantiomer of 5-HPETE with 5-LOX was performed under the same conditions. UV spectroscopy of the hexane phase after vortex mixing showed the appearance of a less well-defined spectrum in the 280 nm region, comprised of a mixture (Fig. 5D). RP-HPLC analysis of the esterified hexane phase demonstrated two products of the incubation (Fig. 5E). The major product exhibited a dienone chromophore with λmax in MeOH/H2O (90:10) at 281 nm. Its structure was identified as 5-oxo-eicosatetraenoic acid (5-oxo-ETE) by GC-MS analysis of its trimethylsilyl ester methoxime derivative (supplementary Fig. I), and by reduction with NaBH4 to 5-hydroxyeicosatetraenoic acid, identified by comparison to an authentic standard. The less abundant product displayed a conjugated triene chromophore with λmax at 280 nm. The structure of this LTA epoxide derived from 5R-HPETE was established by 1H-NMR (see supplementary data), most significantly defining the 5,6-epoxide configuration as cis (J5,6 = 4 Hz), thus identifying the product as the 5R,6S cis epoxide, 5-epi-LTA4 (Fig. 5F). Its yield was similar to that of LTA4 (the dominant recovery of 5-oxo-ETE from 5R-HPETE being in excess of its production from 5S-HPETE). Comparison of the NMR spectra of LTA4 and its 5-epimer also defined their identical double bond configurations as 7E,9E,11Z,14Z (Fig. 6A). Their UV spectra are almost superimposable, with the difference only discernible with the two overlaid (Fig. 6B).
Fig. 6.
Spectral comparison of LTA4 and 5-epi-LTA4. A: NMR spectra of the olefin protons in LTA4 and 5-epi-LTA4 methyl esters. B: Overlay of the UV spectra of LTA4 and 5-epi-LTA4 (cis-epoxy, 5R,6S). Top: detailed view. Bottom: full spectra, 200–350 nm.
DISCUSSION
The dual purposes of our study were to enable the direct structural analysis of the unstable LTA-type epoxides from lipoxygenase reactions and to further the understanding of factors that control their precise stereochemistry. The structure of the classic 5-lipoxygenase product, LTA4, was established beyond doubt by comparison of transformations of the natural product and leukotriene epoxides prepared by total chemical synthesis (15–17). It has been widely assumed that other LOX-catalyzed LTA-type products will be the direct structural analogs of LTA4, although the equivalent experimental support is lacking. The possibility that LOX reactions could lead to cis-epoxy LTA4 was deduced by Corey and coworkers (32), who identified the stereochemistry of the initial LOX-catalyzed hydrogen abstraction as a determinant of the trans or cis epoxide configuration. Herein we identified the stereochemistry of the HPETE substrate as an additional determinant. In the following discussion we present a model that rationalizes these observations and predicts the LTA epoxide stereochemistry in novel LOX-catalyzed reactions.
To explain the available results, we further developed a conceptual model underlying the mechanistic basis of the LOX-catalyzed transformation of HPETE precursor to LTA4-type epoxide (Fig. 7). The nonheme iron in the LOX active site must be involved in the initial hydrogen abstraction from the HPETE substrate and cleavage of the hydroperoxide moiety (33, 34). Key to our thinking, therefore, is that the hydrogen and hydroperoxide should exhibit a suprafacial relationship. Because the conversion of [10R-3H]5-HPETE to LTA4 is associated with a primary isotope effect resulting in an enrichment in the specific activity of the unreacted substrate (35–37), this provides critical evidence identifying the 10pro-R hydrogen abstraction as the first irreversible step in leukotriene A4 biosynthesis. This implicates the ferric iron (hydroxide) in 5-LOX as the active species catalyzing the first step of the transformation. The observation that reducing inhibitors of the lipoxygenase that leave the active site iron in the ferrous state block the dioxygenase reaction and LTA synthesis (7, 23) provides further support for involvement of the ferric iron in catalyzing the initial hydrogen abstraction:
Fig. 7.
Lipoxygenase-catalyzed transformations to LTA epoxides. LTA synthesis is initiated by the ferric form of the LOX enzyme (A–C), whereas dehydration to the ketone involves the ferrous enzyme (D, below the dividing line).
