Abstract
Six-hundred twenty-one subjects with unilateral asymptomatic severe internal carotid artery (ICA) stenosis were prospectively evaluated with a median follow-up of 27 months (min=6, max=68). Vascular risk profile, plaque characteristic, stenosis progression, and common carotid artery intima-media thickness (IMT) were investigated in all patients. Outcome measures were occurrence of ischemic stroke ipsilateral to ICA stenosis and vascular death, while myocardial infarction, contralateral strokes, and transient ischemic attack were considered as competing events. A total of 99 subjects (15.9%) suffered from a vascular event. Among them, 39 were strokes ipsilateral to the stenosis (6.3%). Degree of stenosis, stenosis progression, and common carotid artery IMT resulted as independent predictive factors of ipsilateral stroke. Considering a stenosis of 60% to 70% as reference, a degree between 71% and 90% increased the risk by 2.45, while a degree between 91% and 99% increased the risk by 3.26. The progression of stenosis was a strong risk factor (hazard ratio=4.32). Finally, the role of carotid IMT was confirmed as crucial additional measure, with an increased risk by 25% for each 0.1 mm IMT increase. Our data suggest that IMT, stenosis progression and severity should be considered as risk factors for cerebrovascular events in asymptomatic subjects with severe ICA stenosis.
Keywords: atherosclerosis, carotid artery, cerebrovascular disease, risk factors, ultrasound
Introduction
Clinical randomized trials showed that the surgical correction of asymptomatic severe carotid stenosis reduces the 3-year incidence of stroke by ∼30%.1 However, the initial enthusiasm about this therapeutic option was gradually extinguished by the low incidence of ipsilateral strokes observed in asymptomatic subjects.2 The opinion that the risk–benefit ratio of revascularization treatments is not satisfactory in the majority of subjects has increasingly emerged,3 also supported by the enlargement of pharmacological options for the control of the main vascular risk factors.4 For this reason, markers of increased risk of ischemic events were extensively researched to select, among asymptomatic subjects with significant carotid stenosis, those who could better benefit from aggressive treatments. The assessment of carotid plaques' consequences on intracranial circulation by transcranial Doppler (TCD) suggested that cerebral microembolic signals5 and impaired vasomotor reactivity6, 7, 8, 9 are related to an increased risk of becoming symptomatic. Also studies investigating the prognostic role of the degree of stenosis and plaque morphology have proposed promising but not definitive risk profiles.10 Other studies have suggested carotid wall thickness as a marker of risk.11, 12, 13
In the present study, we aimed at investigating reliable markers of increased risk of stroke ipsilateral to severe internal carotid artery (ICA) stenosis, by integrating ultrasonographic data concerning the carotid wall and plaque characteristics. For this purpose, we conducted a prospective investigation on asymptomatic subjects who received indication for the best medical therapy for each vascular risk condition.14
Materials and methods
During a 4-year period (January 2005 to December 2008), we diagnosed an ICA stenosis of 60% or more in 819 asymptomatic subjects among patients referred by their primary care physicians or other specialists for an ultrasound screening for carotid atherosclerosis according with international guidelines.15
All subjects were educated on the estimated annual stroke risk ipsilateral to carotid stenosis1, 16 and on the advantages and disadvantages of medical vs. surgical along with medical treatment, according to international guidelines.17 The 158 patients who considered carotid endarterectomy were referred to our Vascular Surgery Department and excluded from the analyses. The remaining 661 subjects underwent neurologic and cardiological examination, including electrocardiogram, transthoracic or transesophageal echocardiography, TCD, and brain-computed tomography or magnetic resonance imaging. Particular attention was paid to exclude previous symptoms of cerebrovascular disease. To reduce the detection in the follow-up of stroke not determined by ipsilateral ICA stenosis,18 the following exclusion criteria were applied: stenosis⩾50% in the carotid contralateral to severe stenosis or in the vertebral arteries (23 subjects), significant stenosis of intracranial arteries (5 subjects), probable embolizing cardiopathy or cardiac failure (12 subjects). Blood analysis and clinical history with particular attention to the major vascular risk factors (hypertension, diabetes, smoking, and hyperlipidemia) were also obtained. Hypertension, diabetes, and hyperlipidemia were defined on the basis of clinical and hematological evaluations performed at baseline and/or considering previous medical records in patients on pharmacological treatment. The diagnostic criteria and the pharmacological treatment of vascular risk factors, in addition to behavioral recommendations (smoking cessation, regular physical exercise, weight control) were planned in accordance with international guidelines.14, 17
