Abstract
Studying communication between the gut and the brain is as relevant and exciting as it has been since Pavlov's discoveries a century ago. Although the efferent limb of this communication has witnessed significant advances, it is the afferent, or sensory, limb that has recently made for exciting news. It is now clear that signals from the gut are crucial for the control of appetite and the regulation of energy balance, glucose homeostasis, and more. Ghrelin, discovered just a few years ago, is the first gut hormone that increases appetite, and it may be involved in eating disorders. The stable analogue of glucagon-like peptide-1 has rapidly advanced to one of the most promising treatment options for type-2 diabetes. Changes in the signalling patterns of these and other gut hormones best explain the remarkable capacity of gastric bypass surgery to lower food intake and excess body weight. Given the enormous societal implications of the obesity epidemic, these are no small feats. Together with the older gut hormone cholecystokinin and abundant vagal mechanosensors, the gut continuously sends information to the brain regarding the quality and quantity of ingested nutrients, not only important for satiation and meal termination, but also for the appetitive phase of ingestive behaviour and the patterning of meals within given environmental constraints. By acting not only on brainstem and hypothalamus, this stream of sensory information from the gut to the brain is in a position to generate a feeling of satisfaction and happiness as observed after a satiating meal and exploited in vagal afferent stimulation for depression.
Keywords: food intake, gut hormones, obesity, taste in the gut, vagal mechanosensors
The prevalence of obesity and the metabolic syndrome is rapidly increasing, with the prospect that more than half of the adult population will be overweight or obese in 2015, and every third child born today will develop diabetes later in life.1 The strong correlation between obesity and development of type 2 diabetes, cardiovascular disease, gall bladder disease, osteoarthritis, sleep and mental disorders make it the major health problem. Current treatment of obesity targets both energy intake and expenditure, and includes dieting and physical exercise (life style changes), as well as surgery, drugs, plant extracts and many scientifically undocumented remedies. Most of these treatments are not very effective, with a typical maximal weight loss of <10%, and not able to stop the epidemic. Obesity surgery is presently the most effective treatment with sustained weight loss of up to 50% and it implicates gut–brain signalling by vagal afferent and hormonal mechanisms as an important factor in the development and prevention of obesity. This article briefly reviews the state of the art in gut–brain communication as it pertains to the control of food intake and regulation of energy balance, identifies weaknesses in our understanding of the underlying mechanisms, and points to promising new approaches to make progress in the translation of basic mechanisms from bench to bedside.
SENSING INGESTED NUTRIENTS: FEED-FORWARD SIGNALS INITIATING APPETITIVE AND MAINTAINING CONSUMMATORY BEHAVIOUR
The gustatory and trigeminal sensors in the mouth act as `gate keepers' at the entrance to the alimentary canal, with the classical four taste modalities representing innate detectors for acceptable foods (sweet), dangerous or toxic foods (bitter and sour) and special needs (salt, water). Considerable progress has been made in just the last 5 years in identifying specific receptor and transduction mechanisms for some of these taste modalities, and there may also be a taste for fat.2 Thus, the oro-sensory system is capable of at least recognizing, if not metering, much of the nutrient content of mixed foods, and this is of crucial importance for ingestive behaviour and the regulation of energy balance. It generates the positive hedonic feeling and reward that initially augments and maintains eating and helps prepare the nutrient handling organs for efficient assimilation through cephalic phase reflexes.
Until recently, the gustatory system was the only mechanism known to act in this positive feed-forward fashion, but the newly discovered gut hormone ghrelin also fits this description perfectly (Fig. 1). Ghrelin is secreted mainly from the gastric mucosa with plasma ghrelin levels at their peak just before a meal is taken and rapidly decreasing when nutrients are emptied into the duodenum.3 Similar to taste input, ghrelin has direct effects on components of the reward system in the brain, and together these two peripheral signals can powerfully augment the drive to eat. Ghrelin is actually ahead of the gustatory signal and is involved in pre-ingestive foraging and appetitive behaviour. By acting on hippocampal neurons involved in spatial learning and memory, ghrelin facilitates the process of finding food in the environment.4 Thus, the empty stomach tells the brain to engage in appetitive behaviour, reminiscent of gastric `hunger pangs' long believed to be a feeling of hunger.
Figure 1.
