Abstract
Spontaneous regression of hepatocellular carcinoma (HCC) is an extraordinary phenomenon that occurs rarely. While more than 80 cases have been described, most have been established via radiological findings or examination of biopsy tissues rather than via pathological examination of a resected specimen. The present report describes a purported case of spontaneous regression of HCC as indicated by radiological examination. Subsequent immunostaining of surgically resected specimens revealed viable cancer cells, though only necrotic tissues were seen on hematoxylin and eosin staining. These data indicate that viable cancer cells may still be present even if imaging findings suggest spontaneous regression of HCC. Therefore, these patients should receive aggressive treatment similar to that used for patients with established HCC.
Key words: Hepatocellular carcinoma, Spontaneous regression
Introduction
Hepatocellular carcinoma (HCC) is one of the most common cancers worldwide and is associated with poor prognosis. Patients diagnosed at an early stage may achieve a 5-year survival rate of approximately 50%, while those at an intermediate to advanced stage demonstrate a 20–50% survival at 3 years, and those with terminal stage HCC usually die within 6 months [1].
Spontaneous regression of HCC is rare. Approximately 85 cases of spontaneous regression of HCC have been described in the literature [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21], most of which were established via radiological findings. By contrast, examination of surgically resected specimens following radiologic indications of spontaneous resolution of HCC was conducted in only 21 of these cases [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. Of those, pathological examination revealed complete necrosis in 13 cases [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] and persistence of microscopically viable cancer cells in eight cases [14, 15, 16, 17, 18, 19, 20, 21].
The present report describes a case in which radiologic examination suggested spontaneous resolution of HCC, while hematoxylin and eosin staining of surgically resected specimens showed complete necrosis, but immunological staining revealed a small number of viable HCC cells. The case report is followed by a discussion of the appropriate management of these types of patients.
Case Report
A 79-year-old patient was undergoing routine follow-up at Yamamoto Memorial Hospital for hypertension and chronic heart failure. He had a history of alcohol abuse, consuming approximately 540 ml of the Japanese alcoholic beverage ‘shochu’ (108 g alcohol) every day.
A 20-mm low-density hepatic lesion was incidentally detected on ultrasonography and computed tomography. At that time he did not complain of any symptoms. Laboratory data were as follows: aspartate aminotransferase 40 U/l (normal 5–35), alanine aminotransferase 24 U/l (normal 5–30), alkaline phosphatase 311 U/l (normal 115–359), gamma-glutamyl transferase 121 U/ml (normal 0–50), lactate dehydrogenase 230 U/l (normal 106–211), albumin 4.1 g/dl (normal 3.7–5.5), and prothrombin time-international normalized ratio 1.20. Child-Turcotte-Pugh grade was A. The concentration of serum alpha-fetoprotein was 8.1 ng/ml. Serological evidence of hepatitis B and C was not seen.
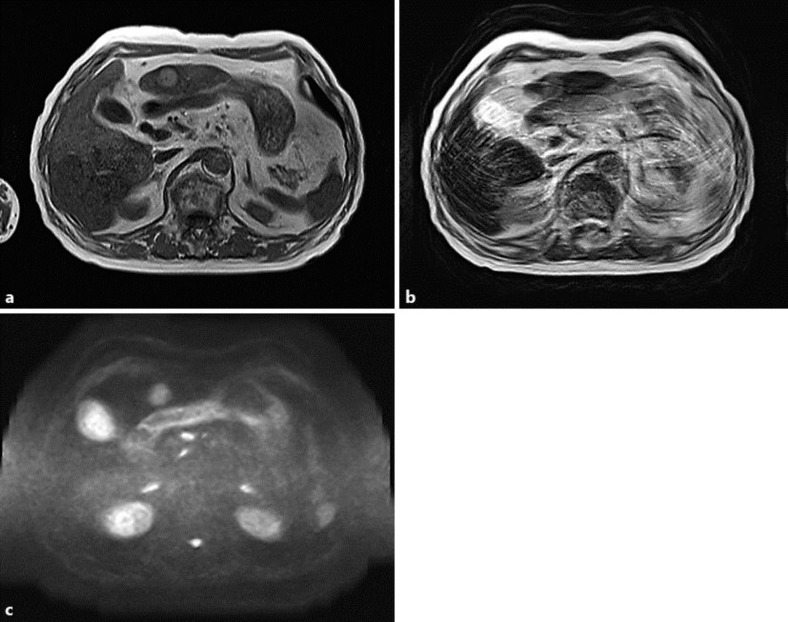
Magnetic resonance imaging (MRI) revealed a faint high-intensity tumor on T1-weighted imaging (T1WI), T2-weighted imaging (T2WI) and diffusion-weighted imaging (DWI) (fig. 1). Contrast-enhanced dynamic MRI showed the tumor as a hyperenhanced lesion in the arterial phase and as a low-intensity lesion in the hepatobiliary phase. These MRI findings were suggestive of a diagnosis of HCC.
