Abstract
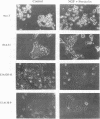
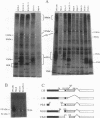
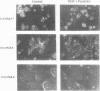
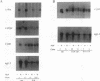
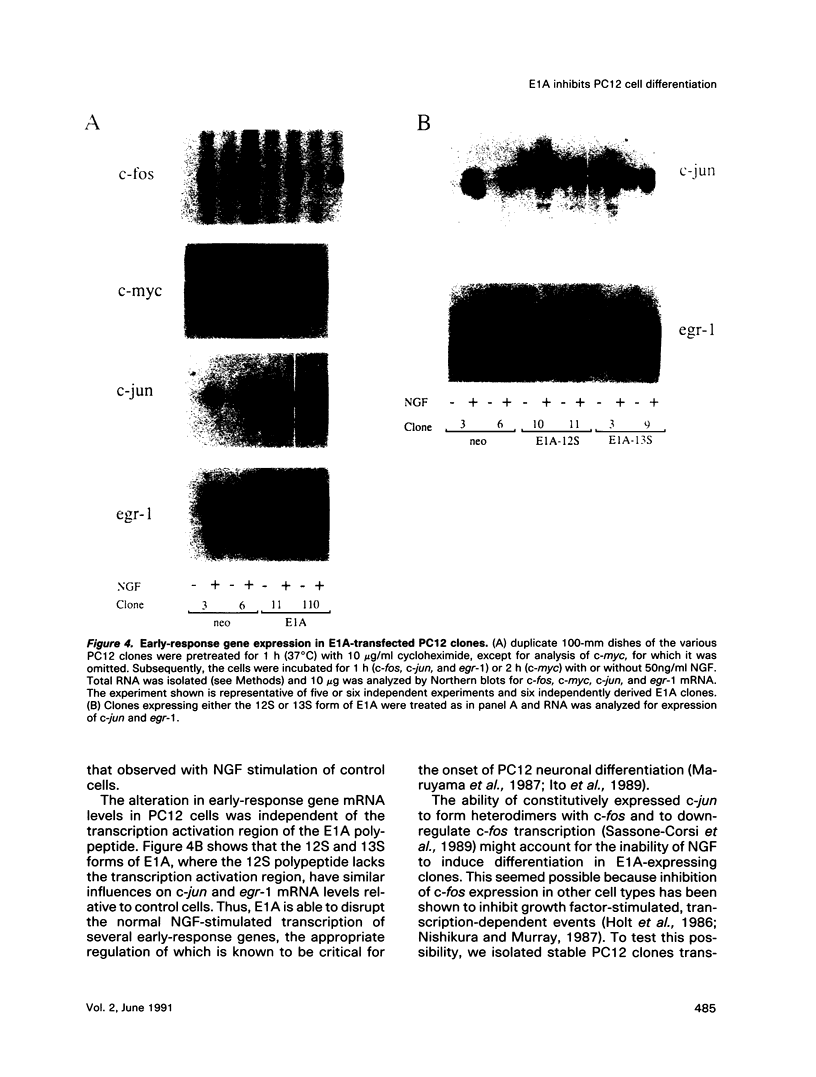
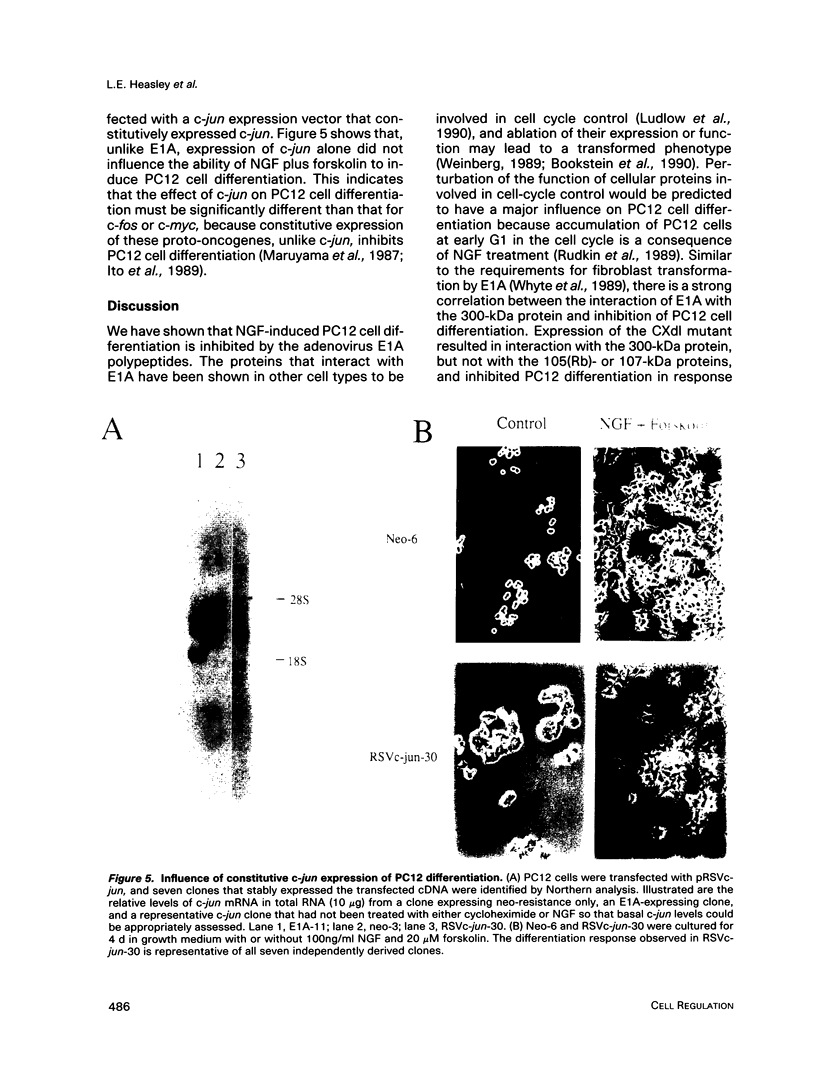
Expression of the adenovirus early gene E1A inhibits the nerve growth factor (NGF)-induced differentiation of PC12 pheochromocytoma cells. Expression of the 12S form of E1A, which lacks the transcription activation region, also inhibited PC12 cell differentiation in a manner similar to the wild-type gene. Three cellular proteins--the retinoblastoma susceptibility gene product referred to as 105(Rb)-, 107-, and 300-kDa proteins--stably interacted with the different E1A polypeptides. Analysis of the association of these cellular proteins with mutant E1A polypeptides demonstrated that a functional domain 1, which is minimally involved in the association of the 300-kDa protein with E1A, was sufficient to inhibit neuronal differentiation. Deletion of transformation domain 2, which encodes sequences necessary for the binding of the 105(Rb)- and 107-kDa proteins, did not influence the ability of the mutant E1A polypeptide to inhibit PC12 cell differentiation. E1A was also shown to alter the expression of mRNAs for the early response genes c-fos, c-myc, egr-1, and c-jun and their regulation in response to NGF. In clones expressing either 12S or 13S E1A, NGF stimulation of c-fos and c-myc was repressed. In contrast, basal mRNA levels for c-jun and egr-1 were constitutively elevated and not significantly affected further by challenge with NGF. Simply expressing c-jun by gene transfer, however, did not mimic the action of E1A because constitutively expressing c-jun clones differentiated in response to NGF. Thus, expression of the E1A polypeptide disrupts NGF control of early transcription events that have been shown to be critical for PC12 cell neuronal differentiation.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagchi S., Raychaudhuri P., Nevins J. R. Adenovirus E1A proteins can dissociate heteromeric complexes involving the E2F transcription factor: a novel mechanism for E1A trans-activation. Cell. 1990 Aug 24;62(4):659–669. doi: 10.1016/0092-8674(90)90112-r. [DOI] [PubMed] [Google Scholar]
- Berk A. J. Adenovirus promoters and E1A transactivation. Annu Rev Genet. 1986;20:45–79. doi: 10.1146/annurev.ge.20.120186.000401. [DOI] [PubMed] [Google Scholar]
- Bookstein R., Shew J. Y., Chen P. L., Scully P., Lee W. H. Suppression of tumorigenicity of human prostate carcinoma cells by replacing a mutated RB gene. Science. 1990 Feb 9;247(4943):712–715. doi: 10.1126/science.2300823. [DOI] [PubMed] [Google Scholar]
