Abstract
20-Hydroxy-5,8,11,14-eicosatetraenoic acid (20-HETE), a product of the cytochrome P450 (CYP)-catalyzed ω-hydroxylation of arachidonic acid, induces oxidative stress and, in clinical studies, is associated with increased body mass index (BMI) and the metabolic syndrome. This study was designed to examine the effects of exogenous 20-HETE on mesenchymal stem cell (MSC)-derived adipocytes. The expression levels of CYP4A11 and CYP4F2 (major 20-HETE synthases in humans) in MSCs decreased during adipocyte differentiation; however, exogenous administration of 20-HETE (0.1–1 μM) increased adipogenesis in a dose-dependent manner in these cells (P < 0.05). The inability of a 20-HETE analog to reproduce these effects suggested the involvement of a metabolic product of 20-HETE in mediating its pro-adipogenic effects. A cyclooxygenase (COX)-1 selective inhibitor enhanced, whereas a COX-2 selective or a dual COX-1/2 inhibitor attenuated adipogenesis induced by 20-HETE. The COX-derived metabolite of 20-HETE, 20-OH-PGE2, enhanced adipogenesis and lipid accumulation in MSCs. The pro-adipogenic effects of 20-HETE and 20-OH-PGE2 resulted in the increased expression of the adipogenic regulators PPARγ and β-catenin in MSC-derived adipocytes. Taken together we show for the first time that 20-HETE-derived COX-2-dependent 20-OH-PGE2 enhances mature inflamed adipocyte hypertrophy in MSC undergoing adipogenic differentiation.
Keywords: adipogenesis, arachidonic acid, mesenchymal stem cells
Metabolic dysfunction of adipose tissue due to oxidative stress is considered the cause of several complications of obesity, such as diabetes and cardiovascular disease (1–3). Dysfunctional adipocytes are associated with the development of insulin resistance, hyperglycemia, atherogenic dyslipidemia, and arterial hypertension, and they favor a prothrombotic and proinflammatory state (4, 5). Oxidative stress in adipose tissue regulates adipogenic response with adipocyte hypertrophy (6, 7). Adipocyte dysfunction is caused by a variety of stimuli, including heavy metals, reactive oxygen species (ROS), nitric oxide (NO), Ang II, and cytokines. The localized inflammation with increased macrophage infiltration in adipose tissue leads to important changes in adipocyte gene expression, with downstream effects on adipocyte lipid metabolism and altered endocrine function (7). Adipocyte differentiation (adipogenesis) is a complex process that includes coordinated changes in hormone sensitivity and gene expression in response to various stimuli, including lipid mediators (8).
20-Hydroxyeicosatetraenoic acid (20-HETE), a product of the cytochrome P450 (CYP)-catalyzed ω-hydroxylation of arachidonic acid, is a primary eicosanoid in the microcirculation that plays a role in the regulation of vascular tone and renal tubular homeostasis (9–12). Perturbations in the levels of 20-HETE in tissues and biological fluids have been observed in multiple pathological states, including hypertension, kidney disease, and metabolic syndrome (13–17). 20-HETE stimulates production of superoxide and inflammatory cytokines, inhibits endothelial nitric oxide synthase (eNOS), and increases oxidative stress (18–20), suggesting a possible role for 20-HETE in the regulation of adipogenesis (21).
Prostaglandins (PG) are known to play a variety of roles in adipocytes and precursor cells that utilize the arachidonate cyclooxygenase (COX) pathway to generate PGs at different stages of the life cycle of adipocytes (8). COX consists of two isozymes, COX-1 and COX-2, and is the rate-limiting enzyme that catalyzes the conversion of arachidonic acid into PGs by specific PG synthases (22). COX-1 is constitutively expressed in most cells, including adipocytes, whereas COX-2 is induced in response to various stimuli, such as cytokines and oxidative stress (23). COX-2 expression is transiently enhanced in the early phase of adipogenesis. Prostaglandin E2 (PGE2) and prostaglandin F2a (PGF2α) are anti-adipogenic factors that suppress the differentiation of adipocytes (23). Arachidonic acid metabolism during adipogenesis is a process governed at multiple levels, suggesting a complex role for PGs during fat cell development (24). The relative contribution of COX-1 and COX-2 to the regulation of adipogenesis remains to be resolved.
20-HETE plays an important role in inducing oxidative stress (21), and as such, we hypothesize that 20-HETE affects adipogenesis. We examined adipogenesis in mesenchymal stem cells (MSC), multipotent stromal cells that can be induced to differentiate into adipocytes (25). 20-HETE is also a substrate for COX metabolites; hence, the goal of the present study was to evaluate the contribution of 20-HETE and its COX-derived metabolites to the regulation of adipogenesis. The results of this study suggest that COX-derived 20-HETE metabolites are a potential therapeutic target in the treatment of obesity and its associated disease states: hypertension, the metabolic syndrome, and diabetes.
MATERIALS AND METHODS
Human bone marrow-derived MSC differentiation into adipocytes
Frozen bone marrow mononuclear cells were purchased from Allcells (Emeryville, CA). After thawing, mononuclear cells were resuspended in an α-minimal essential medium (α-MEM, Invitrogen, Carlsbad, CA) supplemented with 10% heat inactivated fetal bovine serum (FBS, Invitrogen) and 1% antibiotics and antimycotic (Invitrogen). The cells were plated at a density of 1–5 × 106 cells per 100 cm2 dish. The cultures were maintained at 37°C in a 5% CO2 incubator. The medium was changed after 48 h and every 3–4 days thereafter. When the MSCs were confluent, the cells were recovered by the addition of 0.25% trypsin/EDTA (Invitrogen). MSCs (passage 2–3) were plated in either a 75 cm2 flask or a 24-well plate and cultured in α-MEM with 20% FBS up to a density of 2.0 × 104 cells/cm2. The medium was replaced with adipogenic medium, and the cells were cultured for an additional 14 days. The adipogenic media consisted of complete culture medium supplemented with DMEM-high glucose, 10% (v/v) FBS, 10 µg/ml insulin, 0.5 mM dexamethasone (Sigma-Aldrich, St. Louis, MO), and 1% antibiotics and antimycotic (Invitrogen) in the presence and absence of the COX-1 inhibitor (2-valeryloxybenzoic acid, Cayman, Ann Arbor, MI) and the COX-2 inhibitor (3-(4-methylsulphonylphenyl)-4-phenyl-5-trifluoromethylisoxazol, Cayman) with and without 20-HETE, 20-HETE agonist (20-5,14-HEDE), or 20-OH-PGE2. 20-HETE, 20-HETE agonist, or 20-OH-PGE2 were added three times a week at concentrations of 0.1 and 1 μM. Inhibitors of COX-1 and COX-2 were added three times a week at a dose of 100 and 5 μM, respectively.