Further reaction will entail homolytic cleavage of the hydroperoxide, thus cycling the lipoxygenase ferrous iron back to ferric, because radical recombination produces the epoxide product:
It follows from this that the iron must have access to the initial hydrogen abstracted and to the hydroperoxide (i.e., the two are on the same face of the substrate). In achieving this, 5S-HPETE (the natural enantiomer) must assume the transoid conformation at the 5-carbon, thus dictating the natural trans-epoxy configuration of LTA4 (Fig. 7A). The enantiomeric substrate, 5R-HPETE, has to assume the cisoid conformation, which results in its transformation to 5-epi-LTA4, now established experimentally (Fig. 7B).
To complete the picture with related transformations, Fig. 7C illustrates the result reported by Corey and coworkers (32) in which an 8R-LOX activity, which catalyzes pro-S hydrogen abstraction from C-10 (38), converted 5S-HPETE to the cis epoxide 6-epi-LTA4 (32); by contrast, 5S-LOX, which abstracts the 10pro-R hydrogen (30), produced trans-epoxy LTA4 from 5S-HPETE, as expected (32). The epoxides were identified indirectly based on a difference in pattern of the hydrolysis products in comparison to hydrolysis of the synthetic standards (32). Because it is now known that R and S lipoxygenases are closely related enzymes (39, 40) and that substrate is exposed to the same open ligand position of the active site iron (41), we deduce that the 5S-HPETE substrate binds in an opposite head-to-tail orientation in 5S-LOX and 8R-LOX to present the appropriate C-10 hydrogen for abstraction by the nonheme iron (42). Accordingly, the substrate is shown in the reversed orientation in the 8R-LOX reaction illustrated in Fig. 7C. Reactions that appear feasible “on paper” may or may not be executed favorably, depending on steric considerations within the LOX enzyme. This is evident in the reaction of 15R-HPETE with 15-LOX-1 in which LTA epoxide synthesis is formed in only trace amounts. Similarly, in the reaction of 5R-HPETE with 5-LOX, the formation of 5-epi-LTA4 is superseded by 5-oxo-ETE synthesis as the major product in catalysis initiated by the ferrous nonheme iron (Fig. 7D) (43, 44).
The concepts developed here on the determinants of cis or trans LTA4 epoxide configuration establish a rational mechanism underlying the structures of these key biosynthetic intermediates. The concepts can be applied to the enzymatic formation of novel LTA-type epoxides postulated as intermediates in biosynthesis of the resolvin, protectin, and maresin lipid mediators formed from eicosapentaenoic (20:5ω3) and docosahexaenoic (22:6ω3) fatty acids (4, 5). In fact, because S-lipoxygenases predominate in higher animal biology (45) and are implicated in the synthesis of the resolvin/protectin/maresin mediators, it is possible that novel transformations involving the LTA-type epoxides in higher animals follow the same relationships as in the 5S-LOX pathway to the leukotrienes. Isolation and identification of these intermediates are in progress to examine the structures experimentally.
Supplementary Material
Acknowledgments
The authors thank Dr. Don F. Stec for help with the NMR analyses.
Footnotes
Abbreviations:
- HPETE
- hydro(pero)xyeicosatetraenoic acidLT, leukotriene
- LOX
- lipoxygenase
- 5-oxo-ETE
- 5-oxo-eicosatetraenoic acid
- RP-HPLC
- reverse phase high-pressure liquid chromatography
- SP-HPLC
- straight phase high-pressure liquid chromatography
- UV
- ultraviolet
This work was supported by National Institutes of Health grant GM-015431. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of one figure and supplementary data.
