Abstract
Magnetism and multiplets for metal-phthalocyanine (MPc) molecules with transition-metals (M) of Mn and Co were investigated based on the constraint density functional theory calculations by imposing density matrix constraint on the d-orbital occupation numbers. For the MnPc, the ground state is found to be the 4Eg state with the perpendicular magnetic anisotropy with respect to the molecular plane, while for the CoPc, the ground state is the 2A1g state with a planar magnetic anisotropy.
INTRODUCTION
Transition-metal phthalocyanine (MPc), which forms a planar organic molecule with a transition-metal atom (M), is a promising material in applied and industrial fields, since a variety of electric, optical, and magnetic properties with the high chemical and physical stabilities can be designed by substituting the transition-metal in the MPc.1, 2, 3, 4, 5 Moreover, a challenge to miniaturize devices for novel magnetic applications now extends to treating an extreme limit of the single molecule. Importantly, the property of the MPc is governed by the electronic structure, so-called multiplet, which arises from the d-orbitals localized at the transition-metal atom. It is, thus, of importance to understand the multiplet structures of the MPc.
However, the ground state of the multiplets is still debated despite extensive experimental and theoretical investigations spanning decades. For examples, for the FePc, experiments6, 7, 8, 9 have individually proposed the 3Eg and either 3B2g or 3A2g state, and even the density functional theory (DFT) calculations have predicted contradictory.2, 10, 11, 12, 13, 14 For the MnPc, the multiplet for ground state has been suggested either 4Eg or 4B2g state,11, 15, 16 and for the CoPc, the 2Eg or 2A1g state.11, 13 For this, we previously proposed a constraint DFT approach, implemented in the full-potential linearized augmented plane-wave (FLAPW) method,17 by imposing density matrix constraints on the d-orbitals occupation numbers, and our total energy calculations demonstrate that for the FePc, there are three stationary states of the 3Eg, 3B2g, and 3A2g symmetries and that the ground state is the 3A2g state with a planar magnetic anisotropy (MA). Here, we extend our DFT investigations for the MnPc and CoPc, and we demonstrate that for the MnPc (CoPc), the ground state is the 4Eg (2A1g) state with the out-of-plane (planar) MA.
MODEL AND METHOD
An isolated molecule of the MPc has the simple planar structure shown in Fig. 1a, where the transition-metal (M) ion located at the center of the molecule has D4h site symmetry. In the forms, as shown in Fig. 1b, a ligand-field splits the d-orbitals into three singlets (dxy, dz2, and dx2-y2) and one doublet (dxz+yz). Ignoring the high lying dx2-y2 orbital that bonds to the four outer N ions, three multiplets 4A1g (dxy,↑, dz2,↑, dxz+yz,↑2, dz2,↓), 4B2g (dxy,↑, dz2,↑, dxz+yz,↑2, dxy,↓), and 4Eg (dxy,↑, dz2,↑, dxz+yz,↑2, dxz+yz,↓) for MnPc, where the majority-spin states except the dx2-y2 orbital are fully occupied, is approximately constructed, and two multiplets 2A1g (dxy,↑, dz2,↑, dxz+yz,↑2, dxy,↓, dxz+yz,↓2) and 2Eg (dxy,↑, dz2,↑, dxz+yz,↑2, dxy,↓, dz2,↓, dxz+yz,↓) for CoPc when no spin-orbit coupling (SOC) is considered.
Figure 1.
