Abstract
Although an increased leukocyte and platelet adhesion is observed in cerebral venules of mice with either hypertension (HTN) or hypercholesterolemia (HCh), it remains unclear whether the combination of HTN and HCh exerts a comparable effect on leukocyte and platelet recruitment in the cerebral microvasculature. Thus, we examined whether HCh alters platelet and leukocyte adhesion, and blood–brain barrier (BBB) permeability, in cerebral venules in two models of murine HTN: DOCA salt-induced and angiotensin II (Ang II) induced. In both models, the mice were placed on either a normal or cholesterol-enriched diet. An enhanced recruitment of adherent leukocytes and platelets in cerebral venules was noted in both HTN models in the absence of HCh, but not in its presence. The Ang II-induced increase in BBB permeability was attenuated by HCh as well. Both total and high-density lipoprotein (HDL) cholesterol levels were elevated in the HCh mice. The HTN-induced increase in leukocyte and platelet adhesion was attenuated in apolipoprotein A-I transgenic mice (ApoA1-Tg) and blunted in wild-type mice treated with the ApoA1 mimetic peptide, 4F. Our findings indicate that mild HCh significantly blunts the cerebral microvascular responses to HTN and that HDL may have a role in mediating this beneficial effect of HCh.
Keywords: cerebral microvasculature, hypercholesterolemia, hypertension, inflammation, HDL cholesterol
Introduction
A variety of human diseases have been linked to either acute or chronic inflammation. Unlike a localized response to infection or tissue injury, a systemic inflammatory response can result when mediators liberated from injured or inflamed tissue elicits a generalized activation of the innate and adaptive immune systems, which translate into clinical or subclinical manifestations. All of the established risk factors for cardiovascular disease, including hypertension (HTN) and hypercholesterolemia (HCh), are now recognized to induce a systemic inflammatory response that appears to underlie the vascular dysfunction that accompanies these conditions. Hypertensive patients exhibit elevated plasma levels of inflammatory mediators such as high-sensitive C-reactive protein,1, 2 interleukin 6 (IL-6),2 interferon-inducible protein-10, and IL-4, IL-7, and IL-13.3 Similarly, high-sensitive C-reactive protein,4, 5 chemokine CXC ligand 5 (CXCL5),6 xanthine oxidase activity, IL-1 and IL-6 are all increased in plasma of HCh patients.5 In animal models of HCh7 and HTN,8, 9, 10 the risk factor-induced inflammatory phenotype is manifested as an increased adhesion of both leukocytes and platelets in cerebral microvessels.
While it is well known that inflammation and microvascular dysfunction result from the presence of either HTN or HCh, less is known about whether and how combinations of risk factors (HTN and HCh) affects the microvasculature. Epidemiologic studies have revealed a high prevalence (30% to 40%) of coexisting HTN and HCh in the human population,11 and the concurrence of HTN and HCh is believed to increase the incidence of atherosclerosis and cardiac events relative to the changes noted with either risk factor alone.12 The demonstration of accelerated atherosclerotic lesion development and more profound large vessel dysfunction in experimental animals with HTN+HCh (compared with either risk factor alone) tends to support the clinical evidence.13, 14 However, it remains unclear whether the combination of HTN and HCh elicits an altered (exaggerated or dampened) inflammatory response in the microvasculature, compared with either risk factor alone. Hence, the overall objective of this study was to examine the influence of HTN+HCh on the recruitment of adherent leukocytes and platelets in the cerebral microvasculature and on blood–brain barrier (BBB) permeability. Our findings reveal that mild HCh significantly blunts the cerebral microvascular responses to HTN and that high-density lipoproteins (HDLs) may have a role in mediating this effect of HCh.
Materials and methods
Animals
Male C57BL/6 (wild-type, WT) mice and transgenic mice containing the entire human apolipoprotein A-I gene including the promoter, ApoA1 (C57BL/6-Tg(APOA1)1Rub/J), were obtained from Jackson Laboratories (Bar Harbor, ME, USA). The mice were housed under specific pathogen-free conditions and fed standard laboratory chow and water before entering the study. All of the experimental procedures involving the use of animals were reviewed and approved by the Institutional Animal Care and Use Committee of Louisiana State University Health, Shreveport, and performed according to the criteria outlined by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Hypertension Groups
After 2 days of acclimatization, under ketamine (150 mg/kg, intraperitoneally)-xylazine (7.5 mg/kg, intraperitoneally) anesthesia (∼100 μL/mouse), the left kidney was removed from some mice. After surgery, the nephrectomized mice (Uni) were randomly assigned to the following experimental groups: controls (Uni), 3-week-high cholesterol diet (HCh), DOCA salt, or 3-week-high cholesterol diet plus DOCA salt (n=5 to 8/group). A slow release DOCA pellet (50 mg, 21-day release) (Innovative Research of America, Sarasota, FL, USA) was inserted subcutaneously in the DOCA-salt groups and drinking water was replaced by a 1% saline/0.2% potassium chloride drinking solution. Mice in the other groups received tap water. One group of mice was not subjected to any surgical or pharmacological procedure (intact group). The high-cholesterol (HCh) diet (Teklad TD.94059) contained, among other constituents, 1.25% cholesterol, 0.125% choline chloride, and 15.8% fat (Harlan Teklad, Indianapolis, IN, USA). Another group of mice received a 14-day infusion of either saline or angiotensin II (Ang II, Bachem Americas, Inc., Torrance, CA, USA) using microosmotic pumps (Durect Corporation, Cupertino, CA, USA), which were implanted subcutaneously in the intrascapular region under isoflurane anesthesia. The pumps in the control group were loaded with only the saline vehicle, while the experimental groups received Ang II loaded pumps that delivered the peptide at a rate of 2,000 ng/kg per minute. Similarly to the DOCA groups, the Ang II groups were divided as follows: control (saline pump), 3-week-high cholesterol diet with no pump (HCh), Ang II pump, or 3-week-high cholesterol diet plus Ang II pump (n=5 to 8/group). To test the influence of HDL cholesterol on the observed responses, three groups of mice were added: (1) human apolipoprotein A-I transgenic mice (ApoA1-Tg), which have higher levels of plasma HDL cholesterol, plus DOCA salt, (2) WT DOCA-salt mice treated with the ApoA1 mimetic peptide 4F (amino-acid sequence: DWFKAFYDKVAEKFKEAF) (Biosynthesis, Lewisville, TX, USA), and (3) 4F-treated Ang II pump mice. 4F was dissolved in phosphate-buffered saline and administered intraperitoneally (total injection volume of 200 μL) every other day for 2 weeks at a dose of 50 μg/mouse.15
Blood Pressure Measurement
Blood pressure was measured in nonanesthetized mice by tail plethysmography using the Hatteras Instruments System (model SC-1000, Cary, NC, USA). Mice were placed on a heated (40°C) platform and a cuff was placed around the tail and inflated for a period of 60 seconds to record systolic blood pressure. The average of five successive measurements was used as the systolic blood pressure for each animal.
