Abstract
The objective of this study is to delineate whether overexpression of human efflux transporters (P-gp, MRP2, and BCRP) in transfected MDCK cells affect the functional activities, and gene and protein expression of endogenous influx peptide transporter system (PepT). Real-time PCR, immunoblotting, uptake and permeability studies of [3H]Gly-Sar were conducted on transfected MDCKII and wild-type cells to investigate functional differences. Cellular [3H]Gly-Sar accumulation was significantly lower in transfected MDCKII cell lines compared to wild-type cells. Transport efficiency of apical peptide transporters was markedly reduced to around 25%, 30%, and 40% in P-gp-, MRP2-, and BCRP-overexpressed MDCK cell lines, respectively. With ascending cell-passage, transport efficiency was enhanced. A significantly higher Gly-Sar permeability was observed across parental cell-monolayers over transfected cells at all pHs. Levels of mRNA for both canine PepT1 and PepT2 were substantially reduced when efflux transporters overexpressed but enhanced when mRNA-levels of efflux genes diminished with ascending cell-passage of transfected cells. An inverse correlation was evident between endogenous PepT and exogenous efflux transporters in transfected MDCKII cells. Results of protein expression also supported these findings. Overexpression of MDR genes can affect endogenous PepT function which might be due to the phenomenon of transporter-compensation resulting in down-regulation of endogenous genes.
Keywords: Peptide transporters, MDCK cells, Efflux pumps, MDR transfection
1. Introduction
Efflux transporters, including multidrug resistance proteins –P-glycoprotein (P-gp, MDR1, and ABCB1), multidrug resistance-associated protein 2 – MRP2 (ABCC2), and breast cancer resistance protein – BCRP (ABCG2), have been widely accepted as important defense against exogenous compounds encountered in daily life (Ito et al., 2005). Due to their broad substrate specificity and high expression at apical membranes of epithelial barriers like intestine, blood–brain barrier, liver and kidney, these efflux proteins regulate the pharmacokinetic and pharmacodynamic process of numerous therapeutic compounds. Efflux activity of these transporters is the major factor responsible for the poor absorption, lower bioavailability, and high metabolism of a variety of drugs, consequently exhibit a dramatic impact on their therapeutic outcomes and clinical application (Pal et al., 2011; Thuerauf and Fromm, 2006). Therefore a high-throughput screening in vitro model is critical for predicting the in vivo absorption of new promising drug candidates.
Madin–Darby canine kidney (MDCK) cell line has been widely employed for cell-based permeability screening model (Irvine et al., 1999). There are two strains of MDCK cells, both of which are derived from the distal tubule or collecting duct of the nephron (Ojakian and Herzlinger, 1984). In comparison with MDCK strain I cells which are non-ciliated, the strain II cells are ciliated, columnar in shape, and have microvilli on their apical surface when grown on permeable supports (von Bonsdorff et al., 1985). MDCK cells express various endogenous influx transporters, including amino acid transporters (Boerner et al., 1986), organic cation transporters (Shu et al., 2001) and peptide transporters (Putnam et al., 2002; Landowski et al., 2005) and efflux transporters like P-gp (Horio et al., 1989) and the multidrug resistance protein (MRP, ABCC) family (Flanagan et al., 2002). At present, wild-type MDCK strain II cells transfected with the human MDR1, MRP2, or BCRP genes are employed as quick assessment models to estimate in vivo permeability of new drug candidates, which are substrates, inducers, or inhibitors of these efflux pumps, across intestinal mucosa (Agarwal et al., 2007a; Tang et al., 2002a,b; Xiao et al., 2006) or the blood–brain barriers (Wang et al., 2005; Gumbleton and Audus, 2001). However, a large number of these drug candidates are also substrates for influx transporters, and prodrug modification with peptide is a common practice to circumvent efflux. The assumption for drug screening using transfected cell models is that the function and expression of endogenous transporters in transfected cells are comparable with parental cells. Accordingly a proper prediction for the in vivo drug absorption can be obtained from results of permeability assay conducted across these transfected cell models. But recently, gene expression of endogenous canine P-gp in MDCK cells has been reported to be significantly down-regulated after transfection with human MDR1. Transepithelial transport of vinblastine, a substrate for P-gp, was consequently underestimated when comparing the result obtained across human MDR1-transfected versus wild-type MDCK cells (Kuteykin-Teplyakov et al., 2010). Therefore we asked question, does transfection of efflux transporters affect the function of endogenous influx transporters too? If so, it might lead to significant bias in screening dual substrates for influx/efflux transporters, like “peptidomimetics” using these transfected cell lines.
In recent years, considerable research in targeted drug delivery indicate that peptide transporters are attractive targets for new drug discovery because they have broad substrate specificity and high capacity, especially for smaller peptides, i.e. di and tripeptides (Steffansen et al., 2004; Ganapathy and Leibach, 1996). Also “peptidomimic” drugs can be recognized as substrates by the peptide transporter-mediated influx system and ferried across epithelial membrane into systemic circulation (Ganapathy et al., 1995; Wang et al., 2012). Apical peptide transporters in MDCK cells are H+/peptide co-transporters, mainly peptide transporter-2 (PepT2, SLC15A2) (Sawada et al., 2001; Balimane et al., 2007), whereas basolateral peptide transporters are still unknown (Terada et al., 2000). PepT2, a high-affinity and low-capacity nutrient transporter, is expressed in a variety of tissues including kidney, lung, brain, mammary gland, and testis (Lu and Klaassen, 2006). The driving force for PepT2-mediated peptide transport is provided by an inwardly directed H+ gradient and an inside-negative membrane potential. It plays a key role in the uptake and transport of mammalian protein nutrients across biological membranes as well as another important H+/peptide cotransporter PepT1 (SLC15A1). Thus several publications have demonstrated the application of intact or transfected MDCK cell models to characterize permeability and absorption of substrates for peptide transporters (Ouyang et al., 2009; Agarwal et al., 2007b; Jain et al., 2008). However, no previous work has been reported about the alteration of endogenous peptide transporter system expressed in the MDCK cells, especially on apical membrane, after transfection with different human efflux protein genes.
Functional characterization of peptide transporters was evaluated in various MDCKII cell lines in the present study. Uptake and transport of [3H]Gly-Sar were conducted on MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells as well as MDCKII wild-type cells. Real-time PCR and Western blot analyses were also performed to investigate mRNA and protein levels of both exogenous efflux pumps and endogenous peptide transporters, respectively. The aim of this work is to determine whether the function of peptide transporters is compromised by the overexpressed efflux transporter in transfected MDCKII cell lines or not. This study may provide a more accurate assessment for these in vitro models to screen the compounds mediated by peptide transporter/efflux pumps in drug discovery.
2. Experimental materials and methods
2.1. Materials
Transfected MDCKII cells overexpressing human MDR1 (MDCKII-MDR1, passage 3–30), human MRP2 (MDCKII-MRP2, passage 3–30), human BCRP (MDCKII-BCRP, passage 3–30), and wild-type MDCK cells (MDCKII-wt) were generously provided by Dr. P. Borst and Dr. A. Schinkel (The Netherlands Cancer Institute, Amsterdam, Netherlands), respectively. [3H]Glycylsarcosine ([3H]Gly-Sar, 4 Ci/mmol), and [14C]Mannitol (58.8 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA). Dulbecco’s modified Eagle’s medium (DMEM), TrypLE™ Express Stable Trypsin Replacement, and Trizol-LS® reagent were obtained from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawenceville, GA). M-MLV reverse transcriptase, random hexamers, and M-MLV RT 5× reaction buffer were purchased from Promega (Madison, WI). Light Cycler® 480 SYBR Green 1 master kit was obtained from Roche Applied Science (Foster City, CA). NuPAGE® Novex 4–12% Bis–Tris gels and MagicMark™ XP Western Protein Standard (20–220 kDa) were obtained from Invitrogen (Carlsband, CA). Polyclonal canine PepT1 and canine PepT2 primary antibodies were purchased from Biorbyt, Ltd. (Cambridge, Cambridgeshire, UK). BioRad protein estimation kit was purchased from BioRad (Hercules, CA). BCA protein assay kit was obtained from Thermo Scientific (Rockford, IL). Fosinopril and scintillation cocktail reagent was obtained from Fisher Scientific Inc (Fair Lawn, NJ). Glycyl-L-proline (Gly-Pro) was purchased from TCI America (Portland, OR). Glycylsarcosine (Gly-Sar), cefadroxil, Triton X-100, hydroxyl ethyl piperazine ethane sulfonic acid (HEPES), D-glucose and all other chemicals were obtained from Sigma Chemical Co. (St. Louis, MO). All chemicals were products of special reagent grade and used as such. Transwell® inserts and 12-well tissue culture plates were purchased from Corning Costar Corp. (Cambridge, MA).
2.2. Cell culture
Wild-type and transfected MDCKII cells were seeded at a density of 40,000 cells/cm2 in 75 cm2 cell culture flasks and maintained in DMEM containing 10% heat-inactivated FBS, 20 mM HEPES, 29 mM sodium bicarbonate, 100 units/mL penicillin, and 100 μg/mL streptomycin. Medium was replaced every other day. Cells were harvested and passaged at 70–80% confluence using TrypLE™ express stable trypsin replacement at 5–7 days post seeding, and plated at 7 × 104 cells/cm2 on 12-well tissue culture plates for apical uptake studies, and 2 × 105 cells/cm2 on 12-well Transwell® inserts (diameter 12 mm, pore size 0.4 μm) for baso-lateral uptake and transport studies. Medium was changed every alternate day. All experiments except passage dependent studies, including passage dependent Gly-Sar uptake, inhibitory studies and real-time PCR assay, were conducted using cells with low cell-passage number (less than 6).
