Abstract
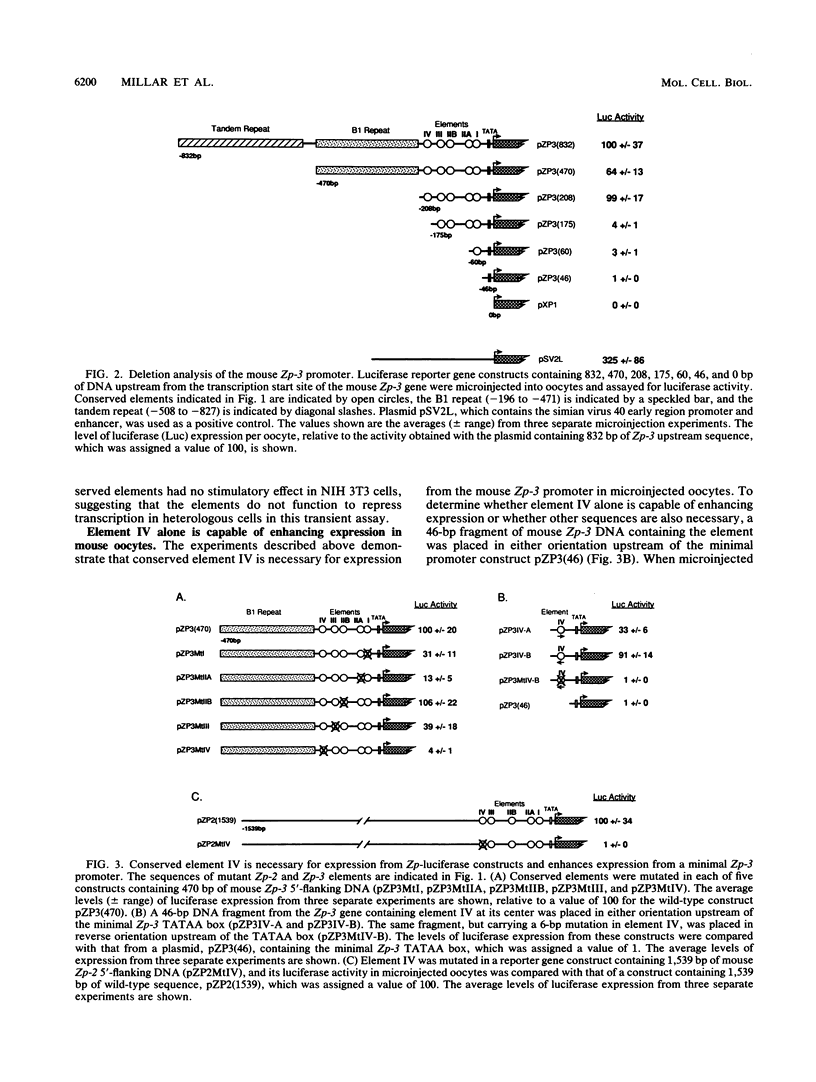
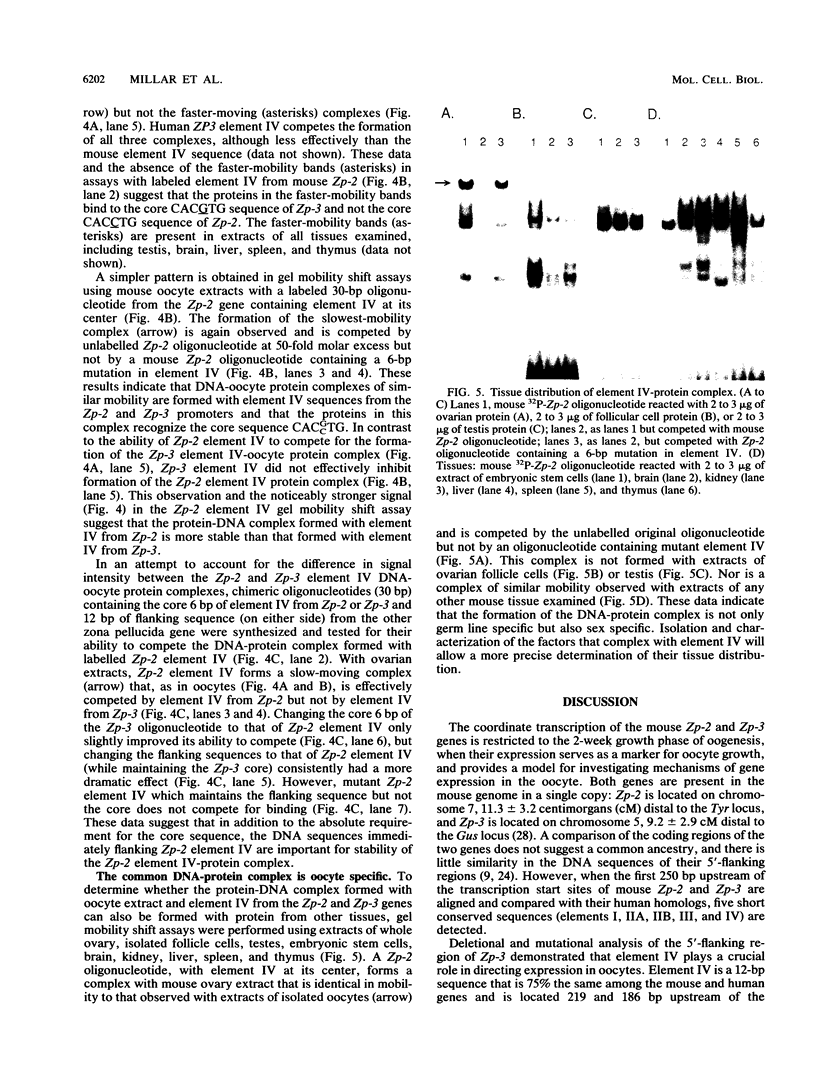
The zona pellucida of mouse oocytes, composed of three major glycoproteins (ZP1, ZP2, and ZP3), performs crucial functions at fertilization and in early development. The transcripts encoding mouse ZP2 and ZP3 are coordinately expressed and accumulate in oocytes during a 2-week growth phase prior to ovulation. The 5'-flanking regions of mouse Zp-2 and Zp-3 genes and their human homologs contain five short DNA sequences (4 to 12 bp) that are 60 to 100% identical and are approximately equidistant upstream of the TATAA box in the four genes. Mutation of these five elements (I, IIA, IIB, III, and IV) in Zp-luciferase constructs demonstrates that the 12-bp element IV, positioned approximately 200 bp upstream from the TATAA box, is necessary for high-level expression from the mouse Zp-2 and Zp-3 promoters after microinjection into the nuclei of 50-microns-diameter oocytes. Injection of minimal Zp-3 promoter constructs containing element IV in either orientation also resulted in high levels of reporter gene activity, suggesting that the element is not only necessary but also sufficient for expression from zona pellucida promoters. Oligonucleotides containing the conserved element from either Zp-2 or Zp-3 form DNA-protein complexes of identical mobility in gel retardation assays using extracts of oocytes but not other tissues. These data are consistent with the hypothesis that common factors binding to conserved element IV are involved in coordinate expression of the oocyte-specific Zp-2 and Zp-3 zona pellucida genes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avigan M. I., Strober B., Levens D. A far upstream element stimulates c-myc expression in undifferentiated leukemia cells. J Biol Chem. 1990 Oct 25;265(30):18538–18545. [PubMed] [Google Scholar]
- Beckmann H., Su L. K., Kadesch T. TFE3: a helix-loop-helix protein that activates transcription through the immunoglobulin enhancer muE3 motif. Genes Dev. 1990 Feb;4(2):167–179. doi: 10.1101/gad.4.2.167. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Weintraub H. Differences and similarities in DNA-binding preferences of MyoD and E2A protein complexes revealed by binding site selection. Science. 1990 Nov 23;250(4984):1104–1110. doi: 10.1126/science.2174572. [DOI] [PubMed] [Google Scholar]
- Bleil J. D., Wassarman P. M. Structure and function of the zona pellucida: identification and characterization of the proteins of the mouse oocyte's zona pellucida. Dev Biol. 1980 Apr;76(1):185–202. doi: 10.1016/0012-1606(80)90371-1. [DOI] [PubMed] [Google Scholar]