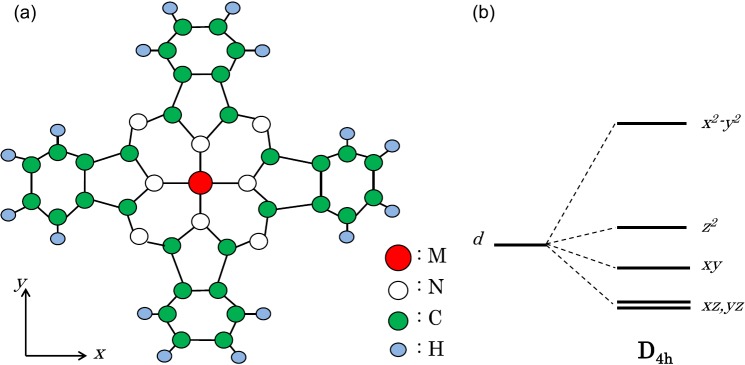
(a) Atomic structure of MPc molecule. Large closed circle (red) represents a transition-metal (M) ion located at the center of the molecule, which has D4h site symmetry. (b) Energy level splitting for M2+ with a D4h site symmetry in MPc.
As the model of the MPc, we adopted a monolayer slab with infinite vacuum on both sides, and a large in-plane lattice constant of 27 a.u. and atomic positions given by experiments18, 19 were employed.
Calculations were performed in the scalar relativistic approximation (SRA), i.e., excluding the SOC, based on the generalized gradient approximation (GGA)20 for exchange-correlation, by using the FLAPW method with film geometries by including fully the additional vacuum regions outside of a single slab.21, 22 The LAPW basis with cutoffs of |k + G| ≤ 3.6 a.u.−1 and muffin-tin (MT) sphere radii of 2.3 a.u. for transition metal (Mn and Co), 1.2 a.u. for N and C, and 0.8 a.u. for H were used. Lattice harmonics with angular momenta up to l = 8 for Mn and Co, 6 for N and C, and 4 for H are employed to expand the charge density, potential, and wave functions.
To constraint electronic configurations of multiplet structures in the MPc without a loss of the site symmetry, we introduce an appropriate total energy functional with constraint fields,
| (1) |
where EGGA[ρ(r)] is the usual total energy functional of the GGA, is a density matrix of d-orbitals of an atom α, and is an occupation number that should be constrained. In the LAPW basis, is given by the projection of the wave function onto the Ylm subspace23 as
| (2) |
| (3) |
where k and b refer to a k-point in the Brillouin zone and a band index, respectively. The is electron occupation (Fermi) function, and and are the radial wave function and its energy derivative in the basis function. The corresponding Kohn-Sham equation can be written as
| (4) |
where the constraint term in the Hamiltonian effectively projects out the chosen irreducible representation for the overall wave function, in which one can pick up an electronic configuration of corresponding multiplet. In practice, we specify a set of constraint fields, , along directions of the eigenvectors in the , consistent with the site symmetry. Then, the , which are rotated back from the , are introduced into Eq. 4, and the corresponding are determined self-consistently. The self-consistent calculations were carried out using the second variation scheme, i.e., the diagonalization of Eq. 4 was carried out in a basis of the eigenfunctions, φk,b, of HGGA. Full self-consistency was achieved for the density matrix as well as the charge and spin densities. The total energy is calculated using Eq. 1, with = .
To determine the MA, the second variational method24 for treating the SOC was performed by using the calculated eigenvectors in the SRA, and the MA energy, EMA, was determined by the force theorem,25, 26, 27 which is defined as the eigenvalue difference for the magnetization oriented along the planar direction and the out-of-plane direction with respect to the molecule plane.
RESULTS AND DISCUSSION
Mn-phthalocyanine
Calculated total energy differences, ΔE, of the three multiplets of 4A1g, 4B2g, and 4Eg states for the MnPc with respect to a variation of the constraint field μxz(yz),↓ are shown in Fig. 2a, where the constraint is imposed in the occupation number of the dxz(yz),↓ orbitals. First, we started self-consistent calculations by using an initial charge density superimposed by those of spherical atoms and with no constraint field, μxz(yz),↓ = 0, as carried out in the standard (usual) self-consistent calculations. We obtained the 4Eg solution [closed square in Fig. 2a], where the doublet dxz+yz,↓ orbitals located at the top of the valence states are occupied by one electron, as shown in Fig. 2b. When the constraint field μxz(yz),↓ is introduced in the positive direction, the multiplet structure changes to the 4B2g state [closed triangle in Fig. 2a]. However, as the μxz(yz),↓ sets to zero (zero constraint field), the system cannot remain in a stationary solution. Also, for 4A1g state, no stationary solution is obtained at zero constraint field. Thus, the ground state may be concluded to the 4Eg state.
