Abstract
Dysregulated protein synthesis is thought to be a core phenotype of fragile X syndrome (FXS). In a mouse model (Fmr1 knockout (KO)) of FXS, rates of cerebral protein synthesis (rCPS) are increased in selective brain regions. We hypothesized that rCPS are also increased in FXS subjects. We measured rCPS with the ℒ-[1-11C]leucine positron emission tomography (PET) method in whole brain and 10 regions in 15 FXS subjects who, because of their impairments, were studied under deep sedation with propofol. We compared results with those of 12 age-matched controls studied both awake and sedated. In controls, we found no differences in rCPS between awake and propofol sedation. Contrary to our hypothesis, FXS subjects under propofol sedation had reduced rCPS in whole brain, cerebellum, and cortex compared with sedated controls. To investigate whether propofol could have a disparate effect in FXS subjects masking usually elevated rCPS, we measured rCPS in C57Bl/6 wild-type (WT) and KO mice awake or under propofol sedation. Propofol decreased rCPS substantially in most regions examined in KO mice, but in WT mice caused few discrete changes. Propofol acts by decreasing neuronal activity either directly or by increasing inhibitory synaptic activity. Our results suggest that changes in synaptic signaling can correct increased rCPS in FXS.
Keywords: brain, Fmr1 knockout mouse, leucine, positron emission tomography, propofol anesthesia, protein synthesis
Introduction
Fragile X syndrome (FXS) is the most common inherited form of intellectual disability and a recognized monogenic cause of autism. The gene responsible, FMR1, is on the long arm of the X chromosome.1 It contains a CGG repeat sequence in the 5'-untranslated region that, on expansion to greater than 200 repeats, results in gene methylation and transcriptional silencing. The absence of its protein product, fragile X mental retardation protein (FMRP), is responsible for the clinical symptoms and pathologic findings of FXS. It is an RNA-binding protein2, 3, 4, 5 that associates with actively translating ribosomes.6 Fragile X mental retardation protein suppresses the translation of specific messages in in vitro preparations7, 8 and in Drosophila.9 The absence of FMRP has been shown to affect rates of brain protein synthesis in awake and functioning animals. In adult Fmr1 knockout (KO) mice, regional rates of cerebral protein synthesis (rCPS) were significantly elevated over those measured in wild-type (WT) mice,10 most remarkably in hippocampus, thalamus, and hypothalamus. Moreover, in a fly model of FXS, excess protein synthesis has been shown to be associated with memory impairment.11
Based on findings in the FXS mouse model, we hypothesized that rCPS are elevated in FXS subjects. We used the quantitative ℒ-[1-11C]leucine positron emission tomography (PET) method12, 13, 14 to measure rCPS in FXS subjects. As most subjects with FXS could not remain motionless in the PET scanner for 2 hours while tracer was injected intravenously and arterial blood samples were drawn, PET studies of FXS subjects were conducted during deep sedation with propofol, a potent hypnotic that has no effect on rCPS in normal subjects.15 Contrary to our hypothesis, we found as reported herein that rCPS were not increased in FXS subjects, but rather decreased in the brain as a whole, cerebellum and cortex compared with age-matched controls studied under identical conditions. This unexpected result led us to reexamine rCPS in the FXS mouse model, both during basal conditions and with propofol sedation. Herein, we report that in Fmr1 KO mice, propofol sedation resulted in significant and widespread decreases in rCPS, whereas in WT mice, as in control human subjects, rCPS were largely unaffected. Our study highlights the importance of appreciating possible interference of psychotropic medications, including hypnotics, on results of studies of developmental disorders. Most importantly, our results suggest that altered cerebral protein synthesis, which may be pathogenic in FXS, is altered by a medication that affects changes in neuronal activity, synaptic signaling and in the balance of excitation and inhibition.
Materials and methods
Human Studies
All procedures on human subjects were performed as described in a protocol approved by the National Institutes of Health Combined Neurosciences Institutional Review Board, the National Institutes of Health Radioactive Drug Research Committee, and the National Institutes of Health Radiation Safety Committee. All subjects or, in the case of subjects with FXS, their legal guardians gave written informed consent before study enrollment.
Subjects
Male full mutation FXS subjects 18 to 24 years of age who had taken no psychotropic medication during the previous year and who had no recent history of seizures were recruited to participate in the study. Diagnosis was confirmed by DNA analysis (Mayo Clinic, Rochester, MN, USA). Control subjects were healthy males 18 to 24 years old who were enrolled after clinical history, physical examination, and a structured clinical interview16 yielded no evidence of neurologic or psychiatric illness. Inclusion criteria were (1) no current or past diagnoses of psychiatric, neurologic, or chronic medical condition, (2) no history of neurologic trauma, (3) no family history of genetically transmissible neurologic syndrome, (4) no history of harmful/paradoxical reactions to general and local anesthetics, (5) no allergy to egg and soy proteins, and (6) HIV negative. Subjects were excluded if they did not meet all inclusion criteria. Subjects were tested for drugs of abuse at screening and again on the day of PET scanning; none of our subjects had any evidence of drug use. Fifteen healthy volunteers and seventeen FXS subjects met the inclusion criteria and were enrolled in the study. Results from five of the studies were not included as follows: we had technical difficulties with the PET scanner in two studies of healthy volunteers and one study of an FXS subject. One control subject was administered atropine after an episode of fainting, and in the case of one FXS subject the dose of tracer was too low to obtain good counting statistics. Good quality data sets were obtained for 12 healthy volunteers and 15 subjects with FXS. These subjects were predominantly Caucasian (13 FXS and 12 healthy volunteers). Two of the FXS subjects were African Americans. All of the healthy volunteers were living in the United States, whereas one of the FXS subjects came from the Netherlands and three came from Italy to participate in our study. Results from 10 of the healthy volunteers were reported previously.15
Psychological Testing
A trained psychologist tested subjects for Intelligence Quotients (IQs) by means of the Wechsler Adult Intelligence Scale 3rd edition (WAIS-III). Both performance and verbal IQs were measured and a full scale IQ computed for each subject. For the three FXS subjects who were not speakers of English, tests were administered through a translator. Parents of subjects with FXS were interviewed by a child psychiatrist to assess the presence of Diagnostic and Statistical Manual of Mental Disorders-IV symptoms of autism in their sons.17 The child psychiatrist also administered the Kiddie-Schedule for Affective Disorders and Schizophrenia Questionnaire.18 These psychiatric assessments pertained to the lifetime of the subject.
Anesthesia/Sedation Regimen
Anesthesia/sedation for imaging studies was administered by anesthesiologists and adhered to monitor and practice standards recommended by the American Society of Anesthesiologists. While a number of sedation regimens are available for imaging studies, given the cognitive impairment of FXS deep sedation or anesthesia was needed to ensure 2 hours of motionlessness required for the PET studies. To that end, the anesthesiologists involved recommended the use of propofol given its safety profile and record of efficacy in completing such imaging studies. For studies with sedation, on the morning of the study, an intravenous line was inserted in one arm, and anesthesia/deep sedation was induced by infusion of propofol (2,6-diisopropylphenol) (Diprovan, Astra-Zeneca Pharmaceuticals LP, Wilmington, DE, USA) intravenously. Deep sedation was maintained with a continuous intravenous infusion of propofol, which was titrated to assure motionlessness. To ensure airway patency, a nasopharyngeal airway was inserted in most subjects. Subjects maintained spontaneous ventilation and received supplemental oxygen via nasal cannula throughout the study. During anesthesia/deep sedation, heart rate, arterial blood pressure, oxygen saturation, capnography, and depth of anesthesia (Bispectral Index, A1050 BIS monitor; Aspect Medical Systems, Newton, MA, USA) were continuously monitored. Bispectral Index values were maintained below 60.19 After completion of the emission scan, propofol was discontinued in healthy volunteer subjects. In FXS subjects, propofol infusion was continued for the conduct of the brain magnetic resonance imaging (MRI). After propofol infusion, all subjects were monitored in the postanesthesia care unit until fully awake.
