Abstract
Increased susceptibility to energy imbalance and anorexia in old age are risk factors for malnutrition during aging, but the underlying mechanisms are not well understood. Here, we explored changes in taste-guided hedonic value (“liking”) and motivation to obtain (“wanting”) palatable foods as potential mediators of age-associated anorexia and weight loss in old Fischer-344 rats. “Liking” as measured by the number of positive hedonic orofacial responses to sucrose and corn oil was not different in old compared with young rats. Taste-guided, low effort “wanting” as measured by the number of licks per 10 seconds was also not different, although old rats exhibited a slight oromotor impairment as revealed by significantly increased interlick intervals. Medium effort “wanting” as measured by performance in the incentive runway was significantly decreased in old versus young rats. Although decreased net running speed was partially accountable, significantly increased duration of distractions suggested additional deficits in motivation and/or reinforcement learning. Together with early satiation on corn oil but not sucrose in aged rats, these changes are likely to have resulted in the significantly greater sucrose preference of old rats in 12-hour tests, and may ultimately lead to reduced energy intake and weight loss.
Keywords: Aging, Anorexia, Palatability, Appetite, Gustation, Reward, Wanting, Licking, Learning
1. Introduction
Age-associated dysregulation of energy balance and anorexia may be 1 of the risk factors for malnutrition during aging (Morley, 1997; Roberts and Rosenberg, 2006). The physiological causes of anorexia of aging are largely unknown and likely multifactorial, involving both peripheral and central mechanisms, such as reduced sensory function, alterations in the hedonic qualities of food, increased gastrointestinal satiation signals, reduced metabolic rate and physical activity, changes in hormonal status, and alterations in central neurotransmitter control over hunger (Chapman, 2004; Morley, 1997). There are also changes in patterns of dietary intake and a reduction in the variety of food consumed in old age that are thought to further reduce energy intake (Fanelli and Stevenhagen, 1985; Roberts et al., 2005). Of particular interest is the fact that elderly individuals frequently complain of a loss of appetite (Morley and Silver, 1988; Olsen-Noll and Bosworth, 1989). Decreased appetite may be associated with a decrease in the pleasantness of food, which is indicative of inappropriate neural signaling within food-reward pathways.
Berridge and Robinson (2003) have outlined the potential psychological components that constitute the reward mechanisms in learning, liking, and wanting, corresponding to partially distinguishable neural mechanisms. With aging, alterations in gustatory evaluation, preference conditioning, appetitive motivation, or any combination may occur. There are data showing a decline in the hedonic qualities of food with aging in humans. This appears to result more from age-associated alterations in olfaction than alterations in taste (for review see Morley, 1997; Roberts and Rosenberg, 2006). Fewer data are available on changes in pleasantness of food with aging (de Graaf et al., 1994; de Jong et al., 1996). Although anecdotal, behavioral, and biochemical evidence suggest that the neural substrate for motivation might deteriorate in old age (for review see Dowling et al., 2008). These alterations can result in altered food preferences, which may reduce the quality and quantity of nutrients ingested.
In humans, age-related decline in food intake has been related to social, psychological, and medical causes. Control of appetite has an important psychological component, where food-reward pathways are modulated by cortical inputs derived from environment factors. This makes it difficult to distinguish between the effects of the environment and physiological factors implicated in age-associated decreases in food intake, mainly in those related to reward for food. Animal models of aging also reflect the decline in food intake and the deregulation of energy balance observed in humans both spontaneously and in response to disturbances in feeding (Gruenewald and Marck, 1996; Mattison et al., 2005; Toshinai et al., 2007; Wolden-Hanson, 2006).
In both humans (De Castro, 1993) and animals (Veyrat-Durebex and Alliot, 1997), the decrease in energy intake with aging is predominantly due to a decrease in fat calories with a small increase in the percent of calories ingested as carbohydrates (Islam et al., 1993; Whichelow and Prevost, 1996). These changes in macronutrient preference are associated with changes in metabolic requirements, but the possible implication of reward mechanism in this change, like changes of the hedonic impact of different nutrients with aging, is unknown. Other mechanisms that may have a significant impact on appetite and food intake in older people are physiological changes in gastrointestinal function (Parker and Chapman, 2004).
The present study was designed to evaluate the possible alterations in pleasantness and reward mechanisms for food in aged rats, which could reflect possible alterations in neural signaling within food reward pathways, and their potential relationship with age-associated decrease in food intake. Using several behavioral test paradigms to measure specific aspects of reward, including brief access lick behavior, orofacial taste reactivity as a measure for core “liking”, and incentive runway behavior as a measure for “wanting”, the relationship between age and food motivated behavior was assessed. In addition, micro- and macrostructure of licking sucrose solutions and corn oil emulsions was assessed in drinking-to-satiation tests and preference in 12-hour choice tests.
2. Methods
2.1. Animals
Young (3-month-old, n = 10) and old (18-month-old, n = 10) male Fischer-344 rats (F344; Harlan Industries, Indianapolis, IN, USA) were housed individually in stainless steel cages with a wire mesh floor in a climate-controlled room (22 ± 2 °C) on a 12:12-hour light-dark cycle with lights on at 7:00 am. Food and water were available ad libitum except as specified below. Animals were fed a standard rodent diet (5001 Laboratory Rodent Diet, Lab-Diet, St. Louis, MO, USA; fat, 13.5 kcal%, carbohydrate, 58 kcal%; protein, 28.5 kcal%) as pellets.
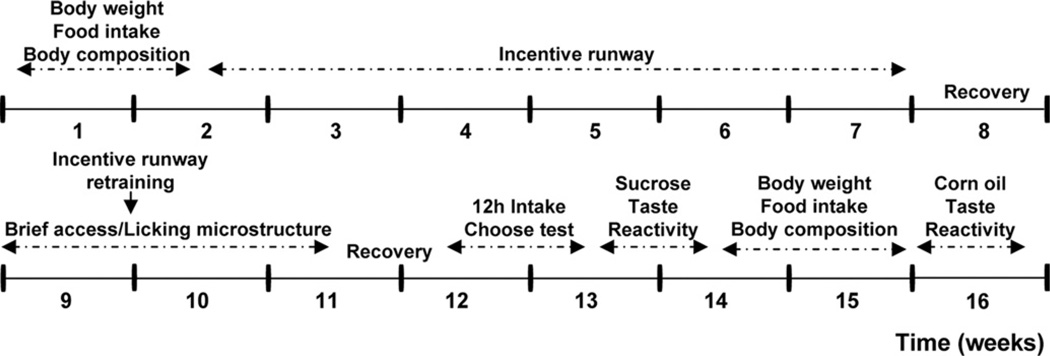
The work was carried out over 16 weeks as outlined in Fig. 1; thus, at the end of the experiments the animals were 7 and 22 months old. Health status of the old animals was monitored throughout the experimental period by checking posture, mobility, tooth length, food intake, and body weight. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center, and conformed to the guidelines of the National Institutes of Health.
Fig. 1.
Temporal sequence of measurements and test procedures.
2.2. Measurement of food intake, body weight, and body composition
Food intake was measured for either 12 or 24 hours. Chow intake was measured by weighing the food jars at the beginning and the end of the 12 or 24 hour periods. Spillage was subtracted from the intake. For each animal, body weight and chow intake were calculated as the average of 3 independent measurements.
Body composition of animals (% fat mass, fluid, and lean mass) was measured by using a Minispec LF 90 NMR Analyzer (Bruker BioSpin Corporation, The Woodlands, TX, USA). This method uses whole body magnetic resonance relaxometry in unanesthetized rodents with excellent linearity and reproducibility (Kunnecke et al., 2004).
2.3. Two- and 3-choice preference tests
Sucrose versus chow preference and corn oil versus chow preference were assessed in a 2-choice preference test and sucrose versus chow, versus corn oil in a 3-choice preference test. All solutions were prepared fresh daily. Sucrose (Sigma, St. Louis, MO, USA) was dissolved in deionized water. Corn oil emulsions (Albertsons, Inc., Boise, ID, USA) were prepared in deionized water (% wt/mL) and stabilized with 2% sodium stearoyl lactylate (Emplex, American Ingredients, Grandview, MO, USA) and 0.18% xanthan gum (ICN Biomedicals, Inc., Aurora, OH, USA). Preferences were assessed in alternate 12-hour dark periods. First, rats were adapted to drinking from 2 bottles, 1 containing deionized water and the other containing 8% corn oil for 2 nights. The 2-choice preference tests were conducted over the next 2 alternate nights. Animals were randomly divided in 2 groups, 1 group was first exposed to sucrose and the other to corn oil, and the flavors were changed the second night. Animals were provided access to 1 bottle containing deionized water and the other containing the test solution (0.5 M sucrose or 8% corn oil, identified in the brief-access lick test as maximal or submaximal preferred concentrations) and chow was provided ad libitum.
