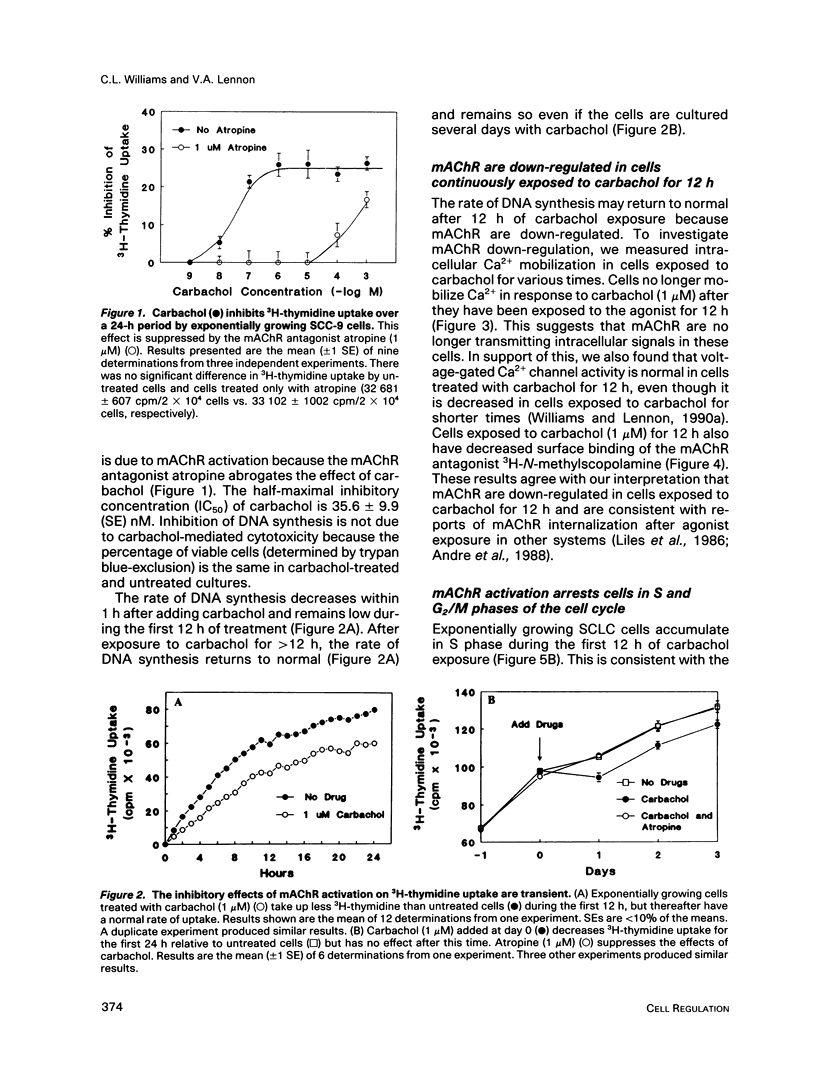
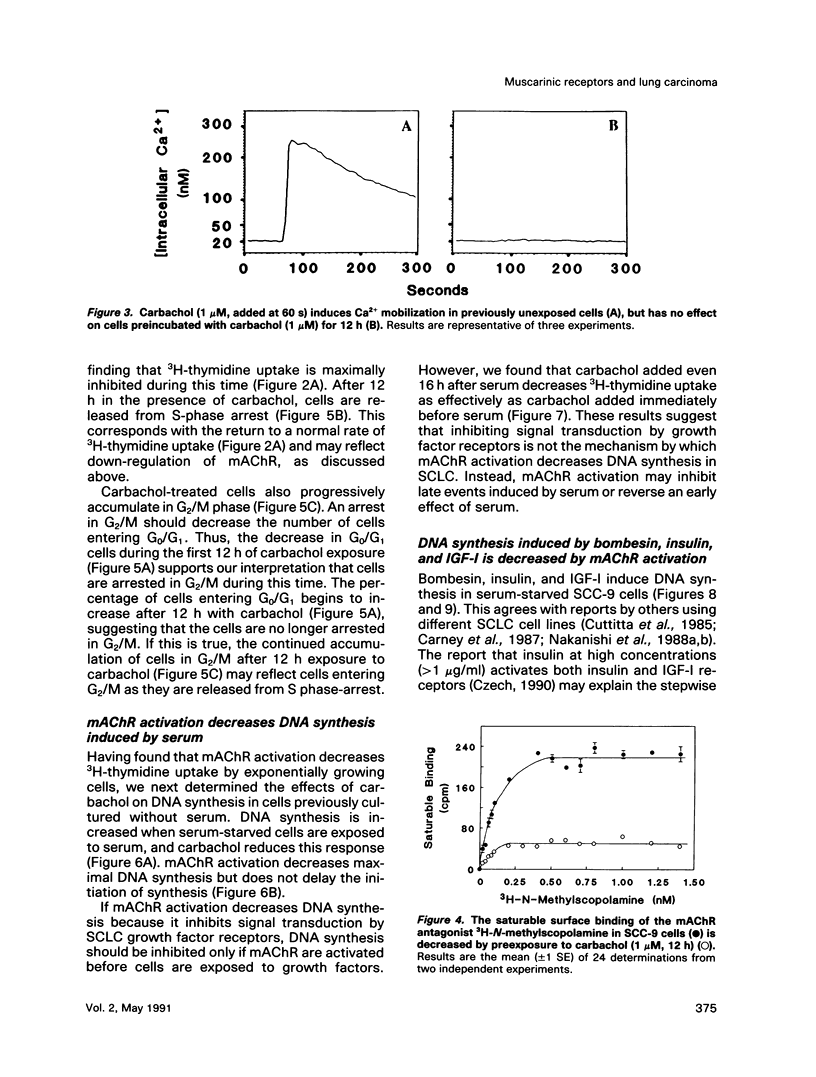
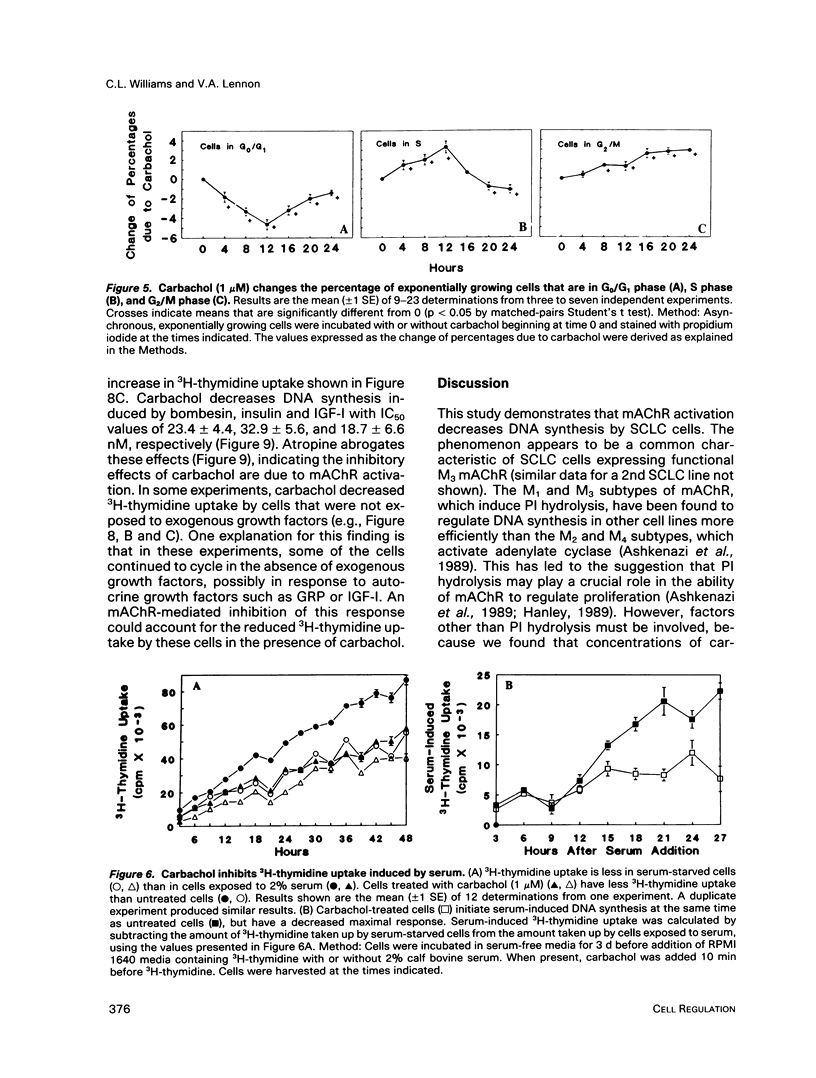
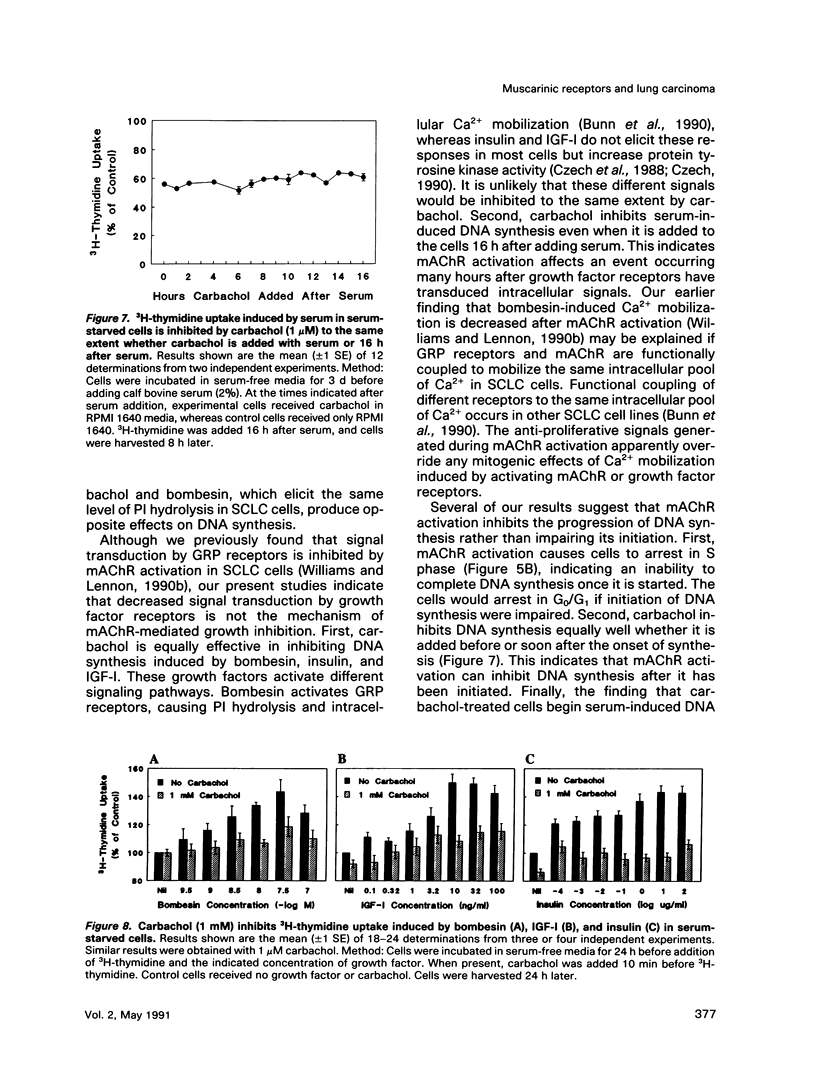
Abstract
We previously reported that activation of muscarinic acetylcholine receptors (mAChR) of M3 subtype causes hydrolysis of phosphoinositides and inhibits voltage-gated Ca2+ channel activity in small cell lung carcinoma (SCLC) cells. We now report that mAChR activation causes exponentially growing SCLC cells to arrest in S and G2/M phases of the cell cycle, concomitant with a decrease in DNA synthesis. Cell cycle progression and DNA synthesis resume when mAChR are down-regulated. In serum-starved SCLC cells, mAChR activation inhibits DNA synthesis induced by serum, bombesin, insulin, or insulin-like growth factor-I. The finding that DNA synthesis is inhibited even when mAChR are activated after exposure of cells to growth factors indicates that decreased signal transduction by growth factor receptors is not the mechanism of mAChR-mediated growth inhibition. Our data suggest that mAChR activation disrupts a common event that is induced by different growth factors and is fundamental for cell cycle progression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André C., Marullo S., Convents A., Lü B. Z., Guillet J. G., Hoebeke J., Strosberg D. A. A human embryonic lung fibroblast with a high density of muscarinic acetylcholine receptors. Eur J Biochem. 1988 Jan 15;171(1-2):401–407. doi: 10.1111/j.1432-1033.1988.tb13804.x. [DOI] [PubMed] [Google Scholar]
