Abstract
Sunitinib malate (SutentTM; Pfizer Inc., New York, N.Y., USA) is a small molecule kinase inhibitor with activity against a number of tyrosine kinase receptors, including vascular endothelial growth factor receptors, stem-cell factor receptor, and platelet-derived growth factor receptors alpha and beta. Sunitinib, registered for the treatment of renal cell carcinoma and gastrointestinal stromal tumors, has recently been approved for the treatment of patients with advanced pancreatic neuroendocrine tumors. Peripheral primitive neuroectodermal tumor (pPNET), paraganglioma (PGL) and epithelioid hemangioendothelioma (EHE) are rare tumors in which there is an overexpression of pro-angiogenic factors and in which a high intratumoral microvessel density is a significant poor prognostic factor. On the basis of this preclinical rationale and the lack of effective treatments in pre-treated advanced stages of these rare diseases, we report our interesting experience of pPNET, PGL and EHE treatment with sunitinib.
Key words: Sunitinib, Antiangiogenic therapy, Peripheral primitive neuroectodermal tumor, Paraganglioma, Epithelioid hemangioendothelioma
Introduction
Solid tumors require the development and expansion of a vascular network to support their growth; angiogenesis is a multistep, complex process by which new microvessels are formed from pre-existing vasculature. The formation of vasculature is regulated by several factors, including angiogenic factors such as vascular endothelial growth factor (VEGF), basic fibroblast growth factor, platelet-derived growth factor (PDGF) and non-angiogenic influences such as hypoxia, necrosis and metabolic rate of tumor [1].
Sunitinib malate (SutentTM; Pfizer Inc., New York, N.Y., USA) is an oral multitargeted tyrosine kinase inhibitor with activity against VEGF receptors (VEGFR 1, 2 and 3), stem-cell factor receptor, PDGF receptors (PDGFR alpha and beta), fetal liver tyrosine kinase receptor 3 and glial cell-line derived neurotrophic factor receptor, whose broad spectrum of targets may result in potential antitumor as well as antiangiogenic effects in tumors [2]. Sunitinib is currently approved for the treatment of metastatic renal cell carcinoma, imatinib-resistant gastrointestinal stromal tumors and, recently, for the treatment of advanced well-differentiated pancreatic neuroendocrine tumors [3, 4].
Ewing's sarcoma, peripheral primitive neuroectodermal tumors (pPNET) and Askin tumor of the chest wall belong to the Ewing's sarcoma/PNET category of tumors. Ewing's sarcoma/PNET is frequently of osseous origin, but in 10% of cases it arises in extra skeletal soft tissues. Treatment paradigms involve combinations of chemotherapy, surgery and sometimes radiation. Treatment of metastatic disease remains unsatisfactory: patients with recurrent disease also fare poorly, particularly if disease recurs early [5].
Paragangliomas (PGLs) are rare tumors that arise from neural crest-derived paraganglia. Approximately 20% of PGLs are malignant and surgical resection, when possible, is the only potentially curative treatment. Most of these tumors are sporadic, but 25% of cases occur as part of a familial syndrome such as von Hippel-Lindau disease (VHL), multiple endocrine neoplasia type 2, hereditary paraganglioma/pheochromocytoma syndromes (succinate dehydrogenase, SDH, subunit B, C or D), neurofibromatosis type 1 and Carney's syndrome. The percentage of malignant PGLs is higher if related to SDH B (SDH-B) gene mutations. Genetic investigation is, therefore, mandatory in all patients with paraganglioma, because specific genetic mutations are correlated with tumor aggressiveness. The most widely therapeutic approaches utilized are 131I-metaiodobenzylguanidine (MIBG) or a combination of cyclophosphamide, vincristine and dacarbazine, with comparable rates of response and toxicity [6], but no standard of care is established for patients with metastatic PGLs.
Epithelioid hemangioendothelioma (EHE) is a rare malignant soft-tissue tumor of vascular origin, found most frequently in extremities, bone, liver or lung, usually with low mitotic activity; however, in roughly 30% of cases, it is associated with more aggressive clinical behavior. Surgery is the most common form of treatment, but clinical outcome is variable and unpredictable. The few data in the literature seem to indicate a lack of effectiveness of chemotherapy in EHE treatment [7].
