Abstract
Although the assessment of dynamic cerebral autoregulation (CA) based on measurements of spontaneous fluctuations in arterial blood pressure (ABP) and cerebral blood flow (CBF) is a convenient and much used method, there remains uncertainty about its reliability. We tested the effects of increasing ABP variability, provoked by a modification of the thigh cuff method, on the ability of the autoregulation index to discriminate between normal and impaired CA, using hypercapnia as a surrogate for dynamic CA impairment. In 30 healthy volunteers, ABP (Finapres) and CBF velocity (CBFV, transcranial Doppler) were recorded at rest and during 5% CO2 breathing, with and without pseudo-random sequence inflation and deflation of bilateral thigh cuffs. The application of thigh cuffs increased ABP and CBFV variabilities and was not associated with a distortion of the CBFV step response estimates for both normocapnic and hypercapnic conditions (P=0.59 and P=0.96, respectively). Sensitivity and specificity of CA impairment detection were improved with the thigh cuff method, with the area under the receiver–operator curve increasing from 0.746 to 0.859 (P=0.031). We conclude that the new method is a safe, efficient, and appealing alternative to currently existing assessment methods for the investigation of the status of CA.
Keywords: arterial blood pressure, cerebral autoregulation, cerebral blood flow, pseudo-random binary sequences
Introduction
Cerebral autoregulation (CA) is a complex homeostatic mechanism whose action maintains a relatively constant flow of blood in the face of perturbations in arterial blood pressure (ABP), and protects the cerebral parenchyma from hyper- or hypoperfusion injury.1, 2
The advent of instrumentation that allows continuous non-invasive monitoring of cerebral blood flow (CBF) and ABP, with excellent temporal resolution, has caused a shift in our understanding of CA dynamics.3 Instead of relying on vasoactive drugs to induce large stable changes in ABP, as required by the traditional ‘static' approach,1, 4 the dynamic CA response can be identified using different maneuvers such as the sudden release of compressed thigh cuffs,3 rhythmic hand grip,5 Valsalva maneuver,6 changes in posture,7, 8 and others that can induce transient alterations in ABP. Most of these maneuvers are difficult to implement in a clinical setting owing to the need for patient cooperation, parallel increases in sympathetic activity, or safety of protocols in vulnerable patients. For these reasons, one important alternative, which has been favored by many centers, is the use of spontaneous fluctuations in ABP and CBF coupled with system identification techniques to derive parameters that can reflect the efficacy of dynamic CA.9 In particular, transfer function analysis using ABP as input and CBF velocity (CBFV) as output yields estimates of the gain and phase frequency responses, which have been shown to be markers of dynamic CA in multiple clinical conditions such as stroke, carotid artery disease, severe head injury, diabetes, and subarachnoid haemorrhage.2, 10 Moreover, the autoregulation index (ARI), an index of dynamic CA initially validated using thigh cuff maneuvers,4 can also be derived from spontaneous fluctuations.11 The ARI ranges from 0 (absence of CA) to 9 (best observed CA) and has also been shown to discriminate between different groups of patients.10
Despite encouraging results, most studies described in the literature on the use of spontaneous fluctuations in ABP and CBF to assess dynamic CA were based on differences between groups rather than individual subjects. Consequently, very limited information is available about the sensitivity and specificity of this approach and hence its overall diagnostic accuracy. One ongoing concern about the use of spontaneous fluctuations for assessment of dynamic CA is the potential lack of substantive variability in ABP and CBF, which could lead to poor signal-to-noise ratio and limitations in reproducibility.8, 12, 13, 14, 15 After the recent development of a new approach to increase ABP variability,16 we have made use of the well-known effect of hypercapnia as a surrogate of CA impairment1, 3, 17 to test the hypothesis that detection of impaired CA is improved by increases in ABP variability.
Materials and methods
Subjects and measurements
Ethical approval was obtained from the Southampton and South West Hampshire Research Ethics Committee A (10/H0502/1) before commencing the study. Volunteers were recruited if they were normotensive, their medical history was free of known cardiovascular and neurological disorders, and were not on any medication known to alter cardiovascular parameters. On the day of the trial, participants were reminded of the protocol and written informed consent was obtained.