2.3. Uptake studies
Cells were washed twice with 2 mL Dulbecco’s phosphate-buffered saline (DPBS, pH 7.4) before the experiments. Uptake was initiated by adding 1 mL DPBS with 0.5 μCi/mL of [3H]Gly-Sar into the wells. Incubation was conducted at 37 °C or 4 °C for 15 min. The tracer solution was aspirated and cells were rinsed three times with ice-cold stop solution (200 mM KCl and 2 mM HEPES) to determine drug uptake. Then the cells were lysed by adding 1 mL of 0.3 N NaOH containing 0.1% Triton-X 100 solution to each well and left overnight at room temperature. Aliquots from each well were transferred to scintillation vials containing 3 mL of scintillation cocktail. Cellular radioactivity was quantified using a scintillation counter (Model LS-6500; Beckman Counter, Fullerton, CA) and then was normalized by amount of protein measured using BioRad protein estimation kit in each well.
2.3.1. Growth, temperature and pH dependence
Uptake of [3H]Gly-Sar (0.5 μCi/mL) was conducted at 37 °C for different time in culture (3–8 days) to determine the optimum culture period for uptake studies. Temperature and pH dependent uptake of [3H]Gly-Sar in various MDCK cell lines at low cell-passage was evaluated at 4 °C and 37 °C, respectively. During final calculation of [3H]Gly-Sar uptake at 37 °C, the passive diffusion component (i.e. at 4 °C) was subtracted. To study pH dependency of Gly-Sar uptake, incubation media DPBS with different pHs from 4.0 to 7.4 were prepared, and permeant solutions (0.5 μCi/mL [3H]Gly-Sar) were made accordingly. Cells were pre-incubated with DPBS (pH 7.4) for 20 min before adding permeant solutions with various pHs.
2.3.2. Concentration dependence
[3H]Gly-Sar solutions with various concentrations (5–250 μM) of unlabeled Gly-Sar were used in this study to determine concentration dependent Gly-Sar cellular accumulation in both wild-type and transfected MDCK cell lines. Uptake was carried out at 4 °C and 37 °C, respectively. Then the data was fitted to a Michaelis–Menten equation as shown in Section 2.8 to determine the kinetic parameters of peptide transporter-mediated specific Gly-Sar uptake.
2.3.3. Passage dependence
In order to illustrate whether various transfected MDCK cell lines exhibit passage dependency for Gly-Sar uptake, concentration dependent studies at both 4 °C and 37 °C were conducted on MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells with different passage number (3–25). Then the uptake kinetic parameter transport efficiency for peptide transporters-mediated Gly-Sar uptake was estimated using Michaelis–Menten equation.
2.3.4. Inhibitory Gly-Sar uptake
To determine the substrate specificity, apical [3H]Gly-Sar (0.5 μCi/mL) in the absence or presence of peptide transporter inhibitors, fosinopril (100 μM), cefadroxil (20 mM) and Gly-Pro (20 mM), was evaluated in various MDCKII cell lines with low (<6) or high (>25) cell-passages.
2.4. Transport studies
Transepithelial transport of Gly-Sar was evaluated across MDCKII cell monolayers (cell-passage number < 6) grown on Transwell® inserts. Medium was aspirated before experiments and cell monolayers were washed twice with DPBS (pH 7.4). Working volumes of the apical (AP) and basolateral (BL) compartments were 0.5 and 1.5 mL, respectively. The monolayer integrity was evaluated by measuring transepithelial electrical resistance (TEER) values using volt–ohm meter (EVOM-G, World Precision Instruments, Sarasota, FL). Only the monolayers with TEER values of about 600 ± 60 ohm cm2 was used in this study. Transport was initiated when 0.5 μCi/mL [3H]Gly-Sar or 0.25 μCi/mL of [14C]Mannitol in DPBS was added in donor chambers and only fresh DPBS in receiving chambers. The pH of incubation media varied from 4.0 to 7.4 in AP chambers only, but remained consistent in BL chambers to pH 7.4. Aliquots (200 μL) were withdrawn from receiving chambers at predetermined time intervals over a period of 60 min and replaced with same volume of fresh DPBS to maintain sink conditions. Samples were analyzed using a liquid scintillation counter described in uptake studies. At the end of experiments, the integrity of monolayers was checked by measuring TEER values. Cell monolayers with less than 10% of initial TEER values dropped was considered as integrity unchanged. Specific Gly-Sar transport across both parental and transfected MDCKII cell lines was calculated by subtracting nonspecific transport (passive diffusion) estimated from [14C]Mannitol from [3H]Gly-Sar transport.
2.5. Tight junction determination
To evaluate the intercellular tight junctions of different MDCKII cell lines, transport of [14C]Mannitol (0.125 μCi/mL) in DPBS (pH 7.4) was performed across MDCK cell monolayers for up to 60 min, as outlined above.
2.6. Real-time PCR assay
Total RNA from different MDCK cells was isolated using Trizol reagent according to the manufacturer’s protocol. Concentration and purity of RNA were estimated using spectrophotometer at 260 and 280 nm. Total RNA (1.5 μg) was reverse transcribed to cDNA using random hexamers and M-MLV Reverse Transcriptase Reagent. After the first strand cDNA synthesis, 100 ng of cDNA was amplified by real-time PCR using LightCycler® 480 SYBR Green-1 Master mix on an ABI Prism 5700 Sequence Detection System (PE Applied Biosystems, Foster City, CA) to evaluate the gene expression of hMDR1 (human), hMRP2 (human), hBCRP (human), cPepT1 (canine), and cPepT2 (canine). Sequences of primers used in this study are summarized in Table 1. GAPDH gene was used as endogenous reference to normalize target gene mRNAs in samples. Comparative threshold method was used to calculate the relative amount of mRNA in comparison with control (cells with lowest passage number).
Table 1.
PCR primers used in real-time PCR assay of transporter expression.
| General names | Species | Primer directions | Primer sequences |
|---|---|---|---|
| PepT1 | Canine | Forward | 5′-GCTTGTTACCCACTGGCATT-3′ |
| Reverse | 5′-GCAAAACCAATGCACTTGAC-3′ | ||
| PepT2 | Human and canine | Forward | 5′-CAGCTTTTGGTGGAGACCAG-3′ |
| Reverse | 5′-AAATCAAGCTCCCTGCATTG-3′ | ||
| MDR1 | Human | Forward | 5′-GAAGCCAGAACATTCCTCCTGGAA-3′ |
| Reverse | 5′-AGCCGCTACTCGAATGAGCTC-3′ | ||
| MRP2 | Human | Forward | 5′-GGTCATCCTTTACGGAGAACATCA-3′ |
| Reverse | 5′-GGACTGCGTCTGGAACGAAG-3′ | ||
| BCRP | Human | Forward | 5′-CCGCCACTCCCACTGAGATT-3′ |
| Reverse | 5′-CTCGGAGGCAGCGCTTTAAC-3′ |
2.7. Western blot
Confluent cells grown on 75 cm2 cell culture flask were washed twice with ice-cold PBS and lysed on ice in RIPA buffer containing freshly added protease inhibitor cocktail. Protein lysate was collected by centrifugation at 15,000 × g, 4 °C for 10 min and the total protein content was determined using BCA protein assay kit. Subsequently an equal amount of total protein (40 μg/lane) was loaded and separated on ready-made Novex 4–12% Bis–Tris gels. Proteins then were transferred to a polyvinylidene fluoride (PVDF) membrane, blocked with 5% non-fat dry milk and 1% BSA for nonspecific binding and probed with primary antibodies specific to canine PepT1 and PepT2, respectively. Following three 5-min washes with TBST (Tris buffered saline + 0.1% Tween 20), blots were probed with secondary antibody in TBST (1:20,000 anti-rabbit IgG-HRP) and visualized by Fujifilm LAS 4000 imaging system (FUJIFILM Medical Systems USA, Inc., Stamford, CT).
2.8. Data analysis
Affinity and capacity of Gly-Sar to peptide transporters were fit to Michaelis–Menten equation by nonlinear regression to determine the kinetic parameters Km and Vmax. Data modeling was performed using KaleidaGraph (Synergy Software). Transport efficiency of peptide transporters was subsequently represented by dividing Vmax by Km:
where V is the rate of Gly-Sar uptake, Vmax is the maximum uptake rate for transporter-mediated process, Km (Michaelis–Menten constant) is the concentration at half-saturation, and [S] is the Gly-Sar concentration.
Apparent permeability coefficients Papp (cm/s) were calculated by linear regression analysis on the time course plot of amount of drugs transported across cell monolayers:
where TRcum/dt is the flux rate of Gly-Sar or mannitol obtained from the slope of transport profile. A is the surface area of cell monolayers. C0 is the initial concentration of Gly-Sar or mannitol in the donor chambers.