Brain Magnetic Resonance Imaging
All subjects underwent a noncontrast T1 weighted MRI of the brain for region of interest (ROI) placement. Magnetic resonance imaging procedures for 10 of the control subjects were previously reported.15 MRI examinations in the present study were performed on a 1.5 or 3.0 Tesla Achieva scanner (Philips Healthcare, Andover, MA, USA). Images were reconstructed with voxel dimensions of 0.87 × 0.87 × 1 mm or 0.94 × 0.94 × 1 mm, and interpolated to isotropic voxel dimensions of (0.87 mm)3 or (0.94 mm)3. One control subject's MRI was reconstructed with isotropic voxel dimensions of (1.0 mm)3. Mean voxel dimensions in FXS and control subjects were (0.87 mm)3 and (0.92 mm)3, respectively. Region of interests were manually placed on each MRI by visually identifying anatomic landmarks.
Positron Emission Tomography Studies
ℒ-[1-11C]Leucine was prepared from H11CN with a modified Strecker-Bucherer reaction as previously described.15 Before each PET study, subjects were instructed to consume a high protein snack and then fast for 8 hours. After sedation with propofol a second intravenous and an arterial catheter were inserted in the antecubital fossa and the radial artery of the contralateral arm, respectively. A headband with targets for the Polaris optical tracking system (Northern Digital, Inc., Waterloo, Ontario, Canada) was positioned on each subject, and subjects were placed in the scanner for a 3-minute transmission scan to determine optimal bed positioning within the scanner field of view. This was followed by a 6-minute transmission scan for attenuation correction. Head position information was continuously recorded throughout the scanning procedure by the Polaris System, which has an accuracy of 0.5 to 0.6 mm, to allow for motion correction of the emission data during image reconstruction.20 Ninety-minute dynamic emission scans were then initiated coincident with the intravenous 2-minute infusion of 794 to 1,143 MBq ℒ-[1-11C]leucine as previously described.14 Injected doses (mean±s.e.m.) administered to control and FXS subjects were 995±33 and 1,004±28 MBq, respectively. The administered doses (means±s.e.m.) were based on each subject's body weight (control: 11.4±0.6, FXS subjects: 12.1±0.4 MBq/kg).
Positron emission tomography studies were performed on the ECAT High Resolution Research Tomograph (CPS Innovations, Knoxville, TN, USA). Data were acquired in list mode and reconstructed using the motion-compensated 3D ordinary Poisson ordered subset expectation maximum algorithm.20 Images were reconstructed as 42 frames of data (16 × 15 seconds, 4 × 30 seconds, 4 × 60 seconds, 4 × 150 seconds, 14 × 300 seconds). All activities were decay corrected to the time of radiotracer injection. 3D frames of data were reconstructed to 207 slices 1.22 mm thick (no interleaved slices) with a pixel size of 1.21 × 1.21 mm. The full width at half maximum of the High Resolution Research Tomograph at the center of the field of view is ∼2.5 mm, the transverse and axial dimensions of the field of view are 31.2 and 25.2 cm, respectively.21
Analysis of Blood Samples
Arterial blood sampling was initiated concurrently with the start of the [11C]leucine infusion to determine the time courses of the concentrations of unlabeled and labeled leucine in arterial plasma and total 11C and 11CO2 activities in arterial whole blood. Timed samples were hand drawn continuously (∼one sample/10 seconds), for the first 4 minutes, and at increasing intervals thereafter for a total of ∼40 samples per study. Blood samples were processed as previously described.14 11C activities in all samples were decay corrected to the time of injection.
Positron Emission Tomography Data Analysis
For each study, a 3D volume was constructed from the average of the emission data acquired between 30 and 60 minutes. This volume was isotropically smoothed with a Gaussian filter (full width at half maximum 3 mm) and aligned to the MRI volume by use of the Flexible Image Registration Toolbox22 with a 3D rigid body transformation. The resliced average 30 to 60 minutes PET image was visually reviewed for correct alignment with the MRI by use of Vinci (Volume Imaging in Neurological Research, CoRegistration and ROIs Included; the Max Planck Institute for Neurological Research, Cologne, Germany). The transformation parameters were then applied to each frame of the PET study (without prior smoothing) to effect their alignment with the MRI volume.
The kinetic model for the behavior of leucine in brain has been described in our previous work.12, 23 The parameters of the model were estimated for each voxel in the whole brain volume by means of the Basis Function Method of Tomasi et al23 with a slightly modified algorithm to avoid negative parameter estimates. Use of voxelwise estimation helps to reduce errors in model parameter estimates because of kinetic heterogeneity in the ROIs. Results for the subset of control subjects previously reported had been based on analyses at the ROI level; for the current study, PET data were reanalyzed voxelwise by the Basis Function Method. Images of each parameter were constructed, and ROIs drawn on MRIs were transferred to parametric images to compute average values of all parameters of the method.
Statistical Analyses
Values are reported as means±s.e.m. Differences between groups in physiologic variables measured and regional estimates of rCPS were tested for statistical significance by means of Student's t-tests. No corrections were made for multiple comparisons. Healthy volunteers were studied twice, once fully conscious and once under propofol sedation. The order of and the interval between the two studies varied among subjects. Comparisons between awake and propofol-sedated states were tested for statistical significance by means of paired Student's t-tests.
Animal Studies
Male WT and Fmr1 KO mice on a C57Bl/6J background, generated by heterozygous female and WT male breeding pairs, were used. The generation of Fmr1 KO mice and their genotyping by PCR amplification of tail DNA were described previously.10 All mice were group housed in a central facility and maintained under controlled conditions of normal humidity and temperature with standard alternating 12-hour period of light and darkness. All procedures were performed in accordance with the National Institutes of Health Guidelines on the Care and Use of Animals and approved by the National Institute of Mental Health Animal Care and Use Committee. Four groups of mice were studied: (1) WT studied awake and treated with vehicle (WT-C) (n=7); (2) KO studied awake and treated with vehicle (KO-C) (n=8); (3) WT sedated with propofol (WT-P) (n=7); and (4) KO sedated with propofol (KO-P) (n=9).
We used the autoradiographic ℒ-[1-14C]leucine method for the in vivo determination of rCPS.24 We prepared mice for studies by insertion of polyethylene catheters (PE-10) into a femoral artery and both femoral veins while under light isoflurane anesthesia. Mice recovered from the surgery overnight with food and water available ad libitum. Mice were permitted to move freely throughout the recovery and experimental periods. We measured mean arterial blood pressure, hematocrit, and arterial plasma glucose concentrations during the 60-minute rCPS experiments to monitor the physiologic state of each mouse. Animals were administered glycopyrrolate (10 μg/kg, subcutaneously) 10 minutes before the propofol bolus to reduce salivary and respiratory secretions. Glycopyrrolate is a muscarinic receptor antagonist that does not cross the blood–brain barrier and consequently does not affect the central nervous system. Propofol (20 mg/kg) was injected intravenously 5 to 10 minutes before injection of [14C]leucine. Thereafter, propofol was infused at 8 to 10 μL/min to achieve a total dose of 50 mg/kg per hour. Akin to the human studies, the infusion rate was titrated to maintain motionlessness in the sedated mice. In two animals, [14C]leucine was administered 20 to 30 minutes after the bolus of propofol during the time of the propofol infusion. Vehicle-injected control mice were administered an intravenous infusion of 5% Intralipid (Baxter Healthcare, Deerfield, IL, USA), a suspension of essential fatty acids in glycerol and soybean oil.