Animal Preparation
Mice were anesthetized with intraperitoneal ketamine (150 mg/kg) and xylazine (7.5 mg/kg) (∼100 μL/mouse). The left femoral vein was cannulated for intravenous administration of 6G-rhodamine, labeled platelets and supplemental doses of anesthesia (15 mg/kg ketamine and 0.75 mg/kg xylazine, intravenously), administered as needed. Body temperature was maintained at 36°C during the experiment with a homeothermic blanket and monitored with a rectal temperature probe. The head of each mouse was fixed on the acrylic frame before the cranial window was created. After skull fixation, a circular skin incision was made, and a craniotomy was created 3 mm lateral and 2 mm posterior to the bregma. The exposed brain tissue was immersed in artificial cerebrospinal fluid and covered with a glass slide. Cerebral vessels were observed through the dura mater.
Intravital Videomicroscopy
The procedures used to monitor blood cell–vessel wall interactions in murine cerebral venules are described elsewhere in detail.16 Briefly, the cerebral microcirculation was visualized with an upright fluorescent microscope using a 20 × water immersion lens. Color images were captured with a 3 charge coupled device (CCD) color video camera. Randomly selected segments of pial venules (20 to 70 μm diameter, 100 μm long) were chosen for observation. Approximately 100 × 106 platelets were isolated from donor mice, labeled (green) ex vivo with carboxyfluorescein diacetate succinimidyl ester,17 and administered to recipient mice through the left femoral vein (this extracorporeal staining produces minimal activation of platelets18). This was followed by the continuous infusion of 0.02% rhodamine 6G, which was used to fluorescently label (red) circulating leukocytes. Adherent leukocytes and platelets were defined as cells remaining stationary within venules for 30 seconds. Cell adhesion data are expressed as number of cells per millimeter squared of venular surface, calculated from venular diameter and length, assuming cylindrical geometry.
Blood–Brain Barrier Dysfunction
Permeability of BBB was assessed using the Evans blue (EB) extravasation method.19 A 2% solution of EB (Sigma-Aldrich, St Louis, MO, USA) was injected (4 mL/kg) into the femoral vein. Twenty-four hours later, 0.4 mL of blood was obtained by cardiac puncture, then the mouse was transcardially perfused with phosphate-buffered saline (100 mm Hg) for 5 minutes. The brain was removed and separated from the dura mater and cerebellum. The cerebrum was divided into two hemispheres, each of which was homogenized and sonicated in 1 mL of 50% trichloroacetic acid (Sigma-Aldrich) and centrifuged at 12,100 g for 20 minutes. The supernatant was diluted with ethanol and the concentrations of EB in brain tissue and plasma were measured using a fluorescence spectrophotometer (FLUOstar Optima microplate reader; BMG LABTECH, Inc, Ortenberg, Germany). The BBB permeability was determined by dividing tissue EB concentration (μg/g brain weight) by the plasma concentration (μg/g).
Serum Cholesterol Levels
At the end of the experiments, blood was drawn from the tail vein, centrifuged, and the plasma was frozen for the subsequent measurement of cholesterol levels using a spectrophotometric assay kit (Stanbio Laboratory, Boerne, TX, USA).
Statistical Analysis
All data were expressed as mean±s.e. Statistical difference between the different groups was determined by a one-way analysis of variance with the Tukey post hoc test. All analyses were performed using Prism 5 software (GraphPad Software, Inc, La Jolla, CA, USA). Statistical significance was set at P<0.05.
Results
Effects of DOCA Salt- or Angiotensin II-Induced Hypertension, When Combined with a High Cholesterol Diet, on Blood Pressure and Total Plasma Cholesterol Levels
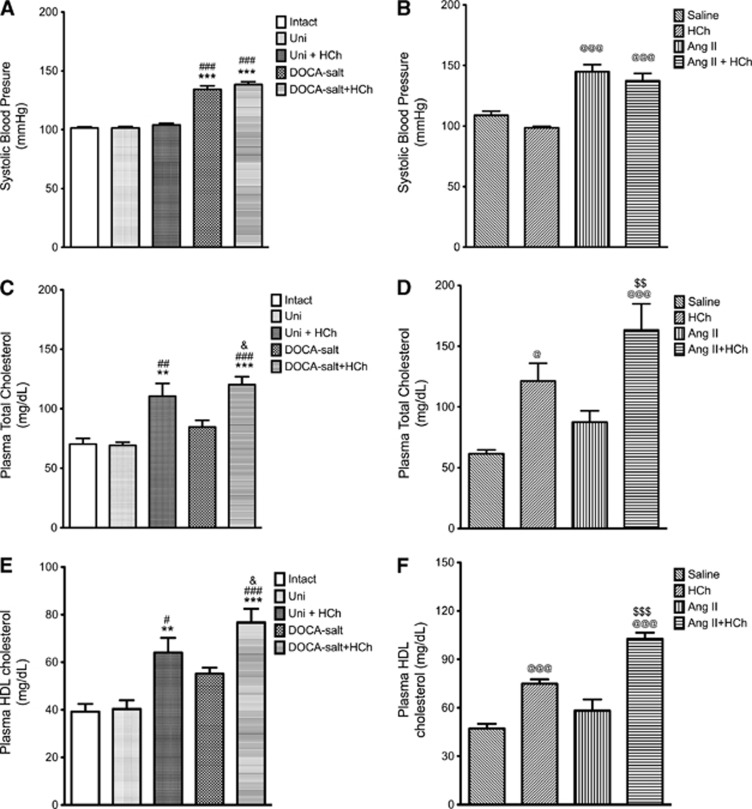
Blood pressure was increased by 30% in both DOCA-salt (Figure 1A) and Ang II mice (Figure 1B) and these values were not significantly altered by a high cholesterol diet. Total plasma cholesterol concentration was elevated in all mice placed on a cholesterol-enriched diet, with increases ranging between 60% and 130%. The presence of HTN did not alter the cholesterol levels (Figures 1C and 1D).
Figure 1.