2.9. Statistical analysis
All experiments were conducted at least in triplicate and repeated independently three or four times. The results were expressed as mean ± SD. One-way analysis of variance (ANOVA) followed by Tukey post hoc test was performed to test for statistically significant differences. Statistical analysis and data fitting were performed using PASW Statistics 17.0 (SPSS, Inc., Chicago, IL). Difference between mean values was considered statistically significant at p < 0.05 and very statistically significant at p < 0.01.
3. Results
3.1. Tight junction determination of MDCKII cell lines
The change of tight junction of MDCK cells after transfection with various human efflux genes was evaluated by determining AP–BL mannitol permeability across different cell lines. Results in Fig. 1 indicate that [14C]Mannitol permeability across wild-type MDCK cells significantly reduced from (4.21 ± 0.05) × 10−5 cm/s to (1.84 ± 0.09) × 10−5 cm/s, (2.18 ± 0.22) × 10−5 cm/s, and (1.07 ± 0.12) × 10−5 cm/s after transfection with human MDR1, MRP2, and BCRP genes, respectively.
Fig. 1.
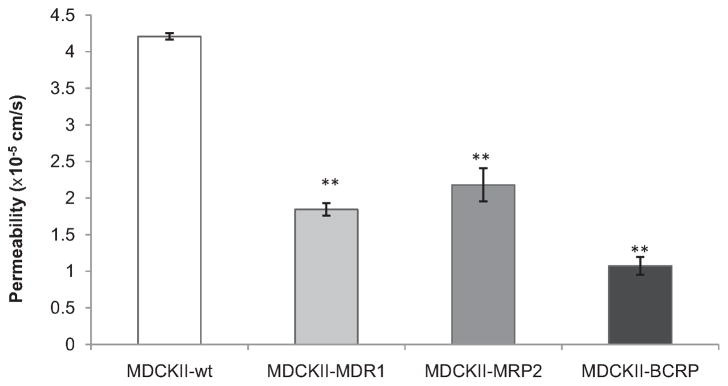
AP–BL permeability of [14C]Mannitol (0.125 μCi/mL) across various MDCKII cell lines at pH 7.4. Each point represents mean ± SD (n = 4). **P < 0.01 compared with MDCKII-wt cells.
3.2. Growth dependence of Gly-Sar uptake in MDCKII cell lines
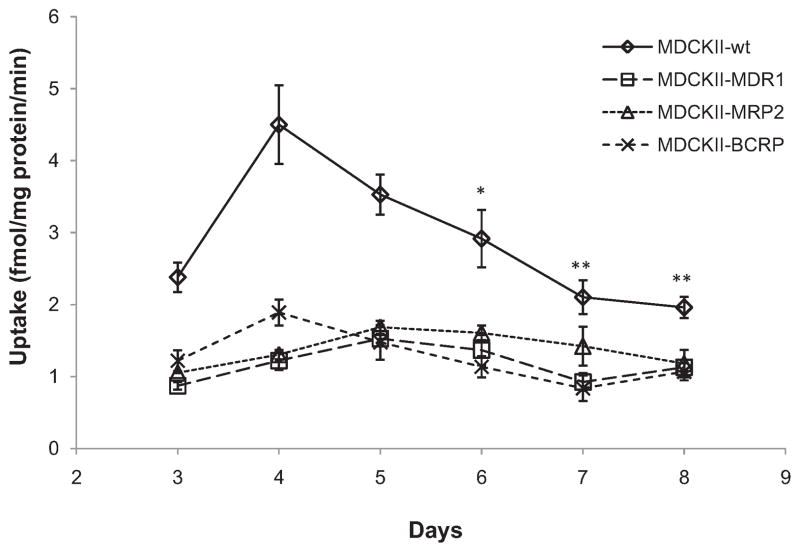
Results in Fig. 2 demonstrate growth dependent Gly-Sar uptake by apical peptide transporters in different MDCKII cell lines through 3–8 days of culture period. The most [3H]Gly-Sar uptake was observed after 4–5 days post seeding in MDCKII-wt, MDCKII-MRP2 and MDCKII-BCRP cell lines. The transfected MDCKII-MDR1 cell line did not show statistically significant different in growth dependent Gly-Sar uptake study. Therefore all subsequent experiments were conducted on the 5th day of seeding to normalize and compare results. Apical uptake of [3H]Gly-Sar was diminished significantly in all transfected MDCKII cell lines compared to wild-type cells.
Fig. 2.
Growth dependent [3H]Gly-Sar (0.5 μCi/mL) uptake at pH 7.4 for 15 min in various MDCKII cell lines. Each point represents mean ± SD (n = 4). *P < 0.05 and **P < 0.01 compared with uptake of same cell line at day 4.
3.3. Temperature dependence of Gly-Sar uptake in MDCKII cell lines
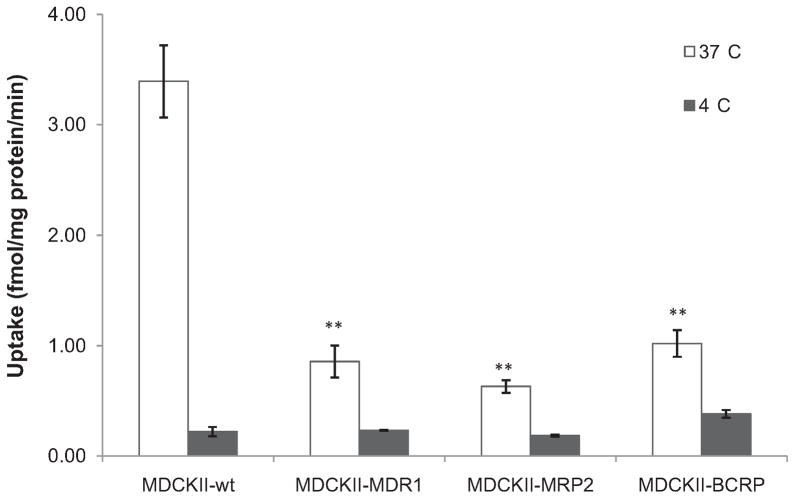
Results of temperature dependent Gly-Sar uptake by apical peptide transporters in different MDCKII cell lines are described in Fig. 3. Around 3–5-fold higher [3H]Gly-Sar uptake was obtained in MDCKII-wt cells in comparison with transfected cells at 37 °C. Whereas the Gly-Sar cellular accumulation at 4 °C, which was mainly achieved via nonspecific uptake by transcellular passive diffusion, exhibited similar extent in all four MDCKII cell lines. Subsequently, apical peptide transporters-mediated specific [3H]Gly-Sar uptakes, calculated by subtracting nonspecific cellular accumulation at 4 °C from total cellular uptake at 37 °C, were found to be 3.17 ± 0.33, 0.62 ± 0.14, 0.45 ± 0.06, and 0.64 ± 0.12 fmol/mg protein/min in MDCKII-wt, MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells, respectively.
Fig. 3.
Temperature dependent [3H]Gly-Sar (0.5 μCi/mL) uptake at pH 7.4 for 15 min in various MDCKII cell lines. Each bar represents mean ± SD (n = 4). **P < 0.01 compared with MDCKII-wt cells at 37 °C.
3.4. pH dependence of Gly-Sar uptake in MDCKII cell lines
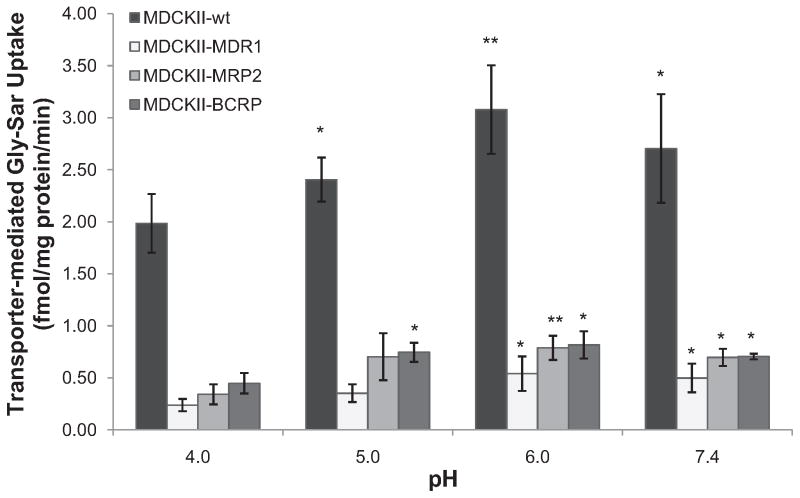
Peptide transporter-mediated apical [3H]Gly-Sar uptake in wild-type and transfected MDCKII cell lines was determined by subtracting nonspecific cellular accumulation at 4 °C from total cellular uptake at 37 °C. Results were compared at different extra-cellular pH and summarized in Fig. 4. Uptake in wild-type cells was 3–5-fold higher than that in transfected MDCKII cells at all pHs determined. The specific Gly-Sar uptake by apical peptide transporters in MDCKII-wt cells showed gradient enhancement when extracellular pH increased from 4.0 to 6.0, then decreased when environmental pH keeps increasing to 7.4. Similar trends were observed for MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells. The maximal apical Gly-Sar uptake was obtained at pH 6.0 in all MDCKII cell lines.
Fig. 4.
The pH dependent specific [3H]Gly-Sar (0.5 μCi/mL) uptake by apical peptide transporters for 15 min in various MDCKII cell lines. Each point represents mean ± SD (n = 4). *P < 0.05 and **P < 0.01 compared with same cell line at pH 4.0.