The experimental period was initiated by an intravenous pulse injection of 3.7 MBq/kg of ℒ-[1-14C]leucine (specific activity, 2.22 GBq/mmol, Moravek Biochemicals and Radiochemicals, Brea, CA, USA). Timed arterial samples were collected during the following 60 minutes for determination of the time courses of plasma concentrations of leucine and [14C]leucine. Labeled and unlabeled leucine concentrations in the acid-soluble fractions of arterial plasma were assayed by liquid scintillation counting and amino-acid analysis, respectively. At the end of the experimental interval, brains were removed and frozen, and serial sections, 20 μm in thickness, were prepared for quantitative autoradiography by means of a Leica cryostat (Leica Microsystems, Inc., Buffalo Grove, IL, USA). Sections were mounted on gelatin-coated slides, fixed and washed in 10% formalin, and exposed to Ektascan B/RA film (Eastman Kodak, Rochester, NY, USA) along with calibrated [14C]methylmethacrylate standards as described previously.10 Autoradiograms were digitized (MCID Analysis, Interfocus Imaging Ltd, Linton, Cambridge, UK), the concentration of 14C in each ROI was determined, and rCPS were calculated by means of the operational equation of the method.24 The value of lambda in the equation was 0.603 (ref. 10). Brain regions were identified by reference to a mouse brain atlas.25
The rCPS data were analyzed by 3-way repeated-measures analysis of variance with genotype (WT, KO) and treatment (vehicle, propofol) as between-subjects factors and brain region as a within-subjects factor. Post hoc pairwise comparisons were further conducted with Bonferroni t-tests. The significance level was set at P⩽0.05. For the comparisons between vehicle-treated WT and vehicle-treated KO mice, we used a one-tailed t-test. All other comparisons were by means of two-tailed tests. We used the SPSS program (IBM, Armonk, NY, USA) for statistical computations.
Results
Human Studies
Control subjects were recruited from local universities and the NIH. Healthy volunteer and FXS subjects were well matched with respect to age and body weight (Table 1). Presence of a full mutation allele was confirmed in the FXS subjects by analysis of the CGG repeat length; all had greater than 200 CGG repeats. In control subjects repeat lengths ranged from 20 to 32 with a mean±s.e.m. of 27±1. Control subjects (n=10) had IQ scores above average (Table 1). The FXS subjects (n=13) had IQ scores in the cognitively impaired range. Of the 15 FXS subjects, 2 met criteria for an anxiety disorder and 4 for Attention Deficit Hyperactivity Disorder (combined type). All of the FXS subjects met at least 1 of 12 Diagnostic and Statistical Manual of Mental Disorders-IV criteria for the diagnosis of autism17 and 6 subjects met the diagnostic criterion for autism.
Table 1. Subject population, psychological testing, and physiologic variables measured during PET studies of propofol-sedated subjects.
| Healthy volunteers | Fragile X subjects | |
|---|---|---|
| Age (years) | 22.5±0.5 | 21.6±0.6 |
| Body weight (kg) | 89±4 | 85±4 |
| WAIS full scalea | 130±5 | 53±1** |
| WAIS verbala | 134±4 | 58±1** |
| WAIS performancea | 118±5 | 55±1** |
| Arterial plasma leucine (μmol/L) | 126±4 | 119±4 |
| ∑[LNAA]i/kmi | 12.4±0.3 | 11.3±0.4 |
| Arterial plasma glucose concentration (mmol/L) | 5.9±0.1 | 5.6±0.2 |
| Hematocrit (%) | 47±1 | 49±1* |
| BiSpectral index (BIS) | 33±2 | 29±2 |
| MABP (mm Hg) | 69±2 | 70±2 |
| Heart rate (beats/min) | 76±3 | 74±3 |
| Arterial blood pH | 7.31±0.01 | 7.28±0.01 |
| Arterial pCO2 (mm Hg)b | 52±2 | 56±3 |
| Arterial pO2 (mm Hg)c | 142±9 | 194±24 |
| Arterial plasma propofol concentration (μmol/L) | 36±4 | 51±7 |
LNAA, large neutral amino acids; MABP, mean arterial blood pressure; PET, positron emission tomography; WAIS, Wechsler Adult Intelligence Scale.
Physiologic variables are time weighted.
Statistically significantly different from healthy volunteers, two-tailed t-tests; *P⩽0.05; **P<0.0001.
Values are means±s.e.m. for 12 healthy volunteers and 15 Fragile X subjects, except where indicated.
10 healthy volunteers and 13 Fragile X subjects.
10 healthy volunteers and 15 Fragile X subjects.
11 healthy volunteers and 15 Fragile X subjects.
Physiologic variables were averaged across the PET scan duration (Table 1). Values of the BIS indicate that, on average, subjects in both groups were deeply sedated and levels of sedation were similar in both groups. The mean time-weighted average arterial plasma concentration of propofol was 41% higher in the fragile X subjects, but the difference was not statistically significant (Table 1). Except for hematocrit which was slightly, but statistically significantly, higher in the fragile X subjects, values of other variables were similar between groups. Plasma leucine concentrations measured over the 90-minute PET scan remained fairly constant. Coefficient of variation for the 12 studies of healthy volunteers ranged from 2.0% to 6.9% (mean 4.6%) and for the 15 fragile X subjects ranged from 1.4% to 7.9% (mean 3.5%). Time-weighted average leucine concentrations were similar in both groups. The concentrations of large neutral amino acids that comprise the primary competitors for entry into brain via the ℒ-amino acid transporter (methionine, valine, isoleucine, leucine, tyrosine, and phenylalanine) were also measured and the sums of the average concentration of each of the amino acids weighted by its Km (the amino-acid concentration at which transport is half maximal) for the transporter were found to be similar in both groups.26
The following ROIs were drawn on MRIs from each subject: whole brain, cerebellum, frontal cortex, parietal cortex, thalamus, hippocampus, amygdala, hypothalamus, caudate nucleus, putamen, and corona radiata. The corona radiata was sampled, but other ROIs encompassed entire regions. We compared volumes of whole brain (not including the ventricles) and the 9 regions that were completely outlined in the 12 healthy volunteers and 15 FXS subjects (Table 2). Results show statistically significant differences in three regions. The volumes of putamen (−21.3%, P<0.01), thalamus (−14.6%, P<0.01), and hippocampus (−15.4%, P=0.01) were smaller in the FXS subjects. The volume of the caudate nucleus (+14.0%, P=0.052) tended to be larger in the FXS subjects. Other volumes were within 9% of control values.