Figure 2.
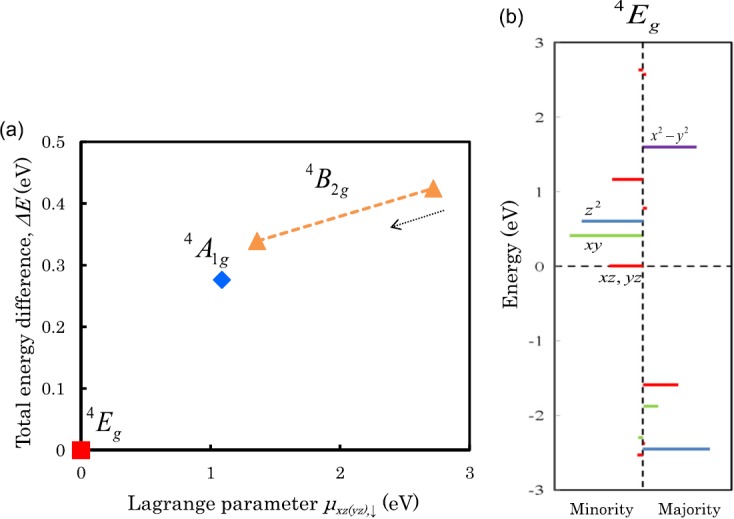
(a) Calculated total energy difference, ΔE, with respect to the constraint field, μxz(yz),↓, for MnPc, where the other μn's are set to zero. Closed square, rhombus, and triangle indicate solutions of 4Eg, 4A1g, and 4B2g states, respectively, obtained by self-consistent calculations. (b) Multiplet structures of the 4Eg state for the MnPc at zero constraint field. Bars (in color) represent weights of d-orbital contribution in muffin-tin Mn sphere. Negative and positive energies indicate occupied and unoccupied orbitals, respectively.
For the 4Eg ground state of the MnPc, the calculated EMA has a positive value of 3.5 meV/molecule, indicating that the spin moments energetically favor pointing out-of-plane (out-of-plane MA). According to perturbation theory,28 the SOC interaction between occupied and unoccupied d states with the same (different) m magnetic quantum number through the Lz (Lx, Ly) operator gives a positive (negative) contribution to the EMA. Thus, for the 4Eg state, the half-occupied state (the occupied and unoccupied states) in the doublet dxy+yz,↓ orbitals (m = ±1) yields a positive contribution to the EMA.
Co-phthalocyanine
Calculated ΔE with respect to variations of μxz(yz),↓ for the CoPc are shown in Fig. 3a. Self-consisted calculations with an superimposed initial charge density and no constraints yield the 2A1g state, where the singlet dxy,↓ and doublet dxz+yz,↓ orbitals are fully occupied, while the singlet dz2,↓ orbital locates in energy above the valence top, as shown in Fig. 3b. When the negative μxz(yz),↓ is introduced, the stationary solution of the 2Eg state is obtained, where the two singlets dxy,↓ and dz2,↓ orbitals are fully occupied, and the doublet dxz+yz,↓ orbitals are occupied by one electron. The total energy difference indicates that the ground state is the 2A1g state, whose energy is lower than that of the 2Eg state by 150 meV/molecule.
Figure 3.
