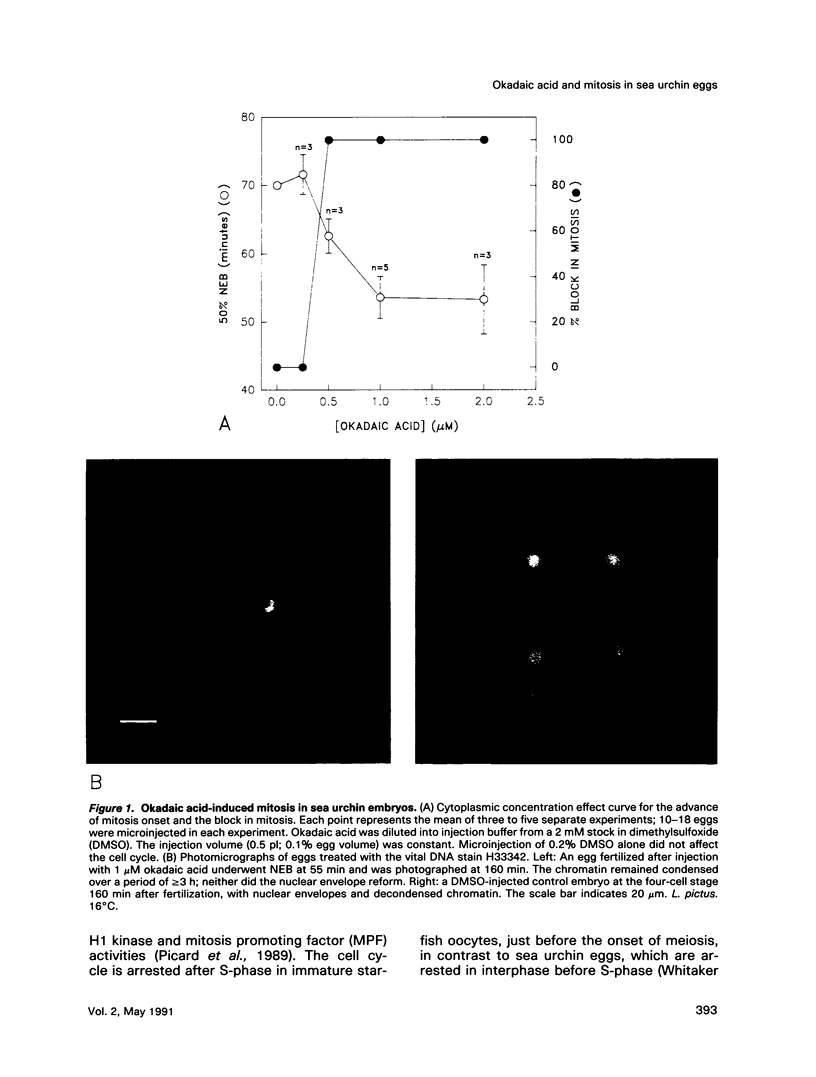
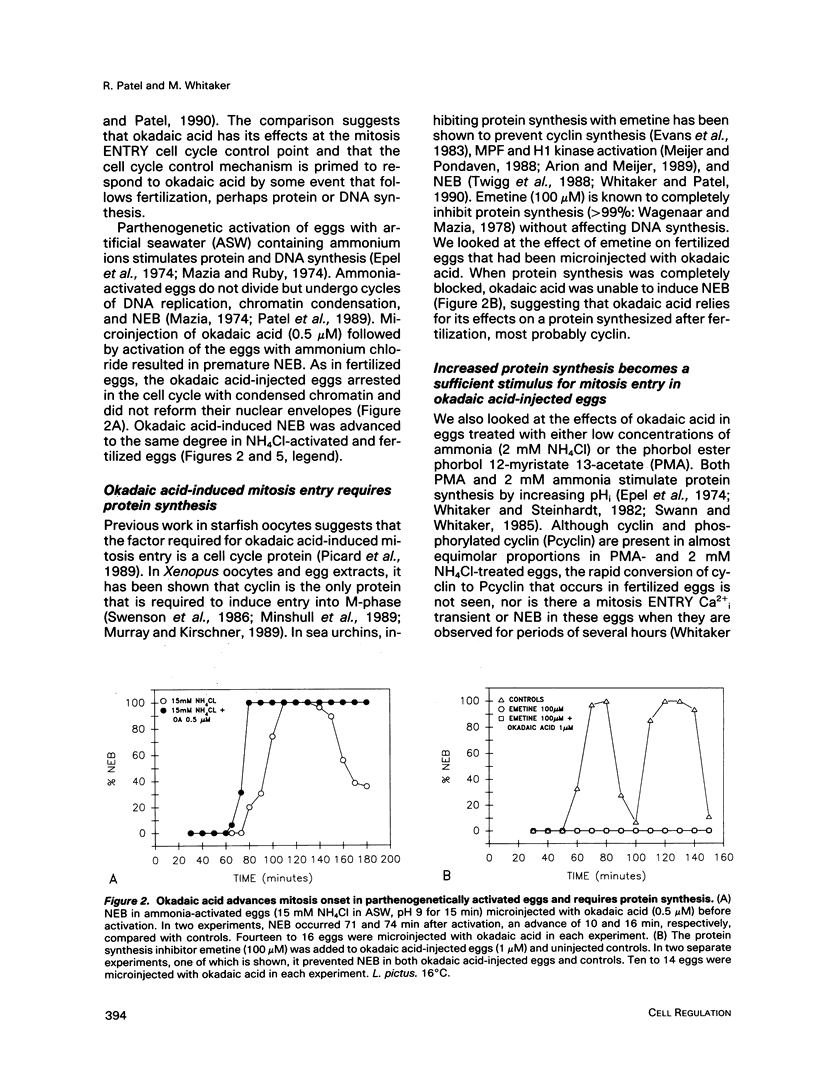
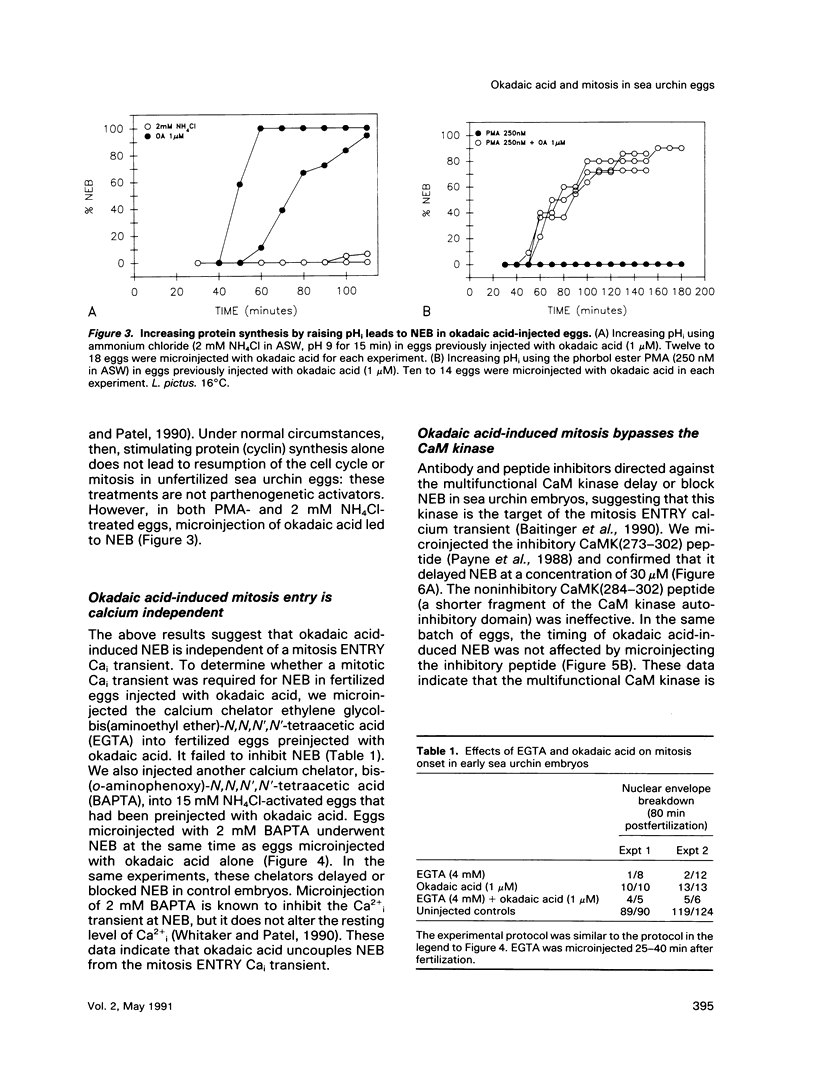
Abstract
We show that a phosphatase inhibitor, okadaic acid, induces premature and persistent mitosis during the first cell cycle in sea urchin embryos. Okadaic acid-induced mitosis requires protein synthesis, suggesting that it activates the protein synthesis-requiring mitotic H1 kinase. By microinjecting the calcium chelators BAPTA and EGTA and by measuring Cai using fura-2, an indicator dye, we show that okadaic acid-induced mitosis is independent of the calcium signal that usually triggers mitosis onset in sea urchin embryos. Disabling the calmodulin kinase II that is thought to respond to the mitotic Cai signal using a peptide inhibitor fails to prevent mitosis in response to okadaic acid. These data suggest that okadaic acid bypasses calcium regulation of mitosis by inducing constitutive phosphorylation of a site on the H1 kinase that is normally under the control of the calmodulin-regulated kinase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arion D., Meijer L. M-phase-specific protein kinase from mitotic sea urchin eggs: cyclic activation depends on protein synthesis and phosphorylation but does not require DNA or RNA synthesis. Exp Cell Res. 1989 Aug;183(2):361–375. doi: 10.1016/0014-4827(89)90397-2. [DOI] [PubMed] [Google Scholar]
- Axton J. M., Dombrádi V., Cohen P. T., Glover D. M. One of the protein phosphatase 1 isoenzymes in Drosophila is essential for mitosis. Cell. 1990 Oct 5;63(1):33–46. doi: 10.1016/0092-8674(90)90286-n. [DOI] [PubMed] [Google Scholar]
- Baitinger C., Alderton J., Poenie M., Schulman H., Steinhardt R. A. Multifunctional Ca2+/calmodulin-dependent protein kinase is necessary for nuclear envelope breakdown. J Cell Biol. 1990 Nov;111(5 Pt 1):1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R., Beach D. Involvement of a type 1 protein phosphatase encoded by bws1+ in fission yeast mitotic control. Cell. 1989 Jun 16;57(6):1009–1016. doi: 10.1016/0092-8674(89)90339-5. [DOI] [PubMed] [Google Scholar]