Table 2. Region-of-interest volume and regional rates of cerebral protein synthesis.
| Region | ROI volume (cc) | rCPS (nmol/g per minute) | |||
|---|---|---|---|---|---|
| |
Healthy volunteers |
Fragile X subjects |
Healthy volunteers conscious |
Healthy volunteers sedated |
Fragile X subjects sedated |
| Whole brain | 1,412.3±32.1 | 1,448.7±32.7 | 1.68±0.04 | 1.67±0.03 | 1.52±0.03 *** |
| Cerebellum | 146.6±3.5 | 155.8±4.2 | 1.93±0.05 | 1.95±0.04 | 1.79±0.04 * |
| Cortex | |||||
| Frontal cortex | 50.4±1.6 | 50.6±0.8 | 2.04±0.06 | 2.07±0.04 | 1.86±0.04 *** |
| Parietal cortex | 35.7±1.2 | 32.6±1.1 | 2.02±0.07 | 2.01±0.04 | 1.82±0.04 ** |
| Subcortical | |||||
| Thalamus | 8.0±0.4 | 6.9±0.2** | 1.70±0.05 | 1.65±0.05 | 1.68±0.04 |
| Hippocampusa | 4.7±0.2 | 4.0±0.1*** | 1.48±0.04 | 1.55±0.05 | 1.56±0.04 |
| Amygdalaa | 1.9±0.1 | 1.9±0.1 | 1.42±0.07 | 1.49±0.09 | 1.45±0.05 |
| Caudate | 8.0±0.4 | 9.2±0.4 | 1.09±0.03 | 1.13±0.04 | 1.09±0.03 |
| Putamen | 8.6±0.4 | 6.8±0.4*** | 1.38±0.05 | 1.43±0.06 | 1.31±0.03 |
| Hypothalamus | 0.8±0.04 | 0.8±0.04 | 1.34±0.06 | 1.34±0.06 | 1.27±0.05 |
| White matter | |||||
| Corona radiate | 8.5±0.4 | 6.9±0.3 | 0.83±0.02 | 0.81±0.02 | 0.75±0.02 |
MRI, magnetic resonance imaging; rCPS, rates of cerebral protein synthesis; ROI, region of interest.
Statistically significantly different from sedated healthy volunteers, two-tailed t-tests; *P⩽0.05; **P⩽0.01; ***P⩽0.005.
Note: Volumes of corona radiata are volumes sampled rather than entire regional volume; no statistical comparison between groups was performed.
Values are means±s.e.m. for 12 healthy volunteers and 15 fragile X subjects, except where indicated.
11 healthy volunteers and 15 fragile X subjects. In one healthy volunteer the quality of the MRI was poor, and we were unable to decipher the edges of the hippocampus and amygdala.
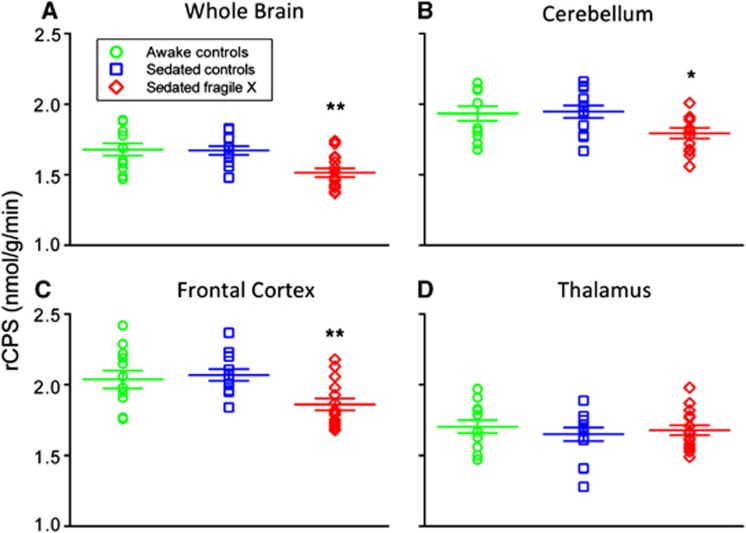
Rates of cerebral protein synthesis were measured with low intersubject variability: the coefficient of variation was 6% to 12% except in the very small (<5 cc) subcortical regions where it was 9% to 20% (Table 2). Figure 1 illustrates the distribution of individual rCPS measurements in whole brain, cerebellum, frontal cortex, and thalamus; one notes the low variability in the measurements and the differences in mean rCPS between the sedated groups.
Figure 1.
Effects of propofol sedation on rates of cerebral protein synthesis (rCPS) in (A) whole brain, (B) cerebellum, (C) frontal cortex, and (D) thalamus in awake (green open circles) and sedated (blue open squares) healthy volunteers and sedated subjects with fragile X syndrome (FXS) (red open diamonds). Each point represents the result from a single subject. Long horizontal lines represent the means and shorter lines the s.e.m. obtained in 12 healthy volunteers and 15 subjects with fragile X syndrome. There were no differences between sedated and awake states in healthy volunteers (paired t-tests) in any region. Mean rCPS in sedated FXS subjects were statistically significantly lower compared with sedated healthy volunteers (*P<0.05; **P<0.01, Student's t-tests). The color reproduction of this figure is available at the Journal of Cerebral Blood Flow and Metabolism journal online.
Each healthy volunteer was studied twice with the [11C]leucine PET method, once awake and once under deep propofol sedation. We compared rCPS in the two states with paired t-tests, and we found no difference in whole brain or in any of the regions examined (Table 2; Figure 1). Since FXS subjects could only be studied under propofol sedation, we compared rCPS in these subjects with the results of the sedated healthy volunteers (Table 2; Figure 1). Contrary to our hypothesis, rCPS were not increased in the FXS subjects in any region examined. Moreover, rCPS were statistically significantly decreased by 8% to 10% in some brain regions in the FXS subjects. The regions affected were cerebellum and frontal and parietal cortex as well as the brain as a whole. In the hippocampus, amygdala, thalamus, and caudate nucleus, the mean values of rCPS in the healthy volunteers and FXS subjects were almost the same. In the putamen and corona radiatum, mean values were lower in the FXS group, but effects did not reach statistical significance. If we take into account that rCPS in 11 regions were analyzed and we apply the very conservative Bonferroni correction for multiple comparisons, then whole brain and frontal cortex remain statistically significantly lower, parietal cortex is very close to significance, but cerebellum is not. Parametric images of representative subjects (Figure 2) illustrate these results and show the patterns of protein synthesis activity. Clearly in both groups higher rates of protein synthesis are evident in gray-matter regions, e.g., hippocampus and cortex and the rates in these regions are not uniformly distributed.
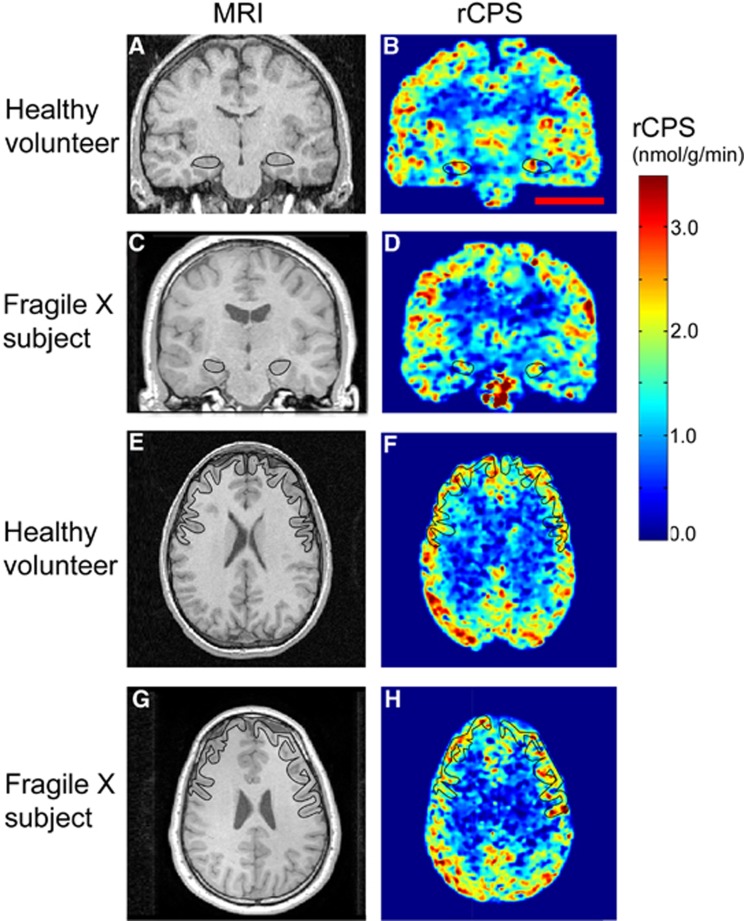
Figure 2.