3.5. Concentration dependence of Gly-Sar uptake in MDCKII cell lines
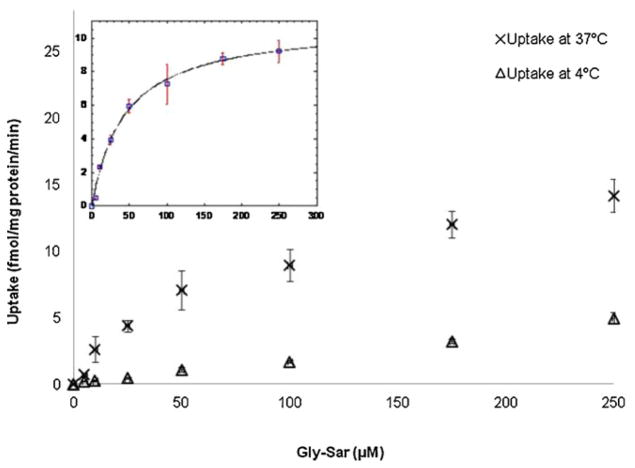
Concentration dependent uptake of [3H]Gly-Sar was carried out at both 37 °C and 4 °C to evaluate the uptake kinetics of Gly-Sar by apical peptide transporters in different MDCKII cell lines. Concentration dependence of apical peptide transporter-mediated specific Gly-Sar uptake was calculated by subtracting nonspecific uptake estimated at 4 °C from total uptake determined at 37 °C (Fig. 5). Uptake kinetic parameters including apparent Km, Vmax, and transport efficiency (Vmax/Km) are summarized in Table 2. The value of Vmax for apical peptide transporters in MDCKII-wt cells was 10.76 ± 1.68 fmol/mg protein/min. After transfection with human MDR1, MRP2, and BCRP genes, it decreased to 1.30 ± 0.36, 4.62 ± 0.76, and 4.50 ± 0.83 fmol/mg protein/min, respectively. Transport efficiency values, which represent the amount of Gly-Sar translocated by unit peptide transporters in unit time, were estimated to 0.180 ± 0.009, 0.045 ± 0.010, 0.055 ± 0.011, and 0.074 ± 0.016 nL/mg protein/min for apical Gly-Sar uptake in MDCKII-wt, MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cell lines, respectively. It indicates that transport efficiency for apical peptide uptake was also diminished in all transfected MDCKII cell lines.
Fig. 5.
Concentration dependence of [3H]Gly-Sar uptake by apical peptide transporters for 15 min in MDCKII-wt cells at 37 °C and 4 °C, respectively. Insert: Concentration dependence of apical peptide transporter-mediated specific Gly-Sar uptake in MDCKII-wt cells. Data (□) were calculated by subtracting nonspecific uptake estimated at 4 °C (△) from total uptake at 37 °C (×). Solid line represents the calculated fit of the data to Michaelis–Menten equation. Each point represents mean ± SD (n = 3).
Table 2.
Kinetic parameters for Gly-Sar uptake mediated by apical or basolateral peptide transporters in various MDCKII cell lines.
| Apical uptake
|
Basolateral uptake
|
|||||||
|---|---|---|---|---|---|---|---|---|
| MDCKII-wt | MDCKII-MDR1 | MDCKII-MRP2 | MDCKII-BCRP | MDCKII-wt | MDCKII-MDR1 | MDCKII-MRP2 | MDCKII-BCRP | |
| Km (μM) | 59.69 ± 1.10 | 29.13 ± 3.29 | 84.75 ± 18.59 | 60.67 ± 8.56 | 13.71 ± 2.22 | 50.87 ± 7.86 | 64.56 ± 4.09 | 46.15 ± 2.26 |
| Vmax (fmol/mg protein/min) | 10.76 ± 1.68 | 1.30 ± 0.36 | 4.62 ± 0.76 | 4.50 ± 0.83 | 4.45 ± 0.04 | 8.52 ± 0.81 | 8.03 ± 1.78 | 13.07 ± 2.61 |
| Vmax /Km (nL/mg protein/min) | 0.180 ± 0.009 | 0.045 ± 0.010** | 0.055 ± 0.011** | 0.074 ± 0.016** | 0.324 ± 0.050 | 0.167 ± 0.058 | 0.124 ± 0.016 | 0.283 ± 0.098 |
Uptake of [3H]Gly-Sar were conducted in the presence of different concentrations of unlabeled Gly-Sar (0–250 μM) at 4 °C and 37 °C, respectively. Each value represents mean ± SD (n = 3).
P < 0.01 compared with apical uptake observed in MDCKII-wt cells.
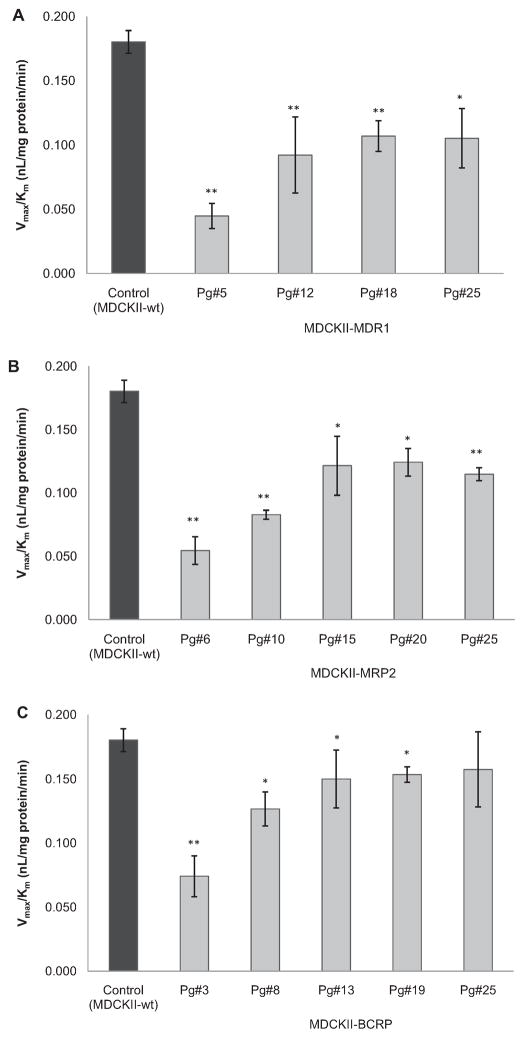
3.6. Passage dependence of transport efficiency for apical Gly-Sar uptake in MDCKII cell lines
Since the expression of transfected efflux transporters varies with ascending passage number, transport efficiency of apical peptide transporters-mediated specific [3H]Gly-Sar uptake was investigated in various MDCKII cell lines with different passage number. Fig. 6A–C illustrates that in comparison to wild-type cells with the value of 0.180 ± 0.009 nL/mg protein/min, transport efficiency of [3H]Gly-Sar uptake mediated by apical peptide transporters was reduced to different extents after transfection. Lowest transport efficiency values were observed at lowest cell passage. With passage number increasing, an increment on the transport efficiency for Gly-Sar uptake was achieved for all transfected cell lines. Finally apical peptide transporters showed relatively invariable transport efficiency values at higher cell passage. At passage 25, these values were obtained to be around 0.10 nL/mg protein/min for MDCKII-MDR1 cells, 0.12 nL/mg protein/min for MDCKII-MRP2 cells, and 0.15 nL/mg protein/min for MDCKII-BCRP cells, respectively.
Fig. 6.
Estimation of transport efficiency (Vmax/Km) for specific [3 H]Gly-Sar uptake by apical peptide transporters in various transfected MDCKII cell lines with different passage number. Uptake of [3H]Gly-Sar were conducted in the presence of different concentrations of unlabeled Gly-Sar (0–250 μM) at 4 °C and 37 °C, respectively: A, MDCKII-MDR1 cells; B, MDCKII-MRP2 cells; C, MDCKII-BCRP cells. Each bar represents mean ± SD (n = 3). *P < 0.05 and **P < 0.01 compared with control.
3.7. Effects of peptide transporter inhibitors on apical Gly-Sar uptake in MDCKII cell lines
Competitive inhibition studies were conducted on MDCKII cell lines in the presence of both PepT1 and PepT2 inhibitors to evaluate the substrate specificity. Results in Fig. 7 indicate that all the observed inhibitors showed significantly reduced apical Gly-Sar uptake compared to control, in which both PepT2 inhibitors fosinopril (100 μM) and cefadroxil (20 mM) exhibited more remarkable inhibitory effects than PepT1 inhibitor Gly-Pro (20 mM) in all four MDCKII cell lines. Moreover, transfected cell lines with low cell passage (lower than passage number 6) exhibited less Gly-Sar uptake than high cell passage (higher than passage number 25). In comparison with MDCKII-wt cells, all transfected MDCKII cell lines showed reduced apical cellular accumulation of Gly-Sar, no matter of the cell passages.
Fig. 7.