Patients were followed-up by telephone interviews every 3 months and reevaluated clinically every 6 months by one designated investigator, who was masked to the clinical data as well as to the degree and side of carotid stenosis. At the end of the follow-up period, based on information provided by patients and relatives, adherence to the therapeutic plan for the control of vascular risk factors was verified with a short questionnaire. Two investigators assigned patients to good or scarce compliance categories (irregular assumption or discontinuation of drugs from entry to the end of the follow-up period), to evaluate the effect of the pharmacological treatments. Patients underwent follow-up neck vessels' ultrasound after 6 and 12 months to evaluate the possible progression of ICA stenosis. Neurosonographers performing follow-up evaluations were masked to clinical data.
Endpoints were stroke ipsilateral and contralateral to ICA stenosis, transient ischemic attack (TIA), myocardial infarction (MI), and vascular death. If the endpoints were not observed in our hospitals, clinical records were acquired to better define the vascular event. Stroke or TIA diagnoses had to be confirmed by a brain-computed tomography or a magnetic resonance imaging.19 Neurologist defining primary events on the basis of clinical charts were masked to neurosonological findings.
The study was approved by the ethics committees of the Marche Polytechnic University and Campus Bio-Medico University. All participants and/or caregivers gave their informed written consent according to the Declaration of Helsinki.
Ultrasonographic Examination
Carotid arteries were assessed and defined by continuous wave Doppler and Color flow B-mode Doppler ultrasound (Philips iU22, Bothell, WA, USA). Best images were digitized and stored for central reading and interpretation. Degree of stenosis was defined according to validated criteria20 in three categories: 60% to 70%, 71% to 90%, and 91% to 99% by taking into consideration the maximal stenosis with respect to the carotid lumen measured distally to plaque.21
Intracranial vessels were examined by means of a Multidop X/TCD instrument (DWL Elektronische SystemeGmbH, Sipplingen, Germany).
Because of the ICA stenosis, we measured common carotid (CCA) intima-media thickness (IMT) according to guidelines.22 In brief, a longitudinal image of the distal 10-mm CCAs was acquired with subjects lying in supine position and the head turned 45° to the left or right. Intima-media thickness was measured at the thickest plaque-free point on the near and far walls with a specially designed computer program. CCA wall thickness was defined as the mean of the maximum wall thickness of the near and far walls on both the left and right side. To measure IMT, a semiautomatic software (QLAB version 8, Philips Medical Systems, Andover, MA, USA) was used to improve measurement reliability and reproducibility.13, 22 Examinations were performed by the same two experienced operators. Interreader reproducibility was assessed by having the two sonographers blindly selected and redigitized a B-mode CCA image from the 20-second videotape recording of a randomly selected set of 200 studies originally read by a third sonographer. Each sonographer then performed an IMT measurement. One sonographer reread 128 studies for an interreader correlation coefficient of 0.87, and the second sonographer reread 72 studies for an interreader correlation coefficient of 0.85. Intrareader variability was also assessed by having the two sonographers reread 70 studies randomly selected and resulted 0.91 and 0.93, respectively. Even if IMT values in the two sides were significantly associated (P<001), their agreement was not optimal (intra-class correlation=0.75), and the ipsilateral IMT values was slightly (1.04 vs. 1.01 mm) yet significantly (P=0.014) higher than contralateral IMT values. For this reason, we considered both measures as well as their arithmetic mean to potentially reduce the misleading effect of univariate outliers.
