Abstract
Signaling through sphingosine-1-phosphate receptor1 (S1P1) promotes blood vessel barrier function. Degradation of S1P1 results in increased vascular permeability in the lung and may explain side effects associated with administration of FTY720, a functional antagonist of the S1P1 receptor that is currently used to treat multiple sclerosis. Ulcerative colitis (UC) is characterized by an increased density of abnormal vessels. The expression or role of S1P1 in blood vessels in the colon has not been investigated. In the present study, we show that S1P1 is overexpressed in the colonic mucosa of UC patients. This increase in S1P1 levels reflects increased vascular density in the inflamed mucosa. Genetic deletion of S1pr1 in mice increases colonic vascular permeability under basal conditions and increases bleeding in experimental colitis. In contrast, neither FTY720 nor AUY954, two S1P receptor-targeting agents, increases bleeding in experimental colitis. Taken together, our findings demonstrate that S1P1 is critical to maintaining colonic vascular integrity and may play a role in UC pathogenesis.
Keywords: inflammatory bowel disease, Fingolimod, endothelial cells, sphingosine-1-phosphate, gastrointestinal bleeding
Sphingosine-1-phosphate (S1P) receptors are a family of five G protein-coupled receptors that induce cellular responses through interactions with S1P (1, 2). S1P receptors expressed in immune cells enable egress from lymph nodes in response to the increasing S1P concentration gradient from lymph nodes to lymph and plasma (3–5). In addition to playing a critical role in controlling immune cell egress, S1P receptors regulate vascular function (6, 7). They are strongly expressed in endothelial cells, with S1P1 being the most abundant of the receptors (8–10). Genetic deletion of S1pr1 results in lethality in utero due to edema and hemorrhage, an effect that has been attributed to loss of S1P1 in endothelial cells (11–13). Recently, the ability of S1P1 to stabilize nascent vascular networks during developmental scenarios was shown (14, 15).
The S1P receptor-targeting drug FTY720 is phosphorylated by sphingosine kinase-2, and the phosphorylated form (FTY720-P) binds to four out of five S1P receptors, but with highest affinity for S1P1 (16, 17). Binding of FTY720-P induces internalization and degradation of S1P1 (5, 18–20), resulting in lymphopenia, because lymphocytes can no longer respond to the high S1P levels in lymph and plasma (5, 21). FTY720 has been approved for the treatment of multiple sclerosis (MS), and its ability to limit lymphocyte trafficking, resulting in attenuation of neural inflammation, demyelination, and neurodegeneration, is thought to be its primary mechanism of action (22, 23). However, this agent caused dose-dependent adverse events, including macular edema and reduced pulmonary function (24). Subsequent mechanistic studies using FTY720 and the S1P1-selective agent AUY954 (25) in mice found that degradation of S1P1 resulted in pulmonary vascular leakage (26).
Ulcerative colitis (UC) is a disease of the colorectum whereby patients manifest cyclical bouts of inflammation, which can result in severe morbidity. The damage that occurs in the colonic mucosa of UC patients is associated with an intense lymphocytic influx (27). The colonic vasculature also plays an important role in the pathogenesis of both human and experimental colitis. During inflammation, vascular density increases and blood vessels become more permeable coincident with immune cell extravasation and infiltration of the colonic mucosa (28, 29). Abnormal vasculature in UC is believed to contribute to the chronic inflammatory state that damages the colon (29).
Although it is well established that S1P1 controls vascular integrity and that dysfunctional vasculature is a feature of UC, the expression or role of S1P1 in UC is unknown. In the present study, we have shown that S1P1 localized to the colonic vasculature in UC. Genetic deletion of S1pr1 increased colonic vascular permeability in control mice and colitis-associated bleeding. These findings suggest an important role for S1P1 in the colonic vasculature under both normal and inflamed conditions.
EXPERIMENTAL PROCEDURES
Patient samples
Samples were obtained at the time of colonoscopic examination from both normal subjects and patients with active UC. Normal subjects and UC patients were matched for age, gender, and smoking status. In patients with UC, biopsies were taken from mucosa that was macroscopically inflamed (30). All samples were immediately placed into RNAlater (Life Technologies; Grand Island, NY) and stored at 4°C for 24 h. RNAlater was then removed, and samples were stored at −80°C until RNA extraction. The study was approved by the Weill Cornell Medical College Institutional Review Board, and all subjects provided informed consent for participation.
Studies of dextran sodium sulfate-induced colitis
For drug intervention studies, male C57BL6/J mice (Jackson Labs; Bar Harbor, ME) aged 8 weeks were administered 2% dextran sodium sulfate (DSS) (MP Biochemical; Irvine, CA) dissolved in drinking water for 7 days, then switched to plain drinking water for an additional 7 days. For FTY720 studies, mice were administered FTY720 (Cayman Chemical; Ann Arbor, MI) (3 mg/kg) dissolved in 2% 2-hydroxypropyl-β-cyclodextrin (HBC) (Sigma-Aldrich; St. Louis, MO) or 2% HBC alone, daily by oral gavage. For AUY954 studies, mice were administered AUY954 (1 mg/kg) (gift from Novartis Pharmaceuticals) dissolved in 2% HBC containing 0.05% DMSO or 2% HBC/0.05% DMSO alone daily by oral gavage. Both drugs were given during the entire 14 day experimental period, during which body weights were measured and bleeding and diarrhea scores recorded as a measurement of disease severity. Bleeding was assessed by detection of heme in stool using the Hemoccult Sensa test (Beckman Coulter; Fullerton, CA) or evidence of gross bleeding on a scale from 0 to 3. Diarrhea was assessed by measuring the softness or appearance of the stool on a scale from 0 to 3.
For studies using S1pr1f/f Rosa26-Cre-ERT2 mice or S1pr1f/f littermates, tamoxifen (Sigma-Aldrich) was administered by oral gavage (200 mg/kg), and 1 week later, 1.5% DSS was given for 7 days or 7 days then switched to plain drinking water for 14 days. A separate group of mice was given 2% DSS for 7 days followed by 10 days of plain drinking water. Measurements of disease severity as described above were recorded during the entire experimental period. Blood platelet counts were determined at the end of each experimental period by the Center for Comparative Medicine and Pathology at Weill Cornell Medical College. Cre status and verification of gene deletion after tamoxifen administration was performed on tail DNA using primers previously described (15).