Two days later the 3-choice preference test was administered where the rats were given access to 1 bottle containing 0.5 M sucrose, the other containing 8% corn oil, and chow. Bottles and food jars were weighed at the beginning and the end of the 12-hour test period. Food intake was expressed as kcal per 100 g body weight after correction for the density and concentration of each solution. Preference percentages were calculated by the equation [(kcal ingested of the test solution/total kcal consumed) × 100].
2.4. Lick to satiation tests
Licking behavior of 8% corn oil emulsion and 0.5 M sucrose solution was assessed in an MS-160 Davis Rig lickometer (DiLog Instruments, Tallahasse, FL, USA) (Smith et al., 2001). Each solution was tested on the day after brief-access tests for the same solution after 15–19 hours of food deprivation. Bottles were weighed at the beginning and the end of the experiment. Ingestion rates were expressed as mL per minute and as cumulative kcal after correction for the density and concentration of each solution. The sucrose and corn oil concentrations were those identified in the brief-access lick test as maximal or submaximal preferred concentrations with similar caloric value (0.5 M sucrose: 0.706 kcal/g; 8% corn oil: 0.648 kcal/g). Bursts were defined as groups of licks separated by <500 ms. Lick efficiency was calculated by dividing the total amount of sucrose or corn oil ingested by the total number of licks emitted during that time.
2.5. Brief-access tests
Rats were first trained to lick from the spout (Smith et al., 2001). To avoid excessive weight loss, they were water deprived overnight only every third day. On test days, nondeprived animals were presented with increasing or decreasing concentrations of either sucrose (0, 0.0078, 0.0312, 0.0625, 0.125, 0.5, and 1 M in distilled water) or corn oil (0.0, 0.063, 0.25, 1, 4, 8, and 16%, in 1% Emplex emulsifier and distilled water) in counterbalanced order. Each concentration was available for 10 seconds with 5-second intervals in 1 ascending and 1 descending series in counterbalanced order, and the number of licks per 10 seconds was averaged for each concentration.
To control for differences in intrinsic lick rate, individual response rates were transformed into standardized lick ratios (SLR) by dividing the mean number of licks per trial for a given stimulus by the maximum number of licks that the same rat could potentially emit across the 10-second trial as described by Glendinning et al. (2002). Interlick intervals (ILIs) <70 ms and >250 ms were excluded from the assessment of frequency distribution of ILI, mean ILI, and total licks. ILIs >250 ms are thought to represent either short (e.g., missed licks) or longer (e.g., deprivation state) pauses between bursts.
2.6. Taste reactivity test (“liking”)
A voluntary drinking procedure was used to assess taste reactivity to sucrose or corn oil in order to avoid potential confounding due to age differences between the 2 age groups in reaction to stress from oral cannula implantation, similar to several previous studies (Gray and Cooper, 1995; Pecina et al., 2003). Prior to the evaluation of taste reactivity, overnight food and water deprivation was instituted to ensure that all animals drank sucrose freely when placed on the floor of the test cage. Positive affective orofacial reactions were elicited from each rat by 2 sucrose solutions (0.1 and 1 M) presented in counterbalanced order on trials spaced 48 hours apart; or corn oil solutions (0.8 and 8%) presented to the animals in the same way 15 days later than the sucrose test.
For taste reactivity testing, each rat was placed in a plastic cylindrical chamber (diameter, 25 cm) with a transparent floor and walls. A mirror positioned beneath the transparent floor reflected a view of the rat’s face and mouth into a close-up lens of a video camera, which videotaped spontaneous affective facial and body reactions that occurred during and after voluntary intake of sucrose. Rats were habituated to the testing environment for 2 days before the beginning of the testing. On each test day, rats were habituated to the testing chamber for 15 minutes before testing. To prevent taste reactivity data from being biased by interrat variation in intake, 3 bouts of a 300 µL sucrose (0.1 or 1 M) or corn oil (0.8 or 8%) solution, that were completely ingested by the animals, were placed on the floor with a 30-second intertrial interval, during which behavior was videotaped for subsequent analysis. The results were expressed as the average of the hedonic reactions obtained in the 3 bouts. The use of 2 different concentrations, varying by an order of magnitude (0.1 and 1 M), allowed assessment of whether taste reactivity measures were sensitive to palatability “liking” differences for rats by comparing affective reactions to markedly different taste levels.
Behavioral analysis of positive (rhythmic midline tongue protrusions, lateral tongue protrusion, and paw licking) and negative (gapes, headshakes, forelimb flails, face washing, and chin rubs) affective reactions was accomplished by viewing the recorded video in slow motion (1/30 of actual speed) following procedures described previously (Berridge, 2000). As measures of general locomotor activity and exploration, we also measured rearing and locomotion as discrete single actions; and grooming scored in 5-second bins.
2.7. Incentive runway test (“wanting”)
The test apparatus consisted of 3 compartments: a start box (20 × 16 × 45 cm), a runway (160 × 16 × 45 cm), and a goal box (21 × 23 × 45). Both start and goal boxes were separated from the alley by retractable doors. The start box could be moved anywhere along the alley. A piece (180–200 mg) of sweet breakfast cereal (Froot Loops; Kellog, Co., Battle Creek, MI, USA) was placed on the floor at the distal end of the goal box. To extinguish any neophobia, rats were habituated to Froot Loops for 2 days in their home cage before training. A video tracking system (Viewpoint Lifesciences, Inc., Montreal, QC, Canada) recorded the movement of the rats within the whole apparatus, and recorded the latency to eat the reward for subsequent analysis.
To avoid excessive impact of food deprivation in old rats, animals were food deprived and tested on alternate days, or sessions, and were partially food deprived in the dark period. Tests were conducted from 8 am to 2 pm resulting in the animals being food deprived for 8–14 hours. Three habituation sessions occurred prior to the training sessions. During the habituation rats were simply placed directly in the closed goal box and allowed to eat the reward for 5 minutes. The following training sessions consisted of 2 trials per day and an intertrial interval of 45–60 minutes. For each trial, the rat was placed into the start box. After 30 seconds the start box door was opened, and the rat was allowed to run down the runway to obtain the food reward. Once in the goal box, the goal box door was closed, and the rat was allowed to consume the reward for 30 seconds before being retrieved. On training sessions 1 and 2, the start box door was placed at 32 cm from the goal box door; on sessions 3–4 the start box was moved to 64 cm, to 98 cm on sessions 5–6, to 128 cm on sessions 7–8, and to 160 cm on sessions 9–14. If the rat did not reach the goal box before the end of the trial, it was gently pushed to the goal box by the experimenter, and the latency was not recorded.
Incentive runway behavior was recorded for subsequent analysis of: (1) latency to leave the start box; (2) latency to reach the goal box; (3) number and duration of pauses in the runway; (4) reversals of direction in the runway (stop in forward locomotion followed by a return toward the start box of a distance equivalent at least the rat’s body length); (5) motor running speed (completion speed minus all pauses and reversals); and (6) latency to began eating the reward once the rats reach goal box.
2.8. Retention test
Fifteen days after the last training session, an additional retention session was conducted consisting of 5 trials with start box located 160 cm from the goal box.
2.9. Data analysis
Body weight, body composition, intake, and preference data were analyzed by Student t test. The rests of the experiments were analyzed using 2-factor repeated measures analysis of variance (ANOVA). Significant main effects were followed-up with multiple comparisons using Tukey’s post-hoc test.
3. Results
3.1. Food intake, body weight, and body composition
Chow intake expressed in g/day was not different between young and old rats both at the beginning and end of the study (Table 1). However, because old rats weighed significantly more, chow intake corrected for body weight (t(18) = 5.71; p < 0.001) or lean mass (t(18) = 5.30, p < 0.001) was significantly decreased in aged rats compared with young rats at the beginning of the study. As rats continued growing with a high rate from young adult (3 months) to middle age (7 months) the body weight difference narrowed at the end of the study, corrected food intake was no longer significantly different (Table 1).
Table 1.