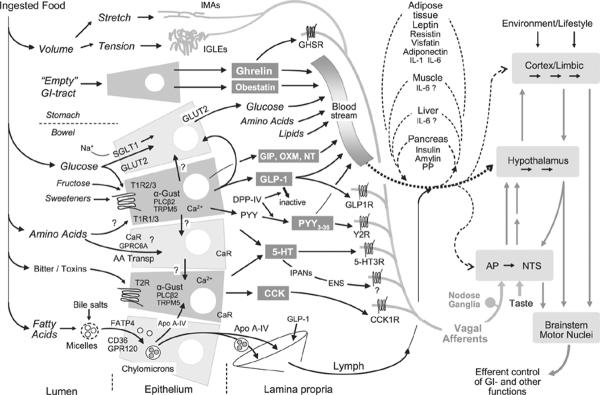
The sensory limb of gut–brain communication. Simplified schematic diagram shows the major transduction sites and mechanisms for the detection of ingested food and its macronutrient components. Ordinary enterocytes are shown in light grey and enteroendocrine cells in darker grey. Note that the molecular machinery given for a particular epithelial cell is not completely known and does, therefore, not define specific fixed configurations. In particular, it is not completely clear to what extent ordinary enterocytes and certain enteroendocrine cells express the different types of G-protein-coupled receptors of the T1R and T2R families, the amino acid-sensing calcium receptor and GPCR6, and the fatty acid transporters FATP4, CD36 and GPR120. After release of nutrients and hormones into the lamina propria, they are either taken up by capillaries and sent to the brain and other organs through the general circulation and/or the lymphatic system. Circulating nutrients and hormones have access to the brain at all levels. Hormones and transmitters in the lamina propria can also interact with relevant receptors on mucosal endings of vagal afferent neurons and enteric neurons as well as dorsal root afferents (not shown). Vagal afferent information reaches the brain through the nucleus tractus solitarius and area postrema in the caudal brainstem and is then disseminated to hypothalamus and forebrain as indicated by grey arrows. Note that intestinal epithelial cells can also communicate with each other through paracrine or humoral mechanisms, and with other organs involved in energy balance regulation such as the pancreas, liver, adipose tissue and muscle, through humoral mechanisms.
As exciting as the discovery of this new hormone is, there are still important unanswered questions that limit its therapeutic potential. For one, we know virtually nothing regarding the paracrine, neural, and hormonal mechanisms determining stimulation and inhibition of its synthesis and release. What is the involvement of the enteric nervous system? What is the role of the autonomic nervous system? Another difficulty for understanding the effects on brain functions is the still controversial issue of ghrelin production within the brain.5 How far is the brain-internal ghrelin pool responsible for the control of food intake and other functions? Systematic inquiries addressing these questions will be necessary for a more complete understanding of the potential of the ghrelin-signalling system for dietary manipulation and pharmacological intervention.
FEED-BACK SIGNALS LEADING TO SATIATION AND MEAL TERMINATION
Once ingested and swallowed, nutrients are up for an exciting journey to the many final destinations, travelling through the upper gastrointestinal tract and, after absorption, through the circulation. On this journey, they interact with a plethora of sensory mechanisms that all seem to ultimately inform the brain about the state of nutritional sufficiency (Fig. 1). Here, I will discuss mainly signals originating from the gastrointestinal tract and only briefly mention other signals like leptin and insulin. Also, the discussion will focus mainly on the effects of these sensory mechanisms on ingestive behaviour, with only brief mention of effects on the regulation of gastrointestinal and other physiological functions.
Except for ghrelin, increased signalling through all of the known sensory mechanisms originating in the gut leads to decreases in food intake. They are considered negative feedback signals, implying that they are only activated by the presence of nutrients in the gastrointestinal tract and do not have suppressible tonic activity in the absence of nutrients that could lead to the initiation of food intake. In other words, lowering signalling activity of negative feedback mechanisms in the fasted state is not expected to stimulate eating. This is an important distinction from longer-term negative feedback signals such as leptin. The power of leptin is best revealed when decreased leptin levels or decreased leptin signalling activity powerfully stimulates appetitive behaviour and food intake above and beyond normal levels.6 The upshot of this difference is that negative feedback signals from the gastrointestinal tract have been thought to only determine meal size but have little to do with meal frequency, as they cannot initiate eating. This rigid distinction will be challenged in the following discussion by arguing that both vagally mediated and hormonal signals affect not only the caudal brainstem but also higher brain areas, where they can change cognitive and reward functions determining overall food intake and food choice.
The stomach, far from being a passive reservoir, is a highly regulated organ with elaborate neural and hormonal control mechanisms. The presence of ingested food is detected by vagal afferent fibres in the mucosa sensitive to mechanical touch, and the volume of ingested food is detected by vagal afferents in the external muscle layers sensitive to stretch and tension [for review see Ref. 7 (Fig. 1)]. Intraganglionic laminar vagal afferent endings (IGLEs) are located in the connective tissue capsule of myenteric plexus ganglia, between the longitudinal (outer) and circular (inner) muscle layers8 (Fig. 2). They thus respond to muscle tension generated by both passive stretch and active contraction of the muscle layers.9
Figure 2.
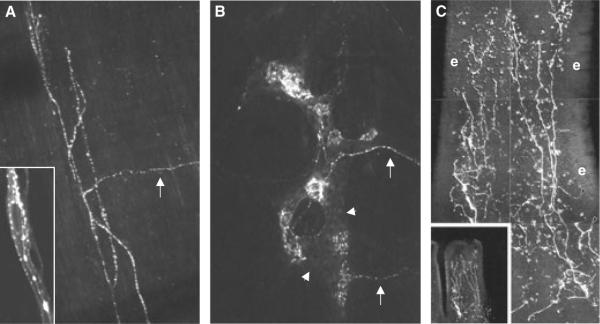
Vagal afferent mechano- and nutrient-sensors in the rat gastrointestinal tract. Vagal afferent fibres and terminal structures were anterogradely traced with the fluorescent dye DiI (bright white) injected into nodose ganglia. A: Intramuscular array (IMA) in longitudinal muscle layer of gastric fundus. Arrow indicates parent axon entering the muscle layer from myenteric plexus. The inset shows vagal afferent fibres in intimate anatomical contact with interstitial cell of Cajal. B: Intraganglionic laminar endings (IGLE) in myenteric plexus of gastric fundus. Two different parent axons are indicated by arrows. Myenteric ganglion is indicated by arrowheads. C: Mucosal endings close to epithelium (e) in villus of proximal duodenum.