For bone marrow transplant experiments, male C57BL6/J mice aged 5 weeks were whole-body irradiated with 9 Gy for 10 min using a Gammacell 40 Exactor Cesium source irradiator (MDS Nordion Inc.; Kanata, Canada). Mice were then retro-orbitally injected with bone marrow isolated from the femurs and tibias of either S1pr1f/f Rosa26-Cre-ERT2 or S1pr1f/f mice (n = 8/genotype). Eight weeks following the bone marrow transplant, all mice were administered tamoxifen and verified for Cre status and deletion of the gene in DNA isolated from blood. Both groups of mice were then challenged with 2% DSS for 7 days followed by 7 days of plain drinking water, and measurements of disease severity were recorded during the experimental period. All animal studies were approved by the Institutional Animal Care and Use Committee at Weill Cornell Medical College.
Quantitive real-time PCR
Total RNA was isolated from frozen human and mouse tissues using the RNeasy mini kit (Qiagen; Valencia, CA). RNA was reverse transcribed to make cDNA using murine leukemia virus reverse transcriptase and oligo (dT)16 primer. The resulting cDNA was used for amplification using QuantiTect Primer Assays (Qiagen) for the following genes: S1P1 (Hs_S1PR1_1_SG) (human); platelet/endothelial cell adhesion molecule 1 (PECAM1) (Hs_PECAM1_1_SG) (human); S1pr1 (Mm__S1pr1_1_SG) (mouse). GAPDH was used as an endogenous normalization control for both human (Hs_GAPDH_2_SG) and mouse samples (Mm_Gapdh_3_SG). Quantitive real-time PCR (qRT-PCR) was performed using 2× SYBR green PCR master mix on a 7500 Real-time PCR system (Applied Biosystems; Carlsbad, CA). Relative fold induction was determined using the ddCT (relative quantification) analysis protocol.
Immunofluorescence
To generate mouse tissues, C57BL6/J mice were given 2% DSS for 7 days then euthanized, and colons were flushed with ice-cold PBS then placed into O.C.T. compound (Sakura; Torrance, CA) and stored at −80°C until sectioning. Human biopsies were collected as described above and immediately placed into O.C.T. Frozen sections were placed into ice-cold methanol-acetone (1:1) for 20 min, washed in PBS, then incubated in 2% BSA for 30 min. Sections were then incubated in anti-S1P1 (H60) (1:100), anti-PECAM1 (1:100) (Santa Cruz Biotechnology; Santa Cruz, CA), or anti-CD3 (1:100) (Abcam; Cambridge, MA) primary antibodies for 1.5 h. After incubation, sections were washed in PBS and blocked in 2% BSA for 30 min, followed by incubation with anti-rabbit-FITC (1:500) and anti-goat-Cy3 (1:500) fluorescent secondary antibodies (Jackson ImmunoResearch; West Grove, PA) for 30 min. Slides were then washed in PBS and dried, and one drop of Prolong Gold anti-fade reagent (Life Technologies) was applied. 4′,6-Diamidino-2-Phenylindole, Dihydrochloride (DAPI) (1:100) (Life Technologies) was applied to mouse tissues for 1 min following the final PBS wash. Slides were coverslipped and allowed to stand for 24 h before imaging on a Fluoview FV10i confocal microscope (Olympus; Center Valley, PA).
Vascular permeability assay
Untreated S1pr1f/f Rosa26-Cre-ERT2 and S1pr1f/f littermates were administered tamoxifen as described above. One week later, mice were given tail vein injections of 0.5% Evans Blue dye (Sigma-Aldrich) dissolved in saline in a volume of 100 µl. Ninety minutes after injection, mice were euthanized, and colons were harvested and flushed with PBS. Colons were dried overnight at 56°C, weighed, then incubated in formamide (Sigma-Aldrich) at 37°C for 24 h, and the extravasation of dye was quantified spectrophotometrically at 595 nm. Concentrations were calculated by using a standard curve of known concentrations of Evans Blue dye and normalized by dry tissue weight (26).
Blood lymphocyte quantification
Male C57BL6/J mice aged 8 weeks were administered FTY720 (3 mg/kg) or AUY954 (1 mg/kg) or their respective vehicles daily by oral gavage for 14 days. Fifteen microliters of blood was collected by tail nick and immediately mixed with 0.6% EDTA. One milliliter of 0.83% NH4CL was added to samples to lyse red blood cells. Samples were centrifuged, and resulting pellets were washed twice in HBSS. Cells were counted on a hemocytometer, transferred to a 96-well plate, and stained for fluorescence-activated cell sorting (FACS) analysis. Cells were incubated with Mouse BD Fc Block (BD Biosciences; San Jose, CA) blocking solution for 5 min then incubated with anti-CD4-APCcy7 (BD Biosciences) (1:200), anti-CD8b-FITC (ebioscience; San Diego, CA) (1:200), and anti-B220-efluor (ebioscience) (1:100) antibodies on ice for 20 min. After staining, cells were washed and fixed in 0.5% formaldehyde. Cell populations were quantified using an LSRII flow cytometer (BD Biosciences). Individual cell populations were calculated as a percent of the total cells in each sample, and the percent decrease in the drug-treated group was calculated relative to the vehicle-treated group.
Statistical analysis
Human sample characteristics were summarized in terms of mean plus or minus the standard deviation for continuous variables and count/frequency for categorical variables. Expression of S1P1 and PECAM1 in human biopsies from UC patients was determined relative to biopsies from healthy controls using the ddCT (relative quantification) analysis protocol. Correlation in expression level between the two genes in the UC samples was quantified using Spearman's method. The nonparametric Wilcoxon rank-sum test was used to examine differences in various end-points, including S1P1 and PECAM1 expression in human biopsies, S1pr1 expression in mouse colons, vascular permeability by the Evans Blue dye assay, maximum DSS-induced weight loss, platelet counts, and the numbers of circulating lymphocytes between FTY720/AUY954-treated versus vehicle-treated mice. The generalized linear mixed-effects model was used to evaluate differences in the probability of having severe DSS-induced diarrhea or colonic bleeding. The log-rank test was used to determine whether genetic deletion of S1pr1 resulted in significantly increased death due to DSS administration.