Parametric images of rates of cerebral protein synthesis (rCPS) in a typical sedated healthy volunteer and sedated fragile X subject. Magnetic resonance images (MRIs) on the left correspond to rCPS positron emission tomography (PET) images on the right. All PET images were resliced to match the subject's MRI, and kinetic model parameters were estimated voxelwise by the Basis Function Method (BFM). Resultant parametric images were smoothed with a 3D Gaussian filter (kernel 2 mm full width at half maximum (FWHM)). Parametric images are color coded for rCPS according to the colorbar on the right. Images at the level of the hippocampus (A–D) are in the coronal plane, and the hippocampi are outlined in each image. Images at the level of the frontal cortex (E–H) are in the axial view and the frontal cortex is outlined in each image. Outlines were drawn on the MR images and transferred to resliced PET images. Scale bar in (B) represents 5 cm and pertains to all eight images.
Animal Studies
The findings in human subjects were contrary to our hypothesis that rCPS would be higher in FXS subjects compared with age-matched healthy volunteers. We considered the possibility that propofol could have a disparate effect on rCPS in FXS subjects thereby masking a baseline elevation in rCPS. We examined this possibility in the Fmr1 KO mouse model with the quantitative autoradiographic ℒ-[1-14C]leucine method24 with which mice could be freely moving during the study. We compared rCPS in WT and KO mice studied either awake or under deep propofol sedation. Awake control mice were administered Intralipid, the vehicle for propofol. Mice were well matched with respect to age, body weight, and arterial plasma leucine and glucose concentrations (Table 3). Levels of sedation were maintained at the extinction of corneal reflexes and motionlessness. Both KO and WT mice under propofol sedation showed similar and expected changes in physiologic variables. Arterial plasma propofol levels were not statistically different from each other in WT and KO mice, but variability in the measurements was high. Average plasma concentrations of propofol were several fold higher in mice than in human subjects possibly indicating that in mice the effective dose is higher.
Table 3. Effects of propofol sedation on physiologic variables in Fmr1 KO mice.
| WT-control (7) | KO-control (8) | WT-propofol (8) | KO-propofol (9) | |
|---|---|---|---|---|
| Age (days) | 132±2 | 138±2 | 135±2 | 136±1 |
| Body weight (g) | 34±1 | 36±1 | 34±1 | 34±1 |
| Hematocrit (%) | 42.2±0.6 | 40.5±1.0 | 38.7±0.5** | 39.0±1.0 |
| Arterial plasma glucose (mmol/L) | 8.16±0.29 | 7.73±0.43 | 6.90±0.64 | 7.39±0.55 |
| Arterial plasma leucine (μmol/L) | 127±5 | 111±4 | 104±10 | 105±7 |
| Arterial blood | ||||
| pCO2 (mm Hg) | 35.3±0.8 | 38.8±1.3 | 56.0±2.4** | 56.3±2.3†† |
| pO2 (mm Hg) | 80.0±0.9 | 81.5±0.8 | 269.7±17.1** | 259.3±26.6†† |
| pH | 7.41±0.01 | 7.39±0.01 | 7.23±0.01** | 7.26±0.01†† |
| Plasma propofol (μmol/L) 60 min | ND | ND | 211±62 | 111±18 |
| Arterial blood pressure 0 time | 115±2 | 115±2 | 96±9 | 81±4†† |
| 45 minutes | 111±3 | 105±2 | 79±5** | 82±4†† |
| Heart rate (beats/min) 0 time | 330±33 | 342±14 | 314±27 | 297±20 |
| 45 minutes | 350±18 | 380±55 | 250±14** | 254±8 |
KO, knockout; WT, wild type.
Values are the means±s.e.m. for the number of mice indicated in parentheses except for plasma propofol concentrations which were measured in four WT and eight KO mice. Hematocrit and arterial plasma glucose concentration were measured immediately before the infusion of either vehicle or propofol. Arterial blood gas concentrations were measured 45 minutes after the [14C]leucine administration. Arterial plasma leucine concentration is the time-weighted average over the duration of the leucine study.
Post hoc Student's t-tests (two-tail): statistically significantly different from WT-C; *0.01⩽P⩽0.05; **0.001⩽P⩽0.01.
Post hoc Student's t-tests (two-tail): Statistically significantly different from KO-C; ††0.001⩽P⩽0.01.
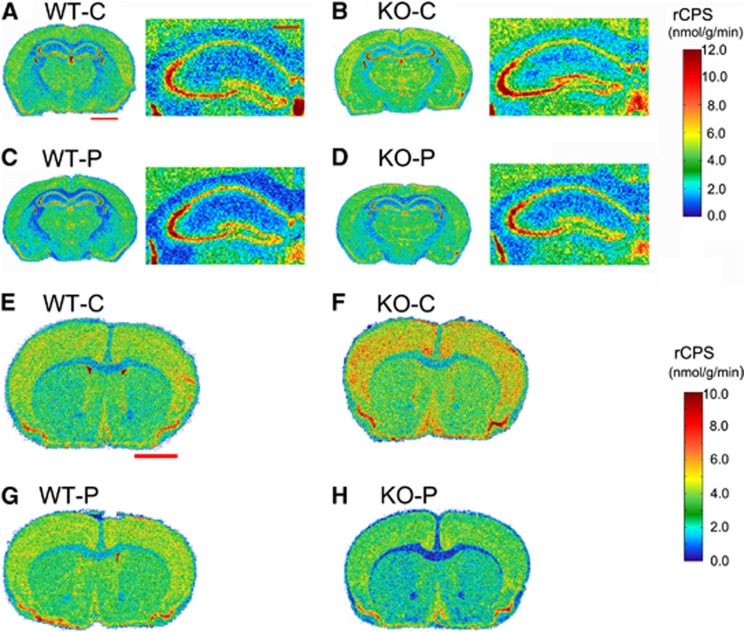
We confirm in the vehicle-treated mice our previous finding that rCPS were increased in the absence of FMRP in selective brain regions (Table 4). Statistically significantly (P⩽0.05, one-tailed t-test) affected regions (% increase) were basolateral amygdala (12%), dorsal hippocampus (12%), stratum radiatum of the dorsal hippocampus (12%), stratum radiatum of the ventral hippocampus (12%), thalamus (8%), and primary motor cortex (9%). Effects of propofol sedation on rCPS differed dramatically between WT and KO mice. In WT mice, propofol decreased rCPS in basolateral amygdala and somatosensory cortex by 10% and increased rCPS by 15% in CA1 of dorsal hippocampus. In KO mice, however, propofol treatment decreased rCPS by 14% to 26% (average decrease of 19%) in all regions examined except CA1 (both ventral and dorsal hippocampus). Comparison of propofol-sedated WT and propofol-sedated KO mice shows that rCPS were lower in the KO mice in primary somatosensory cortex by ∼10%. Representative autoradiograms color-coded for rCPS (Figure 3) at the level of the dorsal hippocampus (A–D) and at the level of the somatosensory cortex (E–H) illustrate this disproportionate effect of propofol on rCPS in KO mice.