Fig. 1.
Initial MRI revealed a faint high-intensity tumor on T1WI (a), T2WI (b) and DWI (c).
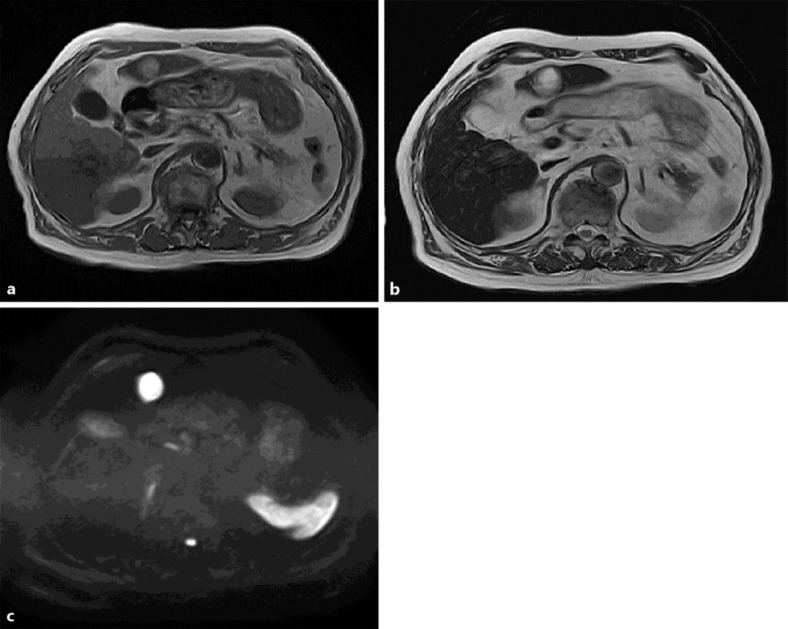
The patient initially refused surgery but ultimately agreed 2 months later. Preoperative MRI revealed a faint high-intensity tumor in T1WI, similar to the results 2 months prior. However, T2WI and DWI showed a lesion with higher intensity than that seen 2 months prior (fig. 2). These findings were suggestive of HCC with spontaneous regression. To exclude the possible presence of viable HCC cells, the patient underwent surgical resection of the liver.
Fig. 2.
Repeat MRI performed 2 months later revealed a faint high-intensity tumor in T1WI (a) which was similar to that seen on previous MRI. However, T2WI (b) and DWI (c) showed a lesion with higher intensity than that seen 2 months prior.
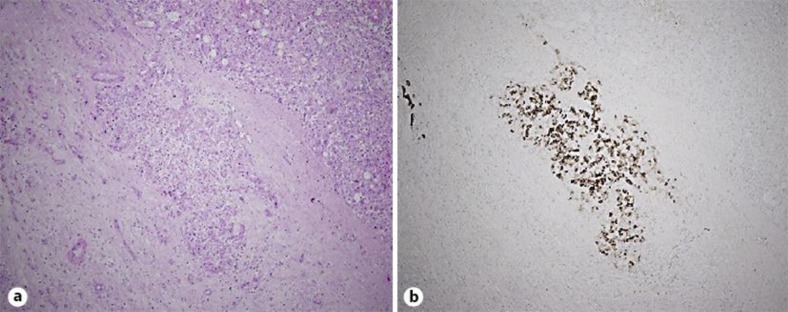
Macroscopic examination of the tumor on the cut specimen showed a 20 × 20 mm lesion that was whitish and necrotic with a fibrous capsule. Microscopically, hematoxylin and eosin staining demonstrated the tumor to consist of extensive coagulative necrosis without viable malignant cells. However, immunological staining using a monoclonal antibody against CD68 demonstrated a small amount of viable HCC cells outside the fibrous capsule of the necrotic tumor, although no viable cells were found inside the margins of the necrotic tumor tissue (fig. 3).