- Ashkenazi A., Ramachandran J., Capon D. J. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989 Jul 13;340(6229):146–150. doi: 10.1038/340146a0. [DOI] [PubMed] [Google Scholar]
- Birrer M. J., Minna J. D. Genetic changes in the pathogenesis of lung cancer. Annu Rev Med. 1989;40:305–317. doi: 10.1146/annurev.me.40.020189.001513. [DOI] [PubMed] [Google Scholar]
- Bunn P. A., Jr, Dienhart D. G., Chan D., Puck T. T., Tagawa M., Jewett P. B., Braunschweiger E. Neuropeptide stimulation of calcium flux in human lung cancer cells: delineation of alternative pathways. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2162–2166. doi: 10.1073/pnas.87.6.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney D. N., Cuttitta F., Moody T. W., Minna J. D. Selective stimulation of small cell lung cancer clonal growth by bombesin and gastrin-releasing peptide. Cancer Res. 1987 Feb 1;47(3):821–825. [PubMed] [Google Scholar]
- Conklin B. R., Brann M. R., Buckley N. J., Ma A. L., Bonner T. I., Axelrod J. Stimulation of arachidonic acid release and inhibition of mitogenesis by cloned genes for muscarinic receptor subtypes stably expressed in A9 L cells. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8698–8702. doi: 10.1073/pnas.85.22.8698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham J. M., Lennon V. A., Lambert E. H., Scheithauer B. Acetylcholine receptors in small cell carcinomas. J Neurochem. 1985 Jul;45(1):159–167. doi: 10.1111/j.1471-4159.1985.tb05488.x. [DOI] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Czech M. P., Klarlund J. K., Yagaloff K. A., Bradford A. P., Lewis R. E. Insulin receptor signaling. Activation of multiple serine kinases. J Biol Chem. 1988 Aug 15;263(23):11017–11020. [PubMed] [Google Scholar]