RESULTS
S1P1 is expressed in the colonic vasculature in UC
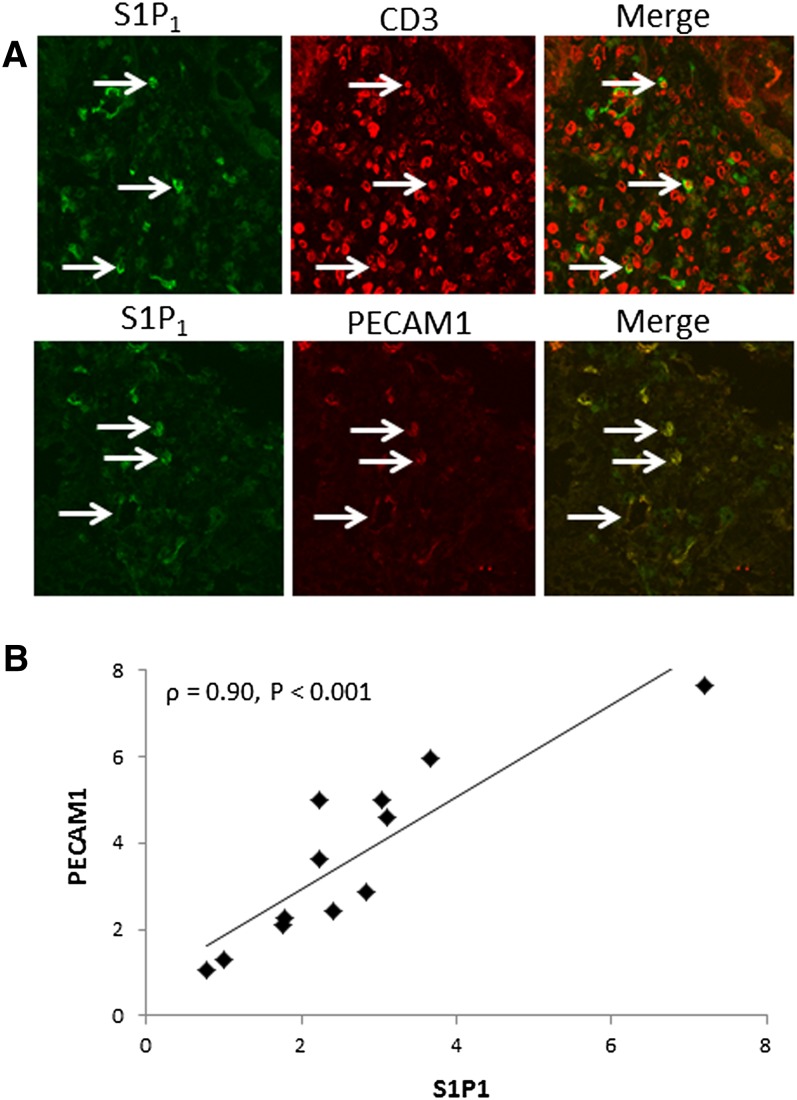
The inflamed mucosa of subjects with active UC is characterized by lymphocytic infiltration. S1P1 is a known regulator of lymphocyte trafficking and could potentially mediate immune cell function in UC. Initially, we examined the expression of S1P1 in lymphocytes in endoscopic biopsies from subjects with active UC. Using coimmunofluorescence, we found a large number of CD3-positive lymphocytes in these samples, but only a small subset also expressed S1P1 (Fig. 1A, top panels, arrows). Increased vasculature is another well-known feature of UC. Notably, S1P1 colocalized with PECAM1, a marker for endothelial cells (Fig. 1A, bottom panels, arrows). In fact, all PECAM1-positive cells were also positive for S1P1. To confirm that the vasculature was a major source of S1P1 in UC biopsies, qRT-PCR was performed on endoscopic biopsies from normal subjects and subjects with active UC. Expression levels of S1P1 and PECAM1 were significantly increased [median (range)] in samples from UC patients relative to normal subjects (S1P1: [2.3 (0.8–7.2)] vs. [0.9 (0.5–3.2)], P = 0.006; PECAM1: [3.2 (1.1–7.6)] vs. [1.0 (0.7–1.4)], P < 0.001). Moreover, a strong correlation between S1P1 and PECAM1 expression was found in UC biopsies (Fig. 1B).
Fig. 1.
S1P1 is expressed in the colonic vasculature in UC. A: Coimmunofluorescence for S1P1 and CD3 (top panels) or S1P1 and PECAM1 (bottom panels) was performed on endoscopic biopsies from subjects with UC. Arrows indicate colocalization of S1P1 and CD3 or PECAM1. Images were acquired using a 63× objective. B: S1P1 and PECAM1 expression was measured by qRT-PCR in endoscopic biopsies from healthy subjects (n = 10) and subjects with active UC (n = 12). After determining expression levels in UC biopsies relative to healthy subjects, the correlation (ρ) of the relative expression of both markers was determined for the same UC samples.
Genetic deletion of S1pr1 disrupts colonic vascular integrity
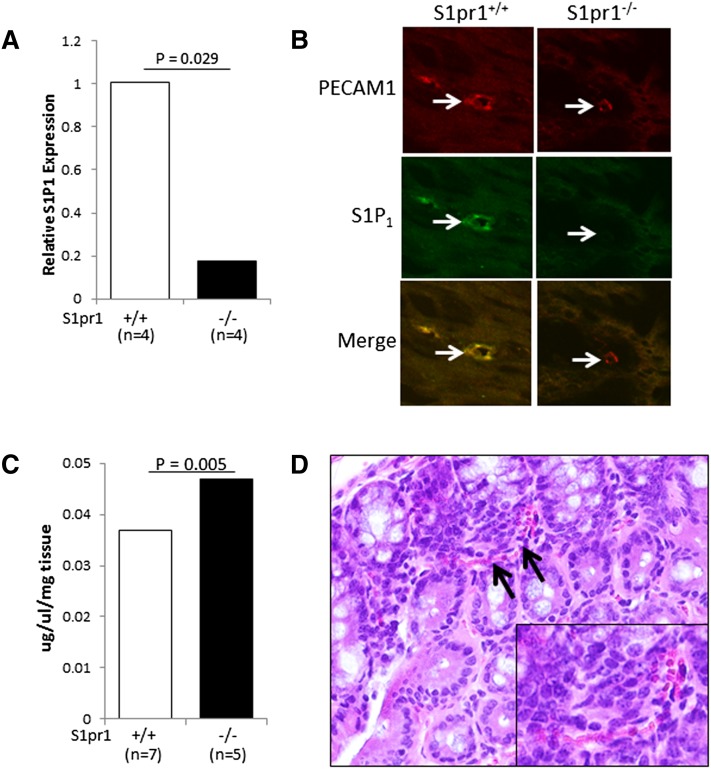
Given the strong expression of S1P1 in the colonic vasculature in human biopsies, we postulated that this receptor could be playing a functional role. To test this idea, a mouse model with a tamoxifen-inducible deletion of S1pr1 (S1pr1f/f Rosa26-Cre-ERT2) was utilized. S1pr1 expression was first measured in colonic tissue from S1pr1f/f Rosa26-Cre-ERT2 (S1pr1−/−) and S1pr1f/f (S1pr1+/+) littermates after tamoxifen administration. S1pr1 levels were reduced by ∼80% in S1pr1−/− mice (Fig. 2A). To determine whether S1P1 was lost in the colonic vasculature of S1pr1−/− mice, coimmunofluorescence was performed for S1P1 and PECAM1. As shown in Fig. 2B, S1P1 colocalized with PECAM1 in the wild-type mice. By contrast, S1P1 expression was lost on PECAM1-positive cells in S1pr1−/− mice.