Fig. 3.
Pathological examination of the tumor. Viable malignant cells were seen outside the fibrous capsule of the necrotic tumor. a Hematoxylin and eosin staining, ×4. b Immunological staining using a monoclonal antibody against CD68, ×4.
Discussion
Spontaneous regression of cancer is defined as a partial or complete disappearance of malignancy in the absence of specific treatment. Androgen withdrawal, abstinence from alcohol, gastrointestinal bleeding and herbal medicine have been reported as possible causes of spontaneous regression of HCC [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. Other studies suggest that spontaneous regression may also result from deprivation of oxygen due to rapid tumor growth or from tumor infarction due to disruption of feeding arteries secondary to subintimal injury, thrombus or tumor invasion [3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. However, the mechanisms of spontaneous regression remain unclear.
Spontaneous regression of cancer is estimated to occur in between 1 in 60,000 and 1 in 100,000 malignancies [6, 8, 13, 17]. To date, 85 cases of apparently spontaneous regression of HCC have been described in the literature, most of which were established via radiological findings. By contrast, only 21 cases were examined pathologically, and 13 of these cases had proven total necrosis of HCC (table 1) [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21]. In the present case, MRI indicated that the HCC lesion had undergone necrosis. Furthermore, hematoxylin and eosin staining of surgically resected specimens also showed completely necrosis. However, immunological staining showed viable tumor cells. These findings suggest that tumors with suspected total necrosis should be examined by hematoxylin and eosin staining as well as by immunological staining. In the 13 cases of total necrosis described in the literature, immunological staining was not conducted.
Table 1.
Characteristics of spontaneous regression of HCC
| Reference (first author) | Year | Age | Sex | Etiology | Radiologic regression | Histologically necrosis (staining method) | Proposed mechanism |
|---|---|---|---|---|---|---|---|
| Andreola [2] | 1987 | 75 | M | alcohol | partial | complete (HE, PAS, Masson's trichrome, Weigert, immunostaining) | venous thrombosis |
| Ozeki [3] | 1996 | 69 | F | unknown | complete | complete (unknown) | herbal medicine |
| Markovic [4] | 1996 | 62 | M | HBV | partial | complete (unknown) | biological effects by cytokines |
| Izuishi [5] | 2000 | 50 | M | HCV | partial | complete (HE, reticulin silver) | ischemia or immune response |
| Matsuo [6] | 2001 | 72 | M | HCV | complete | complete (HE, reticulin silver) | ischemia or immune response |
| Morimoto [7] | 2002 | 73 | M | alcohol | partial | complete (unknown) | hepatic artery thrombosis |
| Iiai [8] | 2003 | 69 | M | HCV | complete | complete (HE) | portal vein tumor thrombus, abstinence from smoking |
| Li [9] | 2003 | 53 | M | HBV | unknown | complete (unknown) | biological effects by cytokines |
| Ohta [10] | 2005 | 74 | M | unknown | complete | complete (HE, reticulin silver) | disturbance of the blood flow (arterial sclerosis) |
| Ohtani [11] | 2005 | 69 | M | HCV, alcohol | partial | complete (HE) | disturbance of the blood flow (a thick capsule) |