- Bradbury E. M., Inglis R. J., Matthews H. R. Control of cell division by very lysine rich histone (F1) phosphorylation. Nature. 1974 Feb 1;247(5439):257–261. doi: 10.1038/247257a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Lowy D. R., Teich N. M., Levine A. S., Rowe W. P. Evidence that the AKR murine-leukemia-virus genome is complete in DNA of the high-virus AKR mouse and incomplete in the DNA of the "virus-negative" NIH mouse. Proc Natl Acad Sci U S A. 1974 Jan;71(1):167–171. doi: 10.1073/pnas.71.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou Y. H., Rebhun L. I. Purification and characterization of a sea urchin egg Ca2+-calmodulin-dependent kinase with myosin light chain phosphorylating activity. J Biol Chem. 1986 Apr 25;261(12):5389–5395. [PubMed] [Google Scholar]
- Cohen P., Klumpp S., Schelling D. L. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett. 1989 Jul 3;250(2):596–600. doi: 10.1016/0014-5793(89)80803-8. [DOI] [PubMed] [Google Scholar]
- Cyert M. S., Thorner J. Putting it on and taking it off: phosphoprotein phosphatase involvement in cell cycle regulation. Cell. 1989 Jun 16;57(6):891–893. doi: 10.1016/0092-8674(89)90325-5. [DOI] [PubMed] [Google Scholar]
- Doonan J. H., Morris N. R. The bimG gene of Aspergillus nidulans, required for completion of anaphase, encodes a homolog of mammalian phosphoprotein phosphatase 1. Cell. 1989 Jun 16;57(6):987–996. doi: 10.1016/0092-8674(89)90337-1. [DOI] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Epel D., Steinhardt R., Humphreys T., Mazia D. An analysis of the partial metabolic derepression of sea urchin eggs by ammonia: the existence of independent pathways. Dev Biol. 1974 Oct;40(2):245–255. doi: 10.1016/0012-1606(74)90127-4. [DOI] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Félix M. A., Cohen P., Karsenti E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: evidence from the effects of okadaic acid. EMBO J. 1990 Mar;9(3):675–683. doi: 