Fig. 2.
Genetic deletion of S1pr1 results in colonic vascular fragility. A: Colonic tissue was harvested from S1pr1+/+ and S1pr1−/− mice, and S1pr1 expression was determined by qRT-PCR. A statistically significant reduction in S1pr1 expression [median (range)] was found in S1pr1−/− [0.2 (0.1–0.3)] compared with S1pr1+/+ [1.0 (0.9–1.1)] mice. B: Tissues from mice described in panel A were examined by coimmunofluorescence for the expression of S1P1 and PECAM1 (63× objective). Note the loss of S1P1 expression on PECAM1-expressing cells in the colons of S1pr1−/− mice. Images were cropped and magnified to enhance visualization of individual blood vessels. C: Evans Blue dye was injected into the tail veins of S1pr1+/+ and S1pr1−/− mice, and the extravasation of dye from colonic tissue was determined, as described in Experimental Procedures. A statistically significant increase in vascular permeability [median (range)] was found in colons from S1pr1−/− [0.047 (0.040–0.051)] compared with S1pr1+/+ [0.037 (0.032–0.040)] mice. D: A representative photomicrograph of a colon from an S1pr1−/− mouse showing red blood cells (arrows) in the colonic mucosa that are not contained within a blood vessel (400×). The inset shows a cropped and magnified version of this image to enhance visualization of red blood cells.
To test whether loss of S1P1 resulted in a vascular defect in the colon, we performed a permeability assay using Evans Blue dye. As shown in Fig. 2C, leakage of dye out of the colonic vasculature was significantly increased in S1pr1−/− mice. We also determined whether loss of S1P1 resulted in colonic bleeding. Three out of 10 S1pr1−/− mice were positive for fecal blood, whereas none of the S1pr1+/+ mice showed evidence of fecal blood. Furthermore, histological analysis of colonic tissue from S1pr1−/− mice revealed red blood cells within the colonic mucosa that were not contained within blood vessels (Fig. 2D).
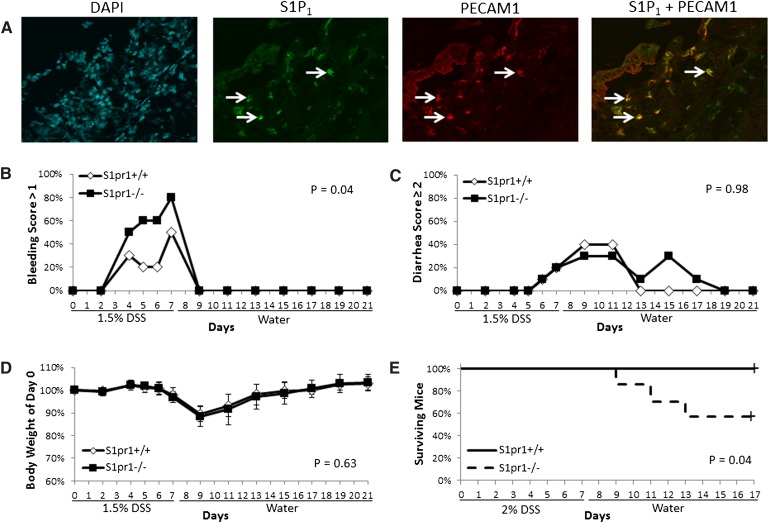
DSS-induced bleeding is enhanced after S1pr1 deletion
To determine whether S1P1 is potentially important in UC, the DSS model of colitis was employed. We tested whether genetic deletion of S1pr1 enhanced colitis-associated bleeding. Consistent with the findings in humans, we first showed that S1P1 colocalized with PECAM1 in ulcerated regions of the colons of wild-type mice given DSS for 7 days (Fig. 3A). To determine whether genetic deletion of S1pr1 enhanced colitis-related bleeding, DSS was administered to S1pr1+/+ and S1pr1−/− mice for 7 days, followed by 14 days of plain drinking water. Figure 3B shows that a significantly greater percent of S1pr1−/− mice had severe bleeding as compared with S1pr1+/+ littermates upon DSS exposure. By contrast, other endpoints of disease severity, including diarrhea severity and weight loss, were unaffected (Fig. 3C, D). Importantly, genetic deletion of S1pr1 sensitized mice to DSS-induced death (Fig. 3E). Recent evidence has shown that loss of S1pr1 in mice impairs platelet production by interfering with pro-platelet release from mature megakaryocytes (31). To determine whether a reduction in the number of platelets may have contributed to the observed increased bleeding in S1pr1−/− mice, platelet counts were quantified in both genotypes after DSS exposure. Platelet counts were not significantly different [median (range)] between S1pr1−/− and S1pr1+/+ mice after 7 days of DSS exposure [1,226 × 103 (1,028 × 103–1,683 × 103)/µl] vs. [1,066 × 103 (850 × 103–1,487 × 103)/µl] (P = 0.11), or 14 days after completing 7 days of DSS administration [1,491 × 103 (1,187 × 103–2,150 × 103)/µl] vs. [1,456 × 103 (1,252 × 103–1,851 × 103)/µl] (P = 0.48).
Fig. 3.