Oil Red O staining
At day 14 of adipogenesis, 0.21% Oil Red O in 100% isopropanol (Sigma-Aldrich, St. Louis, MO) was used. Briefly, adipocytes were fixed in 10% formaldehyde, washed in Oil Red O for 10 min, rinsed with 60% isopropanol (Sigma-Aldrich). Then the Oil Red O was eluted by adding 100% isopropanol for 10 min, and OD was measured at 490 nm for 0.5 s reading. MSC-derived adipocytes were measured by Oil Red O staining (OD = 490 nm) after day 14. Each value of Oil Red O staining was normalized by the cell number.
Adipocyte size
Lipid droplets were measured using an ImagePro Analyzer (Media Cybernetics Corporation, Silver Springs, MD). The MSC-derived adipocytes were treated with increasing concentration of 20-OH-PGE2 (1–1,000 nM) every alternate day for 14 days. To quantify the number and size of the lipid droplets in these images, a proprietary algorithm (patent pending) was developed that segments circular staining patterns. The algorithm was then applied to images obtained from the lipid optical channel for cells exposed to different doses of 20-OH-PGE2.
mRNA isolation and real-time PCR quantification
Total RNA was isolated using the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer's instructions. First-strand cDNA was synthesized with Roche reverse transcription reagents. Total RNA (0.5–1 μg) was analyzed by real-time PCR. The quantitative real-time polymerase chain reaction (qRT-PCR) was performed with the TaqMan gene expression assay on an Applied Biosystems 7500 fast real-time PCR system according to the manufacturer's recommended protocol (Applied Biosystems, Foster City, CA). Each reaction was run in triplicate. The comparative threshold cycle (CT) method was used to calculate the amplification fold as specified by the manufacturer.
Western blot analysis
Western blot analysis of adipocyte cell lysate was carried out as described previously (6, 26). Briefly, cells were placed in a homogenization buffer, and homogenates were centrifuged at 27,000 g for 10 min at 4°C. The supernatant was used for the measurement of COX-1, COX-2, PPARγ, Mest, and β-catenin protein levels (27, 28). The levels were quantified by scanning densitometry using an imaging densitometer, normalized to the levels of total protein.
PGE2 measurement
PGE2 levels were determined in the culture supernatant. Multiple assays were conducted for quantification of the proteins (AssayGate Inc., Ijamsville, MD). All measurements were performed in triplicate.
Statistical analyses
Statistical significance between experimental groups was determined by the Fisher method of analysis of multiple comparisons (P < 0.05 was regarded as significant). For comparison between treatment groups, the null hypothesis was tested by either a single-factor ANOVA for multiple groups or the unpaired t-test for two groups. Data are presented as mean ± SEM. Differences between experimental groups were evaluated with ANOVA with Bonferroni corrections. Statistical significance was regarded as significant at P < 0.05.
RESULTS
CYP4-ω-hydroxylase and COX expression during adipogenesis
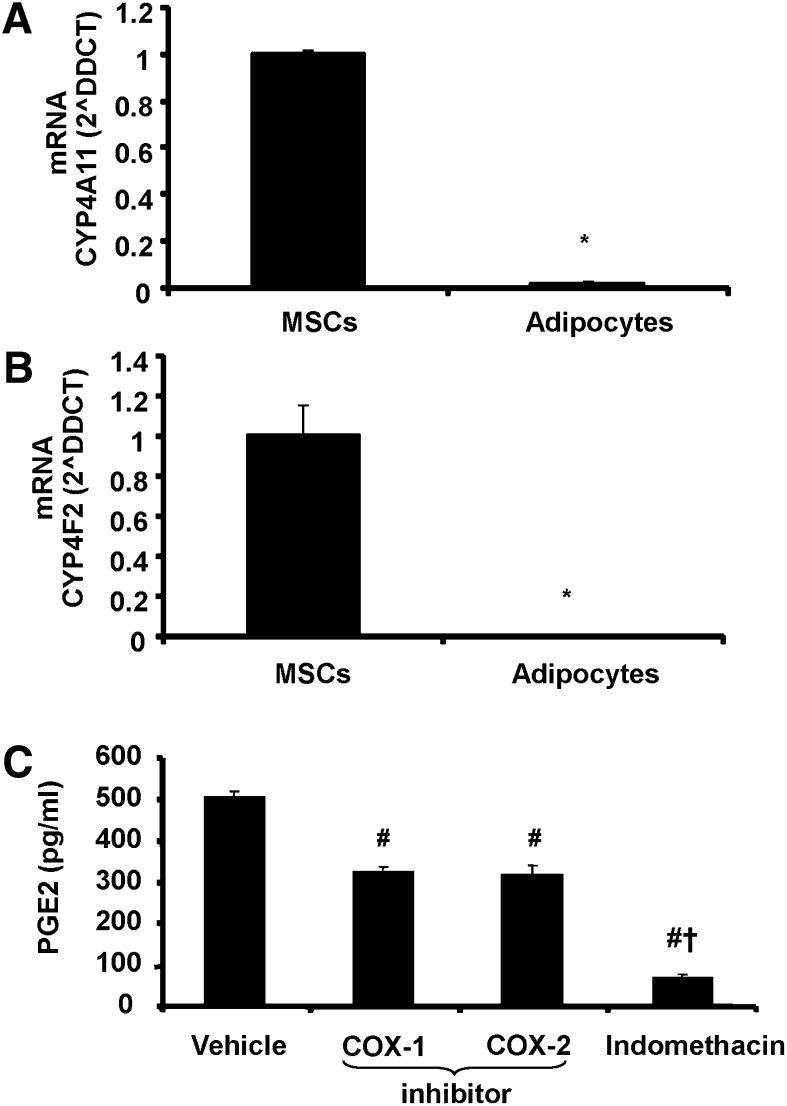
The expression levels of CYP4-ω-hydroxylases were determined in MSC before and after completion of adipogenesis as shown in Fig. 1A, B. MSC expressed relatively high mRNA levels of CYP4A11 and CYP4F2 (the major 20-HETE producing CYP4-ω-hydroxylases in humans) (17) before the start of adipogenic differentiation. In adipocytes derived from MSC, mRNA levels of these hydroxylases were nearly undetectable. To evaluate COX activity in MSC exposed to adipogenic environment, PGE2 levels were determined in conditioned media (Fig. 1C). COX-1 and COX-2 inhibitors decreased PGE2 levels compared with levels in the conditioned media without indomethacin. Addition of indomethacin, which is a dual COX-1 and COX-2 inhibitor, further decreased PGE2 levels (Fig. 1C).