In recent years, an interesting field of biomolecular and clinical research has highlighted the importance of angiogenesis, microvessel density, vascular supply and the crucial role of VEGF and VEGFRs, in each of these three rare diseases [8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18]. Collectively, these findings indicate a potential therapeutic role for targeting components of VEGF and its downstream targets in these malignancies. We report here our experience with anti-VEGFR tyrosine kinase inhibitor sunitinib in the treatment of pPNET, PGL and EHE.
Case Report 1: pPNET
A 46-year-old man, with no significant past medical history, presented at our hospital for right flank and testis pain, associated with dysuria. An ultrasound scan showed an 18 × 15-cm abdominal mass and a computed tomography (CT) scan confirmed a large retroperitoneal patchy mass involving the right kidney; no further sites of disease were found. The patient underwent a surgical right nephro-surreno-ureterectomy with excision of the neoplastic mass. Histopathological examination revealed a morphologic and immunophenotypic oriented framework for an undifferentiated small cell tumor of Ewing's sarcoma/pPNET with high mitotic index, necrosis and vascular embolization. A margin of resection showed infiltration (R1 residual disease). Given his advanced stage, he was treated adjuvantly with 3 cycles of chemotherapy with ifosfamide and etoposide (IE regimen) alternating with vincristine, Adriamycin and cyclophosphamide (VAC regimen).
Meanwhile, genetic testing was performed and fluorescence in situ hybridization analysis, using the LSI EWSR1 break-apart probe, revealed the translocation 22q12 for the EWSR1 gene, translocation characteristic of Ewing's sarcoma/pPNET, confirming diagnosis. Five months later, after onset of right flank pain, a CT scan was performed and it showed recurrence of disease in the right paravertebral lymph nodes from D12 to L3. He received chemotherapy with ifosfamide, carboplatin and etoposide (ICE regimen) for 2 cycles followed by radiation on the prevertebral lesion (dose of 50 Gy in conventional daily fractions of 2 Gy). At the end of treatment, CT and 18F-FDG positron emission tomography (PET) scans showed a complete response in the irradiated site and progressive disease in 2 right paravertebral lymph nodes at D9–D10 level. The patient received 2 further cycles of chemotherapy with ICE regimen and radiotherapy on the paravertebral lesion D9–D10 (40 Gy in daily fractions of 8 Gy). The control CT scan showed a volume reduction of the lesion D9–D10 and the appearance of a solid new lesion with compression of the right-side profile of VI and VIII hepatic segments attributable to recurrent disease (fig. 1a).
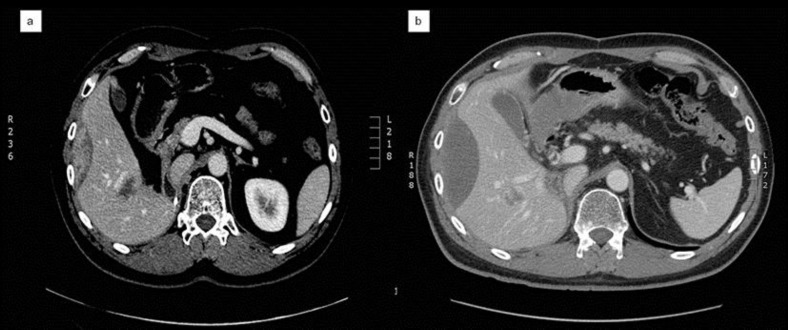
Fig. 1.
a CT scan shows a solid lesion with compression of the right-side profile of VI and VIII hepatic segments attributable to recurrent disease. b After 2 courses of sunitinib treatment, a CT scan documents a change in the consistency of the lesion, which appear predominantly fluid.