- Cho K. O., Skarnes W. C., Minsk B., Palmieri S., Jackson-Grusby L., Wagner J. A. Nerve growth factor regulates gene expression by several distinct mechanisms. Mol Cell Biol. 1989 Jan;9(1):135–143. doi: 10.1128/mcb.9.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Whyte P. RB and the cell cycle: entrance or exit? Cell. 1989 Sep 22;58(6):1009–1011. doi: 10.1016/0092-8674(89)90495-9. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Greene L. A., Ziff E. B. Nerve growth factor and epidermal growth factor induce rapid transient changes in proto-oncogene transcription in PC12 cells. J Biol Chem. 1985 Nov 15;260(26):14101–14110. [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene L. A., Tischler A. S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Franza B. R., Jr, Schley C. Monoclonal antibodies specific for adenovirus early region 1A proteins: extensive heterogeneity in early region 1A products. J Virol. 1985 Sep;55(3):533–546. doi: 10.1128/jvi.55.3.533-546.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Whyte P., Franza B. R., Jr, Schley C. Association of adenovirus early-region 1A proteins with cellular polypeptides. Mol Cell Biol. 1986 May;6(5):1579–1589. doi: 10.1128/mcb.6.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herschman H. R. Extracellular signals, transcriptional responses and cellular specificity. Trends Biochem Sci. 1989 Nov;14(11):455–458. doi: 10.1016/0968-0004(89)90105-9. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E., Sonnenberg J. L., Narayanan R. Nerve growth factor-induced differentiation in PC-12 cells is blocked by fos oncogene. Oncogene. 1989 Oct;4(10):1193–1199. [PubMed] [Google Scholar]
- Kalman D., Wong B., Horvai A. E., Cline M. J., O'Lague P. H. Nerve growth factor acts through cAMP-dependent protein kinase to increase the number of sodium channels in PC12 cells. Neuron. 1990 Mar;4(3):355–366. doi: 10.1016/0896-6273(90)90048-k. [DOI] [PubMed] [Google Scholar]
- Leff T., Elkaim R., Goding C. R., Jalinot P., Sassone-Corsi P., Perricaudet M., Kédinger C., Chambon P. Individual products of the adenovirus 12S and 13S EIa mRNAs stimulate viral EIIa and EIII expression at the transcriptional level. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4381–4385. doi: 10.1073/pnas.81.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludlow J. W., Shon J., Pipas J. M., Livingston D. M., DeCaprio J. A. The retinoblastoma susceptibility gene product undergoes cell cycle-dependent dephosphorylation and binding to and release from SV40 large T. Cell. 1990 Feb 9;60(3):387–396. doi: 10.1016/0092-8674(90)90590-b. [DOI] [PubMed] [Google Scholar]