Genetic deletion of S1pr1 results in enhanced DSS-induced bleeding. A: Wild-type C57BL6/J mice were administered 2% DSS for 7 days, and colons were examined for the expression of S1P1 and PECAM1 by coimmunofluorescence in a region of ulcerated mucosa (as verified by DAPI stain). Arrows indicate positive staining for both S1P1 and PECAM1 (63× objective). B–D: S1pr1+/+ and S1pr1−/− mice (n = 10/group) were administered 1.5% DSS for 7 days, followed by 14 days of plain drinking water; and severity of bleeding (B), diarrhea (C), and weight loss (D) were recorded. Error bars represent SD. (E) S1pr1+/+ (n = 8) and S1pr1−/− (n = 7) mice were administered 2% DSS for 7 days, followed by 10 days of plain drinking water, and death was recorded. Differences in bleeding or diarrhea severity were determined by quantifying the percent of mice with a score >1 or ≥2, respectively, in each group. Differences in body weight loss were determined by quantifying the maximum weight loss of individual mice in each group.
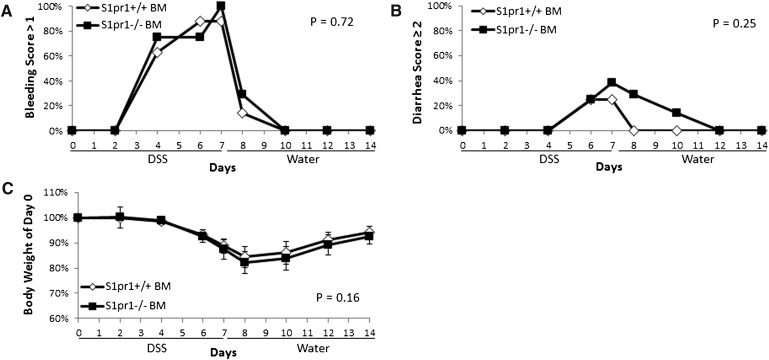
Given that S1P1 has been shown to play a role in immune cell function, we confirmed that the bleeding defect observed in S1pr1−/− mice was not related to loss of S1P1 specifically in myeloid and other hematopoietic/immune cells. To test this, bone marrow from S1pr1f/f Rosa26-Cre-ERT2 or S1pr1f/f mice was transplanted to lethally irradiated wild-type mice and challenged with 2% DSS following tamoxifen administration. Figure 4 shows that deletion of S1pr1 specifically in the bone marrow had no effect on colonic bleeding severity or other endpoints of colitic disease.
Fig. 4.
Genetic deletion of S1pr1 in bone marrow has no effect on the severity of DSS-induced bleeding. Bone marrow from S1pr1f/f Rosa26-Cre-ERT2 and S1pr1f/f mice was transplanted to lethally irradiated C57BL6/J mice. Following tamoxifen administration, recipient mice were given 2% DSS for 7 days, followed by 7 days of plain drinking water, and colitis severity was recorded including colonic bleeding (A), diarrhea (B), and body weight change (C). Error bars represent SD. Differences in bleeding or diarrhea severity were determined by quantifying the percent of mice with a score >1 or ≥2, respectively, in each group. Differences in body weight were determined by quantifying the maximum weight loss of individual mice in each group.
S1P receptor-targeting agents do not disrupt the colonic vasculature
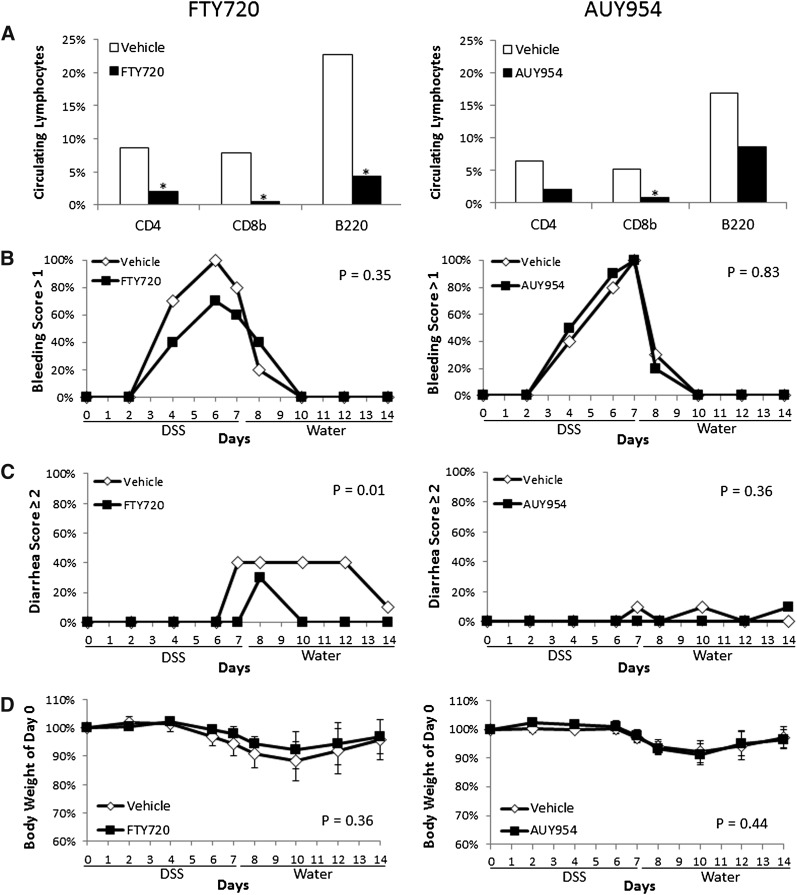
Because genetic deletion of S1pr1 resulted in defective colonic vasculature, we investigated whether administration of the nonselective S1P receptor functional antagonist FTY720 or S1P1-selective agent AUY954 would cause a similar phenotype. To first ensure that these drugs were administered at therapeutic doses, both drugs were given for 14 consecutive days and lymphocyte counts were determined in the peripheral blood by FACS. Compared with vehicle-treated mice, mice given FTY720 (3 mg/kg) had a significant reduction in the number of circulating CD4-, CD8b-, and B220-positive cells, whereas mice given AUY954 (1 mg/kg) had a significant reduction in CD8b-positive cells and a modest reduction in CD4- and B220-expressing cells (Fig. 5A). To test the effect of these drugs on the severity of colitis induced by DSS exposure, mice were given 2% DSS for 7 days then switched to plain drinking water for an additional 7 days. Mice were treated with either FTY720 or AUY954 or their respective vehicles daily during the entire experimental period, and clinical scores of colitis were measured. As shown in Fig. 5B–D, neither drug affected the severity of DSS-induced bleeding or weight loss. However, FTY720 did ameliorate the severity of diarrhea.