- CHIQUOINE A. D. The development of the zona pellucida of the mammalian ovum. Am J Anat. 1960 Mar;106:149–169. doi: 10.1002/aja.1001060207. [DOI] [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Genomic organization of a sex specific gene: the primary sperm receptor of the mouse zona pellucida. Dev Biol. 1989 Jan;131(1):207–214. doi: 10.1016/s0012-1606(89)80052-1. [DOI] [PubMed] [Google Scholar]
- Chamberlin M. E., Dean J. Human homolog of the mouse sperm receptor. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6014–6018. doi: 10.1073/pnas.87.16.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. L., Cheng P. F., Lassar A. B., Weintraub H. The MyoD DNA binding domain contains a recognition code for muscle-specific gene activation. Cell. 1990 Mar 9;60(5):733–746. doi: 10.1016/0092-8674(90)90088-v. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Herman S. A., Martínez-Salas E., Chalifour L. E., Wirak D. O., Cupo D. Y., Miranda M. Microinjecting DNA into mouse ova to study DNA replication and gene expression and to produce transgenic animals. Biotechniques. 1988 Jul-Aug;6(7):662–680. [PubMed] [Google Scholar]
- Goring D. R., Rossant J., Clapoff S., Breitman M. L., Tsui L. C. In situ detection of beta-galactosidase in lenses of transgenic mice with a gamma-crystallin/lacZ gene. Science. 1987 Jan 23;235(4787):456–458. doi: 10.1126/science.3099390. [DOI] [PubMed] [Google Scholar]
- Hall R. K., Taylor W. L. Transcription factor IIIA gene expression in Xenopus oocytes utilizes a transcription factor similar to the major late transcription factor. Mol Cell Biol. 1989 Nov;9(11):5003–5011. doi: 10.1128/mcb.9.11.5003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegemann J. H., Shero J. H., Cottarel G., Philippsen P., Hieter P. Mutational analysis of centromere DNA from chromosome VI of Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jun;8(6):2523–2535. doi: 10.1128/mcb.8.6.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene. 1989 Apr 15;77(1):51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- JONES E. C., KROHN P. L. The relationships between age, numbers of ocytes and fertility in virgin and multiparous mice. J Endocrinol. 1961 Feb;21:469–495. doi: 10.1677/joe.0.0210469. [DOI] [PubMed] [Google Scholar]