Considering the treatments already carried out, the patient started treatment with sunitinib at a standard schedule (50 mg/daily for 4 weeks on/2 weeks off). A CT scan performed after 2 courses of therapy showed a partial response: the lesion remained along the side profile of the right lobe, but there was no longer a recognizable solid component, the alteration was predominantly fluid (fig. 1b). He continued on treatment for a further 4 cycles, showing stable disease after the fourth cycle. He experienced moderate toxicity: grade 2 fatigue, weight loss and dysgeusia. After the sixth course, the control CT scan showed disease progression in the mediastinal and retroperitoneal lymph nodes. Treatment with sunitinib was discontinued and the patient started continuous infusion high-dose ifosfamide for 14 days every 28 days, which is still ongoing.
Case Report 2: PGL
A previously healthy asymptomatic 35-year-old woman underwent surgery for a retroperitoneal mass incidentally found at an abdominal ultrasound performed due to a rise in transaminases. The histological examination revealed the mass to be a PGL with intravascular dissemination. The patient reported a family history negative for neoplastic diseases. Six years later, recurrence of the retroperitoneal lesion was found. Serum and urinary catecholamines were normal. The patient underwent left adrenalectomy and para-aortic lymphadenectomy. After 9 months, an 18F-FDG PET total-body scan showed several metastatic bone lesions and thyroid uptake, while a 123I-MIBG showed only 2 rib lesions. Fine-needle aspiration of the thyroid revealed papillary thyroid carcinoma; therefore, a total thyroidectomy was performed. Simultaneously, genetic tests were performed: DNA sequencing showed an SDH-B mutation, characteristic of familial PGL syndrome 4. Given the low positivity 123I-MIBG imaging, the patient was treated with 6 cycles of chemotherapy with cyclophosphamide, vincristine and dacarbazine, obtaining a partial response.
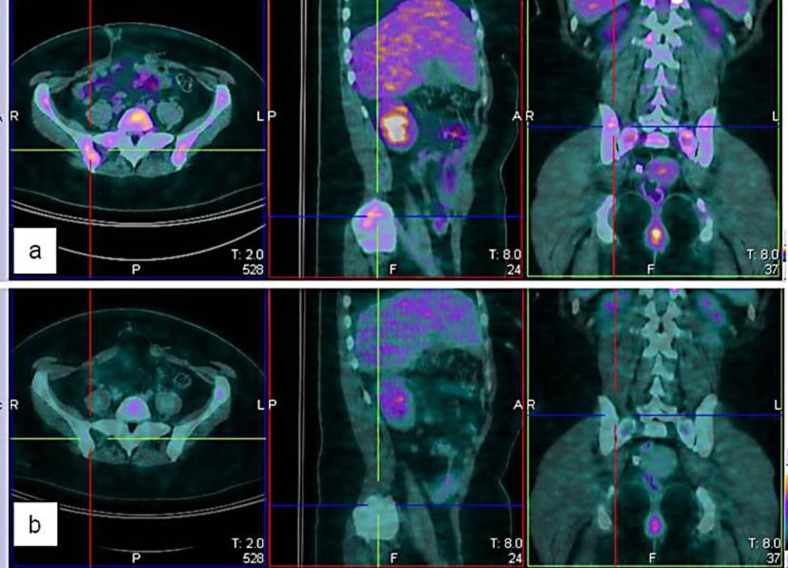
Six months later the patient presented with pelvic pain. An 18F-FDG PET scan revealed new bony lesions (fig. 2a), and treatment with sunitinib was initiated. A standard schedule of 50 mg daily for 4 weeks on/2 weeks off was adopted. After the first cycle, the dose was reduced to 37.5 mg daily and immediately after to 25 mg daily (2 weeks on/1 week off) because the patient developed uncontrolled hypertension as a side effect. Pelvic pain, however, completely disappeared. A PET scan performed 3 months later revealed a partial response (fig. 2b); therefore, the treatment was continued for a further 6 months with stable disease. Subsequent PET scans showed disease progression and the patient started an alternative and still ongoing treatment with temozolomide.
Fig. 2.
a PET scan pretreatment showing the appearance of a new right iliac bone lesion. b PET scan after treatment documents response to sunitinib.