Stenotic lesions were characterized on the basis of their echogenicity and surface. The assessment of plaque echolucency was based on the modified version of the Gray–Weale classification.23 The vessel lumen was used as reference structure for defining echolucency, and the bright echo zone produced by the media–adventitia interface at the far wall was used as the reference structure for defining echogenicity. Plaque echolucency was graded as: (1) uniformly hyperechogenic lesion (2) mixed forms as heterogeneous, hypoechogenic and hyperechogenic, plaques; (3) uniformly hypoechogenic. Plaque surface was defined as (1) smooth and regular; (2) mildly irregular; (3) ulcerated.24
Each patient during follow-up underwent duplex examination at 6 months and, if there was no event, at 12 months from inclusion. Progression of stenosis was defined as any change to a higher category of ICA stenosis from baseline to the 6- or 12-month follow-up evaluations.
All ultrasonographic assessments were performed by sonographers masked to clinical information.
Statistical Analysis
Primary statistical analysis aimed to identify which factors could predict ipsilateral stroke events in patients with carotid stenosis. As no vascular deaths were observed during follow-up, only ipsilateral stroke, contralateral stroke, TIA, and MI defined the typical scenario for competing risks. In this study, we considered ipsilateral stroke as the event of interest. Contralateral stroke, TIA, and MI were defined as competing events because their occurrence brings relevant clinical changes (i.e., diagnostic, therapeutic) that can modify the natural history of ICA stenosis. In particular, patients who had TIA or stroke ipsilateral to carotid stenosis were referred to the Vascular Surgery Department.
Competing risk analysis was applied with R (R version 2.13.2 Copyright 2011 The R Foundation for Statistical Computing, Vienna, Austria). First, each single variable was entered as covariate (continuous or categorical). At the second stage, the univariately significant variables were entered simultaneously to eliminate eventual redundancies. In addition, the nonsignificant variables were added to the multivariable model to assess whether some of them could have a role only after taking into account the effect of other variables. Finally, interactions between the variables of the final model were evaluated to unveil multiplicative interactions that were able to explain the modulation of the risks.
Results
Study participants' baseline characteristics are given in Table 1. A preliminary analysis was performed to compare these subjects with the 158 ones excluded from the study because of their decision to be referred to the Vascular Surgery Department. No difference in demographic characteristics, vascular risk profile, and ultrasound findings emerged. At baseline, subjects with modifiable vascular risk factors were prescribed the corresponding treatment, even if a large variability in compliance was observed. During follow-up, regular therapy assumption was observed in 88% hypertensive, 99% diabetic, and 68% patients with dyslipidemia. Subjects with scarce or lack of compliance were considered as nonusers.
Table 1. Baseline characteristics and percentage of vascular risk factors of the cohort study.
|
Patients (N=621) | ||
| Demographic characteristics | ||
| Follow-up, months (median, min–max) | 27 | 6–68 |
| Age, years (mean, s.d.) | 72.2 | 8.9 |
| Sex (n, % of males) | 348 | 56% |
| Risk factors and therapy (n, %) | ||
| Smoking | 161 | 26% |
| Diabetes | 155 | 25% |
| Dislypidemia | 348 | 56% |
| Hypertension | 416 | 67% |
| Ischemic cardiopathies | 124 | 20% |
| Antihypertensives | 391 | 63% |
| Antidiabetics | 155 | 25% |
| Statins | 279 | 45% |
| Antiplatelets | 329 | 53% |
| Ultrasound findings | ||
| IMT (mean, s.d.) | ||
| Ipsilateral | 1.04 | 0.25 |
| Contralateral | 1.01 | 0.24 |
| Mean | 1.03 | 0.24 |
| Carotid stenosis (n, %) | ||
| 60–70% | 414 | 67% |
| 71–90% | 173 | 28% |
| 91–99% | 34 | 5% |
| Echogenicity (n, %) | ||
| Hyper | 382 | 62% |
| Mixed | 178 | 29% |
| Hypo | 61 | 10% |
| Plaque surface (n, %) | ||
| Regular | 463 | 75% |
| Irregular | 130 | 21% |
| Ulcerated | 28 | 5% |
| Stenosis progression (n, %) | 137 | 22% |
Hyper, hyperechogenic plaque; Hypo, hypoechogenic plaque.