Food intake, body weight, and body composition of rats at beginning and end of study
| Beginning of the experiments | End of the experiments | |||
|---|---|---|---|---|
| Young | Old | Young | Old | |
| Age (months) | 3 | 18 | 7 | 22 |
| Body weight (g) | 302 ± 4.35 | 427 ± 2.94 a | 365 ± 5.30 b | 410 ± 4.26 a,c |
| Chow intake (kcal/day) | 53.56 ± 2.21 | 54.08 ± 2.02 | 48.93 ± 1.7 | 52.38 ± 3.4 |
| Chow intake (kcal/day/100 g body weight) | 17.70 ± 0.65 | 12.66 ± 0.47 a | 13.86 ± 0.43 b | 13.24 ± 0.73 |
| Chow intake (g/day/lean mass) | 0.26 ± 0.01 | 0.19 ± 0.01 a | 0.20 ± 0.01 b | 0.19 ± 0.01 |
| Fat mass (g) | 67.43 ± 2.46 | 100.40 ± 2.36 a | 89.37 ± 0.73 c | 93.34 ± 1.49 d,e |
| Adiposity (%) | 22.64 ± 0.48 | 23.07 ± 0.43 | 24.47 ± 0.37 c | 22.67 ± 0.57 d |
| Lean mass (g) | 203.8 ± 3.19 | 283.9 ± 1.71 a | 240.6 ± 4.13 b | 274.8 ± 2.70 a,c |
| % Lean | 68.68 ± 0.47 | 65.29 ± 0.42 a | 65.89 ± 0.33 b | 65.78 ± 0.65 |
| Fat free mass (g) | 229.5 ± 3.68 | 334.5 ± 1.97 a | 275.9 ± 4.86 b | 323.2 ± 3.83 a,e |
Results are shown as mean ± standard error of the mean (SEM) (old, n = 6–10; young = 8–10).
p < 0.001 old versus young rats based on Student t test.
p < 0.001 versus the same aged group at the beginning of the experiments based on Student t test.
p < 0.01 versus the same aged group at the beginning of the experiments based on Student t test.
p < 0.05 old versus young rats based on Student t test.
p < 0.05 versus the same aged group at the beginning of the experiments based on Student t test.
While the young rats gained 63 g (t(18) = 8.07, p < 0.001) of body weight, the old rats lost 17 g (t(15) = 3.43, p < 0.01) over the 4-month study duration (Table 1). Body weight gain in young rats was due to both increased fat (t(38) = 11.67, p < 0.001) and lean mass (t(18) = 7.06, p < 0.001), and body weight loss in aged rats was due to decreased fat (t(28) = 2.64, p < 0.05) and lean mass (t(14) = 3.00, p < 0.01). Adiposity (percent fat mass) was not different between young and old rats at the beginning of the study, but was slightly but significantly lower (t(14) = 2.77, p < 0.05) in old rats at the end of the study (Table 1).
3.2. Food acceptance and food preference
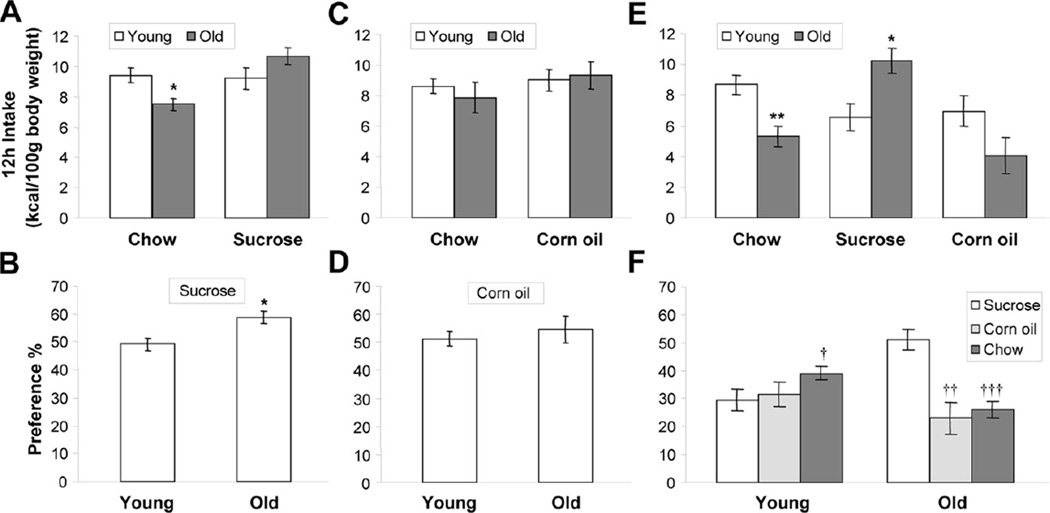
When given a choice between chow and 0.5 M sucrose for 12 hours during the dark period, aged rats ingested significantly less energy per body weight from chow (t(12) = 2.87, p < 0.01) and slightly more from sucrose (t(12) = 1.58, not significant [n.s.]), compared with young rats (Fig. 2A), resulting in a significantly greater sucrose versus chow preference in aged rats (t(12) = 2.91, p > 0.05) (Fig. 2B). When given a choice between chow and 8% corn oil, no significant differences in intake or preference were observed between young and old rats (Fig. 2C and D).
Fig. 2.
Choice tests in young and aged Fischer rats. Body weight-adjusted 12-hours dark period intake of chow and 0.5 M sucrose (A); chow and 8% corn oil (C) in 2-choice tests as well as chow, 0.5 M sucrose and 8% corn oil in a 3-choice test (E). Relative preference for 0.5 M sucrose (B) and 8% corn oil versus chow (D); and 0.5 M sucrose or 8% corn oil versus chow (F). Values represent means ± standard error of the mean (SEM) (n = 6–8 animals per age). * p < 0.05; ** p < 0.01 versus young. †, p < 0.05; ††, p < 0.01 versus sucrose.
When given a choice of all 3 foods, old rats ingested considerably less energy from chow (t(11) = 3.78, p < 0.01), but more from sucrose (t(11) = 3.05, p < 0.05), compared with young rats (Fig. 2E). They also reduced intake of corn oil, but the difference was not statistically significant (t(10) = 1.55, n.s.). Under this 3-choice condition, old rats highly preferred sucrose over both corn oil (t(8) = 4.11, p < 0.01) and chow (t(10) = 5.25; p < 0.001), while the young rats preferred chow (t(14) = 2.17; p < 0.05), but not corn oil (t(14) = 0.36; n.s.) over sucrose (Fig. 2F).
3.3. Lick to satiation tests
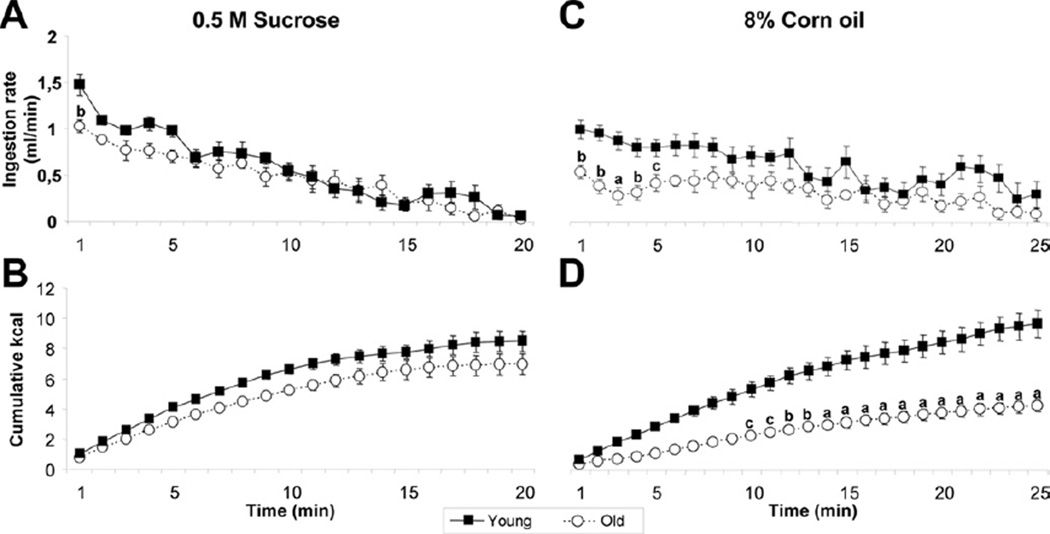
Analysis of lick structure during access to palatable sucrose solutions or corn oil emulsions provides information about the interaction between taste-guided hedonic drive and increasing postingestive satiation signals. Lick (ingestion) rate early during the test mainly indicates positive feedback from taste input, while declining ingestion rate and deceleration of the cumulative intake curve reflects the action of satiety signaling. Because lick efficiency (volume/lick) was reduced in aged rats for both sucrose (young: 5.03 ± 0.25; old: 4.36 ± 0.19; t(13) = 2.14, p = 0.052) and corn oil (young: 4.32 ± 0.32; old: 3.49 ± 0.08; t(13) = 2.37; p < 0.05), ingestion rate was corrected for this lower lick volume.
Ingestion rate for 8% corn oil was reduced in aged compared with young rats (F(1,11) = 21.38 p < 0.001); a similar decrease for 0.5 M sucrose, however, was not statistically significant (F(1,13) = 2.89, n.s.) (Fig. 3A and C). In the case of sucrose, only first-minute ingestion rate was significantly reduced (young: 270.4 ± 3.26 licks; old: 235.0 ± 10.14 licks; t(13) = 3.51, p < 0.01). In the case of corn oil, ingestion rate for each of the first 5 minutes was reduced (first minute, young: 229.0 ± 13.72 licks; old: 159.4 ± 18.44 licks; t(13) = 3.08, p < 0.01). These results indicate decreased positive feedback from taste input, particularly for corn oil.