Table 4. Effects of propofol sedation on rCPS in Fmr1 KO mice.
| Region | rCPS (nmol/g per minute) | |||
|---|---|---|---|---|
| |
WT-C (7) |
KO-C (8) |
WT-Propofol (8) |
KO-Propofol (9) |
| Dorsal hippocampus | 3.54±0.19 | 3.95±0.12* | 3.52±0.12 | 3.42±0.11† |
| CA1 pyramidal cell layer | 6.08±0.41 | 6.49±0.16 | 7.01±0.37¶ | 6.70±0.15 |
| Stratum radiatum | 2.19±0.13 | 2.45±0.09* | 2.07±0.09 | 1.97±0.04†† |
| Ventral hippocampus | 3.34±0.17 | 3.63±0.14 | 3.29±0.16 | 3.07±0.10† |
| CA1 pyramidal cell layer | 6.74±0.34 | 7.27±0.14 | 7.24±0.35 | 7.18±0.15 |
| Stratum radiatum | 2.28±0.14 | 2.57±0.11* | 2.18±0.10 | 2.04±0.06†† |
| Frontal assoc cortex | 3.79±0.23 | 4.14±0.15 | 3.51±0.12 | 3.26±0.11†† |
| Primary motor cortex | 3.94±0.20 | 4.29±0.11* | 3.66±0.10 | 3.31±0.10†† |
| Primary somatosensory cortex | 4.44±0.22 | 4.78±0.13 | 3.97±0.12¶ | 3.53±0.14†† ¥ |
| Parietal cortex | 4.39±0.18 | 4.66±0.11 | 4.13±0.16 | 3.88±0.09†† |
| Anterior cingulate cortex | 4.97±0.24 | 5.29±0.16 | 4.66±0.14 | 4.39±0.09†† |
| Caudate-putamen | 2.96±0.18 | 3.20±0.13 | 2.79±0.09 | 2.55±0.08†† |
| Thalamus | 3.99±0.19 | 4.30±0.11* | 3.76±0.12 | 3.48±0.05†† |
| Basolateral amygdala | 4.81±0.13 | 5.46±0.10** | 4.34±0.16¶ | 4.49±0.08†† |
KO, knockout; rCPS, rates of cerebral protein synthesis; RM-ANOVA, repeated-measures analysis of variance; WT, wild type.
Values are the means±s.e.m. for the number of mice indicated in parentheses. Results were analyzed by means of RM-ANOVA. Neither the genotype × treatment × regions nor the region × genotype interactions were statistically significant, but treatment × region interaction (F(4.11,115.1)=14.21; P<0.001) and genotype × treatment (F(1,28)=5.01; P<0.05) were: post hoc Student's t-tests (one-tail): statistically significantly different from WT-C; *0.01⩽P⩽0.05, **0.001⩽P⩽0.01; Post hoc Student's t-tests (two-tail): statistically significantly different from WT-C; ¶0.01⩽P⩽0.05; post hoc Student's t-tests (two-tail): statistically significantly different from KO-C; †0.001⩽P⩽0.01; ††0.001⩽P; post hoc Student's t-tests (two-tail): statistically significantly different from WT-P; ¥0.01⩽P⩽0.05.
Figure 3.
Effects of propofol sedation on rates of cerebral protein synthesis (rCPS) in wild-type (WT) and knockout (KO) mice. Representative digitized autoradiographic images from the four groups of mice at two levels of brain. Images are color coded for rCPS by the scales shown on the right. The upper colorbar pertains to (A–D), and the lower colorbar pertains to (E–H). The images in (A–D) are autoradiograms at the level of the dorsal hippocampus and the images in (E–H) are autoradiograms at the level of the primary somatosensory cortex. In (A–D), the image on the right is an enlargement of the image on the left. Scale bars shown in red in (A) represent 2 and 0.5 mm in the left and right versions of each figure, respectively, and pertain to the left and right images in all of (A–D). The saclebar shown in red in (E) represents 2 mm and pertains to images (E–H).
Discussion
The main finding of this study is that, during propofol sedation, subjects with FXS have significantly decreased rCPS in the brain as a whole, cerebellum, and parts of cortex compared with controls. Our studies in mice confirm our previous finding10 that, in the absence of FMRP, rCPS is increased over WT in selective brain regions. Further, these studies show that, in rodents and humans without FMRP, propofol is associated with widespread decreases in rCPS whereas, in subjects with FMRP propofol has minimal effects. We do not know whether at baseline, FXS subjects have increased rCPS. The need to sedate FXS subjects added an unanticipated level of complexity to the study. Our results in the mouse model suggest that we would have found increased rCPS in FXS subjects had we been able to study them in the conscious state, assuming that the mouse model reflects the human disease. Moreover, our results suggest that drugs, such as propofol, that decrease neural activity and alter synaptic signaling may reverse the effects of altered rCPS in FXS and are highly therapeutically relevant.
We recognize that our sample was possibly biased toward higher functioning FXS participants as subjects taking psychotropic medication were excluded. However, all FXS subjects enrolled were intellectually impaired with full scale IQ scores between 47 and 63, and 6 of the 15 subjects met the criteria for autism. Moreover, we saw no relationship between rCPS and either IQ or autism scores in our small sample. We are aware, however, that the values of IQ that we measured with the WAIS III may have a ‘floor effect' in this intellectually impaired population. If the phenotype of higher functioning subjects was closer to that of healthy volunteers, then we would have expected rCPS values to have been merely closer to control values, but we found decreased rCPS in these subjects. The direction of the change suggests that there may be an explanation that has more mechanistic implications. Among our control subjects, four had above average IQ scores. This was clearly a result of our successful recruitment efforts among fellows working at the NIH and at local college campuses. We have considered how our above average controls might have affected our results by looking for a relationship between IQ scores and rCPS. We find correlations which are statistically significant (P⩽0.05) in some of the regions (e.g., parietal cortex, amygdala, thalamus, and putamen), but the correlations are negative. We did not see such a relationship in the FXS subjects, but as stated above our measurements of IQ in this group may not be precise. If there is an effect of the skewed sample of controls on our result, then it would likely lead to an underestimation of the decrease in rCPS in FXS subjects.
The primary methodology used in this study, the ℒ-[1-11C]leucine PET method for measuring rCPS in human subjects,12 was based on the autoradiographic ℒ-[1-14C]leucine method for use in animals.24 The PET method was validated in monkeys13 and is characterized by low variability and good reproducibility in controls.14 Whereas the PET method does not have the exquisite spatial resolution of the autoradiographic method, it has the distinct advantage that it can be used to repeatedly study this dynamic process in human subjects under different conditions. Our study shows that the PET method has the sensitivity to detect 8% to 10% changes in rCPS in relatively small numbers of subjects, provided that the subjects are well matched and the study well designed. The method, therefore, may provide a quantitative and objective means of assessing the presence of disease and the effect of proposed treatments.