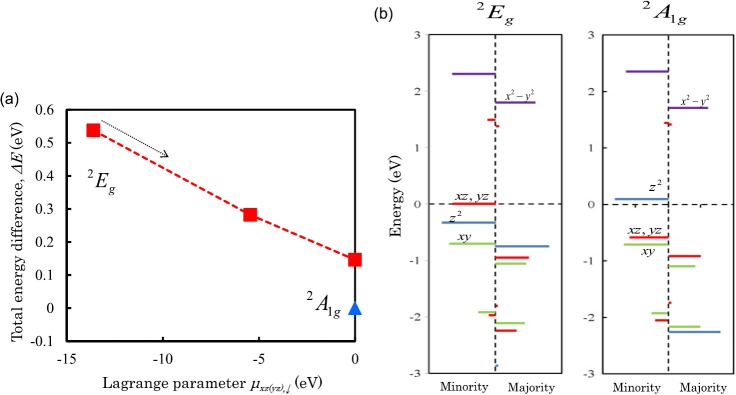
(a) Calculated total energy difference, ΔE, with respect to the constraint field, μxz(yz),↓, for CoPc, where the other μn's are set to zero. Closed triangle and square indicate solutions of 2A1g and 2Eg states, respectively, obtained by self-consistent calculations. (b) Multiplet structures of the 2Eg and 2A1g states for CoPc at zero constraint field. Notations are same as in Fig. 2.
The calculated EMAs for the 2A1g and 2Eg states result in −1.2 and 14.2 meV/molecule, which indicate the planar and out-of-plane MAs, respectively. The planar MA for ground state of the 2A1g state arises from the SOC between the occupied dxy+yz,↓ (m= ± 1) and unoccupied dz2,↓ (m = 0) orbitals, while the out-of-plane MA for the 2Eg state is due to the SOC in the half-occupied state in the doublet dxy+yz,↓ orbitals (m = ±1).
SUMMARY
We carried out the first principles calculations with the FLAPW method, based on constraint DFT, to investigate the electronic structures and MA of the multiplets for the MnPc and CoPc. Results predicted that for the MnPc, the ground state is the 4Eg state, in which the easy axis of magnetization favors pointing out of-plane, while there are no stationary solutions of the 4B2g and 4A1g states. For the CoPc, the 2A1g state with the planar MA is the ground state, whose total energy is lower than that of the 2Eg state.
ACKNOWLEDGMENTS
Work at Mie University was supported by a Grant-in-Aid for Scientific Research (No. 24540344) from the Japan Society for the Promotion of Science, and was performed under the Cooperative Research Program of “Network Joint Research Center for Materials and Devices.” Computations were partially performed at ISSP, University of Tokyo.
References
- Lever A. B. P., Hempstead M. R., Leznoff C. C., Liu W., Melnik M., Nevin W. A., and Seymour P., Pure Appl. Chem. 58, 1467 (1986). 10.1351/pac198658111467 [DOI] [Google Scholar]
- Reynolds P. A. and Figgis B. N., Inorg. Chem. 30, 2294 (1991). 10.1021/ic00010a015 [DOI] [Google Scholar]
- Orti E. and Bredas J. L., J. Am. Chem. Soc. 114, 8669 (1992). 10.1021/ja00048a046 [DOI] [Google Scholar]
- Zeis R., Siegrist T., and Kloc Ch., Appl. Phys. Lett. 86, 022103 (2005). 10.1063/1.1849438 [DOI] [Google Scholar]
- Liao M.-S., Watts J. D., and Huang M.-J., Inorg. Chem. 44, 1941 (2005). 10.1021/ic0401039 [DOI] [PubMed] [Google Scholar]
- Dale B. W., Williams R. J. P., Johnson C. E., and Thorp T. L., J. Chem. Phys. 49, 3441 (1968). 10.1063/1.1670617 [DOI] [Google Scholar]
- Coppens P., Li L., and Zhu N. J., J. Am. Chem. Soc. 105, 6173 (1983). 10.1021/ja00357a046 [DOI] [Google Scholar]
- Barraclough C. G., Martin R. L., Mitra S., and Sherwood R. C., J. Chem. Phys. 53, 1643 (1970). 10.1063/1.1674237 [DOI] [Google Scholar]