10.1002/j.1460-2075.1990.tb08159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gavin A. C., Tsukitani Y., Schorderet-Slatkine S. Induction of M-phase entry of prophase-blocked mouse oocytes through microinjection of okadaic acid, a specific phosphatase inhibitor. Exp Cell Res. 1991 Jan;192(1):75–81. doi: 10.1016/0014-4827(91)90159-r. [DOI] [PubMed] [Google Scholar]
- Goris J., Hermann J., Hendrix P., Ozon R., Merlevede W. Okadaic acid, a specific protein phosphatase inhibitor, induces maturation and MPF formation in Xenopus laevis oocytes. FEBS Lett. 1989 Mar 13;245(1-2):91–94. doi: 10.1016/0014-5793(89)80198-x. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hohmann P. Phosphorylation of H1 histones. Mol Cell Biochem. 1983;57(1):81–92. doi: 10.1007/BF00223526. [DOI] [PubMed] [Google Scholar]
- Kao J. P., Alderton J. M., Tsien R. Y., Steinhardt R. A. Active involvement of Ca2+ in mitotic progression of Swiss 3T3 fibroblasts. J Cell Biol. 1990 Jul;111(1):183–196. doi: 10.1083/jcb.111.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita N., Ohkura H., Yanagida M. Distinct, essential roles of type 1 and 2A protein phosphatases in the control of the fission yeast cell division cycle. Cell. 1990 Oct 19;63(2):405–415. doi: 10.1016/0092-8674(90)90173-c. [DOI] [PubMed] [Google Scholar]
- Mazia D., Ruby A. DNA synthesis turned on in unfertilized sea urchin eggs by treatment with NH4OH. Exp Cell Res. 1974 Mar 30;85(1):167–172. doi: 10.1016/0014-4827(74)90227-4. [DOI] [PubMed] [Google Scholar]
- Meijer L., Arion D., Golsteyn R., Pines J., Brizuela L., Hunt T., Beach D. Cyclin is a component of the sea urchin egg M-phase specific histone H1 kinase. EMBO J. 1989 Aug;8(8):2275–2282. doi: 10.1002/j.1460-2075.1989.tb08353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L., Pondaven P. Cyclic activation of histone H1 kinase during sea urchin egg mitotic divisions. Exp Cell Res. 1988 Jan;174(1):116–129. doi: 10.1016/0014-4827(88)90147-4. [DOI] [PubMed] [Google Scholar]