| Meza-Junco [12] | 2007 | 56 | F | HCV | partial | complete (HE) | disturbance of the blood flow (a thick capsule) |
| Arakawa [13] | 2008 | 78 | F | HBV | partial | complete (HE) | portal vein thrombosis, immune response |
| Storey [14] | 2011 | 52 | M | alcohol | partial | complete (unknown) | abstinence from alcohol |
| Mochizuki [15] | 1991 | 61 | M | unknown | complete | partial (unknown) | radiation |
| Imaoka [16] | 1994 | 65 | M | HCV | partial | partial (HE) | arterial thrombosis |
| Stoelben [17] | 1998 | 56 | M | unknown | partial | partial (HE) | biological effects triggered by infection |
| Stoelben [17] | 1998 | 74 | M | unknown | partial | partial (HE) | biological effects triggered by infection |
| Uenishi [18] | 2000 | 65 | M | HCV | partial | partial (HE) | portal vein thrombosis |
| Blondon [19] | 2004 | 64 | M | alcohol | partial | partial (unknown) | rupture of esophageal varix, tamoxifen |
| Yano [20] | 2005 | 71 | F | HCV | partial | partial (HE, Weigert) | disturbance of the blood flow |
| Park [21] | 2009 | 57 | M | HBV | unknown | partial (HE, immunostaining) | infiltrating lymphocyte |
HBV = Hepatitis B virus infection; HCV = hepatitis C virus infection; HE = hematoxylin and eosin staining; PAS = periodic acid-Schiff stain.
In conclusion, these data indicate that viable cancer cells may still be present even if imaging findings suggest spontaneous regression of HCC. Therefore, these patients should receive aggressive treatment similar to that used for patients with established HCC. Further investigation of purported cases of spontaneous regression is needed to help elucidate the etiology and management of this phenomenon.
References
- 1.Llovet JM, Fuster J, Bruix J. Prognosis of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:7–11. [PubMed] [Google Scholar]
- 2.Andreola S, Audisio RA, Mazzaferro V, Doci R, Milella M. Spontaneous massive necrosis of a hepatocellular carcinoma. Tumori. 1987;73:203–207. doi: 10.1177/030089168707300220. [DOI] [PubMed] [Google Scholar]
- 3.Ozeki Y, Matsubara N, Tateyama K, Kokubo M, Shimoji H, Katayama M. Spontaneous complete necrosis of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:391–392. [PubMed] [Google Scholar]
- 4.Markovic S, Ferlan-Marolt V, Hlebanja Z. Spontaneous regression of hepatocellular carcinoma. Am J Gastroenterol. 1996;91:392–393. [PubMed] [Google Scholar]
- 5.Izuishi K, Ryu M, Hasebe T, Kinoshita T, Konishi M, Inoue K. Spontaneous total necrosis of hepatocellular carcinoma: report of a case. Hepatogastroenterology. 2000;47:1122–1124. [PubMed] [Google Scholar]
- 6.Matsuo R, Ogata H, Tsuji H, Kitazono T, Shimada M, Taguchi K, Fujishima M. Spontaneous regression of hepatocellular carcinoma – a case report. Hepatogastroenterology. 2001;48:1740–1742. [PubMed] [Google Scholar]
- 7.Morimoto Y, Tanaka Y, Itoh T, Yamamoto S, Mizuno H, Fushimi H. Spontaneous necrosis of hepatocellular carcinoma: a case report. Dig Surg. 2002;19:413–418. doi: 10.1159/000065822. [DOI] [PubMed] [Google Scholar]
- 8.Iiai T, Sato Y, Nabatame N, Yamamoto S, Makino S, Hatakeyama K. Spontaneous complete regression of hepatocellular carcinoma with portal vein tumor thrombus. Hepatogastroenterology. 2003;50:1628–1630. [PubMed] [Google Scholar]