An incidental finding of our study is the effect of FXS on regional brain volumes, the subject of several prior studies.27, 28, 29 Increased volume of the caudate nucleus (23% to 25%) is a common finding in two of the prior studies.28, 29 We also found a 14% increase in caudate volume in FXS subjects although it did not quite reach statistical significance. Changes in the caudate nucleus are of interest in light of its involvement in executive function and reports that increased caudate volumes are associated with repetitive behaviors in autism.30 There is general agreement that whole brain and cerebellum volumes are unaffected in FXS (present study; refs. 28 and 29), but differences in other regions analyzed makes comparability impossible. In our study, volumes of hippocampus, thalamus, and putamen were lower (14% to 21%) in the FXS subjects. These effects are of particular interest in view of the involvement of these three regions in many types of learning.
Based on our previous results in the Fmr1 KO mouse,10 we hypothesized that rCPS in FXS subjects are increased compared with age-matched controls. The need to sedate FXS subjects added a potential limitation to the study. We found that, in propofol-sedated FXS subjects, rCPS were not increased, but decreased in the brain as a whole and in selective regions. We can propose three possible explanations for these results. First, it is conceivable, albeit unlikely, that in humans, loss of FMRP may have a different effect on protein synthesis than it has in the mouse. Another possibility is that our selection criteria may have resulted in a bias for higher functioning patients who might have milder (and undetectable) alterations on rCPS. This seems unlikely because our FXS subjects were clearly cognitively impaired and 40% fulfilled the diagnostic criteria for autism. A third possibility is that in the absence of FMRP, propofol may have effects on rCPS not seen in the presence of FMRP. We tested this possibility in a study of Fmr1 KO mice. We used the autoradiographic method for rCPS which can be applied in freely moving animals, so that we could study mice of both genotypes both conscious and sedated. In the mouse study, we found that propofol sedation in WT mice resulted in small changes in rCPS in a few regions, whereas in KO mice it resulted in widespread and highly significant decreases in rCPS. Notably, the CA1 pyramidal cell layer was not affected by propofol in KO animals. Sedated KO compared with sedated WT mice had significantly lower rCPS in one cortical area, an effect similar to our result in sedated human subjects. Taken together, results of human and mouse studies are consistent with the idea that propofol has disparate effects on rCPS in the presence or absence of FMRP.
Propofol is widely used in clinical anesthesia and its primary mechanism of action is thought to be the enhancement of GABAA receptor function to produce hyperpolarization of neurons. Acting on GABAA receptors, propofol can also affect excitatory inputs by inhibiting glutamate release at the synapse.31 Propofol can also block voltage-dependent sodium channels by binding to inactivated channel states,32 and at higher concentrations propofol may directly affect GABAA receptor/chloride channel opening.33 Further, propofol also decreases neural activity as indicated by decreases in cerebral blood flow and energy metabolism.34, 35 Nevertheless, the mechanism by which propofol reduces rCPS in FXS is unknown and will be the subject of future investigation.
We think that the increased rCPS seen in the KO mouse model10 is a core phenotype of FXS and is likely also pathogenic in the human disease. In Drosophila Fmr1 mutants, excess protein synthesis is associated with impairment in long-term memory, and this phenotype can be reversed with inhibitors of protein synthesis.11 In addition, inhibitors of protein synthesis can prevent bicuculline-induced epileptogenic activity in hippocampal slices of FXS mice.36 Our observation that treatment with a drug that alters neuronal and possibly synaptic activity also alters rCPS in KO mice is an important finding and has implications for the development of therapeutic strategies for FXS. We have seen similar effects on rCPS in KO mice after chronic treatment with lithium.37 Lithium treatment reduced rCPS throughout the brain of the KO mice and had very small effects in WT. Others have shown effects of acute treatment of KO mice with the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride on mRNA granules consistent with a normalization of exaggerated translation.38 Moreover, in studies of hippocampal slices from KO mice an elevated rate of incorporation of labeled methionine into protein was normalized by bath application of 2-methyl-6-(phenylethynyl)pyridine hydrochloride.39
Herein, we show that an anesthetic, propofol, reduces rCPS in FXS mouse brain and that in FXS humans sedated with a continuous infusion of propofol, the levels of rCPS are reduced in whole brain compared with control subjects studied under the same conditions. Taken together and extrapolated clinically, our findings suggest that effecting changes in protein synthesis are a viable therapeutic strategy in FXS. This strategy can be accomplished with the use of agents that affect neuronal and synaptic activity, and the effect of strategies that alter protein synthesis can be monitored with the ℒ-[1-11C]leucine PET method.
Acknowledgments
The authors thank Dr E Berry-Kravis, Dr Ben Oostra, Dr G Neri, and Dr R Hagerman who helped with patient recruitment; David L Nelson who gave us the Fmr1 KO mice; Drs N Miao and W Kammerer who administered anesthesia during the PET studies; Dr J Snow who administered IQ tests to subjects; and the following members of the PET Department: G Jacobs, S Sestrich, W Kong, M Der, RPh; S Conant, S Thada, B-K Vuong, M Channing, PhD, and P Herscovitch, MD.
The authors declare no conflict of interest.
Footnotes
This research was supported by the Intramural Research Program, National Institute of Mental Health; Clinical Center, National Institutes of Health; the Fragile X Research Foundation.
References
- Verkerk AJ, Pieretti M, Sutcliffe JS, Fu YH, Kuhl DP, Pizzuti A, et al. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991;65:905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Jensen KB, Jin P, Brown V, Warren ST, Darnell RB. Fragile X mental retardation protein targets G quartet mRNAs important for neuronal function. Cell. 2001;107:489–499. doi: 10.1016/s0092-8674(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Darnell JC, Mostovetsky O, Darnell RB. FMRP RNA targets: identification and validation. Genes Brain Behav. 2005;4:341–349. doi: 10.1111/j.1601-183X.2005.00144.x. [DOI] [PubMed] [Google Scholar]
- Ashley CT, Wilkinson KD, Reines D, Warren ST. FMR1 protein: conserved RNP family domains and selective RNA binding. Science (NY) 1993;262:563–566. doi: 10.1126/science.7692601. [DOI] [PubMed] [Google Scholar]
- Siomi H, Siomi MC, Nussbaum RL, Dreyfuss G. The protein product of the fragile X gene, FMR1, has characteristics of an RNA-binding protein. Cell. 1993;74:291–298. doi: 10.1016/0092-8674(93)90420-u. [DOI] [PubMed] [Google Scholar]
- Feng Y, Gutekunst CA, Eberhart DE, Yi H, Warren ST, Hersch SM. Fragile X mental retardation protein: nucleocytoplasmic shuttling and association with somatodendritic ribosomes. J Neurosci. 1997;17:1539–1547. doi: 10.1523/JNEUROSCI.17-05-01539.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res. 2001;29:2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laggerbauer B, Ostareck D, Keidel EM, Ostareck-Lederer A, Fischer U. Evidence that fragile X mental retardation protein is a negative regulator of translation. Hum Mol Genet. 2001;10:329–338. doi: 10.1093/hmg/10.4.329. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Bailey AM, Matthies HJ, Renden RB, Smith MA, Speese SD, et al. Drosophila fragile X-related gene regulates the MAP1B homolog Futsch to control synaptic structure and function. Cell. 2001;107:591–603. doi: 10.1016/s0092-8674(01)00589-x. [DOI] [PubMed] [Google Scholar]
- Qin M, Kang J, Burlin TV, Jiang C, Smith CB. Postadolescent changes in regional cerebral protein synthesis: an in vivo study in the FMR1 null mouse. J Neurosci. 2005;25:5087–5095. doi: 10.1523/JNEUROSCI.0093-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nature Neurosci. 2008;11:1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KC, Cook MP, Qin M, Kang J, Burlin TV, Smith CB. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: I. Kinetic modeling approach. J Cereb Blood Flow Metab. 2005;25:617–628. doi: 10.1038/sj.jcbfm.9600067. [DOI] [PubMed] [Google Scholar]
- Beebe SmithC, Schmidt KC, Qin M, Burlin TV, Cook MP, Kang J, et al. Measurement of regional rates of cerebral protein synthesis with L-[1-11C]leucine and PET with correction for recycling of tissue amino acids: II. Validation in rhesus monkeys. J Cereb Blood Flow Metab. 2005;25:629–640. doi: 10.1038/sj.jcbfm.9600066. [DOI] [PubMed] [Google Scholar]
- Bishu S, Schmidt KC, Burlin T, Channing M, Conant S, Huang T, et al. Regional rates of cerebral protein synthesis measured with L-[1-11C]leucine and PET in conscious, young adult men: normal values, variability, and reproducibility. J Cereb Blood Flow Metab. 2008;28:1502–1513. doi: 10.1038/jcbfm.2008.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishu S, Schmidt KC, Burlin TV, Channing MA, Horowitz L, Huang T, et al. Propofol anesthesia does not alter regional rates of cerebral protein synthesis measured with L-[1-11C]leucine and PET in healthy male subjects. J Cereb Blood Flow Metab. 2009;29:1035–1047. doi: 10.1038/jcbfm.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Williams JB. Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version, administration booklet. American Psychiatric Pub. 1997.