Participants were asked to assume a supine position, and after a brief settling down period brachial ABP was measured by means of oscillometric sphygmomanometry using a validated device (Omron 705C, Milton Keynes, UK). Bilateral thigh cuffs and the face mask were then attached, and a trial inflation/deflation cycle was performed to familiarize volunteers with the procedure and to ensure that the flow of air to the thigh cuffs was uninterrupted. The thigh cuff inflation system was described in detail in a previous communication.16 In summary, the system comprises a compressor and high-pressure reservoir that holds the air required to inflate bilateral thigh cuffs. The flow of air to the cuffs is controlled by the coordinated action of a pressure regulator, a boost valve, an adaptive controller, and a deflation valve. Cuffs are alternately inflated and deflated (to a maximum pressure of 150 mm Hg) after a pseudo-random binary sequence (PRBS), which increases ABP variability over a wider frequency range (>0.05 Hz) than would be obtained by using a fixed inflation/deflation frequency.16 Delivery of 5% CO2 in air is achieved through a face mask (Vital Signs, Totowa, NJ, USA), which is connected to the CO2 delivery subunit. The subunit comprises a y valve that controls whether carbon dioxide or air is being administered and a 200-l Douglas bag is used to store the CO2/air mixture. Hardware and software included necessary safety features to protect the subjects from unintended exposure to CO2 and excessive thigh cuff inflation pressure.16
Arterial blood pressure was monitored non-invasively using the arterial volume clamping method (Finometer, Finapres Medical Systems, Amsterdam, The Netherlands). Freehand transcranial Doppler (Companion III, Viasys Healthcare, San Diego, CA, USA) identification of both middle cerebral arteries was performed and probes were then held in place with a custom-built head frame. The face mask was connected to the CO2 delivery system and to the capnograph (Datex Normocap 200, Helsinki, Finland) to monitor end-tidal CO2 (EtCO2) levels. A 3-lead surface electrocardiogram was also recorded.
After a 10-minute period of supine rest, participants underwent a 5-minute baseline recording. Three additional 5-minute recordings were then performed in random order to cover all possible combinations of CO2 administration (no CO2 administration and 5% constant CO2 administration) and thigh cuff inflation (no thigh cuff inflation and PRBS-driven thigh cuff inflation).
Data analysis
Signals were sampled at a rate of 500 Hz and stored on a dedicated personal computer for offline analysis. All recordings were visually inspected, the ABP signal was calibrated, and narrow spikes (<100 ms) and artefacts were removed. Subsequently, all signals were filtered in the forward and reverse direction using an eighth-order Butterworth low-pass filter with a cutoff frequency of 20 Hz.
The beginning and end of each cardiac cycle were detected from the electrocardiogram signal, and the heart rate (HR) was estimated to obtain mean beat-to-beat values for the recorded signals. Estimates were then interpolated using a third-order polynomial and resampled at 5 Hz to create a time series with a uniform time base.
Auto- and cross-power spectral densities were estimated using the Welch averaged periodogram method, employing a 102.4s window (512 samples), with a 50% overlap. The complex transfer function H(f) between ABP and CBFV was estimated as:
where Syx(f) is the cross-power spectral density between signals y(n) (CBFV) and x(n) (ABP), and Sxx(f) the auto-power spectral density of signal x(n). The inverse Fourier transform was subsequently used to estimate the CBFV impulse response function from H(f), while the CBFV step response function was obtained by integrating the CBFV impulse response.18 These were in turn used to derive the ARI using Tiecks model, employing the mean square error criterion.4
Statistics
The Shapiro–Wilk test was used to test for normality. Non-normally distributed data being log-transformed. Repeated measures analysis of variance was used to test the effect of different maneuvers on measured and derived parameters (ABP, CBFV, EtCO2, and ARI). Right and left MCA estimates of ARI were averaged if not significantly different as assessed with the paired Student's t-test. To assess whether the use of thigh cuffs led to distorted estimates of dynamic CA (dCA), compared with those obtained from the use of spontaneous fluctuations, the value of the CBFV step response function at 6s was obtained for every volunteer, and paired Student's t-tests were performed between the two estimates for normocapnic and constant hypercapnic conditions.