Fig. 5.
FTY720 and AUY954 administration does not enhance DSS-induced bleeding. A: The percent of circulating lymphocytes was examined after 14 days of daily administration of FTY720 (3 mg/kg) or AUY954 (1 mg/kg) relative to vehicle-treated mice (n = 4–5/group), under control conditions. A statistically significant reduction in the percent of CD4-, CD8b-, and B220-expressing cells [median (range)] was found in FTY720-treated mice [CD4: 2.0 (0.7–5.0)], [CD8b: 0.5 (0.3–0.7)], [B220: 4.3 (3.7–11.9)] compared with controls [CD4: 8.6 (6.5–10.4)], [CD8b: 7.9 (6.0–9.3)], [B220: 22.7 (16.6–26.6)]. A statistically significant reduction in the percent of CD8b-expressing cells [median (range)] was found in AUY954-treated mice [0.8 (0.6–1.4)] compared with controls [5.2 (4.8–5.50] with a trend for a reduction in CD4 and B220-expressing cells [CD4: 2.1 (1.4–8.7)], B220: [8.6 (3.4–18.3)] vs. [CD4: 6.4 (5.5–7.2)], B220: [16.9 (13.9–21.8)]. (B–D) To assess whether either drug had effects on the severity of experimental colitis, 2% DSS was administered to wild-type mice for 7 days, then mice were switched to plain drinking water for an additional 7 days. FTY720, AUY954, or their respective vehicles were administered daily during the entire 14 day experimental period (n = 10/group). Clinical endpoints of colitis severity including bleeding (B), diarrhea (C) and body weight change (D) were measured during disease progression. Error bars represent SD. Differences in bleeding or diarrhea severity were determined by quantifying the percent of mice with a score >1 or ≥2, respectively, in each group. Differences in body weight were determined by quantifying the maximum weight loss of individual mice in each group.
DISCUSSION
S1P1 plays important roles in both lymphocyte trafficking and vascular function. The role or expression pattern of this receptor in the colon under normal or pathological conditions is unknown. The present study shows that S1P1 is strongly expressed in the colonic vasculature in both UC and experimental colitis. Furthermore, we demonstrate that genetic deletion of S1pr1 results in a vascular defect in the colon under basal conditions and during mucosal injury.
The importance of S1P1 in maintaining vascular integrity was first demonstrated by showing that genetic deletion of S1pr1 resulted in embryonic lethality (13). This death was related to a lack of blood vessel maturation owing to a defect in the recruitment of vascular smooth muscle cells to vessel walls (13). Tissue-specific gene deletion studies demonstrated that endothelial cell S1pr1 was critical to this process (11) and that loss of S1pr1 but not S1pr2 or S1pr3 resulted in this phenotype (12). Furthermore, several studies have shown that S1P1 is the main S1P receptor expressed in endothelial cells (8). In addition to vessel formation, S1P1 plays a key role in maintaining endothelial cell junctions. Signaling through S1P1 activates Rac1, which results in vascular endothelial cadherin (VE-cadherin) junction assembly, in addition to other events that enhance vascular barrier function (7, 32). The importance of VE-cadherin expression and assembly is highlighted by studies showing that genetic deletion of VE-cadherin in mice results in embryonic lethality owing to a vascular defect (33). The data presented in the current work demonstrate that genetic deletion of S1pr1 increases vascular permeability in the colon under control conditions and enhances bleeding in experimental colitis (Figs. 2, 3). Although our study does not address the role of other S1P receptors in this process, it is likely that S1P1 is the key receptor, given the results of numerous previous studies highlighting the role of S1P1 in vascular homeostasis (8, 11–13). It is also very likely that the observed phenotype results from loss of S1P1 in endothelial cells, given the strong expression of S1P1 in colonic endothelial cells in wild-type mice, which was lost in S1pr1−/− mice (Fig. 2B). Furthermore, this study demonstrates that whole-body deletion of S1pr1, but not solely in hematopoietic cells, results in an enhanced bleeding phenotype (Fig. 4). Importantly, previously published work has shown a similar phenotype comparing whole-body genetic deletion of S1pr1 and VE-cadherin-specific deletion, in relation to vascular abnormalities (15).
FTY720 (Fingolimod) is FDA-approved for use in MS, owing to its ability to reduce relapse rate (24, 34). FTY720 is believed to be beneficial by reducing lymphocyte homing and subsequent destruction of myelin sheaths in the central nervous system (23). Reduced pulmonary function and macular edema were reported in patients taking this drug (24). In an attempt to explain the observed toxicity, mechanistic studies utilizing supratherapeutic doses of FTY720 or AUY954 were carried out in mice and showed increased lung vascular permeability when S1P1 was degraded (26). In the current study, therapeutic doses of FTY720 and AUY954 (as determined by induction of lymphopenia) failed to enhance colonic bleeding in mice challenged with DSS (Fig. 5). Although the concentration of both agents was sufficient to cause lymphopenia, it is likely that higher doses that cause quantitative and complete degradation of the receptor in the colon are needed to induce a bleeding phenotype. Although we found modest increases in vascular permeability and bleeding in S1pr1−/− mice, pharmacological targeting of a receptor does not typically recapitulate the completeness of genetic deletion. Most importantly, our findings are consistent with the fact that there have been no reports of increased risk of intestinal bleeding in MS patients taking FTY720 (24). Nonetheless, it will be prudent to closely monitor gastrointestinal (GI) bleeding in those MS patients on FTY720 with comorbid conditions such as UC.
Because lymphocytic infiltration into the gut is a major feature of UC, administration of S1P receptor-targeting agents remains a promising therapeutic strategy. Preclinical studies have shown the ability of FTY720 to effectively ameliorate colitis in the trinitrobenzene sulfonic acid (TNBS) and oxazolone haptenating chemical models, which elicit a strong T cell response (35, 36). The drug's effect in these studies was mediated through an increase of T-regulatory cells and suppression of T helper type 2 cytokine production in the TNBS and oxazolone models, respectively (35, 36). In the current study, treatment with neither FTY720 nor AUY954 led to meaningful improvements in DSS-induced colitis (Fig. 5). DSS administration is primarily a model of tissue injury after which an acute inflammatory response occurs, consisting primarily of innate immune cells (37, 38). Although other models of colitis may prove to be more relevant for evaluating the therapeutic potential of S1P receptor-targeting agents as a treatment for inflammatory bowel disease, our findings do not exclude the importance of the S1P signaling axis in DSS-induced colitis. Work by Snider et al. (39) demonstrated that genetic deletion of sphingosine kinase 1, the enzyme that converts sphingosine to S1P, reduced the severity of DSS-induced injury. The beneficial effects of reducing the levels of ligand can potentially be explained by effects mediated by one or more of the five S1P receptors. Alternatively, metabolic function of sphingosine kinase 1 may be relevant (40). Our data suggest that S1P1 does not play an important role in DSS-induced injury. By contrast, genetic deletion of S1pr4 reduced the severity of DSS-induced disease (41).