This type of vagal afferent ending is found in large numbers throughout the oesophagus and gastrointestinal tract7,8 and is likely to be important for generating vagal afferent tone for balanced interoceptive awareness and emotional well-being.10 According to this view, subjective human feelings and emotional awareness are partly based on higher order re-representations of homeostatic sensory activity.11 This may be the neurological explanation of the popular expression `gut-feeling', and may explain the beneficial effects of human cervical vagal (afferent) stimulation on mood12 and the incidence of epileptic seizure activity.13 It is also very likely that re-representations of interoceptive vagal sensory activity at the level of the hypothalamus and limbic forebrain participate in the control of duration of satiety and initiation of the next meal. In other words, the effect of vagal sensory activity is not limited to satiation and termination of a given meal by affecting caudal brainstem circuitry, but extends to the control of meal frequency by affecting the powerful hypothalamic and cortico-limbic circuitries regulating overall energy balance in the context of motivational, emotional and external factors.
Significant progress has been made on the neuro-chemistry,14–16 transduction mechanisms,15,17–19 and morphology20 of IGLEs. Their dependence on neurotrophic factors has also been described.21–23 An exciting idea is the potential crosstalk between IGLEs and the neighbouring enteric neurons via glutamate. It is well known that vagal afferent neurons are glutamatergic, and vesicular glutamate transporter 2 (VGLUT2) is one of the reliable markers for IGLEs.15,19 Because of their abundance throughout the alimentary canal, IGLEs may also be important for the development of visceral hypersensitivity and functional gastrointestinal disorders.24
The other type of vagal ending considered mechanosensory are the intramuscular arrays (IMAs), almost exclusively located in the stomach longitudinal and circular muscle layers7,25 (Fig. 2). They are thought to correspond to electrophysiologically identified stretch receptors firing at the same high rate during prolonged stretch, even when muscle tension decreases due to accommodation, and are the prime candidates for generating the negative feedback from a full stomach that co-determines the level of satiation. Little is known regarding signal transduction, but the acid-sensing ion channel-3 (ASIC3) appears to be involved.26 Further investigation of both of these vagal mechanosensors should be highly rewarding, given the potential to better understand their role in satiation and satiety, gastrointestinal disorders, as well as higher brain functions.
Despite this progress, we are far away from understanding the transduction mechanisms and the functional roles of gastrointestinal mechanoreceptors. Relatively few laboratories are systematically investigating these important sensors. Although mutant mouse models have been used,23,27 the full spectrum of available genetic manipulations will be an important tool for this endeavour.
INTESTINAL CHEMO-SENSORS: TASTE IN THE GUT
Equally exciting are new findings regarding intestinal chemo-sensors and the concept of taste in the gut. Although the idea of taste receptor and taste bud-like cells and structures has been around for many years,28,29 it was only after discovering the molecular basis of the peripheral gustatory taste code (for review see Ref. 2) that serious progress was made in the gut. It is now clear that the same G-protein-coupled receptor families determining sweet (T1R) and bitter (T2R) taste in the mouth, as well as their common G-protein, α-gustducin, are expressed in enteroendocrine cells throughout the bowel in rodents,30–32 and humans33–37 (Fig. 1). One of the reasons for this delay is the dispersed nature of enteroendocrine cells, the cell type that appears to predominantly, if not exclusively, express the molecular machinery for taste perception, at least in the human duodenum.37
As is always the case with new discoveries, they beg for more new questions. One of the fundamental differences between mouth and intestines is that nutrients are absorbed by the entirety of epithelial cells but that according to at least one line of evidence the taste signal is only generated in specialized enteroendocrine cells. In the human duodenum, <5% of epithelial cells and none of the ordinary enterocytes express α-gustducin, the G-protein necessary for sweet, amino acid and bitter taste perception.37 One question is, therefore, whether this control involves simple paracrine diffusion in the lamina propria, the enteric nervous, or even the extrinsic nervous system? If paracrine diffusion is involved, what is the signalling molecule? If the enteric nervous system is involved, what is the role of intestinal primary afferent neurons (IPANs) and what are the downstream transmitters?
However, there might be significant species differences, as indicated by a quite different scenario in the rat intestine. In the rat jejunum, all T1R members are found on the apical membrane of ordinary enterocytes, where they appear to regulate glucose uptake mediated by the sodium glucose co-transporter SGLT1.32 Under this arrangement, no paracrine, hormonal or neural mechanisms are necessary. It will be exciting to follow the development of this area of research in the near future.