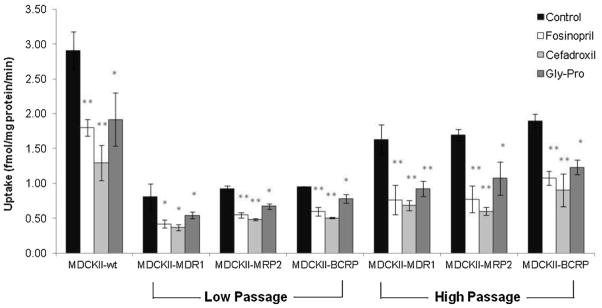
Apical uptake of [3H]Gly-Sar (0.5 μCi/mL) in the absence (control) or presence of peptide transporter inhibitors, fosinopril (100 μM), cefadroxil (20 mM) and glycyl-L-proline (Gly-Pro, 20 mM) at pH 7.4, 37 °C for 15 min in various MDCKII cell lines with different cell passages. Low Passage: passage number is less than 6; High Passage: passage number is more than 25. Each data point represents mean ± SD (n = 3). *P < 0.05 and **P < 0.01 compared with control.
3.8. pH dependent transcellular permeability of Gly-Sar in MDCKII cell lines
Transporter-mediated specific Gly-Sar transport across both parental and transfected cell monolayers of MDCKII cell lines was estimated by subtracting nonspecific transport (passive diffusion) of [14C]Mannitol from [3H]Gly-Sar transport. Results for both AP–BL and BL–AP permeability values are summarized in Table 3. Gly-Sar transport mediated by peptide transporters on AP to BL direction showed significantly higher permeability in MDCKII-wt cells than transfected cells at all pHs examined. Additionally, AP–BL Gly-Sar transport on all MDCKII cells exhibited gradual enhancement when apical pH increased from 4.0 to 7.4, and reached maximal at pH 7.4. However, apparent permeability values of BL–AP Gly-Sar transport across transfected cell monolayers were similar or greater than that on wild-type cells at all pHs observed. Furthermore, no obvious relationship between apical pH and BL–AP transcellular Gly-Sar permeability could be obtained on all MDCKII cells in this study.
Table 3.
pH dependent transporter-mediated Gly-Sar transport on both AP–BL and BL–AP directions across various MDCKII cell monolayers.
| pH in AP chamber | AP–BL permeability (×10−6 cm/s)
|
BL–AP permeability (×10−6 cm/s)
|
||||||
|---|---|---|---|---|---|---|---|---|
| MDCKII-wt | MDCKII-MDR1 | MDCKII-MRP2 | MDCKII-BCRP | MDCKII-wt | MDCKII-MDR1 | MDCKII-MRP2 | MDCKII-BCRP | |
| 4.0 | 13.1 ± 1.4 | 6.0 ± 0.4a | 8.6 ± 1.1a | 7.1 ± 0.3a | 9.4 ± 0.4 | 10.1 ± 1.3 | 8.5 ± 0.6 | 10.9 ± 0.7 |
| 5.0 | 14.9 ± 2.8 | 6.4 ± 0.5a,b | 9.9 ± 0.8a | 7.6 ± 1.2a,c | 6.6 ± 0.6 | 7.6 ± 0.8 | 6.3 ± 0.4 | 6.5 ± 0.7 |
| 6.0 | 22.2 ± 1.9 | 8.0 ± 0.4a | 10.6 ± 0.1a | 10.7 ± 0.7a | 6.0 ± 01.0 | 8.2 ± 0.5 | 7.5 ± 0.5 | 7.2 ± 0.9 |
| 7.4 | 36.7 ± 0.2 | 11.7 ± 0.6a,b,d | 17.7 ± 1.4a | 18.6 ± 1.9a | 8.0 ± 0.9 | 12.9 ± 1.3 | 15.7 ± 0.6 | 15.6 ± 0.1 |
The pH of incubation media varied from 4.0 to 7.4 in apical (AP) chambers, and remained consistent in basolateral (BL) chambers to pH 7.4. Each value represents mean ± SD (n = 4). Statistical analysis was performed to evaluate the difference of AP–BL permeability at each pH within MDCKII cell lines.
P < 0.01 compared with MDCKII-wt cells.
P < 0.01 compared with MDCKII-MRP2 cells.
P < 0.05 compared with MDCKII-MRP2 cells.
P < 0.05 compared with MDCKII-BCRP cells.
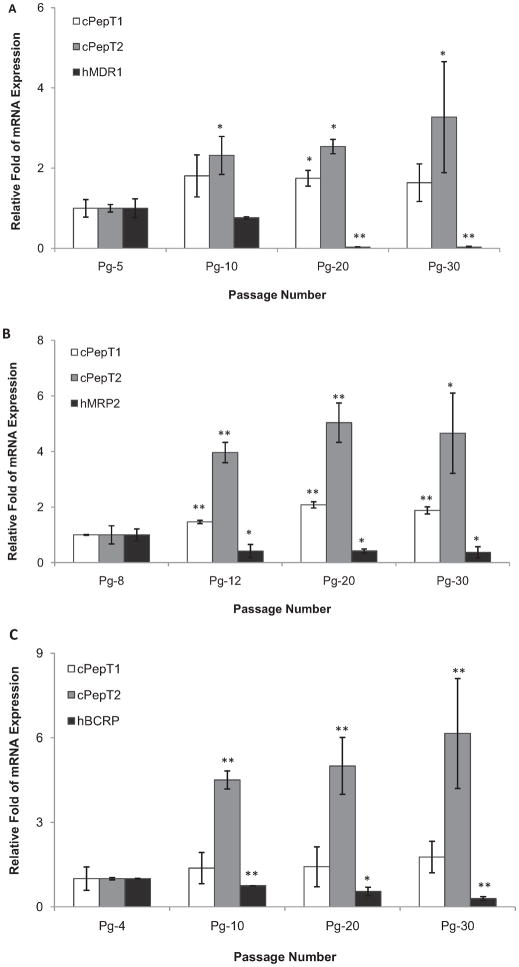
3.9. Real-time PCR assay
Levels of mRNA for both endogenous peptide transporters (cPepT1 and cPepT2) and transfected human efflux transporters (hP-gp, hMRP2, and hBCRP) expressed on various MDCKII cell lines were determined using real-time PCR. The relative amount of mRNA expression at different passage number was illustrated in Fig. 8. All three transfected MDCKII cell lines showed remarkably diminished efflux genes expression with ascending cell passage, and conversely enhanced peptide genes expression was observed on corresponding cell passages from low (passage number 4–8) to high (passage number 30). The relative fold of hMDR1 (transfected) gene expression was reduced from 1.00 ± 0.23 (passage number 5) to 0.03 ± 0.01 (passage number 30) in MDCKII-MDR1 cells. In contrast, endogenous influx cPepT1 and cPepT2 gene expression was enhanced from 1.00 ± 0.22-fold and 1.00 ± 0.09-fold at passage 5 to 1.64 ± 0.47-fold and 3.27 ± 1.38-fold at passage 30, respectively (Fig. 8A). Similar trend was also obtained in hMRP2- and hBCRP-transfected cell lines. Corresponding data in MDCKII-MRP2 cells at passage 30 was found to be 0.37 ± 0.19, 1.88 ± 0.13, and 4.66 ± 1.44, for hMRP2, cPepT1, and cPepT2, respectively (Fig. 8B), and in MDCKII-BCRP cells (passage 30) were 0.29 ± 0.07, 1.77 ± 0.56, and 6.15 ± 1.95 for hBCRP, cPepT1, and cPepT2, respectively (Fig. 8C).
Fig. 8.
Determination of mRNA levels of endogenous influx transporters, canine PepT1 (cPepT1) and canine PepT2 (cPepT2), and exogenous efflux transporters, human P-gp (hMDR1), human MRP2 (hMRP2) and human BCRP (hBCRP) in various transfected MDCKII cell lines with different passage number using real-time PCR: A, MDCKII-MDR1 cells; B, MDCKII-MRP2 cells; C, MDCKII-BCRP cells. Each bar represents mean ± SD (n = 3). *P < 0.05 and **P < 0.01 compared with the lowest cell passage.
3.10. Expression of endogenous PepT1 and PepT2 in MDCKII cells
In order to evaluate protein levels of endogenous PepT1 and PepT2 in MDCKII cells after transfection with different human efflux genes, Western blot analysis was performed using polyclonal primary antibodies specific to PepT1 (78 kDa) and PepT2 (81 kDa) in the present study. Results in Fig. 9 showed that expression of endogenous (canine) PepT2 (Fig. 9A) in MDCK wild-type cells is much higher than PepT1 (Fig. 9B). Moreover, MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells expressed significantly less amounts of PepT2 protein (lanes 3–8) as compared to the MDCKII-wt cells (lanes 1 and 2). Furthermore, PepT2 expression was observed to be enhanced at higher cell-passages (Pg-30) for all transfected MDCKII cells.
Fig. 9.
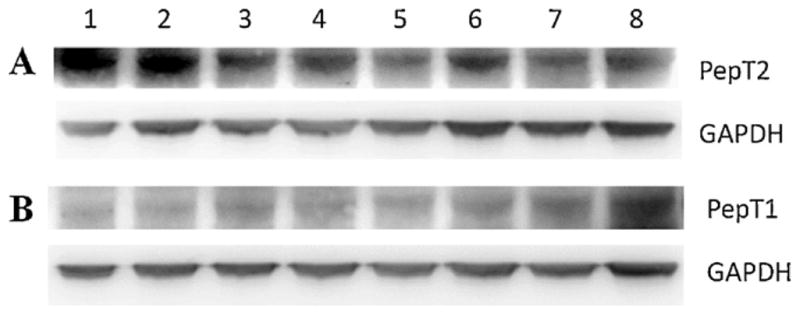
Western blot analysis of endogenous (canine) PepT2 (A) and PepT1 (B) in wild-type and transfected MDCKII cell lines: lane 1, MDCKII-wt (Pg-14); lane 2, MDCKII-wt (Pg-30); lane 3, MDCKII-MDR1 (Pg-5); lane 4, MDCKII-MDR1 (Pg-30); lane 5, MDCKII-MRP2 (Pg-8); lane 6, MDCKII-MRP2 (Pg-30); lane 7, MDCKII-BCRP (Pg-4); lane 8, MDCKII-BCRP (Pg-30).