Inflammatory diseases of the GI tract, including UC, are characterized by increased and dysfunctional vasculature. The data presented here demonstrate the important role of S1P1 in maintaining colonic vascular function and lend insight into a receptor previously unexplored in UC. Whether S1P1 also plays a role in vascular homeostasis in other inflammatory disorders of the GI tract warrants further investigation.
Acknowledgments
The authors thank Dr. Raul Catena for technical assistance in performing bone marrow transplant experiments.
Footnotes
Abbreviations:
- DSS
- dextran sodium sulfate
- FACS
- fluorescence-activated cell sorting
- GI
- gastrointestinal
- HBC
- 2-hydroxypropyl-β-cyclodextrin
- MS
- multiple sclerosis
- qRT-PCR
- quantitive real-time PCR
- S1P1
- sphingosine-1-phosphate receptor1
- TNBS
- trinitrobenzene sulfonic acid
- UC
- ulcerative colitis
- VE-cadherin
- vascular endothelial cadherin
This work was supported by the New York Crohn's Foundation (A. J. D.), National Institutes of Health Grants HL-67330, HL-89934, and HL-70694 (T. H.), and T32 CA-062948 (D. C. M.). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or other granting agencies.
REFERENCES
- 1.Blaho V. A., Hla T. 2011. Regulation of mammalian physiology, development, and disease by the sphingosine 1-phosphate and lysophosphatidic acid receptors. Chem. Rev. 111: 6299–6320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanchez T., Hla T. 2004. Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 92: 913–922 [DOI] [PubMed] [Google Scholar]
- 3.Pappu R., Schwab S. R., Cornelissen I., Pereira J. P., Regard J. B., Xu Y., Camerer E., Zheng Y. W., Huang Y., Cyster J. G., et al. 2007. Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science. 316: 295–298 [DOI] [PubMed] [Google Scholar]
- 4.Rivera J., Proia R. L., Olivera A. 2008. The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat. Rev. Immunol. 8: 753–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thangada S., Khanna K. M., Blaho V. A., Oo M. L., Im D. S., Guo C., Lefrancois L., Hla T. 2010. Cell-surface residence of sphingosine 1-phosphate receptor 1 on lymphocytes determines lymphocyte egress kinetics. J. Exp. Med. 207: 1475–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McVerry B. J., Garcia J. G. 2005. In vitro and in vivo modulation of vascular barrier integrity by sphingosine 1-phosphate: mechanistic insights. Cell. Signal. 17: 131–139 [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Dudek S. M. 2009. Regulation of vascular permeability by sphingosine 1-phosphate. Microvasc. Res. 77: 39–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alewijnse A. E., Peters S. L., Michel M. C. 2004. Cardiovascular effects of sphingosine-1-phosphate and other sphingomyelin metabolites. Br. J. Pharmacol. 143: 666–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chae S. S., Proia R. L., Hla T. 2004. Constitutive expression of the S1P1 receptor in adult tissues. Prostaglandins Other Lipid Mediat. 73: 141–150 [DOI] [PubMed] [Google Scholar]
- 10.Hla T., Maciag T. 1990. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 265: 9308–9313 [PubMed] [Google Scholar]
- 11.Allende M. L., Yamashita T., Proia R. L. 2003. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 102: 3665–3667 [DOI] [PubMed] [Google Scholar]
- 12.Kono M., Mi Y., Liu Y., Sasaki T., Allende M. L., Wu Y. P., Yamashita T., Proia R. L. 2004. The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J. Biol. Chem. 279: 29367–29373 [DOI] [PubMed] [Google Scholar]
- 13.Liu Y., Wada R., Yamashita T., Mi Y., Deng C. X., Hobson J. P., Rosenfeldt H. M., Nava V. E., Chae S. S., Lee M. J., et al. 2000. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 106: 951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaengel K., Niaudet C., Hagikura K., Siemsen B. L., Muhl L., Hofmann J. J., Ebarasi L., Nystrom S., Rymo S., Chen L. L., et al. 2012. The sphingosine-1-phosphate receptor S1PR1 restricts sprouting angiogenesis by regulating the interplay between VE-cadherin and VEGFR2. Dev. Cell. 23: 587–599 [DOI] [PubMed] [Google Scholar]
- 15.Jung B., Obinata H., Galvani S., Mendelson K., Ding B. S., Skoura A., Kinzel B., Brinkmann V., Rafii S., Evans T., et al. 2012. Flow-regulated endothelial S1P receptor-1 signaling sustains vascular development. Dev. Cell. 23: 600–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brinkmann V., Davis M. D., Heise C. E., Albert R., Cottens S., Hof R., Bruns C., Prieschl E., Baumruker T., Hiestand P., et al. 2002. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J. Biol. Chem. 277: 21453–21457 [DOI] [PubMed] [Google Scholar]
- 17.Mandala S., Hajdu R., Bergstrom J., Quackenbush E., Xie J., Milligan J., Thornton R., Shei G. J., Card D., Keohane C., et al. 2002. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science. 296: 346–349 [DOI] [PubMed] [Google Scholar]
- 18.Billich A., Bornancin F., Devay P., Mechtcheriakova D., Urtz N., Baumruker T. 2003. Phosphorylation of the immunomodulatory drug FTY720 by sphingosine kinases. J. Biol. Chem. 278: 47408–47415 [DOI] [PubMed] [Google Scholar]
- 19.Gräler M. H., Goetzl E. J. 2004. The immunosuppressant FTY720 down-regulates sphingosine 1-phosphate G-protein-coupled receptors. FASEB J. 18: 551–553 [DOI] [PubMed] [Google Scholar]
- 20.Sanchez T., Estrada-Hernandez T., Paik J. H., Wu M. T., Venkataraman K., Brinkmann V., Claffey K., Hla T. 2003. Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278: 47281–47290 [DOI] [PubMed] [Google Scholar]