Another fundamental question is what type of enteroendocrine cells express what taste receptor(s)? So far, T1R2 and T1R3, combining to form heterodimeric receptors representing sweet taste have been found on glucagon-like peptide (GLP-1) producing L-cells and glucose-dependent insulinotropic polypep-tide (GIP) producing enteroendocrine cells in the human duodenum.37 Co-localization of α-gustducin with peptide YY (PYY) has been shown in the human colon,36 and with 5-HT in the mouse small intestine.30 No colocalization has yet been reported in cholecystokinin (CCK), neurotensin, gastrin, or ghrelin producing enteroendocrine cells. Of particular interest is the expression of T1R1, which together with T1R3 is responsible for the taste of certain amino acids such as glutamate, the so-called umami taste. Expression of this T1R family member has only been reported in rat enterocytes by one group of researchers.32 It will be interesting to ask the question whether amino acid taste receptors provide a signal regulating the capacity of amino acid absorption in a similar fashion that sweet taste receptors control the absorption of glucose.
Equally interesting are the bitter taste receptors in the gut. Although their most likely function in the gut is a second line of defense against toxins that were able to sneak past the oral gatekeeper, they may have acquired additional functions in intestinal nutrient sensing. For example, intragastric administration of certain bitter-tasting T2R agonists can activate a vagal afferent pathway through CCK1 and Y2 receptor activation.38
As for the ghrelin-producing cells in the stomach, little is known about paracrine, hormonal, and neural modulation of intestinal enteroendocrine cells. In particular, neural modulation by the enteric nervous and extrinsic autonomic nervous system is not well understood. Given the potentially important role of GLP-1 and PYY in the beneficial effect of gastric bypass surgery, a better understanding of their modulation by neural inputs will be crucial. No doubt, continued comparisons with the oral taste system and the use of genetic manipulations will also be helpful.
SIGNALLING TO THE BRAIN: VAGAL AFFERENTS, CIRCULATION, OR BOTH?
It is now clear that ghrelin, CCK, GLP-1 and PYY(3–36) are gut hormones that can powerfully influence the control of food intake and regulation of energy balance.39,40 In addition, obestatin, GIP, oxyntomodulin, neurotensin and 5-HT may also be involved but their status is less clear. All hormonal messengers released from enteroendocrine cells in the gut mucosa can inform the brain either through the circulation or via primary afferent neurons or both, and there is considerable controversy and confusion about the relative importance of these routes. It is possible that a given hormone could use different routes to produce different physiological effects such as changes in gastrointestinal functions or in ingestive behaviour. However, in many instances, different routes are claimed for the same physiological effect, particularly for the effect on food intake. For each of the gut hormones ghrelin,41,42 CCK,43–45 PYY(3–36)46,47 and GLP-1,48–50 there is competing evidence for both vagal afferents and a humoral route to modulate food intake.
Vagal afferent innervation of the duodenal mucosa was demonstrated anatomically using DiI tracing from the rat nodose ganglia7 (Fig. 2). Unlike the idiosyncratic structure of IGLEs which can be easily recognized with VGLUT2 as a marker, anterograde tracing is the only way to recognize the undistinguished mucosal endings. Labelled vagal afferent fibres were present in the lamina propria of duodenal and jejunal villi and crypts of Lieberkühn, but do not cross the basal membrane to innervate the epithelial layer. Thus, vagal afferents are not in a position to sense luminal nutrients directly, but are in close anatomical apposition to the basal membrane of enteroendocrine cells.51 It is also clear that vagal afferent neurons in the nodose ganglia express all the relevant receptors for these gut hormones, such as ghrelin,52 CCK1,53 GLP-1,54 Y246 and 5HT3-receptors.55
BEYOND MEAL SIZE: HORMONAL SIGNALS DETERMINING MEAL INITIATION AND DURATION OF SATIETY
The major components of the distributed neural system controlling food intake and energy balance have been reviewed extensively before.56 The limited view of a few, mainly hypothalamic `centres', that was propagated by the molecular engineers riding the tails of the discovery of leptin, was gradually replaced during the last 10 years by a much more complex and distributed system including various cortico-limbic areas. It is now increasingly recognized that cognitive, hedonic and emotional neural processes play important roles in energy intake and expenditure and the resulting energy balance and that hormones modulate many of these processes. The best studied hormone, leptin, regulates not only hypothalamic feeding circuits, but also acts on many other forebrain mechanisms such as taste57 and olfactory processing,58 learning and memory59 and reward.60
Similarly, the gut hormones ghrelin and PYY(3–36) have recently been demonstrated to act on higher brain functions as well. Ghrelin acts on hippocampal neurons to induce formation of new synapses in the CA1 region correlated with enhanced spatial learning. Ghrelin-deficient mice exhibited impaired spatial learning that was corrected by ghrelin administration.4 These findings are consistent with the idea that ghrelin is involved in the appetitive phase of ingestive behaviour when it is important to find food in the environment. It is plausible that the ghrelin-induced changes in hippocampal function facilitate the recall of stored representations of prior experience with food.61 This is indicated by human subjects reporting a vivid, plastic image of their preferred meal upon intravenous ghrelin infusion.62 Ghrelin also activates dopamine neurons in the ventral tegmental area (VTA), increases dopamine turnover in the nucleus accumbens, and directly stimulates food intake when locally administered to the VTA.63 As local ghrelin receptor blockade in the VTA blunted rebound feeding following fasting,63 these observations suggest that enhancement of reward processing in the mesolimbic dopamine system is an integral part of endogenous ghrelin's orexigenic action.