Systolic blood pressure (A, B), plasma total cholesterol (C, D), and plasma high-density lipoprotein (HDL) cholesterol (E, F) measured in the following groups of mice: intact control (Intact, n=12), uninephrectomized control (Uni, n=9), uninephrectomized hypercholesterolemia (HCh) diet (Uni+HCh, n=12), DOCA salt (n=12), DOCA salt+HCh (n=9), saline control (n=7), HCh diet (HCh, n=7), angiotensin II (Ang II, n=4) and angiotensin II+HCh diet (n=6). All data were expressed as mean±s.e. **P<0.01 and ***P<0.001 versus intact; #P<0.05, ##P<0.01 and ###P<0.001 versus Uni; @P<0.05 and @@@P<0.001 versus saline; &P<0.05 versus DOCA salt; $$P<0.01 and $$$P<0.001 versus Ang II.
Effects of DOCA Salt- or Angiotensin II-Induced Hypertension, When Combined with a High Cholesterol Diet, on the Leukocyte and Platelet Adhesion in Cerebral Venules
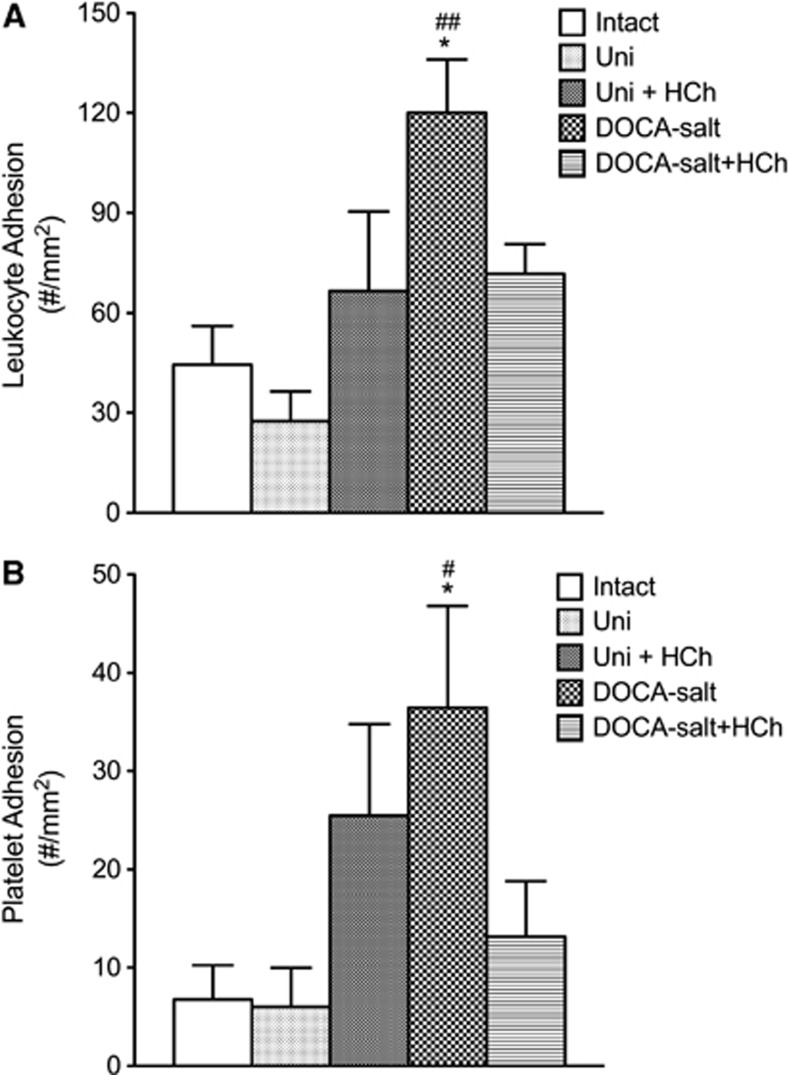
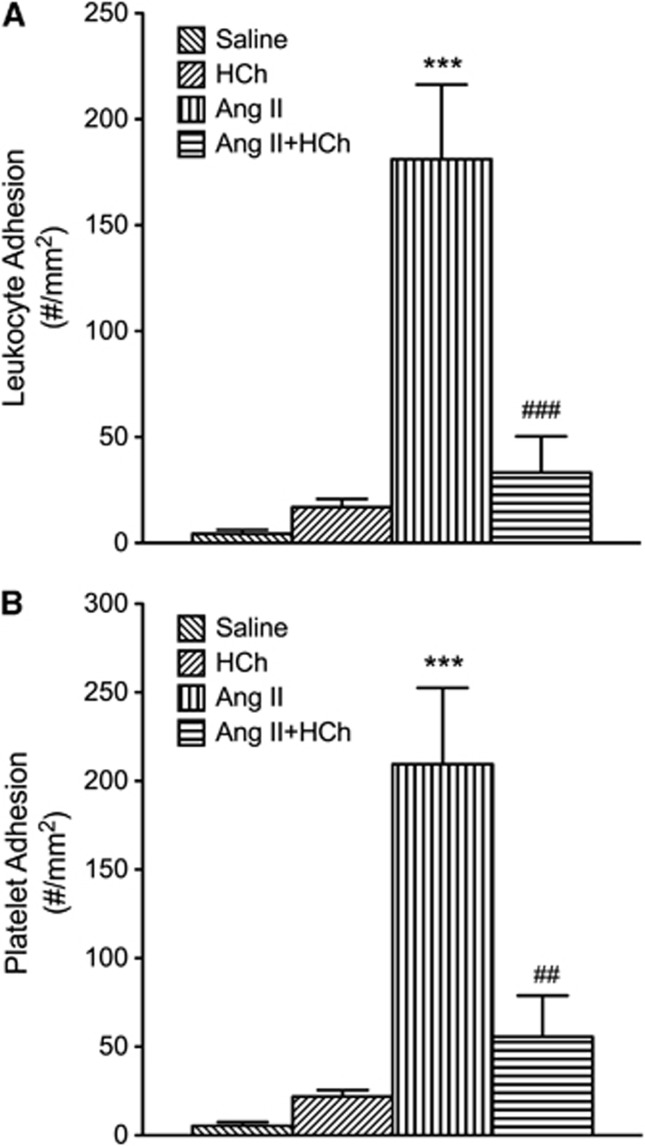
The DOCA-salt HTN was associated with increase in both leukocyte and platelet adhesion in cerebral venules (Figure 2). Placement of the DOCA-salt mice on a cholesterol-enriched diet blunted the blood cell recruitment responses. A similar pattern of responses was noted in mice with Ang II-induced HTN (Figure 3). The adhesion of leukocytes and platelets in cerebral venules was not altered by placement on a high cholesterol diet alone (Figures 2 and 3).