Sensitivity and specificity were used as measures of the performance of ARI in detecting hypercapnia-induced dCA impairment.1, 17 Sensitivity (Sn) was estimated as:
where TP is the number of true-positive volunteers (impaired dCA) and FN the number of false negatives. Specificity (Sp) was estimated as:
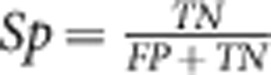 |
where TN is the number of true-negative volunteers (intact dCA) and FP the number of false positives. The receiver–operator characteristic (ROC) curve was plotted as Sn versus (1-Sp) by successively using all discrete values of ARI between 0 and 9 as thresholds (cutpoints) for what was deemed impairment of CA. Values of ARI< threshold were assumed to indicate positive cases (CA impairment). For normocapnia these represented FP and for hypercapnia TP. The reverse classification applied to ARI values above threshold. Receiver–operator characteristic curves were constructed for baseline and also for PRBS-driven thigh cuff inflation and deflation. The improvement in ROC detection characteristics owing to increased ABP variability was assessed by testing the areas under the curve. As subjects were repeated in each curve (air and 5% CO2) and the ROC curves were paired, the nonparametric method proposed by Obuchowski19 to handle clustered data was adopted. Values of P<0.05 were considered to indicate statistical significance.
Results
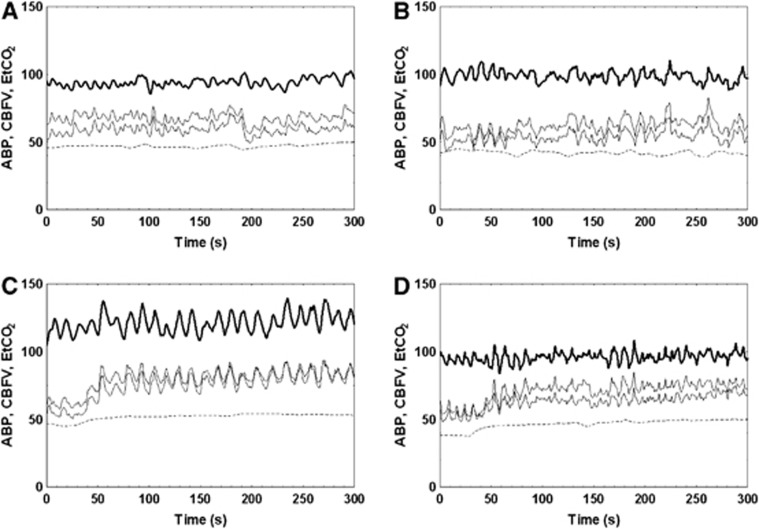
A total of 30 healthy adult volunteers (13 female) aged 22–55 years were recruited. The procedure was well-tolerated, and good quality, complete sets of data were obtained for all subjects. Participant demographics and population-averaged values for the main parameters are given in Tables 1 and 2, respectively. Figure 1 shows representative recordings obtained for baseline and the three different maneuvers. Significant changes in all main parameters in Table 2 were observed as the combined result of hypercapnia and operation of the thigh cuffs (analysis of variance). Post hoc analysis indicated that HR changed owing to hypercapnia but not owing to PRBS inflation and deflation of thigh cuffs.
Table 1. Volunteer demographics (mean±s.d.).
| Age (years) | 31.4±12 |
|---|---|
| Height (cm) | 174±8 |
| Weight (kg) | 72.2±12.8 |
| BMI (kgm−2) | 23.6±3.3 |
| Systolic BP (mm Hg) | 123.0±11.6 |
| Diastolic BP (mm Hg) | 75.8±8.3 |
Abbreviations: BMI, body mass index; BP, blood pressure.
Table 2. Population averages for measured and derived parameters (mean±s.d.), calculated for the entire duration of each maneuver.