- Kaulen H., Pognonec P., Gregor P. D., Roeder R. G. The Xenopus B1 factor is closely related to the mammalian activator USF and is implicated in the developmental regulation of TFIIIA gene expression. Mol Cell Biol. 1991 Jan;11(1):412–424. doi: 10.1128/mcb.11.1.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinloch R. A., Roller R. J., Fimiani C. M., Wassarman D. A., Wassarman P. M. Primary structure of the mouse sperm receptor polypeptide determined by genomic cloning. Proc Natl Acad Sci U S A. 1988 Sep;85(17):6409–6413. doi: 10.1073/pnas.85.17.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Levine J., Spradling A. DNA sequence of a 3.8 kilobase pair region controlling Drosophila chorion gene amplification. Chromosoma. 1985;92(2):136–142. doi: 10.1007/BF00328465. [DOI] [PubMed] [Google Scholar]
- Liang L. F., Chamow S. M., Dean J. Oocyte-specific expression of mouse Zp-2: developmental regulation of the zona pellucida genes. Mol Cell Biol. 1990 Apr;10(4):1507–1515. doi: 10.1128/mcb.10.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lira S. A., Kinloch R. A., Mortillo S., Wassarman P. M. An upstream region of the mouse ZP3 gene directs expression of firefly luciferase specifically to growing oocytes in transgenic mice. Proc Natl Acad Sci U S A. 1990 Sep;87(18):7215–7219. doi: 10.1073/pnas.87.18.7215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan J., Shenk T. In vivo identification of sequence elements required for normal function of the adenovirus major late transcriptional control region. Nucleic Acids Res. 1986 Aug 11;14(15):6327–6335. [PMC free article] [PubMed] [Google Scholar]
- Lunsford R. D., Jenkins N. A., Kozak C. A., Liang L. F., Silan C. M., Copeland N. G., Dean J. Genomic mapping of murine Zp-2 and Zp-3, two oocyte-specific loci encoding zona pellucida proteins. Genomics. 1990 Jan;6(1):184–187. doi: 10.1016/0888-7543(90)90465-7. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Baltimore D. A new DNA binding and dimerization motif in immunoglobulin enhancer binding, daughterless, MyoD, and myc proteins. Cell. 1989 Mar 10;56(5):777–783. doi: 10.1016/0092-8674(89)90682-x. [DOI] [PubMed] [Google Scholar]
- Murre C., McCaw P. S., Vaessin H., Caudy M., Jan L. Y., Jan Y. N., Cabrera C. V., Buskin J. N., Hauschka S. D., Lassar A. B. Interactions between heterologous helix-loop-helix proteins generate complexes that bind specifically to a common DNA sequence. Cell. 1989 Aug 11;58(3):537–544. doi: 10.1016/0092-8674(89)90434-0. [DOI] [PubMed] [Google Scholar]
- Nordeen S. K. Luciferase reporter gene vectors for analysis of promoters and enhancers. Biotechniques. 1988 May;6(5):454–458. [PubMed] [Google Scholar]
- Pedersen T. Follicle kinetics in the ovary of the cyclic mouse. Acta Endocrinol (Copenh) 1970 Jun;64(2):304–323. doi: 10.1530/acta.0.0640304. [DOI] [PubMed] [Google Scholar]