Figure 2.
Effects of high cholesterol diet±DOCA salt-induced-hypertension on leukocyte (A) and platelet (B) adhesion in cerebral venules. Intact (n=7) and uninephrectomized controls (Uni) (n=8), Uni+HCh (n=8), DOCA salt (n=7) and DOCA salt+HCh (n=7) were tested. All data were expressed as mean±s.e. *P<0.05 versus Intact, #P<0.05 and ##P<0.01 versus Uni. HCh, hypercholesterolemia.
Figure 3.
Effects of high cholesterol diet hypercholesterolemia (HCh)±angiotensin II-induced hypertension (Ang II) on leukocyte (A) and platelet (B) adhesion in cerebral venules. Saline control (n=7), HCh (n=11), Ang II (n=9), and Ang II+HCh (n=5) were tested. All data were expressed as mean±s.e. ***P<0.001 versus saline, ##P<0.01 and ###P<0.001 versus Ang II.
Effects of DOCA Salt- or Angiotensin II-Induced Hypertension, When Combined with a High Cholesterol Diet, on the Blood–Brain Barrier Permeability
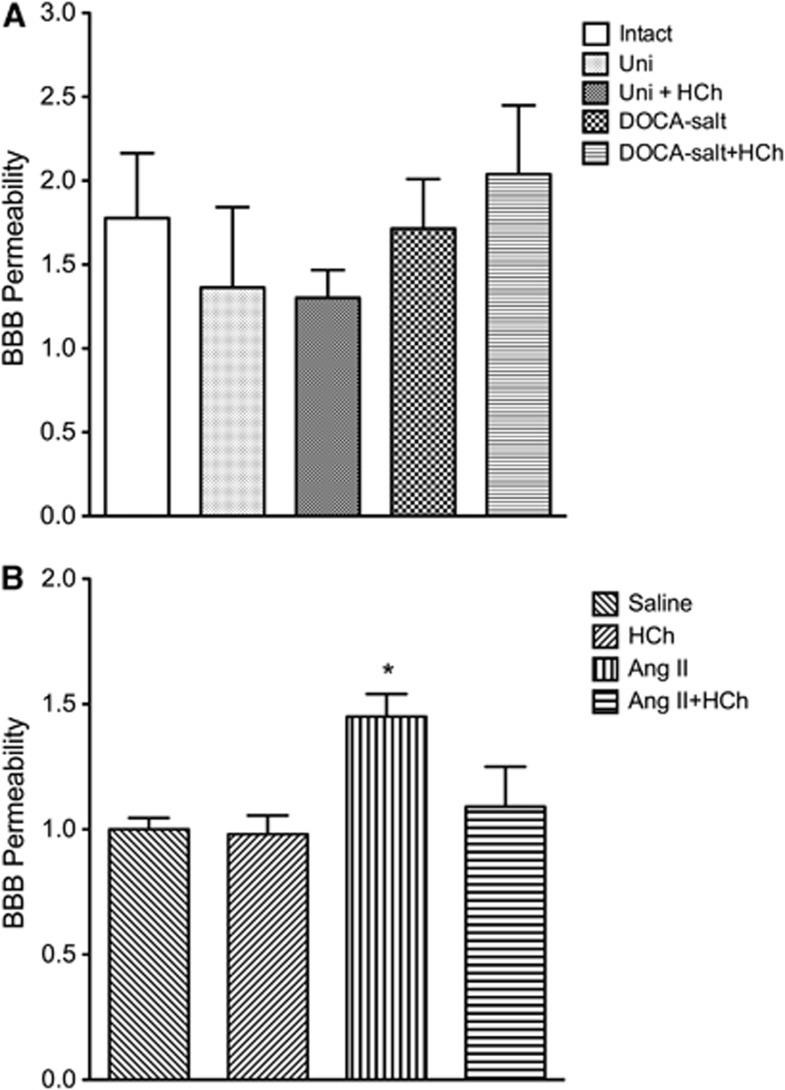
DOCA-salt HTN did not alter the permeability of BBB to EB albumin, either alone or in combination with a high cholesterol diet (Figure 4A). However, Ang II-induced HTN was associated with a significant increase in BBB permeability, a response that was not observed in AngII mice placed on a high cholesterol diet (Figure 4B). Hypercholesterolemia alone had no significant effect on BBB (Figures 4A and 4B).
Figure 4.
Blood brain barrier (BBB) permeability in intact control (Intact, n=6), uninephrectomized control (Uni, n=7), uninephrectomized hypercholesterolemia (HCh) diet (Uni+HCh, n=6), DOCA salt (n=4), and DOCA-salt+HCh diet (n=5) groups of mice (A), and saline control (n=12), HCh diet (HCh, n=12), angiotensin II (Ang II, n=12), and angiotensin II+HCh diet (n=12) mice (B). All data were expressed as mean±s.e. *P<0.05 versus saline.
Effect of DOCA Salt- or Angiotensin II-Induced Hypertension, When Combined with a High Cholesterol Diet, on Plasma High-Density Lipoprotein Cholesterol
Neither model of HTN was associated with a significant change in plasma HDL cholesterol concentration (Figures 1E and 1F). However, placement of mice on a cholesterol-enriched diet resulted in increased plasma HDL, with the increases ranging between 75% and 150% (Figures 1E and 1F).
Effects of Either Genetic Overexpression of Apolipoprotein A-I or 4F Treatment on Blood Pressure and Plasma High-Density Lipoprotein Cholesterol
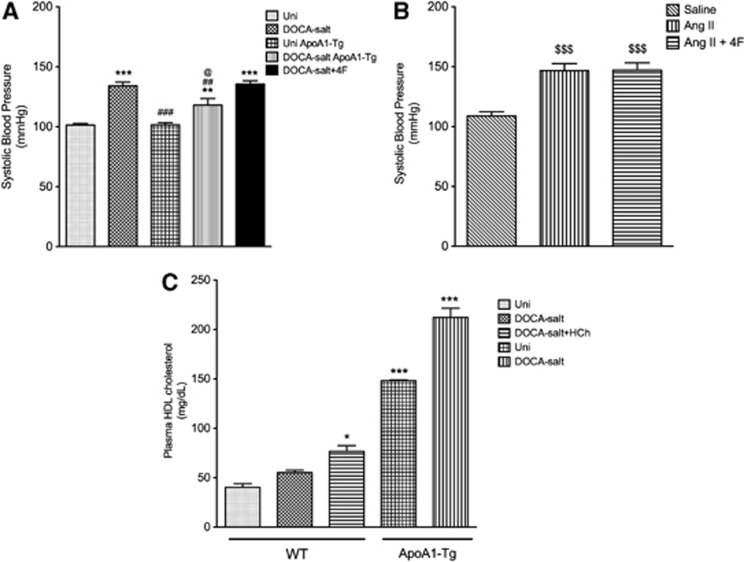
The ApoA1-Tg mice manifested a lower blood pressure after DOCA-salt treatment, compared with WT DOCA-salt mice, but it was still elevated compared with its normotensive littermate control (Uni ApoA1-Tg) (Figure 5A). 4F treatment of WT DOCA-salt mice (or Ang II mice) did not however exhibit a reduced blood pressure response (Figures 5A and 5B). Plasma HDL cholesterol was four times higher in ApoA1-Tg mice compared with WT mice (Figure 5C).