| Parameter | Normocapnia/NTC | Normocapnia/TC | Hypercapnia/NTC | Hypercapnia/TC | P-values (ANOVA) |
|---|---|---|---|---|---|
| mABP (mm Hg) | 88.4±14.7 | 91.0±13.1 | 93.5±16.9 | 94.7±16.1 | P=0.020 |
| CBFVL (cm/s) | 53.6±12.2 | 55.2±12.1 | 65.4±15.6 | 70.7±14.2 | P<10−4 |
| CBFVR (cm/s) | 52.7±14.1 | 54.6±11.9 | 63.9±15.9 | 66.6±14.3 | P<10−4 |
| EtCO2 (mm Hg) | 39.8±3.1 | 38.0±3.1 | 45.9±2.9 | 46.8±2.8 | P<10−4 |
| HR (b.p.m.) | 69.1±8.0 | 68.3±7.3 | 70.9±6.6 | 71.4±6.7 | P=0.001 |
| ARIL | 6.1±1.5 | 6.2±1.0 | 4.4±1.5 | 4.1±1.4 | P<10−4 |
| ARIR | 6.2±1.3 | 6.1±0.9 | 4.8±1.9 | 4.3±1.6 | P<10−4 |
Abberviations: ANOVA, analysis of variance; ARIL, autoregulation index value obtained from CBFV recordings of the left MCA; ARIR, autoregulation index value obtained from CBFV recordings of the right MCA; b.p.m., beats per minute; CBFV, cerebral blood flow velocity; EtCO2, end-tidal CO2; mABP, mean ABP; NTC, no thigh cuffs; TC, thigh cuffs.
Figure 1.
Representative recordings of arterial blood pressure (ABP) (thick line, mm Hg), cerebral blood flow velocity (CBFV) (thin solid lines, cm/second), and end-tidal CO2 (EtCO2) (dotted line, mm Hg) from a 27-year-old male volunteer. Subplots (A) and (C) represent the use of spontaneous variability under normocapnic and hypercapnic conditions, respectively. Subplots (B) and (D) represent recordings obtained from the use of the thigh cuffs under normocapnic and hypercapnic conditions, respectively. Arterial blood pressure, CBFV, and EtCO2 are given in units of mm Hg, cm second−1, and mm Hg, respectively. The increase in CBFV owing to hypercapnia can be observed after 50 seconds.
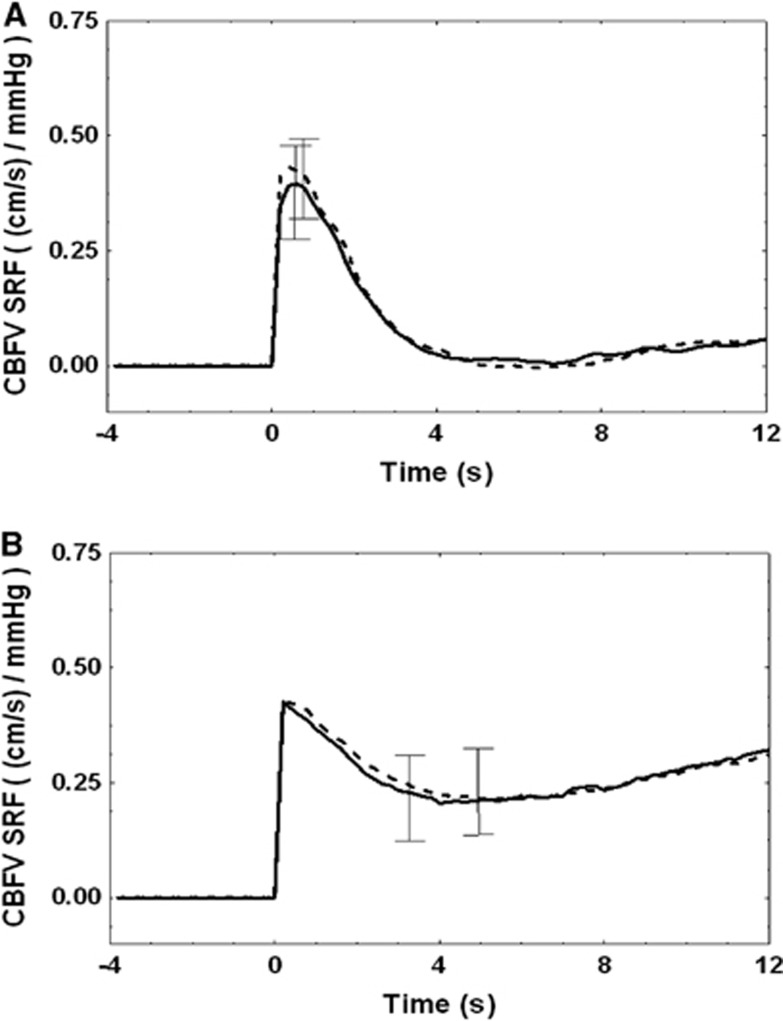
The use of PRBS inflation and deflation of thigh cuffs resulted in increased ABP variability both during normocapnia (9.2±10.5–17.6±14.5 mm Hg2/Hz, P=0.011) and hypercapnia (11.0±8.6–22.3±18.4 mm Hg2/Hz, P=0.001), as assessed by average power spectral values over the frequency range 0–0.1 Hz. The increase in variability did not affect estimates of CBFV step responses (Figure 2). Impairment of dynamic CA owing to hypercapnia was confirmed by the incomplete return of the CBFV step response baseline (Figure 2B) with correspondingly smaller values of ARI (Table 2). Step response values at 6s were not different for baseline compared with thigh cuff operation for both normocapnia (0.03±0.33 versus 0.02±0.25, P=0.56) and hypercapnia (0.42±0.43 versus 0.42±0.37, P=0.96). Autoregulation index values from the right and left MCAs were not significantly different and were averaged for the ROC analysis to follow. Any changes in these averaged ARI values induced by applying the PRBS-controlled thigh cuffs were not significant neither for normocapnia (−0.15±1.19; P=0.49) nor hypercapnia (0.43±1.90; P=0.27).