Fig. 1.
Levels of mRNA for (A) Cyp4F11 and (B) Cyp4F2 and (C) levels of PGE2 in MSC before and after adipogenic differentiation (adipocytes). Data are expressed as means ± SE. *P < 0.05 versus MSC, #P < 0.05 versus vehicle, +P < 0.05 versus COX-1 or COX-2 inhibitor.
Effect of 20-HETE and COX inhibition on adipogenesis
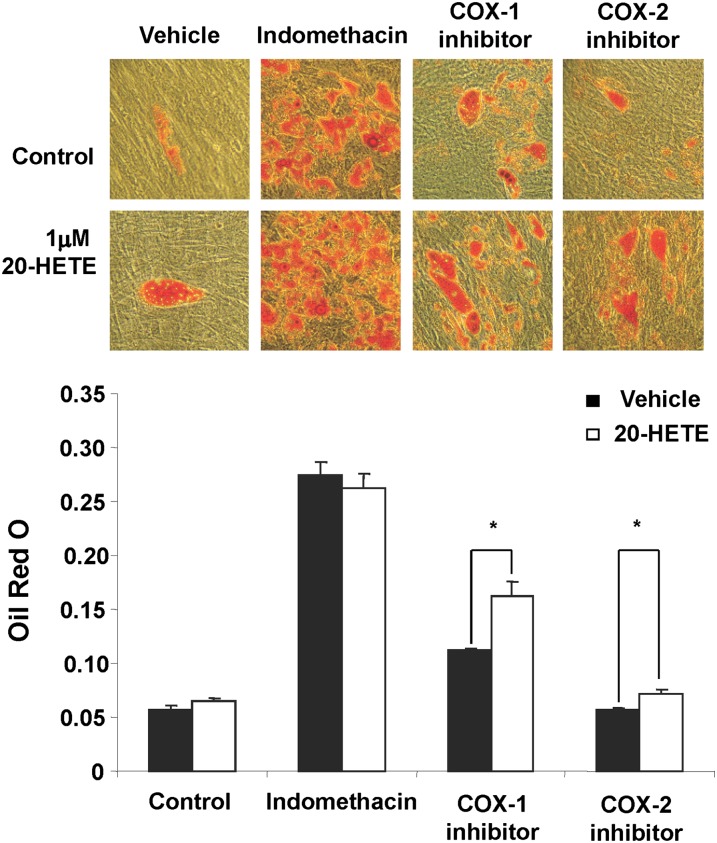
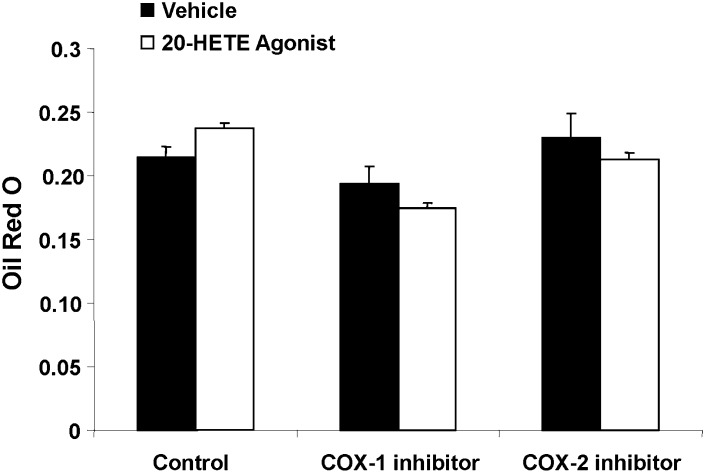
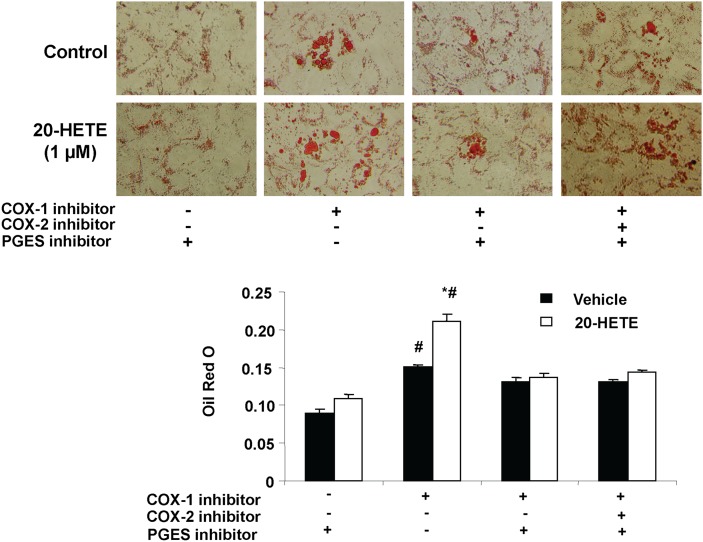
The effect of 20-HETE on lipid accumulation in MSC-derived adipocytes was examined in the presence and the absence of either a COX-1 or COX-2 inhibitor. 20-HETE enhanced lipid accumulation in cells exposed to a COX-1 inhibitor but not in cells exposed to COX-2 inhibitor (Fig. 2). The absence of such an effect of 20-HETE in cells treated with a COX-2 inhibitor alludes to the role of COX-2-derived 20-HETE metabolic product in mediating these enhanced lipogenic effects (Fig. 2). The direct effect of 20-HETE on lipid accumulation in MSCs derived adipocytes was further refuted by the inability of a 20-HETE agonist [sodium 2-((5Z,14Z)-20-hydroxyicosa-5,14-dienamido)acetate, 20-HEDE] to mimic the effects of exogenous 20-HETE as shown in Fig. 3. Results show that in presence of COX-1 and COX-2 inhibitors, the 20-HETE agonist 20-HEDE had no significant effect on adipogenesis, thus further substantiating the notion that a COX-2-derived metabolic product has an enhanced adipogenic effect. Subsequently, addition of the microsomal PGE2 synthase inhibitor CAY10526 abolished the adipogenic effect of 20-HETE in the presence of a COX-1 inhibitor (Fig. 4). Arachidonic acid, which can also be metabolized consecutively by CYP and COX enzymes to a similar product, was less potent than 20-HETE; it had no effect at 0.1 and 1 µM, and at 10 µM, it increased lipid accumulation by 20% (data not shown).