Fig. 3.
Sucrose and corn oil lick macrostructure in young and old rats. Mean minute-by-minute ingestion rate (mL/min) for rats ingesting 0.5 M sucrose (A) or 8% corn oil (C). Same results expressed as cumulative kcal ingested along the meal for sucrose (B) and corn oil (D). Data represent the mean ± standard error of the mean (SEM) (n = 7–8). a, p < 0.001; b, p < 0.01; c, p < 0.05 versus young.
For sucrose, the monotonic decrease of ingestion rate over the 20-minute test period was similar in aged and young rats (time × age: F(19,247) = 1.73; n.s.) (Fig. 3A). The total cumulative intake was also not significantly different (F(1,13) = 5.63; n.s.), and the deceleration of the cumulative intake curve was similar for aged and young rats (time × age: F(19,247) = 1.08; n.s.) (Fig. 3B) indicating that satiety signaling was not different.
For corn oil, after the initial decrease during the first 5 minutes, ingestion rates declined similarly in aged and young rats (time × age: F(24,264) = 0.95; n.s.) (Fig. 3C). However, total cumulative intake of corn oil was greatly reduced in old compared with young rats (F(1,11) = 18.79; p < 0.001), and the deceleration of the cumulative intake curve was faster in old rats (time × age: F(24,264) = 13.75; p < 0.001) (Fig. 3D), suggesting that satiation signals were increased in old rats.
3.4. Brief access lick tests
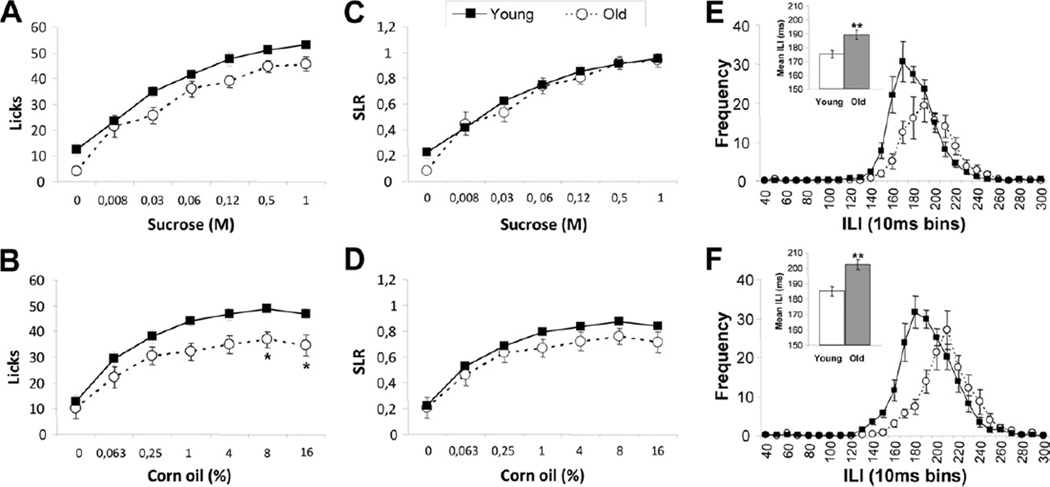
To more specifically evaluate the role of orosensory input in palatable food ingestion, we limited access to taste stimuli to 10 seconds, thus minimizing postingestive satiety signaling. Overall, aged rats licked less of the sucrose (F(1,15) = 8.56, p < 0.05) and corn oil (F(1,15) = 9.96, p < 0.01) solutions compared with young rats. The increase in licks as function of concentration was similar in young and old rats for both sucrose (F(6,90) = 117.96, p < 0.001) and corn oil (F(6,90) = 59.08, p < 0.001), as indicated by the absence of an age-by-dose interaction (sucrose: F(6,90) = 0.86, n.s.; corn oil: F(6,90) = 1.60, n.s.) (Fig. 4A and B).
Fig. 4.
Brief access lick tests. Total licks per 10 seconds for sucrose (A) and corn oil (B) in young and old rats. Data were expressed as standardized lick ratio (licks to the taste stimuli standardized to the maximum licks per trial, SLR) for sucrose (C) and corn oil (D). Rate of licking expressed as frequency distribution of interlick intervals (ILIs) for 1 M sucrose (E) and 8% corn oil (F). Inserts: mean ILI for 1 M sucrose (E) and 8% corn oil (F). Values are mean ± standard error of the mean (SEM) (n = 7–10). * p < 0.05; ** p < 0.01 versus young.
The lick response to water during the third day of sipper tube training was used as a comparison of oromotor function in water-deprived young and old rats. The old rats showed a mean ILI of 205.2 ± 2.1 ms compared with 179.3 ± 2.6 ms in young rats (t(16) = 7.53, p < 0.001), further confirming the orolingual motor impairment in old rats. When lick rate was converted to a standardized lick ratio (SLR, actual number of licks/maximal number of licks per trial) to control for differences in intrinsic lick rate and oromotor function, there were no differences in the response (F(1,15) = 0.74, n.s.) and the slope of the sucrose concentration-response curves (F(6,90) = 1.31, n.s.) (Fig. 4C). For corn oil, uncorrected responses showed that old rats emitted significantly fewer responses to the 2 highest concentrations (Fig. 4B). After SLR correction, there was a tendency for decreased responding to higher concentrations that did not reach statistical significance (F(1,15) = 2.33, n.s.) (Fig. 4D).
Because rats were not water deprived for the sucrose and corn oil experiments, we analyzed the ILI data derived for the brief access 1 M sucrose and 8% corn oil concentration at which the greatest lick responses were observed to clarify whether age differences in the motor control system of licking contributed to the response to palatable stimuli. An ILI distribution was calculated for each rat and averaged by age and solution. For both sucrose and corn oil, the peak ILI for old rats was shifted toward higher values compared with young rats (Fig. 4E and F). Mean ILI was significantly greater in old compared with young rats (sucrose: t(15) = 3.28, p < 0.01; corn oil: t(15) = 3.85, p < 0.01) (Fig. 4E and F inserts). These data indicate that the orolingual motor impairment in old rats was maintained even when animals drank appetizing solutions such as sucrose and corn oil without water deprivation.
3.5. Orofacial positive hedonic reactions as a measure of core “liking”
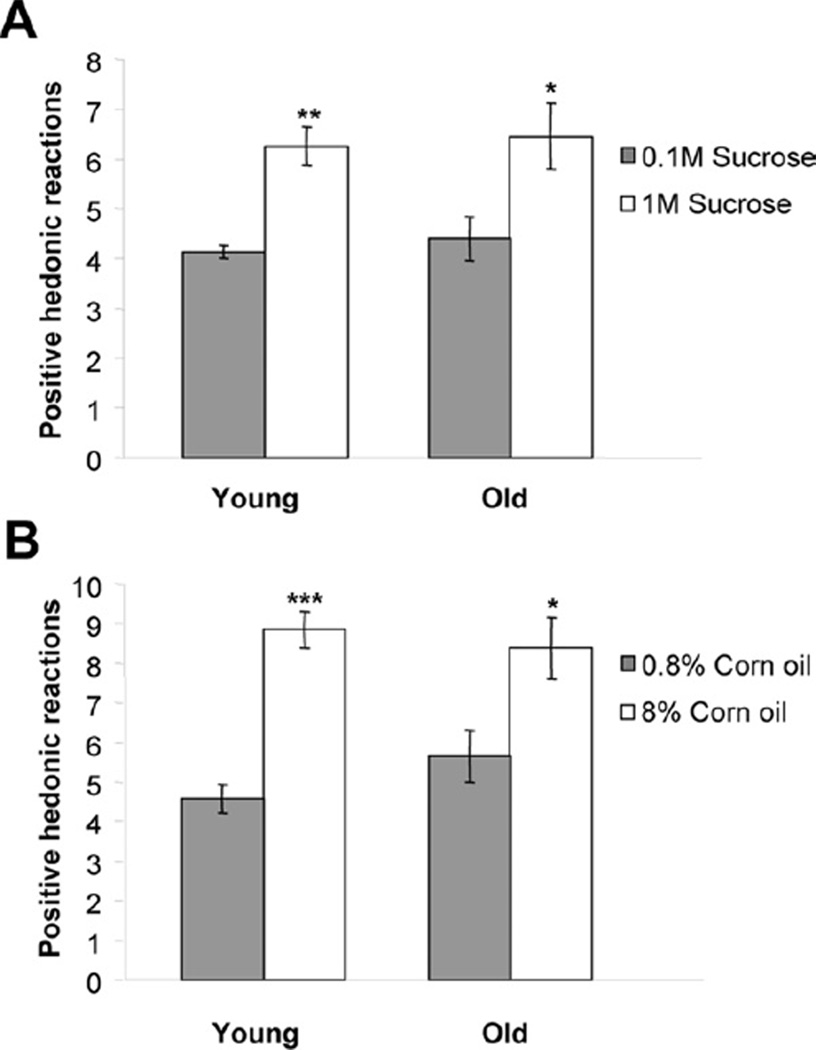
Both young and old rats showed the expected dose-dependent increase in the number of positive hedonic reactions (sucrose: F(1,14) = 34.05; p < 0.001; corn oil: F(1,11) = 26.39; p < 0.001), but there were no differences between young and old rats for both sucrose (F(1,14) = 0.28; n.s.) and corn oil (F(1,11) = 0.57; n.s.) (Fig. 5).