4. Discussion
MDCK cells have been wildly used as an alternative in vitro model to Caco-2 cells which exhibit good correlation with human intestinal mucosa (Irvine et al., 1999; Volpe, 2008). In comparison with Caco-2 cells, MDCK cells have shown common epithelial characteristics and much shorter culture time (5 days for MDCK vs. 21 days for Caco-2). Therefore it has been accepted as an attractive tool for predicting absorption of new drug candidates in drug discovery stage. Especially MDCK strain II cells transfected with different human efflux genes, have been utilized to screen efflux-mediated drug transport (Tang et al., 2002a,b; Wang et al., 2005). However, some functions and biological characteristics of cells might change due to transfection. Our studies demonstrate that [14C]Mannitol transport, an indicator of paracellular permeability, was greatly reduced in MDR1, MRP2, and BCRP-transfected MDCKII cells on apical to basolateral direction in comparison with wild-type cells (Fig. 1), suggesting an enhanced barrier function after transfection. Enhanced tight junctions in transfected MDCKII cells indicate that the function of endogenous active transporters may also be altered. Thus a potential imperfect permeability result may be obtained in transcellular transport of substrates determined on transfected cells when compared with wild-type cells.
MDCK cells express endogenous canine peptide transporters on both apical and basolateral membranes, whereas all the transfected exogenous efflux genes are expressed apically (Tang et al., 2002a,b; An and Morris, 2010). Fig. 2 displays apical uptake of [3H]Gly-Sar, a typical substrate for peptide transporters, in various MDCKII cell lines at different days of culture. A remarkably different Gly-Sar cellular accumulation was obtained between wild-type and transfected cells. Total uptake of [3H]Gly-Sar at 37 °C was much lower in human P-gp, MRP2, and BCRP-overexpressed MDCKII cell lines. These results have been further confirmed by temperature dependence study. It has been reported that passive uptake of dipeptides (non-peptide transporter mediated) can be estimated by measuring uptake at cold temperature, and “transporter-mediated” component can be calculated by subtracting passive uptake component observed at 4 °C from the total uptake measured at 37 °C (Scow et al., 2011). Significant temperature dependent peptide uptake was observed in all four MDCKII cell lines in the present study (Fig. 3). Uptake of [3H]Gly-Sar was much higher at 37 °C than that at 4 °C, suggesting that the active transporter-mediated transport was involved in cellular uptake of Gly-Sar. Additionally, apical Gly-Sar influx via specific transporters in MDCKII-wt cells was 5–7-fold higher than that in MDCKII-MDR1, MDCKII-MRP2, and MDCKII-BCRP cells, indicating a reduced function of apical endogenous peptide transporters after transfection with exogenous efflux genes. This observation suggests that transfection of foreign gene(s) may affect the function of endogenous gene.
H+/peptide co-transporters (PepT1 and PepT2) are generally believed to be functionally expressed at apical membranes of MDCK wild-type cells (Sawada et al., 2001). It suggests that uptake of small peptides from apical side of MDCK cells can be stimulated by an inwardly directed H+ gradient. However, it has also been reported that the binding affinity for charged peptides to peptide transporters can change when extracellular pH altered (Daniel and Kottra, 2004). The neutral substrates like Gly-Sar at brush border or urinary pH (6.0–6.5) were observed to be preferred substrates for PepT1 and PepT2 (Terada et al., 2000; Pan et al., 2001). Specific peptide transporter-mediated Gly-Sar uptake was found to be a “bell-shape” and reached greatest at pH 6.0–6.5 when Terada et al. tested apical [14C]Gly-Sar uptake in MDCK cells with changing environmental pH from 5.0 to 7.5 (Terada et al., 2000). In the present study, apical transporter-mediated specific [3H]Gly-Sar uptake in MDCKII-wt cells was also observed to be maximal at extracellular pH 6.0 (Fig. 4). The pH profiles of uptake in human efflux gene-transfected cell lines appeared to be similar as MDCKII-wt cells, but the uptake in these transfected cells was much lower. The most probable explanation is that characteristic of peptide transporters in the transfected cell lines possibly remains unchanged, but its expression level was possibly too low to facilitate a significant pH-dependent difference in Gly-Sar uptake.
In order to recognize its functional properties, concentration dependence of apical and basolateral peptide transporters-mediated specific Gly-Sar uptake was evaluated in various MDCKII cell lines (Fig. 5 and Table 2). It was observed that mean values of apparent Km from apical side of both wild-type and transfected MDCKII cells varied in the range of 30–85 μM. According to the classification of substrates/inhibitors suggested by previous research (Luckner and Brandsch, 2005; Biegel et al., 2006), this result clearly demonstrates that apical peptide transporters exhibit the significant PepT2 characters of high-affinity (Km < 0.1 mM) for Gly-Sar uptake. The corresponding Vmax values were tremendously diminished to about 12%, 43% and 42% of that in wild-type cells after transfection with hMDR1, hMRP2, and hBCRP efflux genes, respectively. It indicates that the capacity of apical peptide transporters markedly declined. However, Vmax values obtained from different laboratories are usually diverse, since its determination can be affected by different methodology and calculations applied by different investigators. In our current investigation, Vmax values obtained after subtraction of passive diffusion values. Thus determination of transport efficiency (the ratio of Vmax to Km) provides a more reliable way to investigate transporter function (Zhou et al., 2010; Jedlitschky et al., 1996). Transport efficiency values for apical peptide transporters summarized in Table 2 exhibit a significant decrease to around 25% (MDCKII-MDR1), 30% (MDCKII-MRP2), and 41% (MDCKII-BCRP) of that in wild-type cells. In contrast, basolateral peptide transporters in transfected cell lines showed an increased rate for Gly-Sar translocation with higher Vmax values. These results again confirm our hypothesis that alien gene(s) in apical membrane can influence the function of endogenous transporters.
To further investigate the properties of peptide transporter system expressed on apical membrane of MDCKII cell lines after transfection, inhibitory uptake of [3H]Gly-Sar was carried out in the presence of various peptide transporter inhibitors. Gly-Pro showed markedly competitive inhibition for Gly-Sar uptake and was reported as a selected PepT1 inhibitor (International Transporter Consortium et al., 2010; Omkvist et al., 2010). Angiotensin-converting enzyme (ACE) inhibitor fosinopril and aminocephalosporin antibiotic agent sefadroxil have been considered as powerful PepT2 inhibitors due to their high affinity for binding to PepT2 (International Transporter Consortium et al., 2010; Knütter et al., 2008; Ocheltree et al., 2004). In the current study, 20 mM Gly-Pro exhibited a less reduction in [3H]Gly-Sar uptake than 20 mM sefadroxil and 100 μM fosinopril in MDCKII-wt cells indicating less PepT1 than PepT2 expressions on the apical membrane of MDCK cells and similar substrate specificity was observed in transfected cell lines (Fig. 7). These findings provide further evidence that the type of apical peptide transporter system remained unaltered after transfection with exogenous efflux genes, but reduced capacity of influx transporter due to less expression resulting in decreased Gly-Sar uptake compared to wild-type cells.
Functional activities of peptide transporters in MDCK cell lines influences significantly on transepithelial transport of [3H]Gly-Sar across cell monolayers. Previous studies have reported that apical peptide transporter present in MDCKII cells, primarily H+/peptide co-transporter system, is responsible to the luminal uptake into the cells, whereas the basolateral peptide transporter system, a novel peptide transporter which does not appear pH-dependent, is involved in the cellular re-absorption of small peptides from peritubular capillaries (Sawada et al., 2001; Terada et al., 2000). Therefore transcellular transport of Gly-Sar from donor chamber to receiver chamber should be contributed by influx activities mediated by both apical and basolateral peptide transporters. As demonstrated by the present transport data in Table 3, MDCKII-wt cells displayed a significantly higher AP–BL permeability and a similar or slightly lower BL–AP permeability of [3H]Gly-Sar over that in transfected cell lines at all pHs. These findings further confirm the reduced transport efficiencies, particularly the apical endogenous peptide transporters that we reported previously (Agarwal et al., 2007a). Moreover, such inefficiency in apical peptide transporters might be more significant than that in basolateral peptide transporters, as the difference of Gly-Sar transport between wild-type versus transfected cell lines on AP–BL direction seems to be much more than that on BL–AP direction. All human efflux transporter genes are transfected on apical membrane of MDCK cells, thus function of apical peptide transporters has attracted more attention in the present study. We assumed that the transfection of human MDR1, MRP2, and BCRP genes localized in the apical membrane of cells may affect the function of peptide transporters. Overexpression of efflux transporters at the apical cell membrane and their overriding activities may cause differential functional activities and inefficiency of endogenous peptide transporters in transfected MDCKII cell lines.