- De Aizpurua H. J., Lambert E. H., Griesmann G. E., Olivera B. M., Lennon V. A. Antagonism of voltage-gated calcium channels in small cell carcinomas of patients with and without Lambert-Eaton myasthenic syndrome by autoantibodies omega-conotoxin and adenosine. Cancer Res. 1988 Sep 1;48(17):4719–4724. [PubMed] [Google Scholar]
- Dean P. N., Jett J. H. Mathematical analysis of DNA distributions derived from flow microfluorometry. J Cell Biol. 1974 Feb;60(2):523–527. doi: 10.1083/jcb.60.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley M. R. Mitogenic neurotransmitters. Nature. 1989 Jul 13;340(6229):97–97. doi: 10.1038/340097a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B. Growth factors: mechanism of action and relation to oncogenes. Cell. 1984 May;37(1):9–20. doi: 10.1016/0092-8674(84)90296-4. [DOI] [PubMed] [Google Scholar]
- Jaskulski D., Gatti C., Travali S., Calabretta B., Baserga R. Regulation of the proliferating cell nuclear antigen cyclin and thymidine kinase mRNA levels by growth factors. J Biol Chem. 1988 Jul 25;263(21):10175–10179. [PubMed] [Google Scholar]
- Kraus M. H., Pierce J. H., Fleming T. P., Robbins K. C., Di Fiore P. P., Aaronson S. A. Mechanisms by which genes encoding growth factors and growth factor receptors contribute to malignant transformation. Ann N Y Acad Sci. 1988;551:320–336. doi: 10.1111/j.1749-6632.1988.tb22358.x. [DOI] [PubMed] [Google Scholar]
