Abstract
Metabolic syndrome and cardiomyopathies are long-term consequences of chemo- and radiotherapy and develop long after completing the initial tumor treatment. The slow progression of such late effects might be an indication of the involvement of autoimmune processes in the development of such follow-up consequences. Functionally active autoantibodies, which permanently stimulate relevant cell receptors, might be a crucial component. Here, we report the detection of functionally active agonistic autoantibodies such as the autoantibody against the adrenergic alpha1-receptor, the muscarinic M2-receptor, and the newly discovered autoantibody against the Mas-receptor in the plasma of a cancer survivor following chemotherapy treatment.
Key words: Agonistic autoantibodies, G-protein coupled receptor autoantibodies, Chemotherapy, Tumor therapy, Cardiomyopathy, Metabolic syndrome, Long-term disturbances
Introduction
The immune status of patients with tumors is a matter of intense investigation, with the aim to find appropriate markers for improving tumor diagnostics along with fundamental facts about tumor pathophysiology. This also holds true for the autoimmune situation [1, 2]. The occurrence of autoantibodies might even be an early indicator of tumor development. However, to our knowledge, agonistic autoantibodies have not yet been the focus in this context. Thus, it is not known whether a tumor or the subsequent therapy could induce the formation of agonistic autoantibodies.
As the name implies, agonistic autoantibodies activate receptors that are coupled with the G-protein signal cascade. The participation of such autoantibodies in the pathogenesis of a disease has been described for a variety of diseases that are often combined with vascular complications. This holds true for autoantibodies against the adrenergic beta1- and beta2 receptors, adrenergic alpha1-receptor (alpha1-AAB), muscarinic M2-receptor (M2-AAB), AT1-receptor, endothelin receptor, and many more that are implicated in diseases such as malignant hypertension, dilated cardiomyopathy, Chagas’ cardiomyopathy, pulmonary hypertension, vascular necrotic kidney graft rejection, diabetes type 2, and others (excellently reviewed by Xia and Kellems [3]). The problem with these autoantibodies found at low titers is their potential to activate the corresponding receptors without inducing physiologically protective cell and tissue counterregulation responses such as the downregulation of the receptors.
The reason for the occurrence of such autoantibodies is mostly unknown. Only in rare cases such as Chagas’ cardiomyopathy has the pathophysiological context been well investigated. Here, the autoantibodies are a consequence of biological mimicry after a parasite infection [4].
With respect to the tumor situation, the tumor itself and, largely, drastic cell-destroying therapy such as chemotherapy and radiation are situations in which a nonphysiological amount of cell debris has to be cleared by the immune system. The subsequent induction of autoimmune reactions is very likely. We therefore searched for agonistic receptor autoantibodies that have already been described for conditions that are similar to cardiomyopathy and metabolic syndrome caused by tumor therapy.
Case Presentation
A 57-year-old woman, who had previously undergone surgical carcinoma resection, received six cycles of adjuvant cisplatin/doxorubicin/paclitaxel treatment. After therapy, the patient suffered from diarrhea, polyneuropathy, and unregulated high blood pressure episodes that were mostly triggered by stress.
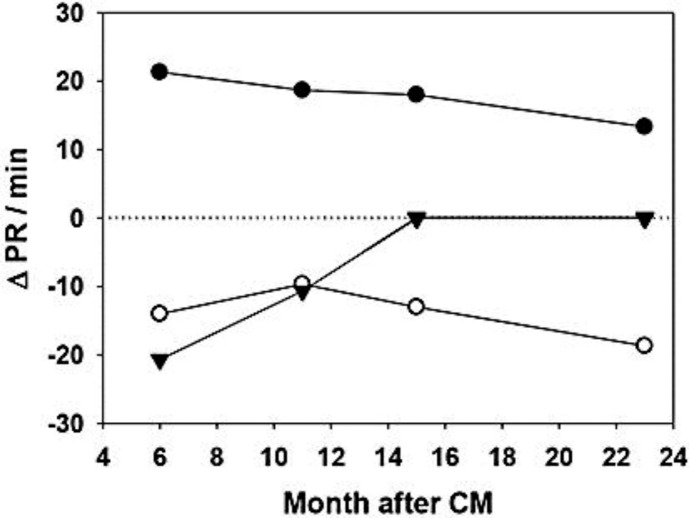
Six months after chemotherapy, the M2-AAB, alpha1-AAB, and Mas-receptor autoantibody (Mas-AAB) were detected in the plasma (fig. 1). M2-AAB activity decreased 1 year after chemotherapy and was paralleled by an improvement in the unregulated bowel movements, and it disappeared 1.5 years after chemotherapy, again paralleled by a further improvement of the situation.