Case Report 3: EHE
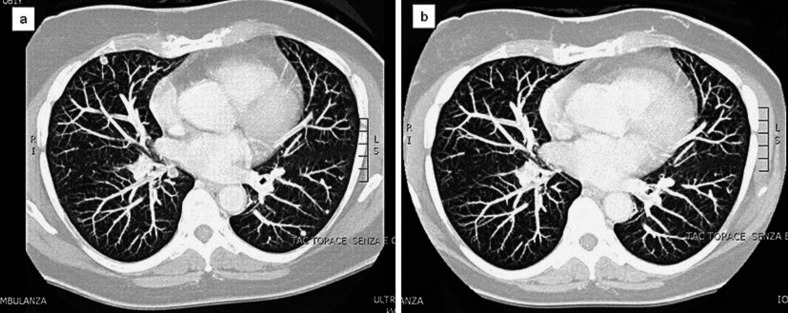
A 72-year-old woman, with a previous history of type 2 diabetes, presented at our hospital with left cervicobrachialgia. Magnetic resonance imaging revealed an intraforaminal solid mass, extending from C7 to D1; furthermore, a chest CT scan showed several bilateral pulmonary nodules. The patient therefore underwent C7 decompressive laminectomy, and the tumor was histologically diagnosed as an EHE. The patient was treated with radiotherapy (45 Gy in daily fractions of 1.5 Gy) on the lesion at the level of C6–C7, followed by chemotherapy with weekly paclitaxel for 12 weeks. After this treatment, a reevaluation CT scan showed clear disease progression, involving lung (fig. 3a), mediastinal lymph nodes and bone (C7; D1–D4). It was decided to start treatment with sunitinib, at a dose of 50/25 mg on alternate days along with zoledronic acid, for 4 weeks on/2 weeks off. After the first 3 weeks, the treatment was interrupted by the appearance of pleural and pericardial effusion, and then re-started at a dose of 25 mg daily continuously. After 2 months of therapy, a chest CT scan showed a partial response with the disappearance of some pulmonary nodules and a reduction in size of some others (fig. 3b). Sunitinib treatment was continued and the patient reported a further response following the CT scan after 4 months and disease stabilization after 7 months. The last CT scan, performed after the tenth month, confirmed stable disease and the patient is continuing treatment with an acceptable degree of tolerance (grade 1 hand-foot syndrome, grade 1 mucositis and grade 1 hypertension).
Fig. 3.
a CT scan shows bilateral lung metastasis. b After 2 months of sunitinib treatment, a CT scan documents a partial response to treatment with the disappearance of some pulmonary nodules.
Discussion
Angiogenesis plays a crucial and necessary role in the pathogenesis of all solid tumors; among many molecules and pathways that regulate this complex process, VEGF and VEGFRs are the main players.
Ewing's sarcoma family tumors (ESFT) have multiple mechanisms by which they develop and maintain their vascular supply. In recent insights into the molecular regulation of vascular development in these tumors, chemotactic ability of VEGF to recruit bone marrow cells and its direct stimulation on local angiogenesis processes were considered crucial [8]. These data and the evidence, in preclinical models, that blocking VEGFR-2 leads to suppression of Ewing's sarcoma tumor growth and tumor vessel formation [9], indicate the VEGF pathway is an important therapeutic target. Also, the translocation t(11;22) is common in ESFT and produces the chimeric EWS/Fli-1 fusion gene found in 85% of patients with ESFT. EWS/Fli-1 is thought to be a transcriptional factor activating the VEGF promoter [10], given that initial clinical data support the hypothesis that the VEGF pathway actually may be a target in these tumors [reviewed by DuBois et al. 11]. Our pPNET patient treated with sunitinib, carrying the translocation 22q12 for the EWSR1 gene, showed a significant radiological response with the almost complete necrosis of the perihepatic metastatic lesion (a typical radiological response of some targeted agents) and a progression-free survival of 9 months. These results are very interesting, considering that the patient was heavily pretreated.