- Laiho M., DeCaprio J. A., Ludlow J. W., Livingston D. M., Massagué J. Growth inhibition by TGF-beta linked to suppression of retinoblastoma protein phosphorylation. Cell. 1990 Jul 13;62(1):175–185. doi: 10.1016/0092-8674(90)90251-9. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Liles W. C., Hunter D. D., Meier K. E., Nathanson N. M. Activation of protein kinase C induces rapid internalization and subsequent degradation of muscarinic acetylcholine receptors in neuroblastoma cells. J Biol Chem. 1986 Apr 25;261(12):5307–5313. [PubMed] [Google Scholar]
- Lindstrom J., Schoepfer R., Whiting P. Molecular studies of the neuronal nicotinic acetylcholine receptor family. Mol Neurobiol. 1987 Winter;1(4):281–337. doi: 10.1007/BF02935740. [DOI] [PubMed] [Google Scholar]
- Luetje C. W., Patrick J., Séguéla P. Nicotine receptors in the mammalian brain. FASEB J. 1990 Jul;4(10):2753–2760. doi: 10.1096/fasebj.4.10.2197155. [DOI] [PubMed] [Google Scholar]
- Macauly V. M., Teale J. D., Everard M. J., Joshi G. P., Smith I. E., Millar J. L. Somatomedin-C/insulin-like growth factor-I is a mitogen for human small cell lung cancer. Br J Cancer. 1988 Jan;57(1):91–93. doi: 10.1038/bjc.1988.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maneckjee R., Minna J. D. Opioid and nicotine receptors affect growth regulation of human lung cancer cell lines. Proc Natl Acad Sci U S A. 1990 May;87(9):3294–3298. doi: 10.1073/pnas.87.9.3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi Y., Mulshine J. L., Kasprzyk P. G., Natale R. B., Maneckjee R., Avis I., Treston A. M., Gazdar A. F., Minna J. D., Cuttitta F. Insulin-like growth factor-I can mediate autocrine proliferation of human small cell lung cancer cell lines in vitro. J Clin Invest. 