Fig. 2.
Effect of 20-HETE on adipogenesis in the presence and absence of indomethacin, COX-1 inhibitor (valeroyl salicylate), and COX-2 inhibitor (CAY10404). Adipogenesis was measured as the relative absorbance of Oil Red O at day 14 after inducing adipogenesis as described in Materials and Methods. Mean ± SE, *P < 0.05 versus vehicle.
Fig. 3.
Effect of a 20-HETE agonist on adipogenesis in the presence and absence of COX-1 or COX-2 inhibitor. Adipogenesis was measured as the relative absorbance of Oil Red O at day 14 after inducing adipogenesis as described in Materials and Methods. Mean ± SE.
Fig. 4.
Effect of 20-HETE on adipogenesis in the presence and absence of COX-1 inhibitor, COX-2 inhibitor, and the PGE2 synthase inhibitor (CAY10526). Adipogenesis was measured as the relative absorbance of Oil Red O at day 14 after inducing adipogenesis as described in Materials and Methods. Mean ± SE, *P < 0.05 versus vehicle, #P < 0.05 versus control.
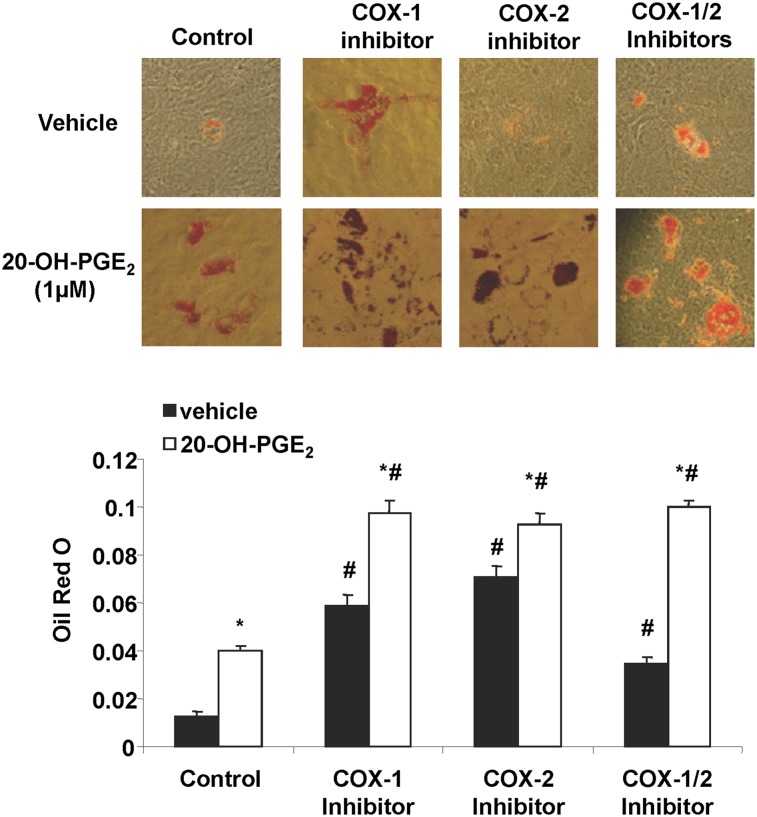
Effect of 20-hydroxy-PGE2 on adipogenesis and adipocyte hypertrophy
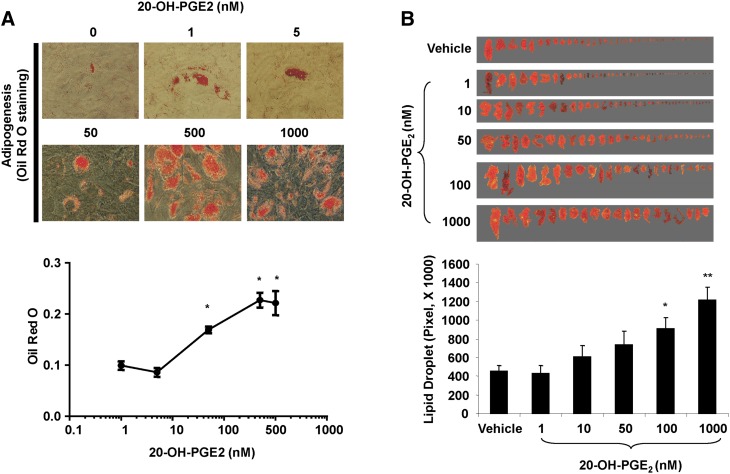
Previous studies have shown that 20-HETE is metabolized by COX to 20-OH-endoperoxides and consequently to 20-OH-PGE2 (29, 30). Therefore, we examined the effects of 20-OH-PGE2 on adipogenesis in the presence or absence of COX-1 and COX-2 inhibitors. 20-OH-PGE2 stimulated (P < 0.05) adipogenesis 4-fold as measured by lipid accumulation (Fig. 5). Neither COX-1 nor COX-2 inhibitor, alone or together, prevented 20-OH-PGE2-mediated increase in adipogenesis, suggesting a COX-independent action of this metabolite (Fig. 5). The effect of 20-OH-PGE2 was concentration dependent (Fig. 6A). It significantly increased lipid accumulation at 50 nM and had maximal effect at 500 nM. Moreover, addition of PGE2 blunted the adipogenic activity of 20-OH-PGE2; PGE2 (10 nM) inhibited the adipogenic activity of 20-OH-PGE2 (500 nM) by 40% as evidenced by a reduction in lipid accumulation measured as absorbance of Oil Red O from 0.38 ± 0.02 to 0.23 ± 0.02 (P < 0.05). In addition, 20-OH-PGE2 stimulated adipocyte hypertrophy as measured by lipid droplet size. As seen in Fig. 6B, lipid droplet size increased 2- and 3-fold in response to 100 and 1,000 nM of 20-OH-PGE2. These results strongly suggest that the COX-2 metabolite 20-hydroxy-PGE2 plays a significant role in inducing adipogenesis and that its effect may be antagonized by PGE2.