REFERENCES
- 1.Samuelsson B. 1983. From studies of biochemical mechanism to novel biological mediators: prostaglandin endoperoxides, thromboxanes, and leukotrienes. Nobel Lecture, 8 December 1982. Biosci. Rep. 3: 791–813 [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson B., Dahlen S. E., Lindgren J. A., Rouzer C. A., Serhan C. N. 1987. Leukotrienes and lipoxins: structures, biosynthesis, and biological effects. Science. 237: 1171–1176 [DOI] [PubMed] [Google Scholar]
- 3.Feltenmark S., Gautam N., Brunnstrom A., Griffiths W., Backman L., Edenius C., Lindbom L., Bjorkholm M., Claesson H. E. 2008. Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells. Proc. Natl. Acad. Sci. USA. 105: 680–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serhan C. N. 2010. Novel lipid mediators and resolution mechanisms in acute inflammation. To resolve or not? Am. J. Pathol. 177: 1576–1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bannenberg G., Serhan C. N. 2010. Specialized pro-resolving lipid mediators in the inflammatory response: an update. Biochim. Biophys. Acta. 1801: 1260–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borgeat P., Samuelsson B. 1979. Arachidonic acid metabolism in polymorphonuclear leukocytes: unstable intermediate in formation of dihydroxy acids. Proc. Natl. Acad. Sci. USA. 76: 3213–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimizu T., Rådmark O., Samuelsson B. 1984. Enzyme with dual lipoxygenase activities catalyzes leukotriene A4 synthesis from arachidonic acid. Proc. Natl. Acad. Sci. USA. 81: 689–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maas R. L., Brash A. R. 1983. Evidence for a lipoxygenase mechanism in the biosynthesis of epoxide and dihydroxy leukotrienes from 15(S)-hydroperoxyicosatetranoic acid by human platelets and porcine leukocytes. Proc. Natl. Acad. Sci. USA. 80: 2884–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rouzer C. A., Matsumoto T., Samuelsson B. 1986. Single protein from human leukocytes possesses 5-lipoxygenase and leukotriene A4 synthase activities. Proc. Natl. Acad. Sci. USA. 83: 857–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marfat A., Corey E. J. 1985. Synthesis and structure elucidation of leukotrienes. Adv. Prostaglandin Thromboxane Leukot. Res. 14: 155–228 [PubMed] [Google Scholar]
- 11.Rokach J., Adams J. 1985. Synthesis of leukotrienes and lipoxygenase products. Acc. Chem. Res. 18: 87–93 [Google Scholar]
- 12.Rokach J., Guindon Y., Young R. N., Adams J., Atkinson J. G. 1988. Synthesis of the leukotrienes. In The total synthesis of natural products. J. ApSimon, editor. John Wiley & Sons, New York. 141–273. [Google Scholar]
- 13.Fitzpatrick F. A., Morton D. R., Wynalda M. A. 1982. Albumin stabilizes leukotriene A4. J. Biol. Chem. 257: 4680–4683 [PubMed] [Google Scholar]
- 14.Rådmark O., Malmsten C., Samuelsson B., Goto G., Marfat A., Corey E. J. 1980. Leukotriene A: isolation from human polymorphonuclear leukocytes. J. Biol. Chem. 255: 11828–11831 [PubMed] [Google Scholar]
- 15.Rådmark O., Malmsten C., Samuelsson B., Clark D. A., Goto G., Marfat A., Corey E. J. 1980. Leukotriene A: stereochemistry and enzymatic conversion to leukotriene B. Biochem. Biophys. Res. Commun. 92: 954–961 [DOI] [PubMed] [Google Scholar]
- 16.Lewis R. A., Austen K. F., Drazen J. M., Clark D. A., Marfat A., Corey E. J. 1980. Slow reacting substances of anaphylaxis: identification of leukotrienes C-1 and D from human and rat sources. Proc. Natl. Acad. Sci. USA. 77: 3710–3714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hammarström S., Samuelsson B., Clark D. A., Goto G., Marfat A., Mioskowski C., Corey E. J. 1980. Stereochemistry of leukotriene C-1. Biochem. Biophys. Res. Commun. 92: 946–953 [DOI] [PubMed] [Google Scholar]