Expression levels of transfected genes are cell-passage-dependent, since transfected cells may lose the cDNA with ascending passage number. Previous research has reported that higher passaged MDCK-MDR1 cells showed lower levels of transfected human MDR1 gene expression than lower passaged cells (Tang et al., 2002a). Thus cell-passage-dependent effects on the determination of transport efficiency of apical peptide transporters were investigated in various MDR gene-transfected MDCKII cell lines. All transfected cell lines demonstrated markedly reduced translocation efficiency for Gly-Sar apical uptake (Fig. 6). Interestingly, this functional inefficiency was found to be clearly passage-dependent, since disparity in transport efficiency between intact and transfected cells attenuated with increasing cell passage. Lower Gly-Sar transport efficiency in transfected cell lines is possibly associated with a corresponding lower expression level of peptide transporters, which might be the results of down-regulation by highly expressed robust efflux genes. When expression of these robust transporters diminishes, the down-regulatory effect also ceases. This assumption is well substantiated by the results of mRNA determination using real-time RT-PCR assay and subsequent protein expression by Western blot analysis.
Expression of transfected human genes decreases quickly as cell passage increases (Fig. 8). P-gp-overexpressed cells exhibited a faster diminishing than MRP2 and BCRP expression levels. This is possibly due to differential transfection stability with different genes. Conversely, expression of both peptide transporters PepT1 and PepT2 present on apical membrane of various transfected MDCK II cell lines has been raised progressively with apical Gly-Sar gradually enhanced uptake along with increasing cell passage in various transfected cells (Fig. 6). Additionally, a more significant enhancement in cPepT2 gene expression over cPepT1 was obtained in all three transfected cell lines. Such inverse correlation between endogenous influx genes and transfected efflux genes suggests that highly active exogenous efflux transporters may influence the protein expression and function of endogenous peptide transporters. This assumption has been further confirmed by the results of Western blot analysis (Fig. 9). Whether this inverse relationship between alien gene and endogenous gene is an adaptation to maintain cellular concord or domination of MDR gene to protect the cells from toxicity is yet to be completely established.
Our study clearly demonstrates that transfected efflux robust genes dominate over endogenous influx transporters. The molecular mechanism of such interaction between transporters is yet to be elucidated. One explanation is that transfected exogenous genes may compete with the endogenous genes for binding sites on transcription factors or transcriptional coactivator and corepressor complexes. Recent studies have shown that transcription factors, Sp1 binding sites play a decisive role in the basal expression of MDR1 (human), MRP2 (rat), BCRP (human) and PepT1 (human) (Cornwell and Smith, 1993; Shimakura et al., 2006; Takakura et al., 2010; Zhang et al., 2012; Kauffmann et al., 2001). Competition with highly expressed exogenous genes might trigger the down-regulation of endogenous gene expression in these cells. Transfection of hMDR1 in MDCKII cells can cause negative feedback and trigger down-regulation of endogenous Mdr1 (Kuteykin-Teplyakov et al., 2010). This down-regulation of endogenous mRNA as well as protein expressions by exogenous gene transfection such as hMDR1 vs. Mdr1 has also been reported by the previous studies, which indicated a feedback-regulatory mechanism from endogenous gene/transcription factor to maintain cellular milieu (Lloyd et al., 1992). The fundamental difference of our work is that we have demonstrated the interplay between two unrelated genes, i.e. hMDR1/hMRP2/hBCRP vs. PepT (canine) controlling efflux of xenobiotics and nutrients, respectively, which was unknown before. The understanding of transporters crosstalk between divergent proteins is critical for developing new peptidomimetics.
This study is the first report illustrating that the function and mRNA expression of endogenous (canine) peptide transporters in MDCKII cells can be influenced by transfection with various human efflux genes like MDR1, MRP2, and BCRP. Our findings demonstrate that endogenous peptide transporters exhibit a reduced function and mRNA expression in efflux genes-transfected cell lines. Moreover, this down-regulation vanishes as the functions of transfected genes diminish.
We have also asked the question what consequences will be when drug-mediated induction of MDR genes occurs (Pal and Mitra, 2006; Pal et al., 2011). Will such induction of MDR arrest the function of nutrient transporters in intestine? Current work in our laboratory has illustrated that rifampicin-induced MDR overexpression in LS180 cells affects endogenous PepT function (unpublished data). Answer to these questions will release a new paradigm of transporter interaction.
Acknowledgments
This study is supported by National Institute of Health Grant R01 AI 07199. Also we would like to thank Dr. P. Borst and Dr. A. Schinkel for their generously providing MDCK cells.
References
- Agarwal S, Jain R, Pal D, Mitra AK. Functional characterization of peptide transporters in MDCKII-MDR1 cell line as a model for oral absorption studies. Int J Pharm. 2007a;332:147–152. doi: 10.1016/j.ijpharm.2006.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal S, Pal D, Mitra AK. Both P-gp and MRP2 mediate transport of lopinavir, a protease inhibitor. Int J Pharm. 2007b;339:139–147. doi: 10.1016/j.ijpharm.2007.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An G, Morris ME. Effects of single and multiple flavonoids on BCRP-mediated accumulation, cytotoxicity and transport of mitoxantrone in vitro. Pharm Res. 2010;27:1296–1308. doi: 10.1007/s11095-010-0108-8. [DOI] [PubMed] [Google Scholar]
- Balimane PV, Chong S, Patel K, Quan Y, Timoszyk J, Han YH, Wang B, Vig B, Faria TN. Peptide transporter substrate identification during permeability screening in drug discovery: comparison of transfected MDCK-hPepT1 cells to Caco-2 cells. Arch Pharm Res. 2007;30:507–518. doi: 10.1007/BF02980227. [DOI] [PubMed] [Google Scholar]
- Biegel A, Knütter I, Hartrodt B, Gebauer S, Theis S, Luckner P, Kottra G, Rastetter M, Zebisch K, Thondorf I, Daniel H, Neubert K, Brandsch M. The renal type H+/peptide symporter PEPT2: structure–affinity relationships. Amino Acids. 2006;31:137–156. doi: 10.1007/s00726-006-0331-0. [DOI] [PubMed] [Google Scholar]
- Boerner P, Evans-Laying MUHS, Saier MH., Jr Polarity of neutral amino acid transport and characterization of a broad specificity transport activity in a kidney epithelial cell line, MDCK. J Biol Chem. 1986;261:13957–13962. [PubMed] [Google Scholar]
- von Bonsdorff CH, Fuller SD, Simons K. Apical and basolateral endocytosis in Madin–Darby canine kidney (MDCK) cells grown on nitrocellulose filters. EMBO J. 1985;4:2781–2792. doi: 10.1002/j.1460-2075.1985.tb04004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell MM, Smith DE. SP1 activates the MDR1 promoter through one of two distinct G-rich regions that modulate promoter activity. J Biol Chem. 1993;268:19505–19511. [PubMed] [Google Scholar]
- Daniel H, Kottra G. The proton oligopeptide cotransporter family SLC15 in physiology and pharmacology. Pflugers Arch. 2004;447:610–618. doi: 10.1007/s00424-003-1101-4. [DOI] [PubMed] [Google Scholar]
- Flanagan SD, Cummins CL, Susanto M, Liu X, Takahashi LH, Benet LZ. Comparison of furosemide and vinblastine secretion from cell lines overexpressing multidrug resistance protein (P-glycoprotein) and multidrug resistance-associated proteins (MRP1 and MRP2) Pharmacology. 2002;64:126–134. doi: 10.1159/000056161. [DOI] [PubMed] [Google Scholar]
- Ganapathy ME, Brandsch M, Prasad PD, Ganapathy V, Leibach FH. Differential recognition of β-lactam antibiotics by intestinal and renal peptide transporters, PEPT 1 and PEPT 2. J Biol Chem. 1995;270:25672–25677. doi: 10.1074/jbc.270.43.25672. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Leibach FH. Peptide transporters. Curr Opin Nephrol Hypertens. 1996;5:395–400. doi: 10.1097/00041552-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Gumbleton M, Audus KL. Progress and limitations in the use of in vitro cell cultures to serve as a permeability screen for the blood–brain barrier. J Pharm Sci. 2001;90:1681–1698. doi: 10.1002/jps.1119. [DOI] [PubMed] [Google Scholar]