Fig. 5.
Effect of 20-OH-PGE2 on adipogenesis in the presence and absence of COX-1 inhibitor and/or COX-2 inhibitor. Adipogenesis was measured as the relative absorbance of Oil Red O at day 14 after inducing adipogenesis as described in Materials and Methods. Mean ± SE, *P < 0.05 versus vehicle, #P < 0.05 versus control.
Fig. 6.
(A) Concentration-dependent effect of 20-OH-PGE2 on adipogenesis. Mean ± SE, *P < 0.05 versus 1 nM concentration of 20-OH-PGE2. (B) Effect of 20-OH-PGE2 on adipocyte size. Mean ± SE, *P < 0.05, and **P < 0.01 versus control.
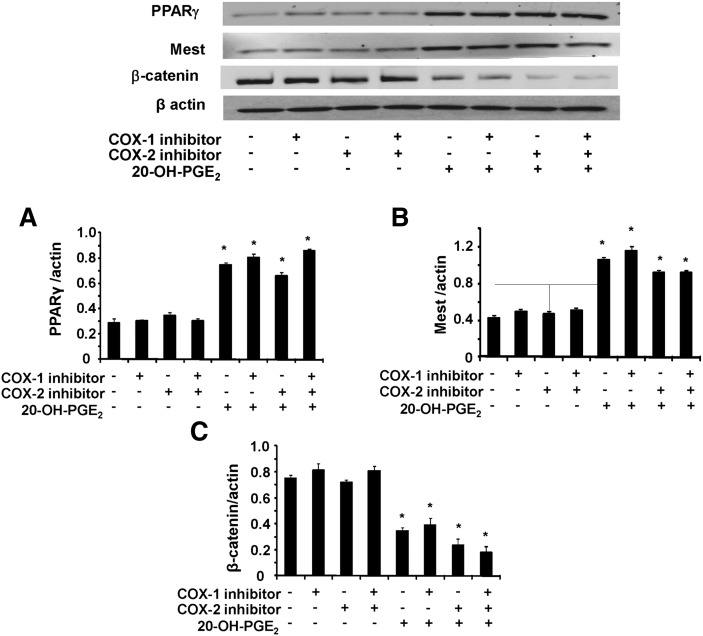
Effect of 20 HETE and 20-OH- PGE2 on adipogenic markers
We examined the effects of 20-HETE and 20-OH-PGE2 on β-catenin, PPARγ, and Mest expression as adipogenic differentiation markers, in the presence and absence of COX-1 and COX-2 inhibitors (Fig. 7). Densitometry analysis showed that the expression of PPARγ and Mest (Fig. 7A, B, respectively) was significantly increased in the presence of 20-OH-PGE2 when the cells were treated with COX-1 or COX-2 inhibitor or both. In contrast, β-catenin expression significantly decreased in the presence of 20-OH-PGE2 when the cells were treated with COX-1 or COX-2 inhibitor or both (Fig. 7C). Similar data were obtained with 20-HETE, except that in the presence of a COX-2 inhibitor, the effect was blunted (data not shown).
Fig. 7.
Effect of 20-OH-PGE2 on adipogenic markers. Expression of (A) PPARγ, (B) Mest, and (C) β-catenin was determined by Western blot analysis in MSC-derived adipocytes. Quantitative densitometry evaluation of the proteins ratio was determined. Data are expressed as means ± SE, *P < 0.05 versus corresponding conditions without 20-OH-PGE2.
DISCUSSION
In the present study, we demonstrated the stimulatory effect of exogenous 20-HETE on lipid accumulation in MSC-derived adipocytes. 20-HETE, a metabolic product of ω-hydroxylases, is produced in physiologically relevant amounts by various tissues and cells, including the vascular wall and hemopoeitic cells (29–31). 20-HETE has been shown to contribute to the regulation of vascular tone and control of blood pressure. Interestingly, along with its potent vasoconstrictor capacity, 20-HETE has been shown to mediate cellular proliferation (32, 33), angiogenesis (34, 35), oxidative stress (19), and inflammation (18), all of which may contribute importantly to the process of adipogenesis. Metabolic imbalance, frequently accompanying pathophysiological conditions traditionally associated with enhanced 20-HETE synthesis, was the focus of this study. The ω-Hydroxylase expression declined in hMSCs as they differentiated into adipocytes, thus limiting their autocrine exposure to endogenously generated 20-HETE. This may be a consequence of in vitro cell culturing conditions. The ability of adipose tissue to produce 20-HETE needs to be further assessed. Nevertheless, this does not preclude nonadipocyte sources of this eicosanoid, including 20-HETE from the vascular smooth muscle cells of the arteries vascularizing the adipose tissues (10, 11, 13, 14) as well as 20-HETE from circulating blood cells (36, 37). We showed a dose-dependent adipogenic response to 20-HETE in hMSCs, which was characterized by increased adipocyte hypertrophy and increased lipid accumulation. These findings highlight a potential role for 20-HETE in promoting lipid accumulation, which in turn has been strongly linked to adipocyte dysfunction and disruption of energy balance.
The inability of a 20-HETE analog to reproduce the enhanced adipogenic effects of 20-HETE led us to examine the hypothesis that a catabolic product of 20-HETE may be responsible for mediating these effects. 20-HETE is a known substrate for COX-2 with the resultant formation of 20-OH-PGG2 (30). Human MSCs have an abundance of microsomal PGE synthase (mPGES-1), the product of which, PGE2, is an inhibitor of adipogenesis (38). Findings in this study suggested that a COX-2-dependent 20-HETE metabolite, most probably 20-OH-PGE2, appears to mediate the adipogenic effect of 20-HETE on hMSCs-derived adipocytes. Blockade of the adipogenic effects of 20-HETE by a selective inhibitor of COX-2 or a specific mPGES-1 inhibitor supports this theory. In addition, administration of 20-OH-PGE2 had a similar effect on hMSCs to that observed during 20-HETE treatment. 20-OH-PGE2 could also be synthesized by the sequential metabolism of arachidonic acid by COX and ω-hydroxylases; however, this seems unlikely due to the nearly absent expression of ω-hydroxylases in maturing adipocytes.