Fig. 1.
Chronotropic activity (ΔPR/min) of the alpha1-AAB (filled circles), M2-AAB (filled triangles), and Mas-AAB (open circles) 6 months, about 1 year, and 2 years after chemotherapy (CM), respectively.
Mas-AAB activity did not change over the 2-year observation period after chemotherapy, while alpha1-AAB activity declined but did not disappear over the same period (fig. 1).
Brief Description of the Measurement Method
To detect the agonistic autoantibodies, a bioassay test system was applied that registers the change in the chronotropic activity of spontaneously beating neonatal rat cardiomyocytes after the addition of the patient's autoantibody containing the immunoglobulin fraction [5]. The autoantibodies target their receptors, inducing the corresponding change in the beating frequency (delta pulse rate/min, ΔPR/min) of the cells. In order to exclude falsified information caused by the combined occurrence of positive chronotropic autoantibodies such as the alpha1-AAB and negative chronotropic autoantibodies such as the muscarinic M2-AAB, which would annihilate the signal, each autoantibody was measured while blocking the other. With respect to the combined occurrence of the alpha1-AAB and muscarinic M2-AAB, atropine was added to block the signal of the muscarinic M2-AAB, while the addition of prazosin/urapidil enabled the measurement of the M2-AAB. For the detection and identification of the Mas-AAB, a specific Mas-receptor antagonist (A779) was added to neutralize the chronotropic effect and verify the specificity.
Discussion
The development of long-term disturbances after chemotherapy, such as cardiomyopathies (especially in the case of doxorubicin treatment [6, 7]) and the development of metabolic syndrome [8], is a sign of the slow progression of the respective disturbance and the potential involvement of autoimmune reactions. We therefore investigated the occurrence of agonistic autoantibodies after chemotherapy.
Initially, one might think of autoantibodies that target intracellular structures, as is the case in lupus. However, Ersvaer et al. [9] found no evidence for such lupus autoantibodies after chemotherapy (ribosomal P autoantibodies). The same was not true for the agonistic autoantibodies against the G-protein coupled receptors, with the M2-AAB being detected in the reported case. The occurrence of the M2-AAB in chronic Chagas’ disease that develops into a megacolon is well accepted [10] and was considered a factor for the reported bowel movement problems observed in the reported case, especially since a relation between titer and the actual condition was observed.
The participation of the alpha1-receptor AAB in hypertension and malignant hypertension is also a well-investigated fact [11]. The alpha1-receptor AAB, which has been detected in our patient, has also been reported to be present in a high percentage of patients with type 2 diabetes [12]. However, a direct relation between the occurrence of the alpha1-AAB and chemotherapy is not possible in this case. Blood pressure dysregulation began about 6–7 months before tumor detection. The tumor-associated induction of the alpha1-AAB cannot be ruled out.
The third AAB, Mas-AAB, has only recently been discovered in diabetic patients [Santos and Wallukat, unpubl. data] and is thought to interfere with blood pressure regulation since its target, the Mas-receptor, is an essential part of this regulatory system. The Mas-receptor is now a considered part of the novel axis of the renin system ACE2/angiotensin-(1–7)/Mas [13]. One of the main consequences of its activation is nitric oxide production in blood vessels and many other tissues [13]. Whether Mas-AAB could lead to nitric oxide production with consequent detrimental or beneficial effects remains to be clarified.
In our case, the Mas-AAB was found 6 months after chemotherapy and persisted over the whole observation period of 2 years.
When a high percentage of gonadal cancer survivors develop therapy-induced disturbances such as metabolic syndrome [8], the possible role of therapy-induced specific agonistic autoantibodies as possible mediators should be considered and become the subject of systematic investigation. First, it should be determined whether tumor therapy or the tumor itself is the reason for the generation of agonistic autoantibodies. In the latter case, nobody would want to interfere with this system on the assumption that these autoantibodies are an essential part of the defense of the body against the tumor if they are more than the product of a ‘bystander effect’. Specific interference should only be considered when the agonistic autoantibodies are a clear side effect of tumor therapy and a cause of persistent side effects. Since tumor survivors rely on an intact immune system as an essential prerequisite for relapse-free survival, nonspecific therapy such as the nonspecific removal of immunoglobulins – as has been successfully applied in the treatment of autoantibody-associated cardiomyopathies [14] – should never be considered. However, recent and ongoing studies have been undertaken to develop highly specific blockers for single agonistic autoantibodies on, e.g., the aptamer basis [15], which would be a more appropriate tool for the tumor situation given that the autoantibodies against G-protein coupled receptors are a consequence of therapy only. Before using such approaches, comprehensive studies clarifying all open questions will be necessary.