Figure 5.
Systolic blood pressure (A, B) measured in uninephrectomized controls (Uni, n=9), wild-type (WT) DOCA salt (n=12), uninephrectomized ApoA1 transgenic (Uni ApoA1-Tg, n=7), ApoA1 transgenic DOCA salt (n=6), 4F-treated DOCA salt (n=6), saline controls (n=5), angiotensin II (Ang II, n=5), and 4F-treated angiotensin II (n=5) mice. Plasma high-density lipoprotein (HDL) cholesterol (C) measured in uninephrectomized control (Uni, n=4), wild-type DOCA salt (n=4), WT DOCA salt+hypercholesterolemia (HCh) (n=4), uninephrectomized ApoA1-Tg controls (n=4), and ApoA1-Tg DOCA-salt mice (n=6). All data were expressed as mean±s.e. *P<0.05, **P<0.01 and ***P<0.001 versus Uni; ##P<0.01 and ###P<0.001 versus WT DOCA salt; @P<0.05 versus Uni ApoA1-Tg; $$$P<0.001 versus saline.
Effects of Either Genetic Overexpression of Apolipoprotein A-I or 4F Treatment on the Recruitment of Leukocytes and Platelets in Cerebral Venules
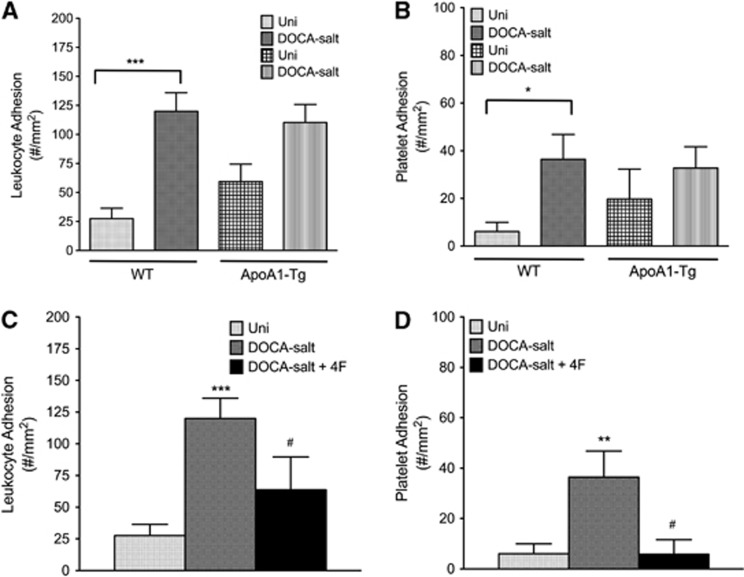
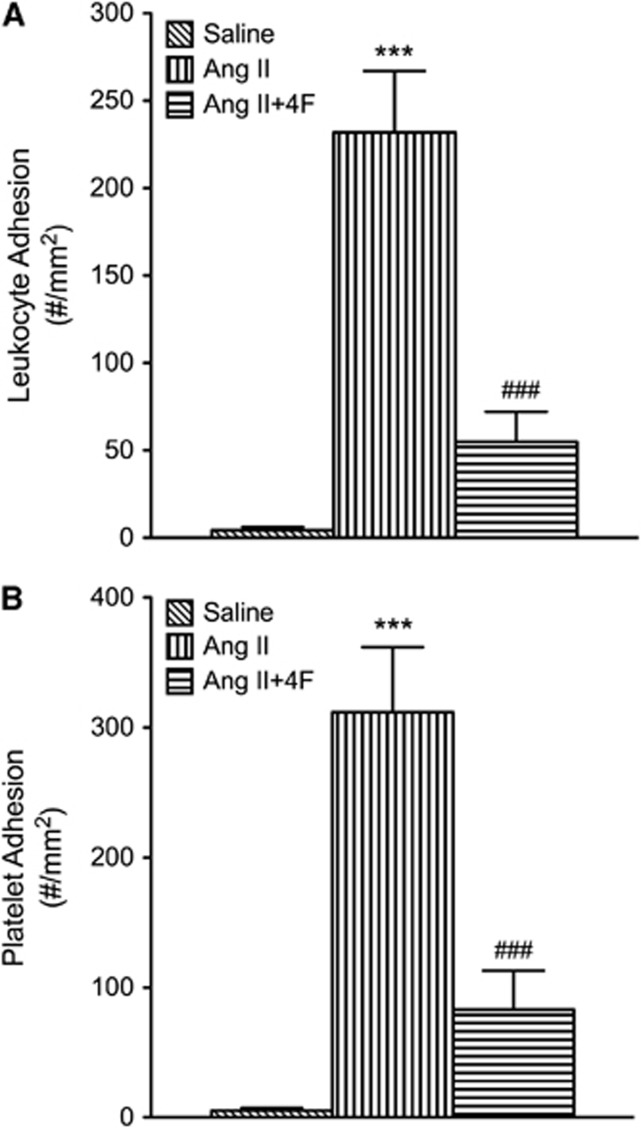
Both ApoA1-Tg DOCA-salt mice and 4F-treated DOCA-salt mice (Figure 6) exhibited reduced leukocyte and platelet adhesion in cerebral venules, compared with the WT DOCA-salt mice. Similarly, 4F treatment significantly reduced leukocyte and platelet adhesion in cerebral venules of Ang II hypertensive mice (Figure 7).
Figure 6.