- Stillman M. J. and Thomson A. J., J. Chem. Soc., Faraday Trans. 2 70, 790 (1974). 10.1039/f29747000790 [DOI] [Google Scholar]
- Bialek B., Kim I. G., and Lee J. I., Surf. Sci. 526, 367 (2003). 10.1016/S0039-6028(03)00002-5 [DOI] [Google Scholar]
- Wang J., Shi Y., Cao J., and Wu R., Appl. Phys. Lett. 94, 122502 (2009). 10.1063/1.3100783 [DOI] [Google Scholar]
- Kuz'min M. D., Hayn R., and Oison V., Phys. Rev B 79, 024413 (2009). 10.1103/PhysRevB.79.024413 [DOI] [Google Scholar]
- Liao M.-S. and Scheiner S., J. Chem. Phys. 114, 9780 (2001). 10.1063/1.1367374 [DOI] [Google Scholar]
- Sumimoto M., Kawashima Y., Hori K., and Fujimoto H., Spectrochim. Acta, Part A 71, 286 (2008). 10.1016/j.saa.2008.02.033 [DOI] [PubMed] [Google Scholar]
- Miyoshi H., Bull. Chem. Soc. Jpn. 47, 561 (1974). 10.1246/bcsj.47.561 [DOI] [Google Scholar]
- Grobosch M., Mahns B., Loose C., Friedrich R., Schmidt C., Kortus J., and Knupfer M., Chem. Phys. Lett. 505, 122 (2011). 10.1016/j.cplett.2011.02.039 [DOI] [Google Scholar]
- Nakamura K., Kitaoka Y., Akiyama T., Ito T., Weinert M., and Freeman A. J., Phys. Rev. B 85, 235129 (2012). 10.1103/PhysRevB.85.235129 [DOI] [Google Scholar]
- Kirner J. K., Dow W., and Sheidt W. R., Inorg. Chem. 15, 1685 (1976). 10.1021/ic50161a042 [DOI] [Google Scholar]
- Williams G. A., Figgis B. N., Mason R., and Fielding P. E., J. Chem. Soc. Dalton Trans. 1990, 1688. 10.1039/DT9800001688 [DOI] [Google Scholar]
- Perdew J. P., Burke K., and Ernzerhof M., Phys. Rev. Lett. 77, 3865 (1996). 10.1103/PhysRevLett.77.3865 [DOI] [PubMed] [Google Scholar]
- Wimmer E., Krakauer H., Weinert M., and Freeman A. J., Phys. Rev. B 24, 864 (1981). 10.1103/PhysRevB.24.864 [DOI] [Google Scholar]
- Weinert M., Wimmer E., and Freeman A. J., Phys. Rev. B 26, 4571 (1982). 10.1103/PhysRevB.26.4571 [DOI] [Google Scholar]
- Shick A. B., Liechtenstein A. I., and Pickett W. E., Phys. Rev. B 60, 10763 (1999). 10.1103/PhysRevB.60.10763 [DOI] [Google Scholar]
- Li C., Freeman A. J., Jansen H. J. F., and Fu C. L., Phys. Rev. B 42, 5433 (1990). 10.1103/PhysRevB.42.5433 [DOI] [PubMed] [Google Scholar]
- Daalderop G. H. O., Kelly P. J., and Schuurmans M. F. H., Phys. Rev. B 41, 11919 (1990). 10.1103/PhysRevB.41.11919 [DOI] [PubMed] [Google Scholar]
- Weinert M., Watson R. E., and Davenport J. W., Phys. Rev. B 32, 2115 (1985). 10.1103/PhysRevB.32.2115 [DOI] [PubMed] [Google Scholar]
- Wang X. D., Wang D. S., Wu R. Q., and Freeman A. J., J. Magn. Magn. Mater. 159, 337 (1996). 10.1016/0304-8853(95)00936-1 [DOI] [Google Scholar]
- Wang D. S., Wu R., and Freeman A. J., Phys. Rev. B 47, 14932 (1993). 10.1103/PhysRevB.47.14932 [DOI] [PubMed] [Google Scholar]