Disclosure Statement
The authors have no conflicts of interest to declare in connection with this paper.
Acknowledgement
A.H. was supported by the ‘Deutsche Gesellschaft für Klinische Chemie und Laboratoriumsmedizin’ (48/2011).
References
- 1.Kobold S, Lütkens T, Cao Y, Bokemeyer C, Atanackovic D. Autoantibodies against tumor-related antigens: incidence and biologic significance. Hum Immunol. 2010;71:643–651. doi: 10.1016/j.humimm.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Conrad K, Bartsch H, Canzler U, Pilarsky C, Grützmann R, Bachmann M. Search for and identification of novel tumor-associated autoantigens. Methods Mol Biol. 2010;576:213–230. doi: 10.1007/978-1-59745-545-9_12. [DOI] [PubMed] [Google Scholar]
- 3.Xia Y, Kellems RE. Receptor-activating autoantibodies and disease: preeclampsia and beyond. Expert Rev Clin Immunol. 2011;7:659–674. doi: 10.1586/eci.11.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari I, Levin MJ, Wallukat G, Elies R, Lebesgue D, Chiale P, Elizari M, Rosenbaum M, Hoebeke J. Molecular mimicry between the immunodominant ribosomal protein P0 of Trypanosoma cruzi and a functional epitope on the human beta 1-adrenergic receptor. J Exp Med. 1995;182:59–65. doi: 10.1084/jem.182.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wallukat G, Wollenberger A. Cultivated cardiac muscle cells – a functional test system for the detection of autoantibodies against the beta-adrenergic receptor (in German) Acta Histochem Suppl. 1988;35:145–149. [PubMed] [Google Scholar]
- 6.Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–575. doi: 10.1038/nrcardio.2010.121. [DOI] [PubMed] [Google Scholar]
- 7.Yoon GJ, Telli ML, Kao DP, Matsuda KY, Carlson RW, Witteles RM. Left ventricular dysfunction in patients receiving cardiotoxic cancer therapies: are clinicians responding optimally? J Am Coll Cardiol. 2010;56:1644–1650. doi: 10.1016/j.jacc.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nuver J, Smit AJ, Wolffenbuttel BH, Sluiter WJ, Hoekstra HJ, Sleijfer DT, Gietema JA. The metabolic syndrome and disturbances in hormone levels in long-term survivors of disseminated testicular cancer. J Clin Oncol. 2005;23:3718–3725. doi: 10.1200/JCO.2005.02.176. [DOI] [PubMed] [Google Scholar]
- 9.Ersvaer E, Bertelsen LT, Espenes LC, Bredholt T, Bøe SO, Iversen BM, Bruserud Ø, Ulvestad E, Gjertsen BT. Characterization of ribosomal P autoantibodies in relation to cell destruction and autoimmune disease. Scand J Immunol. 2004;60:189–198. doi: 10.1111/j.0300-9475.2004.01450.x. [DOI] [PubMed] [Google Scholar]
- 10.Sterin-Borda L, Goin JC, Bilder CR, Iantorno G, Hernando AC, Borda E. Interaction of human chagasic IgG with human colon muscarinic acetylcholine receptor: molecular and functional evidence. Gut. 2001;49:699–705. doi: 10.1136/gut.49.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luther HP, Homuth V, Wallukat G. Alpha 1-adrenergic receptor antibodies in patients with primary hypertension. Hypertension. 1997;29:678–682. doi: 10.1161/01.hyp.29.2.678. [DOI] [PubMed] [Google Scholar]
- 12.Hempel P, Karczewski P, Kohnert KD, Raabe J, Lemke B, Kunze R, Bimmler M. Sera from patients with type 2 diabetes contain agonistic autoantibodies against G protein-coupled receptors. Scand J Immunol. 2009;70:159–160. doi: 10.1111/j.1365-3083.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- 13.Santos RA, Ferreira AJ, Simões E, Silva AC. Recent advances in the angiotensin-converting enzyme2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 14.Dandel M, Wallukat G, Englert A, Lehmkuhl HB, Knosalla C, Hetzer R. Long-term benefits of immunoadsorption in beta(1)-adrenoceptor autoantibody-positive transplant candidates with dilated cardiomyopathy. Eur J Heart Fail. 2012;14:1374–1388. doi: 10.1093/eurjhf/hfs123. [DOI] [PubMed] [Google Scholar]
- 15.Haberland A, Wallukat G, Dahmen C, Kage A, Schimke I. Aptamer neutralization of beta1-adrenoceptor autoantibodies isolated from patients with cardiomyopathies. Circ Res. 2011;109:986–992. doi: 10.1161/CIRCRESAHA.111.253849. [DOI] [PubMed] [Google Scholar]