- 9.Li AJ, Wu MC, Cong WM, Shen F, Yi B. Spontaneous complete necrosis of hepatocellular carcinoma: a case report. Hepatobiliary Pancreat Dis Int. 2003;2:152–154. [PubMed] [Google Scholar]
- 10.Ohta H, Sakamoto Y, Ojima H, Hibi T, Takahashi Y, Sano T, Shimada K, Kosuge T. Spontaneous regression of hepatocellular carcinoma with complete necrosis: case report. Abdom Imaging. 2005;30:734–737. doi: 10.1007/s00261-005-0313-9. [DOI] [PubMed] [Google Scholar]
- 11.Ohtani H, Yamazaki O, Matsuyama M, Horii K, Shimizu S, Oka H, Nebiki H, Kioka K, Kurai O, Kawasaki Y, Manabe T, Murata K, Matsuo R, Inoue T. Spontaneous regression of hepatocellular carcinoma: report of a case. Surg Today. 2005;35:1081–1086. doi: 10.1007/s00595-005-3066-8. [DOI] [PubMed] [Google Scholar]
- 12.Meza-Junco J, Montaño-Loza AJ, Martinez-Benítez B, Cabrera-Aleksandrova T. Spontaneous partial regression of hepatocellular carcinoma in a cirrhotic patient. Ann Hepatol. 2007;6:66–69. [PubMed] [Google Scholar]
- 13.Arakawa Y, Mori H, Ikegami T, Hanaoka J, Kanamoto M, Kanemura H, Morine Y, Imura S, Shimada M. Hepatocellular carcinoma with spontaneous regression: report of the rare case. Hepatogastroenterology. 2008;55:1770–1772. [PubMed] [Google Scholar]
- 14.Storey RE, Huerta AL, Khan A, Laber DA. Spontaneous complete regression of hepatocellular carcinoma. Med Oncol. 2011;28:948–950. doi: 10.1007/s12032-010-9562-8. [DOI] [PubMed] [Google Scholar]
- 15.Mochizuki T, Takehara Y, Nishimura T, Takahashi M, Kaneko M. Regression of hepatocellular carcinoma. AJR Am J Roentgenol. 1991;156:868–869. doi: 10.2214/ajr.156.4.1848389. [DOI] [PubMed] [Google Scholar]
- 16.Imaoka S, Sasaki Y, Masutani S, Ishikawa O, Furukawa H, Kabuto T, Kameyama M, Ishiguro S, Hasegawa Y, Koyama H. Necrosis of hepatocellular carcinoma caused by spontaneously arising arterial thrombus. Hepatogastroenterology. 1994;41:359–362. [PubMed] [Google Scholar]
- 17.Stoelben E, Koch M, Hanke S, Lossnitzer A, Gaertner HJ, Schentke KU, Bunk A, Saeger HD. Spontaneous regression of hepatocellular carcinoma confirmed by surgical specimen: report of two cases and review of the literature. Langenbecks Arch Surg. 1998;383:447–452. doi: 10.1007/s004230050158. [DOI] [PubMed] [Google Scholar]
- 18.Uenishi T, Hirohashi K, Tanaka H, Ikebe T, Kinoshita H. Spontaneous regression of a large hepatocellular carcinoma with portal vein tumor thrombi: report of a case. Surg Today. 2000;30:82–85. doi: 10.1007/PL00010054. [DOI] [PubMed] [Google Scholar]
- 19.Blondon H, Fritsch L, Cherqui D. Two cases of spontaneous regression of multicentric hepatocellular carcinoma after intraperitoneal rupture: possible role of immune mechanisms. Eur J Gastroenterol Hepatol. 2004;16:1355–1359. doi: 10.1097/00042737-200412000-00020. [DOI] [PubMed] [Google Scholar]
- 20.Yano Y, Yamashita F, Kuwaki K, Fukumori K, Kato O, Kiyomatsu K, Sakai T, Yamamoto H, Yamasaki F, Ando E, Sata M. Partial spontaneous regression of hepatocellular carcinoma: a case with high concentrations of serum Lens culinaris agglutinin-reactive alpha fetoprotein. Kurume Med J. 2005;52:97–103. doi: 10.2739/kurumemedj.52.97. [DOI] [PubMed] [Google Scholar]
- 21.Park HS, Jang KY, Kim YK, Cho BH, Moon WS. Hepatocellular carcinoma with massive lymphoid infiltration: a regressing phenomenon? Pathol Res Pract. 2009;205:648–652. doi: 10.1016/j.prp.2009.01.001. [DOI] [PubMed] [Google Scholar]