The leukocyte (A) and platelet (B) adhesion, respectively, in cerebral venules of uninephrectomized wild-type (WT) controls (Uni, n=8), WT DOCA salt (DOCA salt, n=7), uninephrectomized ApoA1 transgenic controls (Uni, n=6) and ApoA1 transgenic DOCA-salt (DOCA salt, n=6) mice. While the leukocyte adhesion was five times higher in wild-type DOCA salt compared with C57 Uni control, the increase was only two times higher in ApoA1-Tg DOCA salt compared with Uni ApoA1-Tg control mice. Similarly, platelet adhesion was five times higher in wild-type DOCA salt compared with its controls and only a 1.5-fold increase in ApoA1 transgenic DOCA salt, compared with its controls. (C, D) The leukocyte and platelet adhesion, respectively, in cerebral venules of uninephrectomized wild-type controls (Uni, n=8), WT DOCA salt (DOCA salt, n=7), and 4F-treated wild-type DOCA salt (DOCA salt+4F, n=6) mice is shown. All data were expressed as mean±s.e. *P<0.05, **P<0.01 and ***P<0.001 versus Uni; #P<0.05 versus DOCA salt.
Figure 7.
Leukocyte (A) and platelet (B) adhesion in cerebral venules of saline controls (n=5), angiotensin II (Ang II, n=5), and 4F-treated angiotensin II (n=5) mice (Ang II+4F). All data were expressed as mean±s.e. ***P<0.001 versus saline; ###P<0.001 versus Ang II.
Discussion
There is a large body of epidemiologic evidence, which suggests that combinations of risk factors exert a synergistic effect on the incidence of ischemic diseases of the heart and brain, compared with individual risk factors.20 Less is known about whether this synergistic response of risk factor combinations also translates into an amplified inflammatory response or enhanced microvascular dysfunction. The limited evidence in the literature on this issue is inconsistent, with some studies showing synergism between HTN and HCh on vascular reactivity13, 21 and others describing an absence of synergism.22, 23 The absence of synergism might be expected since both HTN and HCh induce very similar phenotypic changes in the vasculature and act through similar signaling pathways. The findings of the present study however indicate that the combination of HTN and HCh induces an attenuated proinflammatory and prothrombogenic phenotype in the cerebral microvasculature, compared with HTN alone. This ‘protection' afforded by HCh was evident in models characterized by either high (Ang II infusion) or low (DOCA salt) Ang II levels. Our findings also suggest that the beneficial effect of HCh in these models may result from the elevated HDL levels induced by a cholesterol-enriched diet in mice.
The attenuation of HTN-induced microvascular dysfunction by HCh does not appear to result from a blood pressure lowering effect of HCh. Both the Ang II and DOCA-salt models of HTN were associated with an approximate 30% increase in blood pressure, which was not significantly changed when the mice were placed on a cholesterol-enriched diet. These observations are consistent with other reports describing no effect of HCh on blood pressure. For example, Arruda et al22 showed that elevated blood pressure detected in 2K1C (2-kidney 1-clip) hypertensive C57Bl/6 mice and apolipoprotein E knockout (apoE)-2K1C hypertensive mice is nearly identical. Similarly, Cappelli-Bigazzi et al24 have reported that spontaneously hypertensive rats fed either a standard or cholesterol-enriched diet exhibit similar elevations in blood pressure.
The results of the present study indicate that HCh alone (in the absence of HTN) does not induce a proinflammatory phenotype in the cerebral microvasculature. This observation is inconsistent with previous reports from our laboratory7 and by others.25 While an explanation for the inconsistency is not readily apparent, it may result from the presence or absence of cholate in the cholesterol-enriched diet used in the different studies. Dietary cholate has been previously shown to induce inflammation and to potentiate the inflammation induced by a high fat diet.26, 27, 28 To avoid this potential confounding variable, we used a diet high in cholesterol and fat, but without cholate supplementation, in the present study.
The role for Ang II in mediating the altered BBB permeability noted in the Ang II pump, but not the DOCA salt, model of HTN remains poorly understood. However, we9 and others10 have previously reported that Ang II-induced HTN in mice is associated with an increased BBB permeability. This Ang II-mediated permeability response appears to be dependent on Ang II type-1 receptors expressed on the vessel wall.9 Monolayers of cerebral microvascular endothelial cells exposed to Ang II also exhibit an increased permeability that is dependent on Ang II type-1 receptors.29
Like previous studies,30 we observed an increased plasma HDL concentration in mice placed on a high-fat, cholesterol-enriched diet. Our study also provides two lines of evidence that are consistent with a role for HDL in mediating the antiinflammatory and antithrombogenic effects of HCh. First, we show that ApoA1 transgenic mice (four times the plasma HDL as WT mice) with DOCA-salt HTN exhibit a significantly attenuated adhesion of leukocytes and platelets in cerebral venules. Second, we observed that treatment of DOCA-salt or Ang II hypertensive mice with the ApoA1 mimetic peptide 4F largely prevents the blood cell recruitment responses normally elicited in untreated WT mice. These findings are consistent with reports describing an antiinflammatory action of HDL cholesterol,31, 32, 33 genetic overexpression of ApoA134, 35 as well as 4F treatment.36, 37 The antiinflammatory effects of HDL and ApoA1 have been attributed to an attenuation of leukocyte activation38 and endothelial cell adhesion molecule expression,39 possibly resulting from enhanced nitric oxide production.40 High-density lipoprotein also appears to exert antiplatelet effects, including inhibition of agonist induced platelet aggregation, fibrinogen binding, granule secretion, and production of thromboxane A2.41
In conclusion, the results of this study indicate that mild HCh significantly blunts the recruitment of leukocytes and platelets in the cerebral microvasculature in both low and high Ang II models of HTN. Our findings are also consistent with a role for HDL and ApoA1 as mediators of this antiinflammatory and antithrombogenic effect of a cholesterol-enriched diet. These observations suggest that HDL and ApoA1 may be effective therapeutic targets for prevention of the systemic inflammatory response that accompanies HTN. Whether our results bear on the responses of intracranial arteries to the combination of HTN and HCh remains unclear. The beneficial effects of ApoA1 mimetic treatment on the cerebral microcirculation suggest that elevations in HDL or administration of the ApoA1 mimetic may also afford some level of protection against any impairment of endothelial function in intracranial arteries that result from chronic arterial HTN. However, additional work is needed to directly address this interesting possibility.
The authors declare no conflict of interest.
Footnotes
This study was supported by a grant from the National Heart Lung and Blood Institute (HL26441).