Figure 2.
Group-averaged cerebral blood flow velocity (CBFV) step response functions obtained from the use of thigh cuffs (dashed line) and spontaneous variability (solid line) under normocapnic (A) and hypercapnic conditions (B). Error bars represent largest ±1 s.e.m. During normocapnia, (A) the rapid return of the step response to baseline, at ∼4 seconds indicates an efficient dynamic cerebral autoregulation (CA), which is not observed during hypercapnia, (B) when CA is significantly impaired (Table 2). SRF, step response function.
Both ROC curves in Figure 3 showed good classification characteristics in comparison with the line of indifference (random choice of impaired/unimpaired). Clearly superior results with improvements in sensitivity for any specificity (except near Sp=0—and of little practical importance) were obtained owing to increased ABP variability induced by PRBS cuff inflation and deflation. The area under the ROC curve increased from 0.746 for baseline to 0.859 during cuff operation (P=0.031). The optimal operating point, often defined as the point closest to Sp=1 Sn=1, also displays higher sensitivity and specificity for the thigh cuff inflations.
Figure 3.
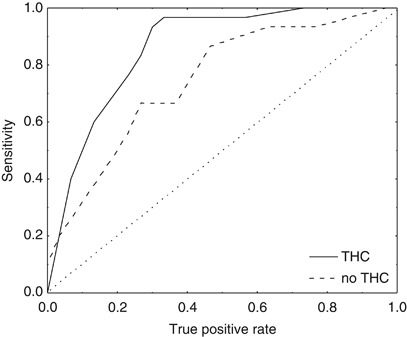
Receiver–operator characteristic (ROC) curves for the detection of cerebral autoregulation (CA) impairment induced by hypercapnia. Using different threshold values for autoregulation index (ARI), sensitivity and specificity (see Materials and methods) were obtained for spontaneous fluctuations in arterial blood pressure (ABP) (dashed line) and for pseudo-random binary sequence (PRBS)-controlled inflation/deflation of thigh cuffs (continuous line). Each curve reflects the discrimination between normal (air) and impaired CA (5%CO2) using the ARI. The increased area under the curve, in relation to the ‘line of indifference' (dotted line), indicates the superior discrimination between normal and impaired CA resulting from the use of thigh cuffs to increase ABP variability. THC, thigh cuff.
Discussion
Main findings
The current study provided confirmation that PRBS-controlled inflation/deflation of bilateral thigh cuffs induces significant increases in ABP variability compared with spontaneous fluctuations recorded at rest. Of note, this study enrolled a much larger population and none of the subjects were the same as those who participated in the original pilot study.16 The main new finding of relevance though is that increased ABP led to improved detection of CA impairment induced by hypercapnia. This improvement did not depend on the selection of a specific value of the ARI, but was clearly apparent from the shift of the ROC curve (Figure 3).