The median follow-up was 27 months (min=6, max=68). During follow-up, 99 subjects (15.9%) experienced a vascular event: 72 (72.7%) cerebrovascular events (39 strokes and 27 TIA ipsilateral to the most stenotic carotid artery, 6 contralateral strokes), and 27 (27.3%) MI.
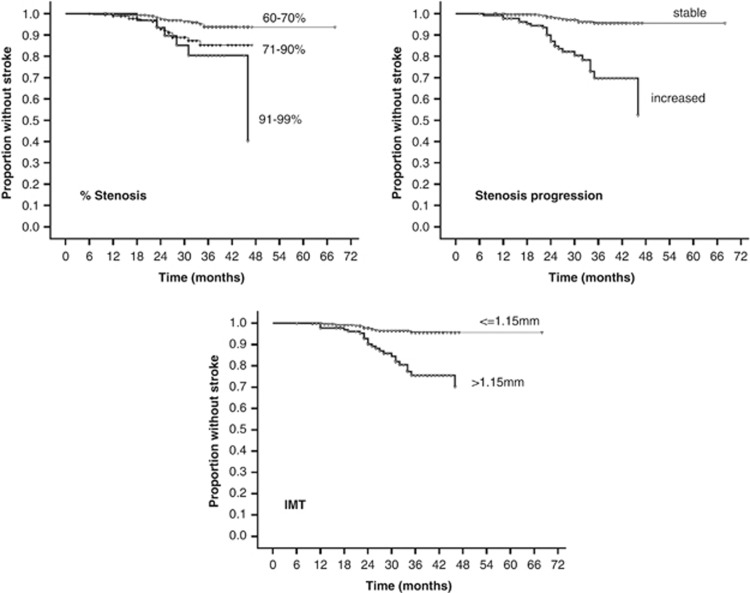
Table 2 summarizes the findings of the Competing Risk Regression, separately for univariable and multivariable models. In the univariable analysis, degree of stenosis, plaque echolucency, surface, and progression and IMT appeared significantly associated with the ipsilateral stroke occurrence. The ‘best' model according to Bayes Information Criterion, comprised degree of stenosis, stenosis progression and IMT as independent predictive factors of ipsilateral stroke. To note, the significant effect of plaque surface in the univariable analysis was clearly attenuated in the multivariable model for its significant association with the degree of stenosis (P=0.004) and stenosis progression (P<001). To describe the effects of these three risk factors, we computed the annual risks for each factor levels. In the whole sample, the annual incidence rate of ipsilateral stroke was 2.6% this rate changed from 1.5% in patients with a 60% to 70% stenosis to 4.2% in those with a 71% to 90% stenosis and to 7.0% in others with a 91% to 99% stenosis; from 1.1% for stable stenosis to 8.3% for progressing stenosis; from 1.3% for IMT<1.15 to 7.1% for IMT>1.15. Kaplan–Meier survival plots graphically confirmed these findings (Figure 1). Hypertension, hyperglycemia, and dyslipidemia were not associated to vascular events, probably because they were often treated with the appropriate therapy. Among the dyslipidemic patients, no significant protective effect of statin use was found (P=0.685).