After considerable controversy regarding its physiological effects on food intake, there is now convincing evidence that PYY(3–36) suppresses food intake in humans and rodents,39 and a recent neuroimaging study points to exciting new neural pathways and mechanisms responsible for this effect. Neural activity in fasted human subjects was compared when either saline or PYY(3–36) was infused for 90 min and at the end of the infusion, food intake was measured.64 As expected, on saline infusion days, the change in neural activity in the posterior hypothalamus associated with fasting correlated positively with the amount of food eaten, suggesting that the level of fasting-induced hunger was encoded in the hypothalamus. However, on PYY(3–36) infusion days, the amount eaten was now negatively correlated to the change in neural activity in the orbitofrontal cortex and the correlation with hypothalamic signal change disappeared. Thus, endogenous PYY released during absorption of a meal appears to switch brain activity predicting meal size, from the hypothalamus to the orbitofrontal cortex and other cortico-limbic brain areas.64 This is the first demonstration that so-called negative feedback signals from the gut act on higher brain areas involved in the cognitive and rewarding aspects of food intake control and it will be interesting to assess the potential of other negative feed back signals such as GLP-1, GIP and oxyntomodulin, to affect these higher brain areas.
That a satiating meal produces a feeling of reward and satisfaction is an old notion, but the signals and mechanisms involved have been elusive.65 Information from the gut during a meal leads not only to decreased hunger, satiation and meal termination, but also to positive or rewarding feelings. This has been convincingly demonstrated in a series of seminal studies by Sclafani et al. (for review, see Ref. 66). When intraduodenal infusions were paired with oral presentation of different flavours, rats learned to prefer the flavour associated with infusion of carbohydrate solutions or fat emulsions over the flavour associated with duodenal saline infusions.66 The exact signal-generation site(s) cannot be controlled with duodenal nutrient infusions and could include the liver, as glucose infusions into the portal vein can also lead to flavour preferences.67 However, the presence of the same transduction mechanisms as in the oral sweet receptor: suggests that the nutrient sensors and their signalling to the brain are critically involved in the rewarding effects of ingested foods.
While CCK and vagal afferent signalling are important for the satiating effects, they are apparently not responsible for mediating postingestive reward as measured by conditioning of flavour preferences, and no other mechanism has yet been identified.66 Therefore, hormonal signals released from intestinal taste cells acting on cortico-limbic brain structures are prime candidates for this function. This is not to say that vagal afferents are not involved. There may be different populations of vagal afferents mediating the satiating and rewarding effects and currently available techniques do not allow selective ablation of functionally specific populations. Satiating and rewarding functions may depend on integrative processes within and/or between vagal afferents or between vagal afferent information and hormonal effects on specific neural circuits.
CONCLUSIONS AND PERSPECTIVES
The major advances in understanding the role of gut–brain communication in homeostatic behaviour have come from a new appreciation for the nutrient-specific release of gut hormones and their diverse actions on the brain. Taste-in-the-gut is no longer just a catch-phrase but is now based on the same genes and transduction mechanisms responsible for oral sweet and bitter taste perception. Together with the abundant vagal mechanosensory innervation of the gut, the system is potentially capable of both registering and metering ingested macronutrients. There are still important gaps and disparities that need to be solved before its full potential can be translated into pharmacological intervention.
On the peripheral side, the more immediate goals include deciphering the nutritional selectivity for the release of each gut hormone. The origin and nature of neural stimuli needs to be identified. The distribution of the taste receptor genes on the various populations of enteroendocrine as well as common enterocytes needs to be clarified. Further down the road are more complete molecular characterizations of the transduction cascades leading to hormone release, as well as the transcription mechanisms and posttranscriptional modifications for each hormone. This will open possibilities for pharmaceutical and dietary approaches to modulate interoceptive mechanisms and their effects on food intake, food choice, and metabolism. We may not be too far away from using drugs, functional foods, and viral constructs that trick the intestinal sensing system into signalling satiation and satisfaction even though fewer calories have been ingested.
On the central side, nutritionally relevant information mediated from the gut to the brain by hormones and vagal afferents acts not only on the energy balance control circuits in the hypothalamus and brainstem, but also impinges on cortico-limbic systems involved in cognitive, reward and executive brain functions. It is thus in a position to modulate higher brain functions that play an important role in coordinating eating behaviour with external conditions and demands that go beyond the controls of an individual meal. Here, progress will be made at a much slower pace, because we do not fully understand which brain areas are receiving and processing information from the gut and how these brain areas are functionally organized. Our neurological explanations for psychological constructs such as appetite, reward, craving, satisfaction, mood, learning and memory are still very sketchy. Thus, continued analysis of these basic mechanisms with modern neuroimaging techniques in humans and more invasive methods in animal models will be necessary. However, such analyses should be multidisciplinary in nature, with gastroenterologists, neurologists, and basic neuroscientists closely working together. Simply producing more data on neural activation in human neuroimaging studies without better understanding what the activation means in functional neurological terms seems futile.