- Minshull J., Blow J. J., Hunt T. Translation of cyclin mRNA is necessary for extracts of activated xenopus eggs to enter mitosis. Cell. 1989 Mar 24;56(6):947–956. doi: 10.1016/0092-8674(89)90628-4. [DOI] [PubMed] [Google Scholar]
- Morla A. O., Draetta G., Beach D., Wang J. Y. Reversible tyrosine phosphorylation of cdc2: dephosphorylation accompanies activation during entry into mitosis. Cell. 1989 Jul 14;58(1):193–203. doi: 10.1016/0092-8674(89)90415-7. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell biology. Cyclins in meiosis and mitosis. Nature. 1987 Apr 9;326(6113):542–543. doi: 10.1038/326542a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W. Cell biology: the cell cycle as a cdc2 cycle. Nature. 1989 Nov 2;342(6245):14–15. doi: 10.1038/342014a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989 May 25;339(6222):275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Néant I., Charbonneau M., Guerrier P. A requirement for protein phosphorylation in regulating the meiotic and mitotic cell cycles in echinoderms. Dev Biol. 1989 Apr;132(2):304–314. doi: 10.1016/0012-1606(89)90227-3. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Patel R., Twigg J., Crossley I., Golsteyn R., Whitaker M. Calcium-induced chromatin condensation and cyclin phosphorylation during chromatin condensation cycles in ammonia-activated sea urchin eggs. J Cell Sci Suppl. 1989;12:129–144. doi: 10.1242/jcs.1989.supplement_12.11. [DOI] [PubMed] [Google Scholar]
- Payne M. E., Fong Y. L., Ono T., Colbran R. J., Kemp B. E., Soderling T. R., Means A. R. Calcium/calmodulin-dependent protein kinase II. Characterization of distinct calmodulin binding and inhibitory domains. J Biol Chem. 1988 May 25;263(15):7190–7195. [PubMed] [Google Scholar]
- Picard A., Capony J. P., Brautigan D. L., Dorée M. Involvement of protein phosphatases 1 and 2A in the control of M phase-promoting factor activity in starfish. J Cell Biol. 1989 Dec;109(6 Pt 2):3347–3354. doi: 10.1083/jcb.109.6.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Tsien R. Y., Steinhardt R. A. Changes of free calcium levels with stages of the cell division cycle. Nature. 