- Horio M, Chin KV, Currier SJ, Goldenberg S, Williams C, Pastan I. Transepithelial transport of drugs by the multidrug transporter in cultured Madin–Darby canine kidney cell epithelia. J Biol Chem. 1989;264:14880–14884. [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM, Hoffmaster KA, Ishikawa T, Keppler D, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan PW, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L International Transporter Consortium. Membrane transporters in drug development. Nat Rev Drug Discov. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. MDCK (Madin–Darby canine kidney) cells: a tool for membrane permeability screening. J Pharm Sci. 1999;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- Ito K, Suzuki H, Horie T, Sugiyama Y. Apical/basolateral surface expression of drug transporters and its role in vectorial drug transport. Pharm Res. 2005;22:1559–1577. doi: 10.1007/s11095-005-6810-2. [DOI] [PubMed] [Google Scholar]
- Jain R, Agarwal S, Mandava NK, Sheng Y, Mitra AK. Interaction of dipeptide prodrugs of saquinavir with multidrug resistance protein-2 (MRP-2): evasion of MRP-2 mediated efflux. Int J Pharm. 2008;362:44–51. doi: 10.1016/j.ijpharm.2008.06.013. [DOI] [PubMed] [Google Scholar]
- Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D. Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res. 1996;56:988–994. [PubMed] [Google Scholar]
- Kauffmann HM, Vorderstemann B, Schrenk D. Basal expression of the rat, but not of the human, multidrug resistance protein 2 (MRP2) gene is mediated by CBF/NF-Y and Sp1 promoter-binding sites. Toxicology. 2001;167:25–35. doi: 10.1016/s0300-483x(01)00455-3. [DOI] [PubMed] [Google Scholar]
- Knütter I, Wollesky C, Kottra G, Hahn MG, Fischer W, Zebisch K, Neubert RH, Daniel H, Brandsch M. Transport of angiotensin-converting enzyme inhibitors by H+/peptide transporters revisited. J Pharmacol Exp Ther. 2008;327:432–441. doi: 10.1124/jpet.108.143339. [DOI] [PubMed] [Google Scholar]
- Kuteykin-Teplyakov K, Luna-Tortós C, Ambroziak K, Löscher W. Differences in the expression of endogenous efflux transporters in MDR1-transfected versus wildtype cell lines affect P-glycoprotein mediated drug transport. Br J Pharmacol. 2010;160:1453–1463. doi: 10.1111/j.1476-5381.2010.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landowski CP, Vig BS, Song X, Amidon GL. Targeted delivery to PEPT1-overexpressing cells: acidic, basic, and secondary floxuridine amino acid ester prodrugs. Mol Cancer Ther. 2005;4:659–667. doi: 10.1158/1535-7163.MCT-04-0290. [DOI] [PubMed] [Google Scholar]
- Lloyd C, Schevzov G, Gunning P. Transfection of nonmuscle beta-and gamma-actin genes into myoblasts elicits different feedback regulatory responses from endogenous actin genes. J Cell Biol. 1992;117:787–797. doi: 10.1083/jcb.117.4.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Klaassen C. Tissue distribution and thyroid hormone regulation of Pept1 and Pept2 mRNA in rodents. Peptides. 2006;27:850–857. doi: 10.1016/j.peptides.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Luckner P, Brandsch M. Interaction of 31 beta-lactam antibiotics with the H+/peptide symporter PEPT2: analysis of affinity constants and comparison with PEPT1. Eur J Pharm Biopharm. 2005;59:17–24. doi: 10.1016/j.ejpb.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Ocheltree SM, Shen H, Hu Y, Xiang J, Keep RF, Smith DE. Mechanisms of cefadroxil uptake in the choroid plexus: studies in wild-type and PEPT2 knockout mice. J Pharmacol Exp Ther. 2004;308:462–467. doi: 10.1124/jpet.103.060400. [DOI] [PubMed] [Google Scholar]
- Ojakian GK, Herzlinger DA. Analysis of epithelial cell surface polarity with monoclonal antibodies. Fed Proc. 1984;43:2208–2216. [PubMed] [Google Scholar]
- Omkvist DH, Brodin B, Nielsen CU. Ibuprofen is a non-competitive inhibitor of the peptide transporter hPEPT1 (SLC15A1): possible interactions between hPEPT1 substrates and ibuprofen. Br J Pharmacol. 2010;161:1793–1805. doi: 10.1111/j.1476-5381.2010.01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang H, Andersen TE, Chen W, Nofsinger R, Steffansen B, Borchardt RT. A comparison of the effects of p-glycoprotein inhibitors on the blood–brain barrier permeation of cyclic prodrugs of an opioid peptide (DADLE) J Pharm Sci. 2009;98:2227–2236. doi: 10.1002/jps.21585. [DOI] [PubMed] [Google Scholar]
- Pal D, Kwatra D, Minocha M, Paturi DK, Budda B, Mitra AK. Efflux transporters- and cytochrome P-450-mediated interactions between drugs of abuse and antiretrovirals. Life Sci. 2011;88:959–971. doi: 10.1016/j.lfs.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal D, Mitra AK. MDR- and CYP3A4-mediated drug–herbal interactions. Life Sci. 2006;78:2131–2145. doi: 10.1016/j.lfs.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Pan Y, Wong EA, Bloomquist JR, Webb KE., Jr Expression of a cloned ovine gastrointestinal peptide transporter (oPepT1) in Xenopus oocytes induces uptake of oligopeptides in vitro. J Nutr. 2001;131:1264–1270. doi: 10.1093/jn/131.4.1264. [DOI] [PubMed] [Google Scholar]
- Putnam WS, Pan L, Tsutsui K, Takahashi L, Benet LZ. Comparison of bidirectional cephalexin transport across MDCK and caco-2 cell monolayers: interactions with peptide transporters. Pharm Res. 2002;19:27–33. doi: 10.1023/a:1013647114152. [DOI] [PubMed] [Google Scholar]
- Sawada K, Terada T, Saito H, Inui K. Distinct transport characteristics of basolateral peptide transporters between MDCK and Caco-2 cells. Pflugers Arch. 2001;443:31–37. doi: 10.1007/s004240100669. [DOI] [PubMed] [Google Scholar]
- Scow JS, Madhavan S, Chaudhry RM, Zheng Y, Duenes JA, Sarr MG. Differentiating passive from transporter-mediated uptake by PepT1: a comparison and evaluation of four methods. J Surg Res. 2011;170:17–23. doi: 10.1016/j.jss.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimakura J, Terada T, Shimada Y, Katsura T, Inui K. The transcription factor Cdx2 regulates the intestine-specific expression of human peptide transporter 1 through functional interaction with Sp1. Biochem Pharmacol. 2006;71:1581–1588. doi: 10.1016/j.bcp.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Shu Y, Bello CL, Mangravite LM, Feng B, Giacomini KM. Functional characteristics and steroid hormone-mediated regulation of an organic cation transporter in Madin–Darby canine kidney cells. J Pharmacol Exp Ther. 2001;299:392–398. [PubMed] [Google Scholar]
- Steffansen B, Nielsen CU, Brodin B, Eriksson AH, Andersen R, Frokjaer S. Intestinal solute carriers: an overview of trends and strategies for improving oral drug absorption. Eur J Pharm Sci. 2004;21:3–16. doi: 10.1016/j.ejps.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Hinoi T, Oue N, Sasada T, Kawaguchi Y, Okajima M, Akyol A, Fearon ER, Yasui W, Ohdan H. CDX2 regulates multidrug resistance 1 gene expression in malignant intestinal epithelium. Cancer Res. 2010;70:6767–6778. doi: 10.1158/0008-5472.CAN-09-4701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa? Pharm Res. 2002a;19:765–772. doi: 10.1023/a:1016140429238. [DOI] [PubMed] [Google Scholar]
- Tang F, Horie K, Borchardt RT. Are MDCK cells transfected with the human MRP2 gene a good model of the human intestinal mucosa? Pharm Res. 2002b;19:773–779. doi: 10.1023/a:1016192413308. [DOI] [PubMed] [Google Scholar]
- Terada T, Sawada K, Ito T, Saito H, Hashimoto Y, Inui K. Functional expression of novel peptide transporter in renal basolateral membranes. Am J Physiol Renal Physiol. 2000;279:F851–F857. doi: 10.1152/ajprenal.2000.279.5.F851. [DOI] [PubMed] [Google Scholar]
- Thuerauf N, Fromm MF. The role of the transporter P-glycoprotein for disposition and effects of centrally acting drugs and for the pathogenesis of CNS diseases. Eur Arch Psychiatry Clin Neurosci. 2006;256:281–286. doi: 10.1007/s00406-006-0662-6. [DOI] [PubMed] [Google Scholar]
- Volpe DA. Variability in Caco-2 and MDCK cell-based intestinal permeability assays. J Pharm Sci. 2008;97:712–725. doi: 10.1002/jps.21010. [DOI] [PubMed] [Google Scholar]
- Wang Q, Rager JD, Weinstein K, Kardos PS, Dobson GL, Li J, Hidalgo IJ. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood–brain barrier. Int J Pharm. 2005;288:349–359. doi: 10.1016/j.ijpharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pal D, Mitra AK. Stereoselective evasion of P-glycoprotein, cytochrome P450 3A, and hydrolases by peptide prodrug modification of saquinavir. J Pharm Sci. 2012;101:3199–3213. doi: 10.1002/jps.23193. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Davidson R, Smith A, Pereira D, Zhao S, Soglia J, Gebhard D, de Morais S, Duignan DB. A 96-well efflux assay to identify ABCG2 substrates using a stably transfected MDCK II cell line. Mol Pharm. 2006;3:45–54. doi: 10.1021/mp050088t. [DOI] [PubMed] [Google Scholar]
- Zhang M, Mathur A, Zhang Y, Xi S, Atay S, Hong JA, Datrice N, Upham T, Kemp CD, Ripley RT, Wiegand G, Avital I, Fetsch P, Mani H, Zlott D, Robey R, Bates SE, Li X, Rao M, Schrump DS. Mithramycin represses basal and cigarette smoke-induced expression of ABCG2 and inhibits stem cell signaling in lung and esophageal cancer cells. Cancer Res. 2012;72:4178–4192. doi: 10.1158/0008-5472.CAN-11-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Duan H, Engel K, Xia L, Wang J. Adenosine transport by plasma membrane monoamine transporter: reinvestigation and comparison with organic cations. Drug Metab Dispos. 2010;38:1798–1805. doi: 10.1124/dmd.110.032987. [DOI] [PMC free article] [PubMed] [Google Scholar]