1988 Jul;82(1):354–359. doi: 10.1172/JCI113594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn L. J., Laufer E. M., Land H. C-MYC: evidence for multiple regulatory functions. Semin Cancer Biol. 1990 Feb;1(1):69–80. [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Sherley J. L., Kelly T. J. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988 Jun 15;263(17):8350–8358. [PubMed] [Google Scholar]
- Sorenson G. D., Pettengill O. S., Cate C. C., Ghatei M. A., Molyneux K. E., Gosselin E. J., Bloom S. R. Bombesin and calcitonin secretion by pulmonary carcinoma is modulated by cholinergic receptors. Life Sci. 1983 Nov 7;33(19):1939–1944. doi: 10.1016/0024-3205(83)90679-3. [DOI] [PubMed] [Google Scholar]
- Stein C., Hille A., Seidel J., Rijnbout S., Waheed A., Schmidt B., Geuze H., von Figura K. Cloning and expression of human steroid-sulfatase. Membrane topology, glycosylation, and subcellular distribution in BHK-21 cells. J Biol Chem. 1989 Aug 15;264(23):13865–13872. [PubMed] [Google Scholar]
- Vostrejs M., Moran P. L., Seligman P. A. Transferrin synthesis by small cell lung cancer cells acts as an autocrine regulator of cellular proliferation. J Clin Invest. 1988 Jul;82(1):331–339. doi: 10.1172/JCI113591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein I. B. Growth factors, oncogenes, and multistage carcinogenesis. J Cell Biochem. 1987 Mar;33(3):213–224. doi: 10.1002/jcb.240330308. [DOI] [PubMed] [Google Scholar]
- Williams C. L., Lennon V. A. Activation of M3 muscarinic acetylcholine receptors inhibits voltage-dependent calcium influx in small cell lung carcinoma. J Biol Chem. 1990 Jan 25;265(3):1443–1447. [PubMed] [Google Scholar]