1985 May 9;315(6015):147–149. doi: 10.1038/315147a0. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Steinhardt R. A., Alderton J. Intracellular free calcium rise triggers nuclear envelope breakdown in the sea urchin embryo. Nature. 1988 Mar 24;332(6162):364–366. doi: 10.1038/332364a0. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. Stimulation of the Na/H exchanger of sea urchin eggs by phorbol ester. Nature. 1985 Mar 21;314(6008):274–277. doi: 10.1038/314274a0. [DOI] [PubMed] [Google Scholar]
- Swann K., Whitaker M. The part played by inositol trisphosphate and calcium in the propagation of the fertilization wave in sea urchin eggs. J Cell Biol. 1986 Dec;103(6 Pt 1):2333–2342. doi: 10.1083/jcb.103.6.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Twigg J., Patel R., Whitaker M. Translational control of InsP3-induced chromatin condensation during the early cell cycles of sea urchin embryos. Nature. 1988 Mar 24;332(6162):366–369. doi: 10.1038/332366a0. [DOI] [PubMed] [Google Scholar]
- Wagenaar E. B. The timing of synthesis of proteins required for mitosis in the cell cycle of the sea urchin embryo. Exp Cell Res. 1983 Apr 1;144(2):393–403. doi: 10.1016/0014-4827(83)90419-6. [DOI] [PubMed] [Google Scholar]
- Whitaker M. J., Steinhardt R. A. Ionic regulation of egg activation. Q Rev Biophys. 1982 Nov;15(4):593–666. doi: 10.1017/s0033583500003760. [DOI] [PubMed] [Google Scholar]
- Whitaker M., Patel R. Calcium and cell cycle control. Development. 1990 Apr;108(4):525–542. doi: 10.1242/dev.108.4.525. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Yasuda H., Pines J., Yasumoto K., Nishitani H., Ohtsubo M., Hunter T., Sugimura T., Nishimoto T. Okadaic acid, a potent inhibitor of type 1 and type 2A protein phosphatases, activates cdc2/H1 kinase and transiently induces a premature mitosis-like state in BHK21 cells. EMBO J. 1990 Dec;9(13):4331–4338. doi: 10.1002/j.1460-2075.1990.tb07882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]