References
- Berni A, Ciani E, Bernetti M, Cecioni I, Berardino S, Poggesi L, et al. Renal resistive index and low-grade inflammation in patients with essential hypertension. J Hum Hypertens. 2012;26:723–730. doi: 10.1038/jhh.2011.93. [DOI] [PubMed] [Google Scholar]
- Chamarthi B, Williams GH, Ricchiuti V, Srikumar N, Hopkins PN, Luther JM, et al. Inflammation and hypertension: the interplay of interleukin-6, dietary sodium, and the renin-angiotensin system in humans. Am J Hypertens. 2011;24:1143–1148. doi: 10.1038/ajh.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stumpf C, Auer C, Yilmaz A, Lewczuk P, Klinghammer L, Schneider M, et al. Serum levels of the Th1 chemoattractant interferon-gamma-inducible protein (IP) 10 are elevated in patients with essential hypertension. Hypertens Res. 2011;34:484–488. doi: 10.1038/hr.2010.258. [DOI] [PubMed] [Google Scholar]
- Espinoza M, Ruiz N, Leal U, Urbaneja H, Rojas S, Orozco U, et al. C-reactive protein is associated to carotid intima media thickness in patients with isolated hypercholesterolemia. Invest Clin. 2010;51:65–75. [PubMed] [Google Scholar]
- Real JT, Martínez-Hervás S, García-García AB, Civera M, Pallardó FV, Ascaso JF, et al. Circulating mononuclear cells nuclear factor-kappa B activity, plasma xanthine oxidase, and low grade inflammatory markers in adult patients with familial hypercholesterolaemia. Eur J Clin Invest. 2010;40:89–94. doi: 10.1111/j.1365-2362.2009.02218.x. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhang Z, Wen J, Wang X, Lu B, Yang Z, et al. Elevated serum chemokine CXC ligand 5 levels are associated with hypercholesterolemia but not a worsening of insulin resistance in Chinese people. J Clin Endocrinol Metab. 2010;95:3926–3932. doi: 10.1210/jc.2009-2194. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Stokes KY, Zhang JH, Nanda A, Granger DN. Cerebral microvascular responses to hypercholesterolemia: roles of NADPH oxidase and P-selectin. Circ Res. 2004;94:239–244. doi: 10.1161/01.RES.0000111524.05779.60. [DOI] [PubMed] [Google Scholar]
- Rodrigues SF, Granger DN. Cerebral microvascular inflammation in DOCA salt-induced hypertension: role of angiotensin II and mitochondrial superoxide. J Cereb Blood Flow Metab. 2012;32:368–375. doi: 10.1038/jcbfm.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vital SA, Terao S, Nagai M, Granger DN. Mechanisms underlying the cerebral microvascular responses to angiotensin II-induced hypertension. Microcirculation. 2010;17:641–649. doi: 10.1111/j.1549-8719.2010.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mao Y, Ramirez SH, Tuma RF, Chabrashvili T. Angiotensin II induced cerebral microvascular inflammation and increased blood-brain barrier permeability via oxidative stress. Neuroscience. 2010;171:852–858. doi: 10.1016/j.neuroscience.2010.09.029. [DOI] [PubMed] [Google Scholar]
- De Bacquer D, De Backer G. The prevalence of concomitant hypertension and hypercholesterolaemia in the general population. Int J Cardiol. 2006;110:217–223. doi: 10.1016/j.ijcard.2005.08.037. [DOI] [PubMed] [Google Scholar]
- Smith GD, Pekkanen J. The cholesterol controversy. BMJ. 1992;304:913. doi: 10.1136/bmj.304.6831.913-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Porcel M, Lerman A, Herrmann J, Schwartz RS, Sawamura T, Condorelli M, et al. Hypertension exacerbates the effect of hypercholesterolemia on the myocardial microvasculature. Cardiovasc Res. 2003;58:213–221. doi: 10.1016/s0008-6363(03)00250-5. [DOI] [PubMed] [Google Scholar]
- Chobanian AV, Lichtenstein AH, Nilakhe V, Haudenschild CC, Drago R, Nickerson C. Influence of hypertension on aortic atherosclerosis in the Watanabe rabbit. Hypertension. 1989;14:203–209. doi: 10.1161/01.hyp.14.2.203. [DOI] [PubMed] [Google Scholar]
- Wool GD, Cabana VG, Lukens J, Shaw PX, Binder CJ, Witztum JL, et al. 4F Peptide reduces nascent atherosclerosis and induces natural antibody production in apolipoprotein E-null mice. FASEB J. 2011;25:290–300. doi: 10.1096/fj.10-165670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M, Vowinkel T, Stokes KY, Arumugam TV, Yilmaz G, Nanda A, et al. CD40/CD40 ligand signaling in mouse cerebral microvasculature after focal ischemia/reperfusion. Circulation. 2005;111:1690–1696. doi: 10.1161/01.CIR.0000160349.42665.0C. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Sekizuka E, Yamaguchi N, Nakadate H, Terao S, Granger DN, et al. Angiotensin II type 1 receptor signaling contributes to platelet-leukocyte-endothelial cell interactions in the cerebral microvasculature. Am J Physiol Heart Circ Physiol. 2007;292:H2306–H2315. doi: 10.1152/ajpheart.00601.2006. [DOI] [PubMed] [Google Scholar]
- Russell J, Cooper D, Tailor A, Stokes KY, Granger DN. Low venular shear rates promote leukocyte-dependent recruitment of adherent platelets. Am J Physiol Gastrointest Liver Physiol. 2003;284:G123–G129. doi: 10.1152/ajpgi.00303.2002. [DOI] [PubMed] [Google Scholar]
- Uyama O, Okamura N, Yanase M, Narita M, Kawabata K, Sugita M. Quantitative evaluation of vascular permeability in the gerbil brain after transient ischemia using Evans blue fluorescence. J Cereb Blood Flow Metab. 1988;8:282–284. doi: 10.1038/jcbfm.1988.59. [DOI] [PubMed] [Google Scholar]
- Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ.Measuring the global burden of disease and risk factors, 1990–2001In: Lopez AD, Mathers CD, Ezzati M, Jamison DT, Murray CJ, (eds).. Global Burden of Disease and Risk Factors World Bank: Washington, DC; 2006. Chapter 1. [Google Scholar]