- Maruyama K., Schiavi S. C., Huse W., Johnson G. L., Ruley H. E. myc and E1A oncogenes alter the responses of PC12 cells to nerve growth factor and block differentiation. Oncogene. 1987;1(4):361–367. [PubMed] [Google Scholar]
- Moran B., Zerler B. Interactions between cell growth-regulating domains in the products of the adenovirus E1A oncogene. Mol Cell Biol. 1988 Apr;8(4):1756–1764. doi: 10.1128/mcb.8.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran E., Zerler B., Harrison T. M., Mathews M. B. Identification of separate domains in the adenovirus E1A gene for immortalization activity and the activation of virus early genes. Mol Cell Biol. 1986 Oct;6(10):3470–3480. doi: 10.1128/mcb.6.10.3470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikura K., Murray J. M. Antisense RNA of proto-oncogene c-fos blocks renewed growth of quiescent 3T3 cells. Mol Cell Biol. 1987 Feb;7(2):639–649. doi: 10.1128/mcb.7.2.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter-Landsberg C., Jastorff B. The role of cAMP in nerve growth factor-promoted neurite outgrowth in PC12 cells. J Cell Biol. 1986 Mar;102(3):821–829. doi: 10.1083/jcb.102.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Rudkin B. B., Lazarovici P., Levi B. Z., Abe Y., Fujita K., Guroff G. Cell cycle-specific action of nerve growth factor in PC12 cells: differentiation without proliferation. EMBO J. 1989 Nov;8(11):3319–3325. doi: 10.1002/j.1460-2075.1989.tb08493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rydel R. E., Greene L. A. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987 Nov;7(11):3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Segerson T. P., Kauer J., Wolfe H. C., Mobtaker H., Wu P., Jackson I. M., Lechan R. M. Thyroid hormone regulates TRH biosynthesis in the paraventricular nucleus of the rat hypothalamus. Science. 1987 Oct 2;238(4823):78–80. doi: 10.1126/science.3116669. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A. Positive and negative controls on cell growth. Biochemistry. 1989 Oct 17;28(21):8263–8269. doi: 10.1021/bi00447a001. [DOI] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Woon C. W., Soparkar S., Heasley L., Johnson G. L. Expression of a G alpha s/G alpha i chimera that constitutively activates cyclic AMP synthesis. J Biol Chem. 1989 Apr 5;264(10):5687–5693. [PubMed] [Google Scholar]
- Wu B. Y., Fodor E. J., Edwards R. H., Rutter W. J. Nerve growth factor induces the proto-oncogene c-jun in PC12 cells. J Biol Chem. 1989 May 25;264(15):9000–9003. [PubMed] [Google Scholar]