Regarding PGLs, as already mentioned, 25% of cases occur as part of a familial syndrome. When associated with a hereditary cancer syndrome, such as VHL or hereditary PGL/pheochromocytoma syndromes (SDH subunit B, C or D), PGLs have been shown to overexpress high levels of hypoxia-inducible factor 1 alpha, which is mainly responsible for inducing VEGF in hypoxic conditions and, consequently, high levels of VEGF and a high level of microvascularity [12, 13]. This state of pseudo-hypoxia is functionally analogous to defects in the VHL protein in renal cell carcinoma, where antiangiogenic therapy and sunitinib are effective. As proof of this, Joshua et al. [14] treated 3 PGL patients with sunitinib, reporting important and long-term responses, inducing them to design a single-arm phase II study with sunitinib in pheochromocytoma/PGL pts. Similarly our patient, with SDH-B mutation, reported a clinical benefit with pain disappearance, an evident PET response and a progression-free survival of 36 weeks, confirming this treatment as very interesting in this setting of patients. Attention must be paid, however, to the possible exacerbation of hypertension.
Given the vascularity and endothelial origin of EHE, it is likely that angiogenic factors are highly expressed in this tumor. Recently, overexpression of VEGF, VEGFR-2 and VEGFR-3 has been observed in pulmonary EHE samples [15]. In a small study, Emamaullee et al. [16] investigated the expression of VEGF by means of immunohistochemical assessment in 6 samples from hepatic EHE patients: VEGF expression was present in 100% of the specimens examined. These data suggest that the VEGF-VEGFR pathway may also be dysregulated in EHE, and anti-VEGF therapies with new agents such as bevacizumab, thalidomide, sorafenib and sunitinib represent promising therapeutic approaches for EHE in early clinical data [17, 18]. Despite the initial side effects reported at the standard dose, in our patient treated with sunitinib at the dose of 25 mg daily continuously, we observed a reduction in the number and size of lung metastases and a disease-free survival of 11 months to date. In accordance with literature case reports, our results also confirm that anti-VEGFR treatment in this rare disease is an approach of considerable interest.
In conclusion, early clinical data suggest that antiangiogenic and antivascular strategies might be beneficial in the treatment of patients with pPNET, PGL and EHE. In our report, sunitinib appears to be a promising treatment for these malignancies. All 3 patients signed an informed consent form clearly outlining the off-label nature of the sunitinib treatment. Additional studies are urgently needed to expand and confirm these findings, to further evaluate the role of an angiogenesis biomarker, to identify predictors of response and to determine how to optimize these strategies in the management of each of these tumors, although this will be difficult in the short time, due to the rarity of these diseases.
Disclosure Statement
None.
References
- 1.De Bock K, Cauwenberghs S, Carmeliet P. Vessel abnormalization: another hallmark of cancer? Molecular mechanisms and therapeutic implications. Curr Opin Genet Dev. 2011;21:73–79. doi: 10.1016/j.gde.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 2.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–337. [PubMed] [Google Scholar]
- 3.Rock EP, Goodman V, Jiang JX, Mahjoob K, Verbois SL, Morse D, et al. Food and Drug Administration drug approval summary: sunitinib malate for the treatment of gastrointestinal stromal tumor and advanced renal cell carcinoma. Oncologist. 2007;12:107–113. doi: 10.1634/theoncologist.12-1-107. [DOI] [PubMed] [Google Scholar]