Our hypothesis that the metabolic product of 20-HETE, most likely 20-OH-PGE2, is primarily a derivative of COX-2 stems from the observation that 20-HETE has no measurable effects on lipid accumulation on adipocytes undergoing COX-2 blockade, either alone or in combination with COX-1 blockade. Absence of a stimulatory effect of 20-HETE on hMSC-derived adipocytes in the absence of any COX blockade indicates the reciprocal effects of arachidonic acid-derived PGE2 and 20-HETE-derived 20-OH-PGE2. PGE2 inhibits adipogenesis, and for this reason, in vitro analysis of adipogenesis is traditionally carried out in a setting of COX blockade. We propose that the COX-2-derived metabolic product of 20-HETE counteracts the anti-adipogenic effects of PGE2. The source of PGE2 appears to be predominantly COX-1, as the basal level of adipogenesis is far greater in the presence of a COX-1 inhibitor than in the presence of a COX-2 inhibitor. The combination treatment of hMSCs with COX-1 and COX-2 inhibitors does not lead to increased adipogenesis compared with cells undergoing COX-1 blockade alone, further strengthening the notion that the inhibitory eicosanoid PGE2 is principally a catabolic product of COX-1.
Our proposal of the occurrence of reciprocal effects of PGE2 and 20-OH PGE2 on adipogenesis in hMSCs is strengthened by the demonstrations of a rightward shift of the dose-dependent adipogenic response in cells concurrently exposed to low concentrations of exogenous PGE2. The adipogenic activity of 20-HETE and 20-OH-PGE2 was associated with a marked increase in the expression of two major pro-adipogenic factors, PPARγ and Mest, and a decrease in β-catenin, which is a key anti-adipogenic regulator (39). 20-HETE and its metabolites were implicated as PPARγ activators (40, 41). In addition to increasing lipid accumulation, 20-HETE also enhanced hMSC COX-2 expression, which could provide a microenvironment more conducive to the formation of 20-OH-PGE2. It is possible that 20-HETE and/or 20-OH-PGE2 stimulate transcription activation of the PPARγ receptor and thereby set in motion a pro-adipogenic program that is further evidenced by increasing the expression of Mest and suppressing β-catenin. Additional studies are needed to fully identify the precise mechanisms of action of this eicosanoid.
20-HETE has been shown to increase in experimental models of diabetes and obesity (42). The clinical relevance of 20-HETE as a pro-adipogenic agent has been suggested in several studies. A report by Laffer et al. (15) showed a correlation between levels of 20-HETE and circulating insulin in essential hypertension with obesity. Croft and colleagues showed a significant correlation between 20-HETE levels and BMI, as well as with oxidative stress and endothelial dysfunction in hypertensive individuals (43–46). A recent study in our laboratory examined associations between circulating 20-HETE levels and the function of endothelial progenitor cells in diabetic and nondiabetic patients undergoing cardiac bypass surgery. This study found that levels of 20-HETE are significantly elevated in diabetic patients and that 20-HETE correlated with BMI in both diabetic and nondiabetic patients (47). Taken together, these studies and the current findings suggest that 20-HETE may play an important role in the regulation of adipose tissue.
In conclusion, we characterize a pro-adipogenic role of 20-HETE in adipocytes that appears to be mediated by a COX-2-dependent catabolic product. Additional analysis suggested that the mediator in question is 20-OH-PGE2. 20-OH-PGE2 could serve as an antagonist to the PGE2-dependent anti-adipogenic effects and contribute toward adipocyte hypertrophy and dysfunctional adipogenesis frequently associated with pathophysiological conditions, such as the metabolic syndrome. A selective COX-2 inhibitor could ablate these effects and could, in part, be used therapeutically in the fight against these chronic pathological conditions.
Footnotes
Abbreviations:
- BMI
- body mass index
- COX
- cyclooxygenase
- CYP
- cytochrome P450
- 20-HETE
- 20-hydroxy-5,8,11,14-eicosatetraenoic acid
- MSC
- mesenchymal stem cell
- PG
- prostaglandin
- PPAR
- peroxisome proliferator-activated receptor
This work was supported by National Institutes of Health Grants HL-34300 (M.L.S.) and HL-55601 (N.G.A.), and by the BrickStreet Foundation (J.S., N.G.A.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Hung W. W., Hsieh T. J., Lin T., Chou P. C., Hsiao P. J., Lin K. D., Shin S. J. 2011. Blockade of the renin-angiotensin system ameliorates apelin production in 3T3-L1 adipocytes. Cardiovasc. Drugs Ther. 25: 3–12 [DOI] [PubMed] [Google Scholar]
- 2.Matsushita K., Wu Y., Okamoto Y., Pratt R. E., Dzau V. J. 2006. Local renin angiotensin expression regulates human mesenchymal stem cell differentiation to adipocytes. Hypertension. 48: 1095–1102 [DOI] [PubMed] [Google Scholar]
- 3.Sodhi K., Puri N., Inoue K., Falck J. R., Schwartzman M. L., Abraham N. G. 2012. EET agonist prevents adiposity and vascular dysfunction in rats fed a high fat diet via a decrease in Bach 1 and an increase in HO-1 levels. Prostaglandins Other Lipid Mediat. 98: 133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allende-Vigo M. Z. 2012. Adipocytes and cardiometabolic risk. Am. J. Ther. 19: 294–299 [DOI] [PubMed] [Google Scholar]
- 5.Espiritu D. J., Mazzone T. 2008. Oxidative stress regulates adipocyte apolipoprotein E and suppresses its expression in obesity. Diabetes. 57: 2992–2998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim D. H., Burgess A. P., Li M., Tsenovoy P. L., Addabbo F., McClung J. A., Puri N., Abraham N. G. 2008. Heme oxygenase-mediated increases in adiponectin decrease fat content and inflammatory cytokines, tumor necrosis factor-alpha and interleukin-6 in Zucker rats and reduce adipogenesis in human mesenchymal stem cells. J. Pharmacol. Exp. Ther. 325: 833–840 [DOI] [PubMed] [Google Scholar]