- 18.Brash A. R., Baertschi S. W., Ingram C. D., Harris T. M. 1988. Isolation and characterization of natural allene oxides: unstable intermediates in the metabolism of lipid hydroperoxides. Proc. Natl. Acad. Sci. USA. 85: 3382–3386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schneider C., Niisuke K., Boeglin W. E., Voehler M., Stec D. F., Porter N. A., Brash A. R. 2007. Enzymatic synthesis of a bicyclobutane fatty acid by a hemoprotein-lipoxygenase fusion protein from the cyanobacterium Anabaena PCC 7120. Proc. Natl. Acad. Sci. USA. 104: 18941–18945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peers K. E., Coxon D. T. 1983. Controlled synthesis of monohydroperoxides by α-tocopherol inhibited autoxidation of polyunsaturated lipids. Chem. Phys. Lipids. 32: 49–56 [Google Scholar]
- 21.Schneider C., Yu Z., Boeglin W. E., Zheng Y., Brash A. R. 2007. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 433: 145–157 [DOI] [PubMed] [Google Scholar]
- 22.Boeglin W. E., Itoh A., Zheng Y., Coffa G., Howe G. A., Brash A. R. 2008. Investigation of substrate binding and product stereochemistry issues in two linoleate 9-lipoxygenases. Lipids. 43: 979–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bryant R. W., Schewe T., Rapoport S. M., Bailey J. M. 1985. Leukotriene formation by a purified reticulocyte lipoxygenase enzyme. Conversion of arachidonic acid and 15-hydroperoxyeicosatetraenoic acid to 14,15-leukotriene A4. J. Biol. Chem. 260: 3548–3555 [PubMed] [Google Scholar]
- 24.Van Os C. P. A., Rijke-Schilder G. P. M., Van Halbeek H., Verhagen J., Vliegenthart J. F. G. 1981. Double dioxygenation of arachidonic acid by soybean lipoxygenase-1. Kinetics and regio-stereo specificities of the reaction steps. Biochim. Biophys. Acta. 663: 177–193 [DOI] [PubMed] [Google Scholar]
- 25.Corey E. J., Arai Y., Mioskowski C. 1979. Total synthesis of (+/−)-5,6-oxido-7,9-trans,11,14-cis-eicosapentaenoic acid, a possible precursor of SRSA. J. Am. Chem. Soc. 101: 6748–6749 [Google Scholar]
- 26.Maas R. L., Ingram C. D., Porter A. T., Oates J. A., Taber D. F., Brash A. R. 1985. Investigation of the chemical conversion of hydroperoxy-eicosatetraenoate to leukotriene epoxide using stereospecifically labeled arachidonic acid. J. Biol. Chem. 260: 4217–4228 [PubMed] [Google Scholar]
- 27.Maas R. L., Brash A. R., Oates J. A. 1981. A second pathway of leukotriene biosynthesis in porcine leukocytes. Proc. Natl. Acad. Sci. USA. 78: 5523–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachs-Olsen C., Sanak M., Lang A. M., Gielicz A., Mowinckel P., Lodrup Carlsen K. C., Carlsen K. H., Szczeklik A.2010. Eoxins: a new inflammatory pathway in childhood asthma. J. Allergy Clin. Immunol.126: 859–867, e859.
- 29.Chen X., Reddanna P., Reddy G. R., Kidd R., Hildenbrandt G., Reddy C. C. 1998. Expression, purification, and characterization of a recombinant 5-lipoxygenase from potato tuber. Biochem. Biophys. Res. Commun. 243: 438–443 [DOI] [PubMed] [Google Scholar]
- 30.Corey E. J., Lansbury P. T., Jr 1983. Stereochemical course of 5-lipoxygenation of arachidonate by rat basophil leukemic cell (RBL-1) and potato enzymes. J. Am. Chem. Soc. 105: 4093–4094 [Google Scholar]
- 31.Mercier J., Agoh B. 1974. Comportement d'hydroperoxydes allyliques a longue chaine en presence de complexes de certains metaux de transition. II. Structure des époxy-alcools formés à partir d'hydroperoxydes d'octadécène-9 oates de méthyle cis et trans en présence d'acétylacétonate de vanadyle. Chem. Phys. Lipids. 12: 239–248 [Google Scholar]