- Javeshghani D, Schiffrin EL, Sairam MR, Touyz RM. Potentiation of vascular oxidative stress and nitric oxide-mediated endothelial dysfunction by high-fat diet in a mouse model of estrogen deficiency and hyperandrogenemia. J Am Soc Hypertens. 2009;3:295–305. doi: 10.1016/j.jash.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Arruda RM, Peotta VA, Meyrelles SS, Vasquez EC. Evaluation of vascular function in apolipoprotein E knockout mice with angiotensin-dependent renovascular hypertension. Hypertension. 2005;46:932–936. doi: 10.1161/01.HYP.0000182154.61862.52. [DOI] [PubMed] [Google Scholar]
- Lorkowska B, Bartus M, Franczyk M, Kostogrys RB, Jawien J, Pisulewski PM, et al. Hypercholesterolemia does not alter endothelial function in spontaneously hypertensive rats. J Pharmacol Exp Ther. 2006;317:1019–1026. doi: 10.1124/jpet.105.098798. [DOI] [PubMed] [Google Scholar]
- Cappelli-Bigazzi M, Rubattu S, Battaglia C, Russo R, Enea I, Ambrosio G, et al. Effects of high-cholesterol and atherogenic diets on vascular relaxation in spontaneously hypertensive rats. Am J Physiol. 1997;273:H647–H654. doi: 10.1152/ajpheart.1997.273.2.H647. [DOI] [PubMed] [Google Scholar]
- Desai MS, Mariscalco MM, Tawil A, Vallejo JG, Smith CW. Atherogenic diet-induced hepatitis is partially dependent on murine TLR4. J Leukoc Biol. 2008;83:1336–1344. doi: 10.1189/jlb.0607390. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- Samokhin AO, Bühling F, Theissig F, Brömme D. ApoE-deficient mice on cholate-containing high-fat diet reveal a pathology similar to lung sarcoidosis. Am J Pathol. 2010;176:1148–1156. doi: 10.2353/ajpath.2010.090857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokhin AO, Wilson S, Nho B, Lizame ML, Musenden OE, Brömme D. Cholate-containing high-fat diet induces the formation of multinucleated giant cells in atherosclerotic plaques of apolipoprotein E−/− mice. Arterioscler Thromb Vasc Biol. 2010;30:1166–1173. doi: 10.1161/ATVBAHA.110.203976. [DOI] [PubMed] [Google Scholar]
- Fleegal-DeMotta MA, Doghu S, Banks WA. Angiotensin II modulates BBB permeability via activation of the AT(1) receptor in brain endothelial cells. J Cereb Blood Flow Metab. 2009;29:640–647. doi: 10.1038/jcbfm.2008.158. [DOI] [PubMed] [Google Scholar]
- Hayek T, Ito Y, Azrolan N, Verdery RB, Aalto-Setälä K, Walsh A, et al. Dietary fat increases high density lipoprotein (HDL) levels both by increasing the transport rates and decreasing the fractional catabolic rates of HDL cholesterol ester and apolipoprotein (Apo) A-I. Presentation of a new animal model and mechanistic studies in human Apo A-I transgenic and control mice. J Clin Invest. 1993;91:1665–1671. doi: 10.1172/JCI116375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimayuga P, Zhu J, Oguchi S, Chyu KY, Xu XO, Yano J, et al. Reconstituted HDL containing human apolipoprotein A-1 reduces VCAM-1 expression and neointima formation following periadventitial cuff-induced carotid injury in apoE null mice. Biochem Biophys Res Commun. 1999;264:465–468. doi: 10.1006/bbrc.1999.1278. [DOI] [PubMed] [Google Scholar]
- Lapergue B, Moreno JA, Dang BQ, Coutard M, Delbosc S, Raphaeli G, et al. Protective effect of high-density lipoprotein-based therapy in a model of embolic stroke. Stroke. 2010;41:1536–1542. doi: 10.1161/STROKEAHA.110.581512. [DOI] [PubMed] [Google Scholar]
- Vaisar T, Pennathur S, Green PS, Gharib SA, Hoofnagle AN, Cheung MC, et al. Shotgun proteomics implicates protease inhibition and complement activation in the anti-inflammatory properties of HDL. J Clin Invest. 2007;117:746–756. doi: 10.1172/JCI26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belalcazar LM, Merched A, Carr B, Oka K, Chen KH, Pastore L, et al. Long-term stable expression of human apolipoprotein A-I mediated by helper-dependent adenovirus gene transfer inhibits atherosclerosis progression and remodels atherosclerotic plaques in a mouse model of familial hypercholesterolemia. Circulation. 2003;107:2726–2732. doi: 10.1161/01.CIR.0000066913.69844.B2. [DOI] [PubMed] [Google Scholar]
- Rubin EM, Krauss RM, Spangler EA, Verstuyft JG, Clift SM. Inhibition of early atherogenesis in transgenic mice by human apolipoprotein AI. Nature. 1991;353:265–267. doi: 10.1038/353265a0. [DOI] [PubMed] [Google Scholar]
- Buga GM, Navab M, Imaizumi S, Reddy ST, Yekta B, Hough G, et al. L-4F alters hyperlipidemic (but not healthy) mouse plasma to reduce platelet aggregation. Arterioscler Thromb Vasc Biol. 2010;30:283–289. doi: 10.1161/ATVBAHA.109.200162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Datta G, Zhang Y, Miller AP, Mochon P, Chen YF, et al. Apolipoprotein A-I mimetic peptide treatment inhibits inflammatory responses and improves survival in septic rats. Am J Physiol Heart Circ Physiol. 2009;297:H866–H873. doi: 10.1152/ajpheart.01232.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ, Suhartoyo A, Stirzaker RA, Shaw J, Sviridov D, et al. Neutrophil activation is attenuated by high-density lipoprotein and apolipoprotein A-I in in vitro and in vivo models of inflammation. Arterioscler Thromb Vasc Biol. 2011;31:1333–1341. doi: 10.1161/ATVBAHA.111.226258. [DOI] [PubMed] [Google Scholar]
- Murphy AJ, Woollard KJ. High-density lipoprotein: a potent inhibitor of inflammation. Clin Exp Pharmacol Physiol. 2010;37:710–718. doi: 10.1111/j.1440-1681.2009.05338.x. [DOI] [PubMed] [Google Scholar]
- Besler C, Lüscher TF, Landmesser U. Molecular mechanisms of vascular effects of high-density lipoprotein: alterations in cardiovascular disease. EMBO Mol Med. 2012;4:251–268. doi: 10.1002/emmm.201200224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nofer JR, Brodde MF, Kehrel BE. High-density lipoproteins, platelets and the pathogenesis of atherosclerosis. Clin Exp Pharmacol Physiol. 2010;37:726–735. doi: 10.1111/j.1440-1681.2010.05377.x. [DOI] [PubMed] [Google Scholar]