- American Psychiatric Association Diagnostic and Statistical Manual of Mental DisordersRevised 4th edWashington, DC; 2000 [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. The Schedule for Affective Disorders and Schizophrenia for School-Age Children. University of Pittsburgh Medical Center Pittsburgh, PA; 1996. [Google Scholar]
- Gan TJ, Glass PS, Windsor A, Payne F, Rosow C, Sebel P, et al. Bispectral index monitoring allows faster emergence and improved recovery from propofol, alfentanil, and nitrous oxide anesthesia. Anesthesiology. 1997;87:808–815. doi: 10.1097/00000542-199710000-00014. [DOI] [PubMed] [Google Scholar]
- Carson RE, Barker WC, Liow J-S, Johnson CA. Design of a motion-compensation OSEM list-mode algorithm for resolution-recovery reconstruction for the HRRT. IEEE Nucl Sci Symp Conf Rec. 2003;5:3281–3285. [Google Scholar]
- Wienhard K, Schmand M, Casey ME, Baker K, Bao J, Eriksson L, et al. The ECAT HRRT: performance and first clinical application of the new high resolution research tomograph. IEEE Trans Nucl Sci. 2002;49:104–110. [Google Scholar]
- Fischer B, Modersitzki J. Intensity-based image registration with a guaranteed one-to-one point match. Methods Inf Med. 2004;43:327–330. [PubMed] [Google Scholar]
- Tomasi G, Bertoldo A, Bishu S, Unterman A, Smith CB, Schmidt KC. Voxel-based estimation of kinetic model parameters of the L-[1-11C]leucine PET method for determination of regional rates of cerebral protein synthesis: validation and comparison with region-of-interest-based methods. J Cereb Blood Flow Metab. 2009;29:1317–1331. doi: 10.1038/jcbfm.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CB, Deibler GE, Eng N, Schmidt K, Sokoloff L. Measurement of local cerebral protein synthesis in vivo: influence of recycling of amino acids derived from protein degradation. Proc Natl Acad Sci USA. 1988;85:9341–9345. doi: 10.1073/pnas.85.23.9341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos DG, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. Academic Press New York, NY; 2001. [Google Scholar]
- Smith CB, Schmidt KC, Bishu S, Channing M, Bacon J, Burlin T, et al. Use of acute hyperphenylalaninemia in rhesus monkeys to examine sensitivity and stability of the L-[1-11C]leucine method for measurement of regional rates of cerebral protein synthesis with PET. J Cereb Blood Flow Metab. 2008;28:1388–1398. doi: 10.1038/jcbfm.2008.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss AL, Lee J, Freund L. Neuroanatomy of fragile X syndrome: the temporal lobe. Neurology. 1994;44:1317–1324. doi: 10.1212/wnl.44.7.1317. [DOI] [PubMed] [Google Scholar]
- Gothelf D, Furfaro JA, Hoeft F, Eckert MA, Hall SS, O'Hara R, et al. Neuroanatomy of fragile X syndrome is associated with aberrant behavior and the fragile X mental retardation protein (FMRP) Ann Neurol. 2008;63:40–51. doi: 10.1002/ana.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, et al. In vivo brain anatomy of adult males with fragile X syndrome: an MRI study. Neuroimage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Llcalzi E, et al. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biol Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Buggy DJ, Nicol B, Rowbotham DJ, Lambert DG. Effects of intravenous anesthetic agents on glutamate release. Anesthesiology. 2000;92:1067–1073. doi: 10.1097/00000542-200004000-00025. [DOI] [PubMed] [Google Scholar]
- Haeseler G, Karst M, Foadi N, Gudehus S, Roeder A, Hecker H, et al. High-affinity blockade of voltage-operated skeletal muscle and neuronal sodium channels by halogenated propofol analogues. Br J Pharmacol. 2008;155:265–275. doi: 10.1038/bjp.2008.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eghbali M, Gage PW, Birnir B. Effects of propofol on GABA (A) channel conductance in rat cultured hippocampal neurons. Eur J Pharmacol. 2003;468:75–82. doi: 10.1016/s0014-2999(03)01641-8. [DOI] [PubMed] [Google Scholar]
- Kaisti KK, Langsjo JW, Aalto S, Oikonen V, Sipola H, Teras M, et al. Effects of sevoflurane, propofol, and adjunct nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology. 2003;99:603–613. doi: 10.1097/00000542-200309000-00015. [DOI] [PubMed] [Google Scholar]
- Dam M, Ori C, Pizzolato G, Ricchieri GL, Pellegrini A, Giron GP, et al. The effects of propofol anesthesia on local cerebral glucose utilization in the rat. Anesthesiology. 1990;73:499–505. doi: 10.1097/00000542-199009000-00021. [DOI] [PubMed] [Google Scholar]
- Chuang S-C, Zhao W, Bauchwitz R, Yan Q, Bianchi R, Wong RKS. Prolonged epileptiform discharges induced by altered group I metabotropic glutamate receptor-mediated synaptic responses in hippocampal slices of a fragile X mouse model. J Neurosci. 2005;25:8048–8055. doi: 10.1523/JNEUROSCI.1777-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z-H, Huang T, Smith CB. Lithium reverses increased rates of cerebral protein synthesis in a mouse model of fragile X syndrome. Neurobiol Dis. 2012;45:1145–1152. doi: 10.1016/j.nbd.2011.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschrafi A, Cunningham BA, Edelman GM, Vanderklish PW. The fragile X mental retardation protein and group I metabotropic glutamate receptors regulate levels of mRNA granules in brain. Proc Natl Acad Sci USA. 2005;102:2180–2185. doi: 10.1073/pnas.0409803102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterweil EK, Krueger DD, Reinhold K, Bear MF. Hypersensitivity to mGluR5 and ERK1/2 leads to excessive protein synthesis in the hippocampus of a mouse model of fragile X syndrome. J Neurosci. 2010;30:15616–15627. doi: 10.1523/JNEUROSCI.3888-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]