Inclusion of patients and animal models with gastric bypass and other obesity surgeries will be important in all these endeavours, as the number of bariatric surgeries is growing exponentially. The research will not only benefit patients through the development of refinements and improvements in the surgical approaches, it is hoped, it may also lead to the replacement of these invasive and irreversible methods by dietary and pharmacological approaches.
Acknowledgments
CONFLICTS OF INTEREST H-RB was supported for this study by NIH grants DK47348 and DK52257.
REFERENCES
- 1.Li C, Ford ES, McGuire LC, Mokdad AH. Increasing trends in waist circumference and abdominal obesity among US adults. Obesity (Silver Spring) 2007;15:216–24. doi: 10.1038/oby.2007.505. [DOI] [PubMed] [Google Scholar]
- 2.Chandrashekar J, Hoon MA, Ryba NJ, Zuker CS. The receptors and cells for mammalian taste. Nature. 2006;444:288–94. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 3.Cummings DE. Ghrelin and the short- and long-term regulation of appetite and body weight. Physiol Behav. 2006;89:71–84. doi: 10.1016/j.physbeh.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 4.Diano S, Farr SA, Benoit SC, et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat Neurosci. 2006;9:381–8. doi: 10.1038/nn1656. [DOI] [PubMed] [Google Scholar]
- 5.Kageyama H, Kitamura Y, Hosono T, et al. Visualization of ghrelin-producing neurons in the hypothalamic arcuate nucleus using ghrelin-EGFP transgenic mice. Regul Pept. 2007;145:116–121. doi: 10.1016/j.regpep.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Farooqi S, O'Rahilly S. Genetics of obesity in humans. Endocr Rev. 2006;27:710–8. doi: 10.1210/er.2006-0040. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci. 2000;85:1–17. doi: 10.1016/S1566-0702(00)00215-0. [DOI] [PubMed] [Google Scholar]
- 8.Neuhuber WL, Kressel M, Stark A, Berthoud HR. Vagal efferent and afferent innervation of the rat esophagus as demonstrated by anterograde DiI and DiA tracing: focus on myenteric ganglia. J Auton Nerv Syst. 1998;70:92–102. doi: 10.1016/s0165-1838(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 9.Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–68. doi: 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig AD. Interoception: the sense of the physiological condition of the body. Curr Opin Neurobiol. 2003;13:500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- 11.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cogn Sci. 2005;9:566–71. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10:1116–24. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murphy JV, Patil AA. Improving the lives of patients with medically refractory epilepsy by electrical stimulation of the nervous system. Expert Rev Med Devices. 2005;2:175–89. doi: 10.1586/17434440.2.2.175. [DOI] [PubMed] [Google Scholar]
- 14.Ewald P, Neuhuber WL, Raab M. Vesicular glutamate transporter 1 immunoreactivity in extrinsic and intrinsic innervation of the rat esophagus. Histochem Cell Biol. 2006;125:377–95. doi: 10.1007/s00418-005-0083-z. [DOI] [PubMed] [Google Scholar]
- 15.Raab M, Neuhuber WL. Intraganglionic laminar endings and their relationships with neuronal and glial structures of myenteric ganglia in the esophagus of rat and mouse. Histochem Cell Biol. 2004;122:445–59. doi: 10.1007/s00418-004-0703-z. [DOI] [PubMed] [Google Scholar]
- 16.Wang ZJ, Neuhuber WL. Intraganglionic laminar endings in the rat esophagus contain purinergic P2X2 and P2X3 receptor immunoreactivity. Anat Embryol (Berl) 2003;207:363–71. doi: 10.1007/s00429-003-0351-4. [DOI] [PubMed] [Google Scholar]
- 17.Zagorodnyuk VP, Lynn P, Costa M, Brookes SJ. Mechanisms of mechanotransduction by specialized low-threshold mechanoreceptors in the guinea pig rectum. Am J Physiol Gastrointest Liver Physiol. 2005;289:G397–406. doi: 10.1152/ajpgi.00557.2004. [DOI] [PubMed] [Google Scholar]
- 18.Lynn P, Zagorodnyuk V, Hennig G, Costa M, Brookes S. Mechanical activation of rectal intraganglionic laminar endings in the guinea pig distal gut. J Physiol. 2005;564:589–601. doi: 10.1113/jphysiol.2004.080879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Raab M, Neuhuber WL. Vesicular glutamate transporter 2 immunoreactivity in putative vagal mechanosensor terminals of mouse and rat esophagus: indication of a local effector function? Cell Tissue Res. 2003;312:141–8. doi: 10.1007/s00441-003-0721-5. [DOI] [PubMed] [Google Scholar]
- 20.Powley TL, Wang XY, Fox EA, Phillips RJ, Liu LW, Huizinga JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil. 2007;20:69–79. doi: 10.1111/j.1365-2982.2007.00990.x. [DOI] [PubMed] [Google Scholar]