- 7.Puri N., Sodhi K., Haarstad M., Kim D. H., Bohinc S., Foglio E., Favero G., Abraham N. G. 2012. Heme induced oxidative stress attenuates sirtuin1 and enhances adipogenesis in mesenchymal stem cells and mouse pre-adipocytes. J. Cell. Biochem. 113: 1926–1935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chu X., Xu L., Nishimura K., Jisaka M., Nagaya T., Shono F., Yokota K. 2009. Suppression of adipogenesis program in cultured preadipocytes transfected stably with cyclooxygenase isoforms. Biochim. Biophys. Acta. 1791: 273–280 [DOI] [PubMed] [Google Scholar]
- 9.Sodhi K., Wu C. C., Cheng J., Gotlinger K., Inoue K., Goli M., Falck J. R., Abraham N. G., Schwartzman M. L. 2010. CYP4A2-induced hypertension is 20-hydroxyeicosatetraenoic acid- and angiotensin II-dependent. Hypertension. 56: 871–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno C., Maier K. G., Hoagland K. M., Yu M., Roman R. J. 2001. Abnormal pressure-natriuresis in hypertension: role of cytochrome P450 metabolites of arachidonic acid. Am. J. Hypertens. 14: 90S–97S [DOI] [PubMed] [Google Scholar]
- 11.Miyata N., Roman R. J. 2005. Role of 20-hydroxyeicosatetraenoic acid (20-HETE) in vascular system. J. Smooth Muscle Res. 41: 175–193 [DOI] [PubMed] [Google Scholar]
- 12.Dunn K. M., Renic M., Flasch A. K., Harder D. R., Falck J., Roman R. J. 2008. Elevated production of 20-HETE in the cerebral vasculature contributes to severity of ischemic stroke and oxidative stress in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 295: H2455–H2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park F., Sweeney W. E., Jr, Jia G., Akbulut T., Mueller B., Falck J. R., Birudaraju S., Roman R. J., Avner E. D. 2009. Chronic blockade of 20-HETE synthesis reduces polycystic kidney disease in an orthologous rat model of ARPKD. Am. J. Physiol. Renal Physiol. 296: F575–F582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minuz P., Jiang H., Fava C., Turolo L., Tacconelli S., Ricci M., Patrignani P., Morganti A., Lechi A., McGiff J. C. 2008. Altered release of cytochrome p450 metabolites of arachidonic acid in renovascular disease. Hypertension. 51: 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laffer C. L., Laniado-Schwartzman M., Nasjletti A., Elijovich F. 2004. 20-HETE and circulating insulin in essential hypertension with obesity. Hypertension. 43: 388–392 [DOI] [PubMed] [Google Scholar]
- 16.Williams J. M., Murphy S., Burke M., Roman R. J. 2010. 20-hydroxyeicosatetraeonic acid: a new target for the treatment of hypertension. J. Cardiovasc. Pharmacol. 56: 336–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lasker J. M., Chen W. B., Wolf I., Bloswick B. P., Wilson P. D., Powell P. K. 2000. Formation of 20-hydroxyeicosatetraenoic acid, a vasoactive and natriuretic eicosanoid, in human kidney. Role of Cyp4F2 and Cyp4A11. J. Biol. Chem. 275: 4118–4126 [DOI] [PubMed] [Google Scholar]
- 18.Ishizuka T., Cheng J., Singh H., Vitto M. D., Manthati V. L., Falck J. R., Laniado-Schwartzman M. 2008. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-kappaB activation and the production of inflammatory cytokines in human endothelial cells. J. Pharmacol. Exp. Ther. 324: 103–110 [DOI] [PubMed] [Google Scholar]
- 19.Singh H., Cheng J., Deng H., Kemp R., Ishizuka T., Nasjletti A., Schwartzman M. L. 2007. Vascular cytochrome P450 4A expression and 20-hydroxyeicosatetraenoic acid synthesis contribute to endothelial dysfunction in androgen-induced hypertension. Hypertension. 50: 123–129 [DOI] [PubMed] [Google Scholar]
- 20.Cheng J., Ou J. S., Singh H., Falck J. R., Narsimhaswamy D., Pritchard K. A., Jr, Schwartzman M. L. 2008. 20-hydroxyeicosatetraenoic acid causes endothelial dysfunction via eNOS uncoupling. Am. J. Physiol. Heart Circ. Physiol. 294: H1018–H1026 [DOI] [PubMed] [Google Scholar]
- 21.Brownlee M. 2001. Biochemistry and molecular cell biology of diabetic complications. Nature. 414: 813–820 [DOI] [PubMed] [Google Scholar]
- 22.Ueno T., Fujimori K. 2011. Novel suppression mechanism operating in early phase of adipogenesis by positive feedback loop for enhancement of cyclooxygenase-2 expression through prostaglandin F2alpha receptor mediated activation of MEK/ERK-CREB cascade. FEBS J. 278: 2901–2912 [DOI] [PubMed] [Google Scholar]
- 23.Fujimori K., Amano F. 2011. Niacin promotes adipogenesis by reducing production of anti-adipogenic PGF2alpha through suppression of C/EBPbeta-activated COX-2 expression. Prostaglandins Other Lipid Mediat. 94: 96–103 [DOI] [PubMed] [Google Scholar]
- 24.Xie Y., Kang X., Ackerman W. E., Belury M. A., Koster C., Rovin B. H., Landon M. B., Kniss D. A. 2006. Differentiation-dependent regulation of the cyclooxygenase cascade during adipogenesis suggests a complex role for prostaglandins. Diabetes Obes. Metab. 8: 83–93 [DOI] [PubMed] [Google Scholar]