One important additional finding was that mean dynamic CA, either expressed by the entire CBFV step response curve or the ARI index, did not change significantly owing to the random inflation and deflation of thigh cuffs, neither in normocapnia nor hypercapnia. However, small changes in ARI estimates led to significantly improved consistency of the ARI drop from hypocapnia to hypercapnia and the observed increase in the area under the ROC curve.
Role of ABP variability
Concerns about the influence of ABP on the reliability of parameters used for assessment of dynamic CA have been raised in previous studies12, 13, 15 with the suggestion that different protocols, such as the sit-to-stand maneuver,8 could be beneficial to address this potential problem. In particular, Liu et al13 demonstrated that selecting recordings with high spontaneous ABP variability led to more robust estimates of dynamic CA parameters.
Apart from our own pilot study based on a smaller and different group of subjects,16 the use of PRBS to drive the inflation and deflation of bilateral thigh cuffs has not been described previously. Aaslid et al20 used a square wave sequence to drive the inflation of thigh cuffs in a group of adult and pediatric neurosurgical patients under mild hypocapnic conditions. Changes in ABP variability owing to the repeated inflation and deflation of thigh cuffs were not reported and neither the use of different square wave frequencies. The influence of thigh cuff inflation on HR or other indicators of sympathetic outflow was not described either.
Compared with the classical single inflation/release of thigh cuffs3 or the fixed frequency square wave inflation/deflation approach,20 PRBS-controlled inflation/deflation of thigh cuffs present several important advantages. First of all, the method allows the time during which the cuffs remain inflated to be much reduced compared with the conventional thigh cuff test,16 thus reducing patient discomfort and minimizing the possibility of increased sympathetic activity, as indicated by the stable HR values in Table 2.21 Several of our subjects expressed some degree of discomfort from the face mask, but none complained about the thigh cuffs. This was in stark contrast with our previous extensive experience with the use of the classical single inflation/release of thigh cuffs, which can be very painful owing to inflation pressures of 20 mm Hg or more above systolic ABP during at least 3 minutes.22 Second, owing to its temporal variability, PRBS have a much broader frequency spectrum than a single frequency square wave provoking increased ABP variability over a broader range of frequencies thus bringing additional improvements to the quality of transfer function analysis estimates.16
Despite potential limitations owing to insufficient ABP variability, estimates of dynamic CA based on spontaneous fluctuations should be regarded as the best ‘physiological reference' method owing to the lack of intervening covariates. For this reason, whenever alternative methods for assessment of CA are proposed, for example, using changes in posture, it is important to demonstrate that these yield at least similar results to the approach based on spontaneous fluctuations. Finally, it should also be highlighted that the PRBS instrumentation system is portable and can thus readily be made available by the patients' bedside.
Accuracy of dynamic CA indices
The ROC curve has been used previously in clinical studies of dynamic CA, but not to describe the diagnostic accuracy of ARI for detection of impaired CA resulting from hypercapnia. In severe head injury, Panerai et al23 obtained the ROC curve for the ARI as a predictor of mortality. Hu et al24 showed ROC curves for two different techniques to derive the phase difference between CBFV and ABP, another indicator of dynamic CA, as predictors of type 2 diabetes. More recently, Budohoski et al25 also presented ROC curves comparing two different indices of CA based on correlation coefficients, as predictors of intracranial hypertension caused by severe head injury. Unfortunately, none of these previous studies reported on levels of ABP variability, thus making comparisons with our results difficult. However, it is possible to speculate that ABP variability could be elevated in patients with diabetes owing to depression of the baroreflex in this patient group, while severe head injury patients in intensive care might have their ABP variability elevated owing to artificial ventilation.
Study limitations
Measurements of CBFV can reflect changes in CBF as long as the diameter of the insonated vessel remains constant. Several studies have demonstrated that the cross-sectional area of the MCA changes minimally during thigh cuff inflation/deflation or owing to large changes in ABP or PaCO2.26, 27, 28
For the purposes of this study, a maximum thigh cuff pressure setting (MTCP) of 150 mm Hg was selected, and was used uniformly for all volunteers. Future studies could include the personalized selection of the MTCP pressure settings in their design, to ensure that maximum ABP variability is induced. This is particularly important when investigating CA function in elderly subjects or patients with elevated systolic BP.
Only healthy volunteers were included in this study, and it is therefore not certain whether similar results would be obtained in patients with cardiovascular conditions. The age range of the volunteers may also constitute a limitation that hinders the extrapolation of our findings to an older population.