- 32.Corey E. J., Wright S. W., Matsuda S. P. T. 1989. Stereochemistry and mechanism of the biosynthesis of Leukotriene A4 from 5(S)-hydroperoxy-6(E),8,11,14(Z)-eicosatetraenoic acid. Evidence for an organoiron intermediate. J. Am. Chem. Soc. 111: 1452–1455 [Google Scholar]
- 33.Kühn H., Schewe T., Rapoport S. M. 1986. The stereochemistry of the reactions of lipoxygenases and their metabolites. Proposed nomenclature of lipoxygenases and related enzymes. Adv. Enzymol. Relat. Areas Mol. Biol. 58: 273–311 [DOI] [PubMed] [Google Scholar]
- 34.Zheng Y., Brash A. R. 2010. Formation of a cyclopropyl epoxide via a leukotriene A synthase-related pathway in an anaerobic reaction of soybean lipoxygenase-1 with 15S-hydroperoxyeicosatetraenoic acid. Evidence that oxygen access is a determinant of secondary reactions with fatty acid hydroperoxides. J. Biol. Chem. 285: 13427–13436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maas R. L., Ingram C. D., Taber D. F., Oates J. A., Brash A. R. 1982. Stereospecific removal of the DR hydrogen atom at carbon 10 of arachidonic acid in the biosynthesis of leukotriene A4 by human leukocytes. J. Biol. Chem. 257: 13515–13519 [PubMed] [Google Scholar]
- 36.Panossian A., Hamberg M., Samuelsson B. 1982. On the mechanism of biosynthesis of leukotrienes and related compounds. FEBS Lett. 150: 511–513 [DOI] [PubMed] [Google Scholar]
- 37.Ueda N., Yamamoto S., Oates J. A., Brash A. R. 1986. Stereoselective hydrogen abstraction in leukotriene A4 synthesis by purified 5-lipoxygenase of porcine leukocytes. Prostaglandins. 32: 43–48 [DOI] [PubMed] [Google Scholar]
- 38.Hughes M. A., Brash A. R. 1991. Investigation of the mechanism of biosynthesis of 8-hydroxyeicosatetraenoic acid in mouse skin. Biochim. Biophys. Acta. 1081: 347–354 [DOI] [PubMed] [Google Scholar]
- 39.Brash A. R., Boeglin W. E., Chang M. S., Shieh B-H. 1996. Purification and molecular cloning of an 8R-lipoxygenase from the coral Plexaura homomalla reveal the related primary structures of R- and S-lipoxygenases. J. Biol. Chem. 271: 20949–20957 [DOI] [PubMed] [Google Scholar]
- 40.Oldham M. L., Brash A. R., Newcomer M. E. 2005. Insights from the X-ray crystal structure of coral 8R-lipoxygenase: calcium activation via a C2-like domain and a structural basis of product chirality. J. Biol. Chem. 280: 39545–39552 [DOI] [PubMed] [Google Scholar]
- 41.Brash A. R. 1999. Lipoxygenases: occurrence, functions, catalysis, and acquisition of substrate. J. Biol. Chem. 274: 23679–23682 [DOI] [PubMed] [Google Scholar]
- 42.Coffa G., Schneider C., Brash A. R. 2005. A comprehensive model of positional and stereo control in lipoxygenases. Biochem. Biophys. Res. Commun. 338: 87–92 [DOI] [PubMed] [Google Scholar]
- 43.Yu Z., Schneider C., Boeglin W. E., Marnett L. J., Brash A. R. 2003. The lipoxygenase gene ALOXE3 implicated in skin differentiation encodes a hydroperoxide isomerase. Proc. Natl. Acad. Sci. USA. 100: 9162–9167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zheng Y., Brash A. R. 2010. Dioxygenase activity of epidermal lipoxygenase-3 unveiled: typical and atypical features of its catalytic activity with natural and synthetic polyunsaturated fatty acids. J. Biol. Chem. 285: 39866–39875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivanov I., Heydeck D., Hofheinz K., Roffeis J., O'Donnell V. B., Kuhn H., Walther M. 2010. Molecular enzymology of lipoxygenases. Arch. Biochem. Biophys. 503: 161–174 [DOI] [PubMed] [Google Scholar]
- 46.Gravel J., Falgueyret J. P., Yergey J., Trimble L., Riendeau D. 1993. Identification of 5-keto-(7E,9E,11Z,14Z)-eicosatetraenoic acid as a novel nonenzymatic rearrangement product of leukotriene A4. Arch. Biochem. Biophys. 306: 469–475 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.