- 25.Salem H. K., Thiemermann C. 2010. Mesenchymal stromal cells: current understanding and clinical status. Stem Cells. 28: 585–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicolai A., Li M., Kim D. H., Peterson S. J., Vanella L., Positano V., Gastaldelli A., Rezzani R., Rodella L. F., Drummond G., et al. 2009. Heme oxygenase-1 induction remodels adipose tissue and improves insulin sensitivity in obesity-induced diabetic rats. Hypertension. 53: 508–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanella L., Kim D. H., Sodhi K., Barbagallo I., Burgess A. P., Falck J. R., Schwartzman M. L., Abraham N. G. 2011. Crosstalk between EET and HO-1 downregulates Bach1 and adipogenic marker expression in mesenchymal stem cell derived adipocytes. Prostaglandins Other Lipid Mediat. 96: 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim D. H., Vanella L., Inoue K., Burgess A., Gotlinger K., Manthati V. L., Koduru S. R., Zeldin D. C., Falck J. R., Schwartzman M. L., et al. 2010. Epoxyeicosatrienoic acid agonist regulates human mesenchymal stem cell-derived adipocytes through activation of HO-1-pAKT signaling and a decrease in PPARgamma. Stem Cells Dev. 19: 1863–1873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai I. J., Croft K. D., Puddey I. B., Beilin L. J., Barden A. 2011. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am. J. Physiol. Heart Circ. Physiol. 300: H1194–H1200 [DOI] [PubMed] [Google Scholar]
- 30.Schwartzman M. L., Falck J. R., Yadagiri P., Escalante B. 1989. Metabolism of 20-hydroxyeicosatetraenoic acid by cyclooxygenase. Formation and identification of novel endothelium-dependent vasoconstrictor metabolites. J. Biol. Chem. 264: 11658–11662 [PubMed] [Google Scholar]
- 31.Abraham N. G., Feldman E., Falck J. R., Lutton J. D., Schwartzman M. L. 1991. Modulation of erythropoiesis by novel human bone marrow cytochrome P450-dependent metabolites of arachidonic acid. Blood. 78: 1461–1466 [PubMed] [Google Scholar]
- 32.Lin F., Rios A., Falck J. R., Belosludtsev Y., Schwartzman M. L. 1995. 20-Hydroxyeicosatetraenoic acid is formed in response to EGF and is a mitogen in rat proximal tubule. Am. J. Physiol. 269: F806–F816 [DOI] [PubMed] [Google Scholar]
- 33.Muthalif M. M., Benter I. F., Karzoun N., Fatima S., Harper J., Uddin M. R., Malik K. U. 1998. 20-Hydroxyeicosatetraenoic acid mediates calcium/calmodulin-dependent protein kinase II-induced mitogen-activated protein kinase activation in vascular smooth muscle cells. Proc. Natl. Acad. Sci. USA. 95: 12701–12706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jiang M., Mezentsev A., Kemp R., Byun K., Falck J. R., Miano J. M., Nasjletti A., Abraham N. G., Laniado-Schwartzman M. 2004. Smooth muscle-specific expression of CYP4A1 induces endothelial sprouting in renal arterial microvessels. Circ. Res. 94: 167–174 [DOI] [PubMed] [Google Scholar]
- 35.Chen P., Guo M., Wygle D., Edwards P. A., Falck J. R., Roman R. J., Scicli A. G. 2005. Inhibitors of cytochrome P450 4A suppress angiogenic responses. Am. J. Pathol. 166: 615–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stec D. E., Gannon K. P., Beaird J. S., Drummond H. A. 2007. 20-Hydroxyeicosatetraenoic acid (20-HETE) stimulates migration of vascular smooth muscle cells. Cell. Physiol. Biochem. 19: 121–128 [DOI] [PubMed] [Google Scholar]
- 37.Wu C. C., Schwartzman M. L. 2011. The role of 20-HETE in androgen-mediated hypertension. Prostaglandins Other Lipid Mediat. 96: 45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Inazumi T., Shirata N., Morimoto K., Takano H., Segi-Nishida E., Sugimoto Y. 2011. Prostaglandin E(2)-EP4 signaling suppresses adipocyte differentiation in mouse embryonic fibroblasts via an autocrine mechanism. J. Lipid Res. 52: 1500–1508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cao J., Peterson S. J., Sodhi K., Vanella L., Barbagallo I., Rodella L. F., Schwartzman M. L., Abraham N. G., Kappas A. 2012. Heme oxygenase gene targeting to adipocytes attenuates adiposity and vascular dysfunction in mice fed a high-fat diet. Hypertension. 60: 467–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fang X., Dillon J. S., Hu S., Harmon S. D., Yao J., Anjaiah S., Falck J. R., Spector A. A. 2007. 20-carboxy-arachidonic acid is a dual activator of peroxisome proliferator-activated receptors alpha and gamma. Prostaglandins Other Lipid Mediat. 82: 175–184 [DOI] [PubMed] [Google Scholar]
- 41.Cowart L. A., Wei S., Hsu M. H., Johnson E. F., Krishna M. U., Falck J. R., Capdevila J. H. 2002. The CYP4A isoforms hydroxylate epoxyeicosatrienoic acids to form high affinity peroxisome proliferator-activated receptor ligands. J. Biol. Chem. 277: 35105–35112 [DOI] [PubMed] [Google Scholar]
- 42.Theken K. N., Deng Y., Schuck R. N., Oni-Orisan A., Miller T. M., Kannon M. A., Poloyac S. M., Lee C. R. 2012. Enalapril reverses high-fat diet-induced alterations in cytochrome P450-mediated eicosanoid metabolism. Am. J. Physiol. Endocrinol. Metab. 302: E500–E509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsai I. J., Croft K. D., Mori T. A., Falck J. R., Beilin L. J., Puddey I. B., Barden A. E. 2009. 20-HETE and F2-isoprostanes in the metabolic syndrome: the effect of weight reduction. Free Radic. Biol. Med. 46: 263–270 [DOI] [PubMed] [Google Scholar]
- 44.Ward N. C., Hodgson J. M., Puddey I. B., Beilin L. J., Croft K. D. 2006. 20-Hydroxyeicosatetraenoic acid is not associated with circulating insulin in lean to overweight humans. Diabetes Res. Clin. Pract. 74: 197–200 [DOI] [PubMed] [Google Scholar]
- 45.Ward N. C., Rivera J., Hodgson J., Puddey I. B., Beilin L. J., Falck J. R., Croft K. D. 2004. Urinary 20-hydroxyeicosatetraenoic acid is associated with endothelial dysfunction in humans. Circulation. 110: 438–443 [DOI] [PubMed] [Google Scholar]
- 46.Ward N. C., Puddey I. B., Hodgson J. M., Beilin L. J., Croft K. D. 2005. Urinary 20-hydroxyeicosatetraenoic acid excretion is associated with oxidative stress in hypertensive subjects. Free Radic. Biol. Med. 38: 1032–1036 [DOI] [PubMed] [Google Scholar]
- 47.Issan Y., Hochhauser E., Guo A., Gotlinger K. H., Kornowski R., Leshem-Lev D., Lev E., Porat E., Snir E., Thompson C. I., Abraham N. G., Laniado-Schwartzman M. 2013. Elevated level of pro-inflammatory eicosanoids and EPC dysfunction in diabetic patients with cardiac ischemia. Prostaglandins Other Lipid Mediat. In press [DOI] [PMC free article] [PubMed] [Google Scholar]