The sample size of the study (n=30) was calculated to detect a change in ARI=1.2, with 80% power at P=0.05.14 Changes in ARI above this target were observed owing to hypercapnia, both during baseline and PRBS-controlled inflation/deflation of thigh cuffs. The thigh cuffs induced a mean change in ARI of −0.15 in normocapnia and 0.43 in hypercapnia (together with reduced s.d.—see Table 2), which were not significant. However, it is possible that with a larger sample size, the effect of random inflation/deflation on dynamic CA would have become statistically significant, but such small changes are unlikely to be of relevance in clinical applications.
To assess the sensitivity and specificity of ARI in detecting impairments of autoregulation, we decided to induce a state of autoregulatory inefficiency by means of administering CO2 at a concentration of 5%, which was used as a surrogate of dynamic CA impairment. Though sufficient evidence exists to suggest that this assumption is not unreasonable,1, 17 caution needs to be exercised before extrapolating our findings to impairment of dCA caused by different pathologies.
Conclusions
We have demonstrated the feasibility and efficacy of using pseudo-random binary stimuli for the integrated assessment of cerebral hemodynamics with good acceptability by the volunteers. This approach resulted in increased ABP variability without distortion of dynamic CA estimates. Moreover, the use of PRBS to drive the inflation of thigh cuffs resulted in improved sensitivity, specificity, and accuracy of the ARI method in detecting dynamic CA impairment. Further work is needed to investigate the reliability of estimates of dynamic CA obtained with this new method of assessment in a larger population and to test its applicability in a clinical setting.
The authors declare no conflict of interest.
Footnotes
This study was supported by the UK EPSRC grants EP/G008787/1and EP/G010420/1.
References
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- van Beek AH, Claassen JA, Rikkert MG, Jansen RW. Cerebral autoregulation: an overview of current concepts and methodology with special focus on the elderly. J Cereb Blood Flow Metab. 2008;28:1071–1085. doi: 10.1038/jcbfm.2008.13. [DOI] [PubMed] [Google Scholar]
- Aaslid R, Lindegaard KF, Sorteberg W, Nornes H. Cerebral autoregulation dynamics in humans. Stroke. 1989;20:45–52. doi: 10.1161/01.str.20.1.45. [DOI] [PubMed] [Google Scholar]
- Tiecks FP, Lam AM, Aaslid R, Newell DW. Comparison of static and dynamic cerebral autoregulation measurements. Stroke. 1995;26:1014–1019. doi: 10.1161/01.str.26.6.1014. [DOI] [PubMed] [Google Scholar]
- Kwan J, Lunt M, Jenkinson D. Assessing dynamic cerebral autoregulation after stroke using a novel technique of combining transcranial Doppler ultrasonography and rhythmic handgrip. Blood Press Monit. 2004;9:3–8. doi: 10.1097/00126097-200402000-00002. [DOI] [PubMed] [Google Scholar]
- Tiecks FP, Douville C, Byrd S, Lam AM, Newell DW. Evaluation of impaired cerebral autoregulation by the Valsalva maneuver. Stroke. 1996;27:1177–1182. doi: 10.1161/01.str.27.7.1177. [DOI] [PubMed] [Google Scholar]
- Lipsitz LA, Mukai S, Hamner J, Gagnon M, Babikian V. Dynamic regulation of middle cerebral artery blood flow velocity in aging and hypertension. Stroke. 2000;31:1897–1903. doi: 10.1161/01.str.31.8.1897. [DOI] [PubMed] [Google Scholar]
- Claassen JA, Levine BD, Zhang R. Dynamic cerebral autoregulation during repeated squat-stand maneuvers. J Appl Physiol. 2009;106:153–160. doi: 10.1152/japplphysiol.90822.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panerai RB. Assessment of cerebral pressure autoregulation in humans—a review of measurement methods. Physiol Meas. 1998;19:305–338. doi: 10.1088/0967-3334/19/3/001. [DOI] [PubMed] [Google Scholar]