Fig. 5.
Taste reactivity measure of positive affective reaction in young and old rats. Rats were presented three bouts of 300 µL of sucrose (0.1 or 1 M) (A) or corn oil (0.8 or 8%) (B). Values represent mean ± standard error of the mean (SEM) of positive hedonic reactions displayed within 30 seconds after voluntary drinking in the 3 bouts, (n = 6–9). * p < 0.05; ** p < 0.01; *** p < 0.001 versus lower doses.
Young rats displayed more exploration-related behaviors than aged rats during the taste reactivity test, including locomotion across the test chamber (sucrose: F(1,14) = 31.05; p < 0.001; corn oil: F(1,11) = 51.32; p < 0.001) and rearing (sucrose: F(1,14) = 20.49; p < 0.001; corn oil: F(1,11) = 29.63; p < 0.001), indicating less motor activity and exploratory tendency in aged rats. These behaviors were not altered by the concentration of sucrose or corn oil (data not shown). There were no significant differences in grooming between young and aged rats (sucrose: F(1,14) = 0.33; n.s; corn oil: F(1,11) = 0.21; n.s.), and the incidence of grooming was not influenced by the dose of sucrose or corn oil (data not shown).
3.6. Incentive runway performance as a measure of “wanting”
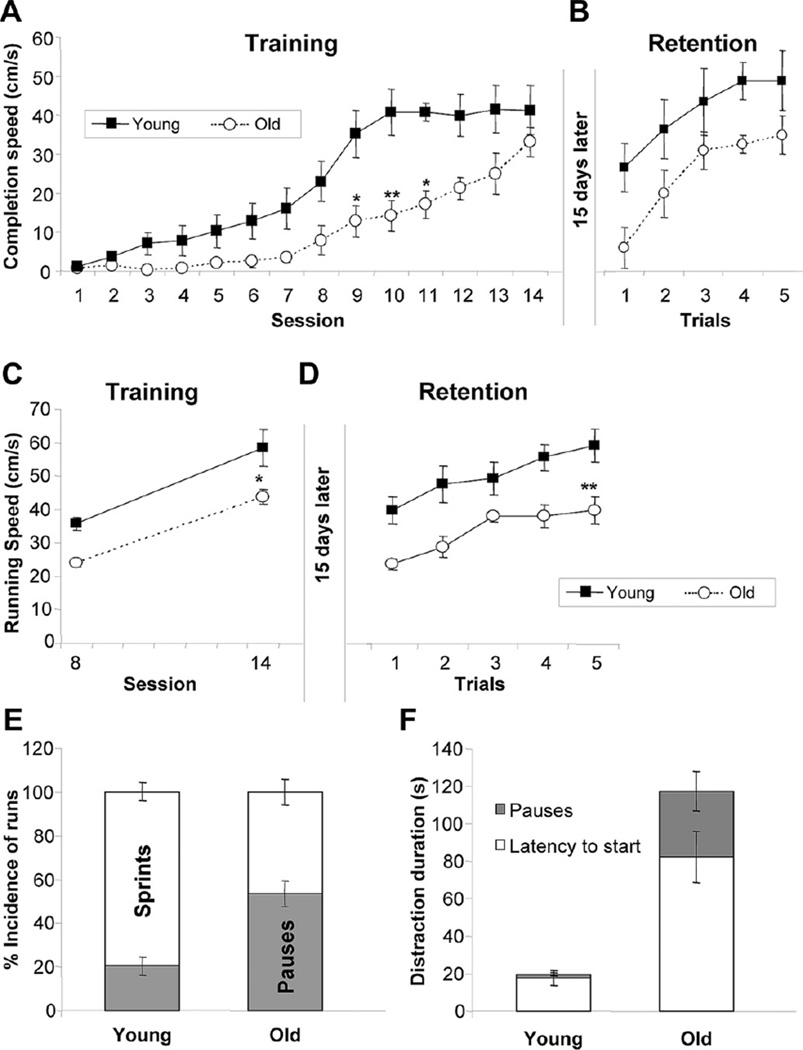
Old rats were slower in learning to get a palatable sweet reward in the goal box both during the initial test and when retested for retention 2 weeks later (Fig. 6A and B). The completion speed, a measure of “wanting”, was significantly lower in old rats during sessions 9–11 (F(1,13) = 24.85; p < 0.001). While the young rats reached maximal completion speed in 10 sessions, it took old rats 14 sessions. Furthermore, in the retention test, young rats started at about half maximal completion speed, while the aged rats started at close to zero, although these differences did not reach statistical significance (Fig. 6B).
Fig. 6.
Incentive runway training and retention tests. Completion speed (cm/s) in young and old rats during the pre-exposure (sessions 1–7), learning (session 8–11), and overtraining (session 12–14) phases of training (A); and during the 5 trials of the retention session conducted 15 days after training (B). Net running speed after subtraction of time spent in distractions for the initial acquisition (sessions 8–14) (C) and the 5 sessions of the retention phase (D). Incidence of sprints (uninterrupted runs without any pause) of young and old rats in session 8–11 (E), and time spent in distractions (latency to start and pauses) (F). Data are mean ± standard error of the mean (SEM) (n = 7–8). * p < 0.05; ** p < 0.01 versus young.
Further analysis showed that net running speed (after subtraction of time spent pausing and engaging in other behaviors) during sessions 8–14 was significantly decreased in the old rats (F(1, 10) = 38.03; p < 0.001) (Fig. 6C) and a similar effect was seen in the retention test (Fig. 6D). In addition, the latency to leave the start box (F(1,13) = 7.58; p < 0.05) and the number (F(1,13) = 17.15; p < 0.001) and duration (F(1,13) = 5.90; p < 0.05) of pauses along the runway were significantly increased in the old compared with the young rats (Fig. 6E and F). No differences were found in the number of other behaviors such as reversals.
4. Discussion
4.1. Age-related decline in feed efficiency but not food intake
In humans, changes in the regulation of energy balance occur during normal aging (Roberts and Rosenberg, 2006), often associated with decreased food intake and body weight (Elahi et al., 1983; Fischer and Johnson, 1990) but the mechanisms leading to these changes are not well understood. Here, we examined a possible role for changed food reward behaviors in these age-related alterations in aged Fischer rats. As previously reported in this and other animal models of aging (Gruenewald and Marck, 1996; Mattison et al., 2005), we confirmed the presence of relative anorexia and declining body weight in 18–22-month-old F344 rats. We further show that food intake normalized to lean body mass was 27% lower in aged compared with 3-month-old rats. However, this significant difference in chow intake was no longer observed at the end of the experimental period, because the younger rats significantly reduced corrected chow intake during the 4-month period while it remained stable in the older rats. The declining food intake in the younger cohort likely reflects the decelerating growth curve starting at about 3 months of age. Thus, based on food intake normalized for lean body mass, our study shows anorexia of early adulthood, rather than anorexia of aging. It is possible that the real anorexia of aging would have revealed itself at a later age, beyond 25 months of age. In spite of maintaining food intake at the level of younger rats, the older rats were, however, unable to maintain body weight, implying significantly reduced feed efficiency and dysregulation of energy balance as previously reported in humans and animals (Roberts and Rosenberg, 2006). Body weight loss was due to loss of both fat and lean mass.
4.2. Intact hedonic evaluation but reduced motor performance in taste-guided intake tests
Age-related declines in the hedonic evaluation or pleasantness of food due to diminished taste and smell has been suggested as a possible mechanism involved in age-related declines in energy intake in humans (Morley, 1997; Roberts and Rosenberg, 2006). To evaluate hedonic impact or “liking” of sweet and oily stimuli, we used Grill and Norgren’s taste reactivity test (Grill and Norgren, 1978) and the brief access lick test (Spector et al., 1998). The taste reactivity test is the purest measure of “liking” in an animal as it is dissociated from motivational and postingestive processes (Berridge, 2000), while the brief access test is a combination of hedonic evaluation (“liking”) and consummation (“wanting”), that in contrast with the incentive runway requires very little effort to obtain the reward. Because of the limited total intake, it also minimizes postingestive effects and can be considered mainly taste-guided.
No deficit in hedonic evaluation by older rats was revealed in these tests, although it is possible that a lower, threshold concentration of sucrose in the taste reactivity test might have revealed a difference. Also, old rats licked slightly less than young rats at all concentrations of both sucrose and corn oil, but this was most likely due to declining orolingual motor performance rather than a deficit in hedonic evaluation. The slightly larger difference observed with increasing corn oil concentrations may reflect aggravation of this motor decline with increasing viscosity.