- 21.Matloubian M., Lo C. G., Cinamon G., Lesneski M. J., Xu Y., Brinkmann V., Allende M. L., Proia R. L., Cyster J. G. 2004. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature. 427: 355–360 [DOI] [PubMed] [Google Scholar]
- 22.Brinkmann V., Billich A., Baumruker T., Heining P., Schmouder R., Francis G., Aradhye S., Burtin P. 2010. Fingolimod (FTY720): discovery and development of an oral drug to treat multiple sclerosis. Nat. Rev. Drug Discov. 9: 883–897 [DOI] [PubMed] [Google Scholar]
- 23.Chun J., Hartung H. P. 2010. Mechanism of action of oral fingolimod (FTY720) in multiple sclerosis. Clin. Neuropharmacol. 33: 91–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cohen J. A., Barkhof F., Comi G., Hartung H. P., Khatri B. O., Montalban X., Pelletier J., Capra R., Gallo P., Izquierdo G., et al. 2010. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N. Engl. J. Med. 362: 402–415 [DOI] [PubMed] [Google Scholar]
- 25.Pan S., Mi Y., Pally C., Beerli C., Chen A., Guerini D., Hinterding K., Nuesslein-Hildesheim B., Tuntland T., Lefebvre S., et al. 2006. A monoselective sphingosine-1-phosphate receptor-1 agonist prevents allograft rejection in a stringent rat heart transplantation model. Chem. Biol. 13: 1227–1234 [DOI] [PubMed] [Google Scholar]
- 26.Oo M. L., Chang S. H., Thangada S., Wu M. T., Rezaul K., Blaho V., Hwang S. I., Han D. K., Hla T. 2011. Engagement of S1P(1)-degradative mechanisms leads to vascular leak in mice. J. Clin. Invest. 121: 2290–2300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Monteleone G., Pallone F., MacDonald T. T. 2011. Emerging immunological targets in inflammatory bowel disease. Curr. Opin. Pharmacol. 11: 640–645 [DOI] [PubMed] [Google Scholar]
- 28.Chidlow J. H., Jr, Langston W., Greer J. J., Ostanin D., Abdelbaqi M., Houghton J., Senthilkumar A., Shukla D., Mazar A. P., Grisham M. B., et al. 2006. Differential angiogenic regulation of experimental colitis. Am. J. Pathol. 169: 2014–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chidlow J. H., Jr, Shukla D., Grisham M. B., Kevil C. G. 2007. Pathogenic angiogenesis in IBD and experimental colitis: new ideas and therapeutic avenues. Am. J. Physiol. Gastrointest. Liver Physiol. 293: G5–G18 [DOI] [PubMed] [Google Scholar]
- 30.Otani T., Yamaguchi K., Scherl E., Du B., Tai H. H., Greifer M., Petrovic L., Daikoku T., Dey S. K., Subbaramaiah K., et al. 2006. Levels of NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase are reduced in inflammatory bowel disease: evidence for involvement of TNF-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 290: G361–G368 [DOI] [PubMed] [Google Scholar]
- 31.Zhang L., Orban M., Lorenz M., Barocke V., Braun D., Urtz N., Schulz C., von Bruhl M. L., Tirniceriu A., Gaertner F., et al. 2012. A novel role of sphingosine 1-phosphate receptor S1pr1 in mouse thrombopoiesis. J. Exp. Med. 209: 2165–2181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee M. J., Thangada S., Claffey K. P., Ancellin N., Liu C. H., Kluk M., Volpi M., Sha'afi R. I., Hla T. 1999. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 99: 301–312 [DOI] [PubMed] [Google Scholar]
- 33.Carmeliet P., Lampugnani M. G., Moons L., Breviario F., Compernolle V., Bono F., Balconi G., Spagnuolo R., Oosthuyse B., Dewerchin M., et al. 1999. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 98: 147–157 [DOI] [PubMed] [Google Scholar]
- 34.Kappos L., Radue E. W., O'Connor P., Polman C., Hohlfeld R., Calabresi P., Selmaj K., Agoropoulou C., Leyk M., Zhang-Auberson L., et al. 2010. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N. Engl. J. Med. 362: 387–401 [DOI] [PubMed] [Google Scholar]
- 35.Daniel C., Sartory N., Zahn N., Geisslinger G., Radeke H. H., Stein J. M. 2007. FTY720 ameliorates Th1-mediated colitis in mice by directly affecting the functional activity of CD4+CD25+ regulatory T cells. J. Immunol. 178: 2458–2468 [DOI] [PubMed] [Google Scholar]
- 36.Daniel C., Sartory N. A., Zahn N., Schmidt R., Geisslinger G., Radeke H. H., Stein J. M. 2007. FTY720 ameliorates oxazolone colitis in mice by directly affecting T helper type 2 functions. Mol. Immunol. 44: 3305–3316 [DOI] [PubMed] [Google Scholar]
- 37.Montrose D. C., Horelik N. A., Madigan J. P., Stoner G. D., Wang L. S., Bruno R. S., Park H. J., Giardina C., Rosenberg D. W. 2011. Anti-inflammatory effects of freeze-dried black raspberry powder in ulcerative colitis. Carcinogenesis. 32: 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yan Y., Kolachala V., Dalmasso G., Nguyen H., Laroui H., Sitaraman S. V., Merlin D. 2009. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE. 4: e6073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snider A. J., Kawamori T., Bradshaw S. G., Orr K. A., Gilkeson G. S., Hannun Y. A., Obeid L. M. 2009. A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J. 23: 143–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kohno M., Momoi M., Oo M. L., Paik J. H., Lee Y. M., Venkataraman K., Ai Y., Ristimaki A. P., Fyrst H., Sano H., et al. 2006. Intracellular role for sphingosine kinase 1 in intestinal adenoma cell proliferation. Mol. Cell. Biol. 26: 7211–7223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze T., Golfier S., Tabeling C., Rabel K., Graler M. H., Witzenrath M., Lipp M. 2011. Sphingosine-1-phospate receptor 4 (S1P(4)) deficiency profoundly affects dendritic cell function and TH17-cell differentiation in a murine model. FASEB J. 25: 4024–4036 [DOI] [PubMed] [Google Scholar]