- Panerai RB. Cerebral autoregulation: from models to clinical applications. Cardiovasc Eng. 2008;8:42–59. doi: 10.1007/s10558-007-9044-6. [DOI] [PubMed] [Google Scholar]
- Panerai RB, White RP, Markus HS, Evans DH. Grading of cerebral dynamic autoregulation from spontaneous fluctuations in arterial blood pressure. Stroke. 1998;29:2341–2346. doi: 10.1161/01.str.29.11.2341. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Levine BD. Deterioration of cerebral autoregulation during orthostatic stress: insights from the frequency domain. J Appl Physiol. 1998;85:1113–1122. doi: 10.1152/jappl.1998.85.3.1113. [DOI] [PubMed] [Google Scholar]
- Liu J, Simpson DM, Allen R. High spontaneous fluctuation in arterial blood pressure improves the assessment of cerebral autoregulation. Physiol Meas. 2005;26:725–741. doi: 10.1088/0967-3334/26/5/012. [DOI] [PubMed] [Google Scholar]
- Brodie FG, Atkins ER, Robinson TG, Panerai RB. Reliability of dynamic cerebral autoregulation measurement using spontaneous fluctuations in blood pressure. Clin Sci. 2009;116:513–520. doi: 10.1042/CS20080236. [DOI] [PubMed] [Google Scholar]
- Gommer ED, Shijaku E, Mess WH, Reulen JP. Dynamic cerebral autoregulation: different signal processing methods without influence on results and reproducibility. Med Biol Eng Comput. 2010;48:1243–1250. doi: 10.1007/s11517-010-0706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsogridakis E, Bush G, Fan L, Birch AA, Simpson DM, Allen R, et al. Random perturbations of arterial blood pressure for the assessment of dynamic cerebral autoregulation. Physiol Meas. 2012;33:103–116. doi: 10.1088/0967-3334/33/2/103. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effects of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas. 1999;20:265–275. doi: 10.1088/0967-3334/20/3/304. [DOI] [PubMed] [Google Scholar]
- Zhang R, Zuckerman JH, Giller CA, Levine BD. Transfer function analysis of dynamic cerebral autoregulation in humans. Am J Physiol. 1998;274:H233–H241. doi: 10.1152/ajpheart.1998.274.1.h233. [DOI] [PubMed] [Google Scholar]
- Obuchowski N. Nonparametric analysis of clustered ROC curve data. Biometrics. 1997;53:567–578. [PubMed] [Google Scholar]
- Aaslid R, Blaha M, Sviri G, Douville CM, Newell DW. Asymmetric dynamic cerebral autoregulatory response to cyclic stimuli. Stroke. 2007;8:1465–1469. doi: 10.1161/STROKEAHA.106.473462. [DOI] [PubMed] [Google Scholar]
- van Lieshout JJ, Secher NH. Point: Counterpoint: sympathetic activity does/does not influence cerebral blood flow. Point: sympathetic activity does influence cerebral blood flow. J Appl Physiol. 2008;105:1364–1366. doi: 10.1152/japplphysiol.90597.2008. [DOI] [PubMed] [Google Scholar]
- Mahony PJ, Panerai RB, Deverson ST, Hayes PD, Evans DH. Assessment of the thigh cuff technique for measurement of dynamic cerebral autoregulation. Stroke. 2000;31:476–480. doi: 10.1161/01.str.31.2.476. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Kerins V, Fan L, Yeoman PM, Hope T, Evans DH. Association between dynamic cerebral autoregulation and mortality in severe head injury. Br J Neurosurg. 2004;18:471–479. doi: 10.1080/02688690400012343. [DOI] [PubMed] [Google Scholar]
- Hu K, Peng CK, Huang NE, Wu Z, Lipsitz LA, Cavallerano J, et al. Altered phase interactions between spontaneous blood pressure and flow fluctuations in type 2 diabetes mellitus: nonlinear assessment of cerebral autoregulation. Physica A. 2008;387:2279–2292. doi: 10.1016/j.physa.2007.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, de Riva N, Smielewski P, Pickard JD, Menon DK, et al. The relationship between cerebral blood flow autoregulation and cerebrovascular pressure reactivity after traumatic brain injury. Neurosurgery. 2012;71:652–661. doi: 10.1227/NEU.0b013e318260feb1. [DOI] [PubMed] [Google Scholar]
- Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery. 1993;32:737–741. [PubMed] [Google Scholar]
- Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]