Similar to humans, a decrease in olfactory sensitivity in rats has been described (Alberts and May, 1980; Kraemer and Apfelbach, 2004), although the mechanisms of taste appear to remain intact in the elderly, because the density and function of taste buds are not altered with aging (Mistretta, 1989). Even among humans, the alterations appear to be due more to alterations that occur in olfaction than in taste, and these alterations do not appear until relatively late in life (Cavanaugh, 1992). There are less data available on changes in pleasantness of food with aging. In humans it has been described that optimally preferred concentrations were greater for some food flavors, but not for others (de Graaf et al., 1994), with a minimal correlation between preferred flavors and the pleasantness of a complex food (de Jong et al., 1996). To our knowledge, this is the first time several behavioral test paradigms measuring the pleasantness of food have been used in aged animals. In agreement with a statement by Morley suggesting that “these changes in hedonic qualities of food are less dramatic and appear to have a minimal association with the alterations in energy intake that occur with aging in humans”, our results suggest it is not likely that age-related alterations in sensory abilities or hedonic qualities of food were involved in age-related decrease in appetite and food intake.
4.3. Increased postingestive negative feedback in older rats
Using the lickometer to monitor the number of licks throughout a meal until satiety reveals the dynamic interactions between successively decreasing taste-mediated feed-forward and increasing postingestive negative feedback signals (Davis and Perez, 1993). Specifically, the initial rate of ingestion was found to covary with taste responsiveness and is thus thought to reflect its hedonic impact (Davis and Perez, 1993). We found that the lick rate during the first minute was significantly decreased in older rats, suggesting a decrease in the hedonic impact of sucrose and corn oil in old rats. However, given the failure to demonstrate such a deficit in the more specific tests for hedonic impact discussed above, the decrease could also be due to the age-related decline in orolingual motor performance observed; consistent with other reports in F344 rats (Stanford et al., 2003), and aged monkeys (Michels et al., 1988). Alterations in orolingual motor function including dysarthria, dysphagia, and masticatory deficits have been linked to morbidity and mortality in the elderly (Baum and Bodner, 1983; Lieu et al., 2001).
With sucrose, the total cumulative intake and the monotonically decelerating cumulative intake curve was similar in young and old rats indicating a normal satiation process in aging. In contrast, with corn oil, the cumulative intake curve decelerated faster in old rats and resulted in an almost 50% reduction in cumulative intake. This scenario suggests changes in negative (gut signals) feedback, although the orolingual motor impairment could potentially account for it. It has been suggested that the increased satiation observed in older persons during the ingestion of a meal (Clarkston et al., 1997; Parker and Chapman, 2004) is due predominantly to signals from the stomach (Cook et al., 1997). It has been shown that the degree of antral distension is directly proportional to the development of satiation after ingestion of a meal (Jones et al., 1997). With aging, the rate of gastric emptying slows (Clarkston et al., 1997; Horowitz et al., 1984), and food tends to pass more rapidly from the fundus into the antrum, remaining longer in the antrum, leading to earlier and greater degrees of antral distention (Clarkston et al., 1997). The differential effects between sucrose and corn oil ingestion in the aged rats may result from several possible postingestive signals including: the increased delaying effect of lipids on gastric emptying observed in the elderly (Nakae et al., 1999); age-related alterations in gastric lipase (Nakae et al., 1999); and, as the viscosity of food ingested has an additive effect in delaying gastric emptying and increasing the sense of satiety during meal consumption and gastric emptying (Marciani et al., 2001), corn oil emulsion ingestion could be more affected by the alterations in gastric motor function observed during aging (Moore et al., 1983) than sucrose ingestion. The fact that liquid preloads producing quicker emptying from the stomach do not appear to decrease, and may even increase, energy intake in older compared with younger persons (Morley, 1997) would be consistent with our observation.
Such differences in postingestive handling of sucrose (and perhaps all carbohydrates) and fat may be the main determinants of food choice behavior. Our 12-hour acceptance/preference tests revealed a significantly greater preference for sucrose over both fat and chow. These findings are consistent with decreased fat consumption in elderly humans (De Castro, 1993) and rats (Veyrat-Durebex and Alliot, 1997), and increased carbohydrate consumption in both (Islam et al., 1993; Whichelow and Prevost, 1996).
4.4. Decline in incentive motivation and learning
The most compelling evidence for decreased motivation to obtain a food reward was revealed in the incentive runway task. It took the older rats significantly longer to get to the food reward; they exhibited more distractions along the way and walked slower. Importantly, they eventually got to the food reward just as fast as the younger rats, but it took them at least 5 more sessions. Because the net running speed was consistently lower in the older rats, involvement of age-related impairment of motor performance cannot be ruled out as a contributing factor. However, it can only partially account for the much larger differences in completion speed during the critical sessions, leaving deficits in incentive motivation and/or learning as the most plausible explanations.
Deficits in learning and memory in healthy older humans (Crook et al., 1987) and animals (Barnes et al., 1990; Bartus and Dean, 1979) have been described. Studies investigating reward association learning in humans and animals showed controversial results, but it seems to be accepted that although older adults can learn stimulus response associations and are flexible to adapt from existing to new situations, they require more effort compared with young adults (Marschner et al., 2005). More specifically, earlier work found deficits in reinforced bar-pressing responses for food (Hamm et al., 1983; Meck et al., 1986) and age-related impairments in acquisition of odor associations (Brushfield et al., 2008) and flavor reward (Marschner et al., 2005; Renteria et al., 2008).
Fewer studies have directly analyzed alterations in the motivation to obtain a food reward as a result of aging. A higher incentive motivation should increase drive to obtain a reward, and result in fewer distractions (Pecina et al., 2003). The old rats took more time to leave the start box, and paused more often than young rats, suggesting they were less focused on obtaining the reward. Similar age-related reductions in motivation for food has been observed in aged rhesus monkeys (Mattison et al., 2005).
It is possible that an operant reinforcement learning test such as progressive ratio lever pressing would have yielded different results, but it was not available to us at the time of this study. Even though a considerable number of lever presses are typically required for determining the break point, lever pressing may have been less vulnerable to motor deficits in the old rats. However, because in both test paradigms measurement of motivation is intricately linked to learning, it will always be difficult to experimentally dissociate the 2 processes.
4.5. Potentially underlying neural systems
A distributed opioid system and the mesolimbic dopamine system have most extensively been linked to food reward mechanisms (Kelley and Berridge, 2002) and there is considerable evidence for age-related alterations in these systems. Mu-opioid receptor binding in the striatum, anterior cortex, and amygdala declines with aging (Messing et al., 1980), and ligands such as β-endorphin and enkephalin are sparser (Dupont et al., 1980; Gambert, 1981; Girardot and Holloway, 1985b). Age-related changes in opioid control of food intake have also been reported (Gambert, 1981; Girardot and Holloway, 1985a; Kavaliers et al., 1985). However, more specific examinations of hedonic functions suggest that the ability of endogenous opioids to mediate the pleasure of eating sweets does not appear to decline in aging humans (Whichelow and Prevost, 1996) or rats (Islam et al., 1993), consistent with our finding that the hedonic impact of sucrose and corn oil was not altered in aged rats.
Age-related deficits in striatal dopamine signaling have been described extensively, including: (1) decreased striatal dopamine release in response to electrical stimulation (Yurek et al., 1998); (2) decreased dopamine receptor expression in nucleus accumbens (Morelli et al., 1990) and other parts of the striatum (Han et al., 1989; Nabeshima et al., 1994; Zhang and Roth, 1997; Zhang et al., 1995); and (3) reduction of amphetamine or selective D2 receptor agonist-induced c-Fos expression in the ventral striatum and nucleus accumbens (Crawford and Levine, 1997). Note, however, that there are also several negative studies (Crawford and Levine, 1997; Sonnenschein and Franklin, 2008; Stanford et al., 2002; Wallace and Booze, 1996).
Deficits in dopaminergic function could potentially explain the reduced incentive runway performance (“wanting”) in the present study. This interpretation is supported by the “opposite” effect in the hyperdopaminergic mouse—increased completion speed and absence of distractions along the way (Pecina et al., 2003). Alternatively, age-related reduction in dopamine signaling could affect reinforcement learning independent of incentive motivational processes.
In conclusion, our data suggest that old F344 rats show differential alterations of reward mechanisms and preference for sucrose and corn oil. There was no change in “liking” of sucrose or corn oil in old rats suggesting that changes in pleasantness might not be related to decreased appetite in aged rats. Nevertheless, “wanting” and learning” of an incentive motivation task for sweet reward were decreased in the older rats. The observations of age-related change in motivation for food, with the significant impaired orolingual motor activity and an increase of inhibitory postingestive feedback from the gut, mainly observed when animals ingest corn oil, might help to explain the decrease in fat and calories ingestion observed with advancing age as well as the greater preference for sucrose observed in old rats.