- 21.Fox EA, Phillips RJ, Baronowsky EA, Byerly MS, Jones S, Powley TL. Neurotrophin-4 deficient mice have a loss of vagal intraganglionic mechanoreceptors from the small intestine and a disruption of short-term satiety. J Neurosci. 2001;21:8602–15. doi: 10.1523/JNEUROSCI.21-21-08602.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chi MM, Fan G, Fox EA. Increased short-term food satiation and sensitivity to cholecystokinin in neurotrophin-4 knock-in mice. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1044–53. doi: 10.1152/ajpregu.00420.2004. [DOI] [PubMed] [Google Scholar]
- 23.Chi MM, Powley TL. NT-4-deficient mice lack sensitivity to meal-associated preabsorptive feedback from lipids. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2124–35. doi: 10.1152/ajpregu.00825.2006. [DOI] [PubMed] [Google Scholar]
- 24.Delgado-Aros S, Camilleri M. Visceral hypersensitivity. J Clin Gastroenterol. 2005;39:S194–203. doi: 10.1097/01.mcg.0000156114.22598.1b. [DOI] [PubMed] [Google Scholar]
- 25.Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. J Comp Neurol. 2000;421:302–24. [PubMed] [Google Scholar]
- 26.Page AJ, Brierley SM, Martin CM, et al. Different contributions of ASIC channels 1a, 2, and 3 in gastrointestinal mechanosensory function. Gut. 2005;54:1408–15. doi: 10.1136/gut.2005.071084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox EA. A genetic approach for investigating vagal sensory roles in regulation of gastrointestinal function and food intake. Auton Neurosci. 2006;127:9–29. doi: 10.1016/j.autneu.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 28.Fujita T, Kobayashi S. Structure and function of gut endocrine cells. Int Rev Cytol Suppl. 1977;6:187–233. [PubMed] [Google Scholar]
- 29.Newson B, Ahlman H, Dahlstrom A, Nyhus LM. Ultrastructural observations in the rat ileal mucosa of possible epithelial “taste cells” and submucosal sensory neurons. Acta Physiol Scand. 1982;114:161–4. doi: 10.1111/j.1748-1716.1982.tb06967.x. [DOI] [PubMed] [Google Scholar]
- 30.Sutherland K, Young RL, Cooper NJ, Horowitz M, Black-shaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1420–8. doi: 10.1152/ajpgi.00504.2006. [DOI] [PubMed] [Google Scholar]
- 31.Margolskee RF, Dyer J, Kokrashvili Z, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc Natl Acad Sci U S A. 2007;104:15075–80. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. J Physiol. 2007;582:379–92. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hofer D, Asan E, Drenckhahn D. Chemosensory Perception in the Gut. News Physiol Sci. 1999;14:18–23. doi: 10.1152/physiologyonline.1999.14.1.18. [DOI] [PubMed] [Google Scholar]
- 34.Dyer J, Salmon KS, Zibrik L. Shirazi-Beechey SP. Expression of sweet taste receptors of the T1R family in the intestinal tract and enteroendocrine cells. Biochem Soc Trans. 2005;33:302–5. doi: 10.1042/BST0330302. [DOI] [PubMed] [Google Scholar]
- 35.Rozengurt E. Taste receptors in the gastrointestinal tract. I. Bitter taste receptors and alpha-gustducin in the mammalian gut. Am J Physiol Gastrointest Liver Physiol. 2006;291:G171–7. doi: 10.1152/ajpgi.00073.2006. [DOI] [PubMed] [Google Scholar]
- 36.Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the alpha-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006;291:G792–802. doi: 10.1152/ajpgi.00074.2006. [DOI] [PubMed] [Google Scholar]
- 37.Jang HJ, Kokrashvili Z, Theodorakis MJ, et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc Natl Acad Sci U S A. 2007;104:15069–74. doi: 10.1073/pnas.0706890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2007;294:R33–38. doi: 10.1152/ajpregu.00675.2007. [DOI] [PubMed] [Google Scholar]
- 39.Chelikani PK, Haver AC, Reeve JR, Jr, Keire DA, Reidelberger RD. Daily, intermittent intravenous infusion of peptide YY(3–36) reduces daily food intake and adiposity in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R298–305. doi: 10.1152/ajpregu.00674.2005. [DOI] [PubMed] [Google Scholar]
- 40.Batterham RL, Heffron H, Kapoor S, et al. Critical role for peptide YY in protein-mediated satiation and body-weight regulation. Cell Metab. 2006;4:223–33. doi: 10.1016/j.cmet.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Date Y, Murakami N, Toshinai K, et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology. 2002;123:1120–8. doi: 10.1053/gast.2002.35954. [DOI] [PubMed] [Google Scholar]
- 42.Arnold M, Mura A, Langhans W, Geary N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci. 2006;26:11052–60. doi: 10.1523/JNEUROSCI.2606-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith GP, Jerome C, Norgren R. Afferent axons in abdominal vagus mediate satiety effect of cholecystokinin in rats. Am J Physiol. 1985;249:R638–41. doi: 10.1152/ajpregu.1985.249.5.R638. [DOI] [PubMed] [Google Scholar]
- 44.Wu XY, Zhu JX, Gao J, Owyang C, Li Y. Neurochemical phenotype of vagal afferent neurons activated to express CFOS in response to luminal stimulation in the rat. Neuroscience. 2005;130:757–67. doi: 10.1016/j.neuroscience.2004.09.060. [DOI] [PubMed] [Google Scholar]