- 4.Raymond E, Dahan L, Raoul JL, Bang YJ, Borbath I, Lombard-Bohas C, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 5.Leavey PJ, Mascarenhas L, Marina N, Chen Z, Krailo M, Miser J, Children's Oncology Group et al. Prognostic factors for patients with Ewing sarcoma (EWS) at first recurrence following multi-modality therapy: a report from the Children's Oncology Group. Pediatr Blood Cancer. 2008;51:334–338. doi: 10.1002/pbc.21618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chrisoulidou A, Kaltsas G, Ilias I, Grossman AB. The diagnosis and management of malignant phaeochromocytoma and paraganglioma. Endocr Relat Cancer. 2007;14:569–585. doi: 10.1677/ERC-07-0074. [DOI] [PubMed] [Google Scholar]
- 7.Dei Tos AP, Wagner AJ, Modena P, Comandone A, Leyvraz S. Epithelioid soft tissue tumors. Semin Oncol. 2009;36:347–357. doi: 10.1053/j.seminoncol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Z, Reddy K, Guan H, Kleinerman ES. VEGF(165), but not VEGF(189), stimulates vasculogenesis and bone marrow cell migration into Ewing's sarcoma tumors in vivo. Mol Cancer Res. 2007;5:1125–1132. doi: 10.1158/1541-7786.MCR-07-0174. [DOI] [PubMed] [Google Scholar]
- 9.Zhou Z, Bolontrade MF, Reddy K, Duan X, Guan H, Yu L, et al. Suppression of Ewing's sarcoma tumor growth, tumor vessel formation, and vasculogenesis following anti vascular endothelial growth factor receptor-2 therapy. Clin Cancer Res. 2007;13:4867–4873. doi: 10.1158/1078-0432.CCR-07-0133. [DOI] [PubMed] [Google Scholar]
- 10.Fuchs B, Inwards CY, Janknecht R. Vascular endothelial growth factor expression is up-regulated by EWS-ETS oncoproteins and Sp1 and may represent an independent predictor of survival in Ewing's sarcoma. Clin Cancer Res. 2004;10:1344–1353. doi: 10.1158/1078-0432.ccr-03-0038. [DOI] [PubMed] [Google Scholar]
- 11.DuBois SG, Marina N, Glade-Bender J. Angiogenesis and vascular targeting in Ewing sarcoma: a review of preclinical and clinical data. Cancer. 2010;116:749–757. doi: 10.1002/cncr.24844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pollard PJ, Brière JJ, Alam NA, Barwell J, Barclay E, Wortham NC, et al. Accumulation of Krebs cycle intermediates and over-expression of HIF1alpha in tumours which result from germline FH and SDH mutations. Hum Mol Genet. 2005;14:2231–2239. doi: 10.1093/hmg/ddi227. [DOI] [PubMed] [Google Scholar]
- 13.Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7:77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 14.Joshua AM, Ezzat S, Asa SL, Evans A, Broom R, Freeman M, et al. Rationale and evidence for sunitinib in the treatment of malignant paraganglioma/pheochromocytoma. J Clin Endocrinol Metab. 2009;94:5–9. doi: 10.1210/jc.2008-1836. [DOI] [PubMed] [Google Scholar]
- 15.Stacher E, Gruber-Mösenbacher U, Halbwedl I, Dei Tos AP, Cavazza A, Papotti M, et al. The VEGF-system in primary pulmonary angiosarcomas and haemangioendotheliomas: new potential therapeutic targets? Lung Cancer. 2009;65:49–55. doi: 10.1016/j.lungcan.2008.10.031. [DOI] [PubMed] [Google Scholar]
- 16.Emamaullee JA, Edgar R, Toso C, Thiesen A, Bain V, Bigam D, et al. Vascular endothelial growth factor expression in hepatic epithelioid hemangioendothelioma: implications for treatment and surgical management. Liver Transpl. 2010;16:191–197. doi: 10.1002/lt.21964. [DOI] [PubMed] [Google Scholar]
- 17.Park MS, Ravi V, Araujo DM. Inhibiting the VEGF-VEGFR pathway in angiosarcoma, epithelioid hemangioendothelioma, and hemangiopericytoma/solitary fibrous tumor. Curr Opin Oncol. 2010;22:351–355. doi: 10.1097/CCO.0b013e32833aaad4. [DOI] [PubMed] [Google Scholar]
- 18.Salech F, Valderrama S, Nervi B, Rodriguez JC, Oksenberg D, Koch A, et al. Thalidomide for the treatment of metastatic hepatic epithelioid hemangioendothelioma: a case report with a long term follow-up. Ann Hepatol. 2011;10:99–102. [PubMed] [Google Scholar]