Acknowledgements
We thank S. Peciña and H. Zheng for technical advice and L.M. Patterson and A.C. Shin for technical assistance. Supported by National Institute of Health grants DK 071082 and DK 47348 to HRB.
Footnotes
Disclosure statement
None of the authors on this manuscript has any actual or potential conflict of interest pertinent to this work.
All animal procedures were approved by the Institutional Animal Care and Use Committee at the Pennington Biomedical Research Center, and conformed to the guidelines of the National Institutes of Health.
References
- Alberts JR, May B. Ontogeny of olfaction: development of the rats’ sensitivity to urine and amyl acetate. Physiol. Behav. 1980;24:965–970. doi: 10.1016/0031-9384(80)90157-2. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Markowska AL, Ingram DK, Kametani H, Spangler EL, Lemken VJ, Olton DS. Acetyl-1-carnitine. 2: Effects on learning and memory performance of aged rats in simple and complex mazes. Neurobiol. Aging. 1990;11:499–506. doi: 10.1016/0197-4580(90)90110-l. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL. Recent memory in aged non-human primates: hypersensitivity to visual interference during retention. Exp. Aging Res. 1979;5:385–400. doi: 10.1080/03610737908257214. [DOI] [PubMed] [Google Scholar]
- Baum BJ, Bodner L. Aging and oral motor function: evidence for altered performance among older persons. J. Dent. Res. 1983;62:2–6. doi: 10.1177/00220345830620010401. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci. Biobehav. Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Brushfield AM, Luu TT, Callahan BD, Gilbert PE. A comparison of discrimination and reversal learning for olfactory and visual stimuli in aged rats. Behav. Neurosci. 2008;122:54–62. doi: 10.1037/0735-7044.122.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh JC. Adult Development and Aging. Brooks/Cole: Washington; 1992. [Google Scholar]
- Chapman IM. Endocrinology of anorexia of ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004;18:437–452. doi: 10.1016/j.beem.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Clarkston WK, Pantano MM, Morley JE, Horowitz M, Littlefield JM, Burton FR. Evidence for the anorexia of aging: gastrointestinal transit and hunger in healthy elderly vs. young adults. Am. J. Physiol. 1997;272:R243–R248. doi: 10.1152/ajpregu.1997.272.1.R243. [DOI] [PubMed] [Google Scholar]
- Cook CG, Andrews JM, Jones KL, Wittert GA, Chapman IM, Morley JE, Horowitz M. Effects of small intestinal nutrient infusion on appetite and pyloric motility are modified by age. Am. J. Physiol. 1997;273:R755–R761. doi: 10.1152/ajpregu.1997.273.2.R755. [DOI] [PubMed] [Google Scholar]
- Crawford CA, Levine MS. Dopaminergic function in the neostriatum and nucleus accumbens of young and aged Fischer 344 rats. Neurobiol. Aging. 1997;18:57–66. doi: 10.1016/s0197-4580(96)00210-2. [DOI] [PubMed] [Google Scholar]
- Crook T, Bahar H, Sudilovsky A. Age-associated memory impairment: diagnostic criteria and treatment strategies. Int. J. Neurol. 1987–1988;21–22:73–82. [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced microstructural changes in ingestive behavior. Am. J. Physiol. 1993;264:R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- De Castro JM. Age-related changes in spontaneous food intake and hunger in humans. Appetite. 1993;21:255–272. doi: 10.1006/appe.1993.1044. [DOI] [PubMed] [Google Scholar]
- de Graaf C, Polet P, Van Staveren WA. Sensory perception and pleasantness of food flavors in elderly subjects. J. Gerontol. 1994;49:93–99. doi: 10.1093/geronj/49.3.p93. [DOI] [PubMed] [Google Scholar]
- de Jong N, de Graaf C, Van Staveren WA. Effect of sucrose in breakfast items on pleasantness and food intake in the elderly. Physiol. Behav. 1996;60:1453–1462. doi: 10.1016/s0031-9384(96)00306-x. [DOI] [PubMed] [Google Scholar]
- Dowling GJ, Weiss SR, Condon TP. Drugs of abuse and the aging brain. Neuropsychopharmacology. 2008;33:209–218. doi: 10.1038/sj.npp.1301412. [DOI] [PubMed] [Google Scholar]
- Dupont A, Barden N, Cusan L, Merand Y, Labrie F, Vaudry H. beta-Endorphin and met-enkephalins: their distribution, modulation by estrogens and haloperidol, and role in neuroendocrine control. Fed. Proc. 1980;39:2544–2550. [PubMed] [Google Scholar]
- Elahi VK, Elahi D, Andres R, Tobin JD, Butler MG, Norris AH. A longitudinal study of nutritional intake in men. J. Gerontol. 1983;38:162–180. doi: 10.1093/geronj/38.2.162. [DOI] [PubMed] [Google Scholar]
- Fanelli MT, Stevenhagen KJ. Characterizing consumption patterns by food frequency methods: core foods and variety of foods in diets of older Americans. J. Am. Diet. Assoc. 1985;85:1570–1576. [PubMed] [Google Scholar]
- Fischer J, Johnson MA. Low body weight and weight loss in the aged. J. Am. Diet. Assoc. 1990;90:1697–1706. [PubMed] [Google Scholar]
- Gambert SR. Interaction of age and thyroid hormone status on beta-endorphin content in rat corpus striatum and hypothalamus. Neuroendocrinology. 1981;32:114–117. doi: 10.1159/000123141. [DOI] [PubMed] [Google Scholar]
- Girardot MN, Holloway FA. Chronic stress, aging and morphine analgesia: chronic stress affects the reactivity to morphine in young mature but not old rats. J. Pharmacol. Exp. Ther. 1985a;233:545–553. [PubMed] [Google Scholar]
- Girardot MN, Holloway FA. Effect of age and long-term stress experience on adaptation to stress analgesia in mature rats: role of opioids. Behav. Neurosci. 1985b;99:411–422. doi: 10.1037//0735-7044.99.3.411. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Gresack J, Spector AC. A high-throughput screening procedure for identifying mice with aberrant taste and oromotor function. Chem. Sens. 2002;27:461–474. doi: 10.1093/chemse/27.5.461. [DOI] [PubMed] [Google Scholar]
- Gray RW, Cooper SJ. Benzodiazepines and palatability: taste reactivity in normal ingestion. Physiol. Behav. 1995;58:853–859. doi: 10.1016/0031-9384(95)00115-y. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Res. 1978;143:263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Gruenewald DA, Marck BT, Matsumoto AM. Fasting-induced increases in food intake and neuropeptide Y gene expression are attenuated in aging male brown Norway rats. Endocrinology. 1996;137:4460–4467. doi: 10.1210/endo.137.10.8828508. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Knisely JS, Dixon CE. An animal model of age changes in short-term memory: the DRL schedule. Exp. Aging Res. 1983;9:23–25. doi: 10.1080/03610738308258415. [DOI] [PubMed] [Google Scholar]
- Han Z, Kuyatt BL, Kochman KA, DeSouza EB, Roth GS. Effect of aging on concentrations of D2-receptor-containing neurons in the rat striatum. Brain Res. 1989;498:299–307. doi: 10.1016/0006-8993(89)91108-6. [DOI] [PubMed] [Google Scholar]
- Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Harding PE, Shearman DJ. Changes in gastric emptying rates with age. Clin. Sci. Lond. 1984;67:213–218. doi: 10.1042/cs0670213. [DOI] [PubMed] [Google Scholar]
- Islam AK, Beczkowska IW, Bodnar RJ. Interactions among aging, gender, and gonadectomy effects upon naloxone hypophagia in rats. Physiol. Behav. 1993;54:981–992. doi: 10.1016/0031-9384(93)90312-4. [DOI] [PubMed] [Google Scholar]
- Jones KL, Doran SM, Hveem K, Bartholomeusz FD, Morley JE, Sun WM, Chatterton BE, Horowitz M. Relation between postprandial satiation and antral area in normal subjects. Am. J. Clin. Nutr. 1997;66:127–132. doi: 10.1093/ajcn/66.1.127. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Teskey GC, Hirst M. The effects of aging on day-night rhythms of kappa opiate-mediated feeding in the mouse. Psychopharmacology (Berl) 1985;87:286–291. doi: 10.1007/BF00432709. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J. Neurosci. 2002;22:3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer S, Apfelbach R. Olfactory sensitivity, learning and cognition in young adult and aged male Wistar rats. Physiol. Behav. 2004;81:435–442. doi: 10.1016/j.physbeh.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Kunnecke B, Verry P, Benardeau A, von Kienlin M. Quantitative body composition analysis in awake mice and rats by magnetic resonance relaxometry. Obes. Res. 2004;12:1604–1615. doi: 10.1038/oby.2004.200. [DOI] [PubMed] [Google Scholar]