- 45.Reidelberger RD, Hernandez J, Fritzsch B, Hulce M. Abdominal vagal mediation of the satiety effects of CCK in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1005–12. doi: 10.1152/ajpregu.00646.2003. [DOI] [PubMed] [Google Scholar]
- 46.Koda S, Date Y, Murakami N, et al. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 2005;146:2369–75. doi: 10.1210/en.2004-1266. [DOI] [PubMed] [Google Scholar]
- 47.Batterham RL, Cowley MA, Small CJ, et al. Gut hormone PYY(3–36) physiologically inhibits food intake. Nature. 2002;418:650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 48.Merchenthaler I, Lane M, Shughrue P. Distribution of prepro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol. 1999;403:261–80. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 49.Yamamoto H, Kishi T, Lee CE, et al. Glucagon-like peptide-1-responsive catecholamine neurons in the area postrema link peripheral glucagon-like peptide-1 with central autonomic control sites. J Neurosci. 2003;23:2939–46. doi: 10.1523/JNEUROSCI.23-07-02939.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abbott CR, Monteiro M, Small CJ, et al. The inhibitory effects of peripheral administration of peptide YY(3–36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 2005;1044:127–31. doi: 10.1016/j.brainres.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 51.Berthoud HR, Patterson LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996;156:123–31. doi: 10.1159/000147837. [DOI] [PubMed] [Google Scholar]
- 52.Burdyga G, Varro A, Dimaline R, Thompson DG, Dockray GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol Gastrointest Liver Physiol. 2006;290:G1289–97. doi: 10.1152/ajpgi.00543.2005. [DOI] [PubMed] [Google Scholar]
- 53.Broberger C, Holmberg K, Kuhar MJ, Hokfelt T. Cocaine- and amphetamine-regulated transcript in the rat vagus nerve: a putative mediator of cholecystokinin-induced satiety. Proc Natl Acad Sci U S A. 1999;96:13506–11. doi: 10.1073/pnas.96.23.13506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kakei M, Yada T, Nakagawa A, Nakabayashi H. Glucagon-like peptide-1 evokes action potentials and increases cytosolic Ca2+ in rat nodose ganglion neurons. Auton Neurosci. 2002;102:39–44. doi: 10.1016/s1566-0702(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 55.Morales M, Wang SD. Differential composition of 5-hydroxytryptamine3 receptors synthesized in the rat CNS and peripheral nervous system. J Neurosci. 2002;22:6732–41. doi: 10.1523/JNEUROSCI.22-15-06732.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Berthoud H-R. Multiple neural systems controlling food intake and body weight. Neurosci Biobehav Rev. 2002;26:393–428. doi: 10.1016/s0149-7634(02)00014-3. [DOI] [PubMed] [Google Scholar]
- 57.Shigemura N, Ohta R, Kusakabe Y, et al. Leptin modulates behavioral responses to sweet substances by influencing peripheral taste structures. Endocrinology. 2004;145:839–47. doi: 10.1210/en.2003-0602. [DOI] [PubMed] [Google Scholar]
- 58.Getchell TV, Kwong K, Saunders CP, Stromberg AJ, Getchell ML. Leptin regulates olfactory-mediated behavior in ob/ob mice. Physiol Behav. 2006;87:848–56. doi: 10.1016/j.physbeh.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Harvey J. Leptin: a diverse regulator of neuronal function. J Neurochem. 2007;100:307–13. doi: 10.1111/j.1471-4159.2006.04205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Farooqi IS, Bullmore E, Keogh J, Gillard J, O'Rahilly S, Fletcher PC. Leptin regulates striatal regions and human eating behavior. Science. 2007;317:1355. doi: 10.1126/science.1144599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng H, Berthoud HR. Eating for pleasure or calories. Curr Opin Pharmacol. 2007;7:607–12. doi: 10.1016/j.coph.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schmid DA, Held K, Ising M, Uhr M, Weikel JC, Steiger A. Ghrelin stimulates appetite, imagination of food, GH, ACTH, and cortisol, but does not affect leptin in normal controls. Neuropsychopharmacology. 2005;30:1187–92. doi: 10.1038/sj.npp.1300670. [DOI] [PubMed] [Google Scholar]
- 63.Abizaid A, Liu ZW, Andrews ZB, et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–39. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Batterham RL, ffytche DH, Rosenthal JM, et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behavior in humans. Nature. 2007;450:106–11. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- 65.Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 66.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–9. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 67.Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol. 1986;251:R192–6. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]