- Philpott C. C., Ringuette M. J., Dean J. Oocyte-specific expression and developmental regulation of ZP3, the sperm receptor of the mouse zona pellucida. Dev Biol. 1987 Jun;121(2):568–575. doi: 10.1016/0012-1606(87)90192-8. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991 Jan 11;251(4990):186–189. doi: 10.1126/science.1987636. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Chamberlin M. E., Baur A. W., Sobieski D. A., Dean J. Molecular analysis of cDNA coding for ZP3, a sperm binding protein of the mouse zona pellucida. Dev Biol. 1988 Jun;127(2):287–295. doi: 10.1016/0012-1606(88)90315-6. [DOI] [PubMed] [Google Scholar]
- Ringuette M. J., Sobieski D. A., Chamow S. M., Dean J. Oocyte-specific gene expression: molecular characterization of a cDNA coding for ZP-3, the sperm receptor of the mouse zona pellucida. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4341–4345. doi: 10.1073/pnas.83.12.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosner M. H., Vigano M. A., Ozato K., Timmons P. M., Poirier F., Rigby P. W., Staudt L. M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990 Jun 21;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawadogo M., Roeder R. G. Interaction of a gene-specific transcription factor with the adenovirus major late promoter upstream of the TATA box region. Cell. 1985 Nov;43(1):165–175. doi: 10.1016/0092-8674(85)90021-2. [DOI] [PubMed] [Google Scholar]
- Shabanowitz R. B. Mouse antibodies to human zona pellucida: evidence that human ZP3 is strongly immunogenic and contains two distinct isomer chains. Biol Reprod. 1990 Aug;43(2):260–270. doi: 10.1095/biolreprod43.2.260. [DOI] [PubMed] [Google Scholar]
- Shabanowitz R. B., O'Rand M. G. Characterization of the human zona pellucida from fertilized and unfertilized eggs. J Reprod Fertil. 1988 Jan;82(1):151–161. doi: 10.1530/jrf.0.0820151. [DOI] [PubMed] [Google Scholar]
- Shea M. J., King D. L., Conboy M. J., Mariani B. D., Kafatos F. C. Proteins that bind to Drosophila chorion cis-regulatory elements: a new C2H2 zinc finger protein and a C2C2 steroid receptor-like component. Genes Dev. 1990 Jul;4(7):1128–1140. doi: 10.1101/gad.4.7.1128. [DOI] [PubMed] [Google Scholar]
- Shimizu S., Tsuji M., Dean J. In vitro biosynthesis of three sulfated glycoproteins of murine zonae pellucidae by oocytes grown in follicle culture. J Biol Chem. 1983 May 10;258(9):5858–5863. [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]
- de Wet J. R., Wood K. V., DeLuca M., Helinski D. R., Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Mol Cell Biol. 1987 Feb;7(2):725–737. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]