- Lieu PK, Chong MS, Seshadri R. Ann. Acad. Med. Vol. 30. Singapore: 2001. The impact of swallowing disorders in the elderly; pp. 148–154. [PubMed] [Google Scholar]
- Marciani L, Gowland PA, Spiller RC, Manoj P, Moore RJ, Young P, Fillery-Travis AJ. Effect of meal viscosity and nutrients on satiety, intragastric dilution, and emptying assessed by MRI. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G1227–G1233. doi: 10.1152/ajpgi.2001.280.6.G1227. [DOI] [PubMed] [Google Scholar]
- Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR. Reward-based decision-making and aging. Brain Res. Bull. 2005;67:382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Black A, Huck J, Moscrip T, Handy A, Tilmont E, Roth GS, Lane MA, Ingram DK. Age-related decline in caloric intake and motivation for food in rhesus monkeys. Neurobiol. Aging. 2005;26:1117–1127. doi: 10.1016/j.neurobiolaging.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Meck WH, Church RM, Wenk GL. Arginine vasopressin innoculates against age-related increases in sodium-dependent high affinity choline uptake and discrepancies in the content of temporal memory. Eur. J. Pharmacol. 1986;130:327–331. doi: 10.1016/0014-2999(86)90287-6. [DOI] [PubMed] [Google Scholar]
- Messing RB, Vasquez BJ, Spiehler VR, Martinez JL, Jr, Jensen RA, Rigter H, McGaugh JL. 3H-Dihydromorphine binding in brain regions of young and aged rats. Life Sci. 1980;26:921–927. doi: 10.1016/0024-3205(80)90112-5. [DOI] [PubMed] [Google Scholar]
- Michels RR, King JE, Hsiao S. Preference differences for sucrose solutions in young and aged squirrel monkeys. Physiol. Behav. 1988;42:53–57. doi: 10.1016/0031-9384(88)90259-4. [DOI] [PubMed] [Google Scholar]
- Mistretta CM. Anatomy and neurophysiology of the taste system in aged animals. Ann. N.Y. Acad. Sci. 1989;561:277–290. doi: 10.1111/j.1749-6632.1989.tb20989.x. [DOI] [PubMed] [Google Scholar]
- Moore JG, Tweedy C, Christian PE, Datz FL. Effect of age on gastric emptying of liquid--solid meals in man. Dig. Dis. Sci. 1983;28:340–344. doi: 10.1007/BF01324951. [DOI] [PubMed] [Google Scholar]
- Morelli M, Mennini T, Cagnotto A, Toffano G, Di CG. Quantitative autoradiographical analysis of the age-related modulation of central dopamine D1 and D2 receptors. Neuroscience. 1990;36:403–410. doi: 10.1016/0306-4522(90)90435-7. [DOI] [PubMed] [Google Scholar]
- Morley JE. Anorexia of aging: physiologic and pathologic. Am. J. Clin. Nutr. 1997;66:760–773. doi: 10.1093/ajcn/66.4.760. [DOI] [PubMed] [Google Scholar]
- Morley JE, Silver AJ. Anorexia in the elderly. Neurobiol. Aging. 1988;9:9–16. doi: 10.1016/s0197-4580(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Yamada K, Hayashi T, Hasegawa T, Ishihara S, Kameyama T, Morimasa T, Kaneyuki T, Shohmori T. Changes in muscarinic cholinergic, PCP, GABAA, D1, and 5-HT2A receptor binding, but not in benzodiazepine receptor binding in the brains of aged rats. Life Sci. 1994;55:1585–1593. doi: 10.1016/0024-3205(94)00320-3. [DOI] [PubMed] [Google Scholar]
- Nakae Y, Onouchi H, Kagaya M, Kondo T. Effects of aging and gastric lipolysis on gastric emptying of lipid in liquid meal. J. Gastroenterol. 1999;34:445–449. doi: 10.1007/s005350050294. [DOI] [PubMed] [Google Scholar]
- Olsen-Noll CG, Bosworth MF. Anorexia and weight loss in the elderly. Causes range from loose dentures to debilitating illness. Postgrad. Med. 1989;85:140–144. doi: 10.1080/00325481.1989.11700603. [DOI] [PubMed] [Google Scholar]
- Parker BA, Chapman IM. Food intake and ageing – the role of the gut. Mech. Ageing Dev. 2004;125:859–866. doi: 10.1016/j.mad.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Pecina S, Cagniard B, Berridge KC, Aldridge JW, Zhuang X. Hyperdopaminergic mutant mice have higher “wanting” but not “liking” for sweet rewards. J. Neurosci. 2003;23:9395–9402. doi: 10.1523/JNEUROSCI.23-28-09395.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renteria AF, Silbaugh BC, Tolentino JC, Gilbert PE. Age-related changes in conditioned flavor preference in rats. Behav. Brain Res. 2008;188:56–61. doi: 10.1016/j.bbr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SB, Hajduk CL, Howarth NC, Russell R, McCrory MA. Dietary variety predicts low body mass index and inadequate macronutrient and micronutrient intakes in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:613–621. doi: 10.1093/gerona/60.5.613. [DOI] [PubMed] [Google Scholar]
- Roberts SB, Rosenberg I. Nutrition and aging: changes in the regulation of energy metabolism with aging. Physiol. Rev. 2006;86:651–667. doi: 10.1152/physrev.00019.2005. [DOI] [PubMed] [Google Scholar]
- Smith BK, Volaufova J, West DB. Increased flavor preference and lick activity for sucrose and corn oil in SWR/J vs. AKR/J mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;281:R596–R606. doi: 10.1152/ajpregu.2001.281.2.R596. [DOI] [PubMed] [Google Scholar]
- Sonnenschein B, Franklin KB. The rewarding efficacy of brain stimulation and its modulation by dopaminergic drugs in young adult and old BN F344F1 rats. Pharmacol. Biochem. Behav. 2008;90:735–741. doi: 10.1016/j.pbb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. 1. Behav. Neurosci. 1998;112:678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Gash CR, Gerhardt GA. Aged F344 rats exhibit an increased proportion of dopamine agonist-excited striatal neurons. Neurobiol. Aging. 2002;23:263–270. doi: 10.1016/s0197-4580(01)00284-6. [DOI] [PubMed] [Google Scholar]
- Stanford JA, Vorontsova E, Surgener SP, Gerhardt GA, Fowler SC. Aged Fischer 344 rats exhibit altered orolingual motor function: relationships with nigrostriatal neurochemical measures. Neurobiol. Aging. 2003;24:259–266. doi: 10.1016/s0197-4580(02)00083-0. [DOI] [PubMed] [Google Scholar]
- Toshinai K, Mondal MS, Shimbara T, Yamaguchi H, Date Y, Kangawa K, Nakazato M. Ghrelin stimulates growth hormone secretion and food intake in aged rats. Mech. Ageing Dev. 2007;128:182–186. doi: 10.1016/j.mad.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Veyrat-Durebex C, Alliot J. Changes in pattern of macronutrient intake during aging in male and female rats. Physiol. Behav. 1997;62:1273–1278. doi: 10.1016/s0031-9384(97)00304-1. [DOI] [PubMed] [Google Scholar]
- Wallace DR, Booze RM. Dopamine D3 receptor density elevation in aged Fischer-344 x Brown-Norway (F1) rats. Eur. J. Pharmacol. 1996;308:283–285. doi: 10.1016/0014-2999(96)00354-8. [DOI] [PubMed] [Google Scholar]
- Whichelow MJ, Prevost AT. Dietary patterns and their associations with demographic, lifestyle and health variables in a random sample of British adults. Br. J. Nutr. 1996;76:17–30. doi: 10.1079/bjn19960006. [DOI] [PubMed] [Google Scholar]
- Wolden-Hanson T. Mechanisms of the anorexia of aging in the Brown Norway rat. Physiol. Behav. 2006;88:267–276. doi: 10.1016/j.physbeh.2006.05.032. [DOI] [PubMed] [Google Scholar]
- Yurek DM, Hipkens SB, Hebert MA, Gash DM, Gerhardt GA. Age-related decline in striatal dopamine release and motoric function in brown Norway/Fischer 344 hybrid rats. Brain Res. 1998;791:246–256. doi: 10.1016/s0006-8993(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ravipati A, Joseph J, Roth GS. Aging-related changes in rat striatal D2 receptor mRNA-containing neurons: a quantitative nonradioactive in situ hybridization study. J. Neurosci. 1995;15:1735–1740. doi: 10.1523/JNEUROSCI.15-03-01735.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Roth GS. The effect of aging on rat striatal D1 receptor mRNA-containing neurons. Neurobiol. Aging. 1997;18:251–255. doi: 10.1016/s0197-4580(97)00011-0. [DOI] [PubMed] [Google Scholar]