Table 2. Competing risk regression analysis for ipsilateral stroke presented separately for univariable and multivariable models.
| Univariable | Multivariable | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HR |
95% CI for HR |
P-value | HR |
95% CI for HR |
P-value | ||||
| |
|
Lower |
Upper |
|
|
Lower |
Upper |
|
|
| Age | 0.99 | 0.95 | 1.03 | 0.560 | |||||
| Sex (M vs. F) | 1.47 | 0.77 | 2.82 | 0.240 | |||||
| Smoking (yes vs. no) | 0.78 | 0.37 | 1.64 | 0.510 | |||||
| Diabetes (yes vs. No) | 1.79 | 0.93 | 3.45 | 0.079 | |||||
| Dyslipidemia (yes vs. no) | 0.53 | 0.28 | 1.01 | 0.055 | |||||
| Hypertension (yes vs. no) | 1.96 | 0.90 | 4.24 | 0.089 | |||||
| Cardiopathies (yes vs. no) | 0.52 | 0.20 | 1.31 | 0.160 | |||||
| Stenosis | 0.001a | 0.005a | |||||||
| 71–90% vs. 60–70% | 2.73 | 1.38 | 5.38 | 0.004 | 2.45 | 1.26 | 4.78 | 0.008 | |
| 91–99% vs. 60–70% | 4.37 | 1.76 | 10.88 | 0.002 | 3.26 | 1.41 | 7.57 | 0.006 | |
| Echogenicity | 0.001a | ||||||||
| Mixed vs. Hyper | 2.90 | 1.76 | 9.74 | 0.003 | |||||
| Hypo vs. Hyper | 4.15 | 1.76 | 9.74 | 0.001 | |||||
| Plaque surface | 0.000a | ||||||||
| Irregular vs. Regular | 3.36 | 1.66 | 6.81 | 0.000 | |||||
| Ulcerated vs. Regular | 8.24 | 3.80 | 17.90 | 0.000 | |||||
| Stenosis progression (yes vs. no) | 6.87 | 3.58 | 13.20 | 0.000 | 4.32 | 2.31 | 8.08 | 0.000 | |
| Antihypertensives (yes vs. no) | 1.31 | 0.66 | 2.57 | 0.440 | |||||
| Antidiabetics (yes vs. no) | 1.78 | 0.93 | 3.42 | 0.083 | |||||
| Statins (yes vs. no) | 0.71 | 0.37 | 1.36 | 0.300 | |||||
| Antiplatelets (yes vs. no) | 1.86 | 0.96 | 3.61 | 0.066 | |||||
| IMT (unit 0.1 mm) | |||||||||
| Ipsilateral | 1.33 | 1.21 | 1.45 | 0.000 | |||||
| Contralateral | 1.20 | 1.13 | 1.28 | 0.000 | |||||
| Mean | 1.28 | 1.19 | 1.38 | 0.000 | 1.25 | 1.16 | 1.35 | 0.000 | |
CI, confidence interval; HR, hazard ratio; Hyper, hyperechogenic plaque; Hypo, hypoechogenic plaque.
Significant correlations are evidenced in bold.
Wald omnibus test.
Figure 1.
Kaplan–Meier plots of the three ultrasonographic findings that showed a significant association with an increased risk of ipsilateral stroke. IMT, intima-media thickness.
When the above factors were adjusted for the others, considering a stenosis of 60% to 70% as reference, a lumen narrowing between 71% and 90% increased the risk by 2.45, while a 91% to 99% degree increased the risk by 3.26. Taking into account the degree of stenosis, the progression turned out to be a strong risk factor (hazard ratio=4.32). Finally, the role of IMT was confirmed as additional crucial measure, with an increased risk by 25% for each 0.1 mm IMT increase. As such, this model proved to be the best among all the tested models (minimal Bayesion Information Criterion (BIC) value). We also considered the classification based on a cutoff of 1.15.14 When IMT continuous measure was replaced by IMT binary classification in the multivariable model, patients with values of IMT>1.15 had three times more risk than patients below this threshold, after adjusting for the other relevant factors (hazard ratio=3.01, 95% CI: 1.48 to 6.11). The interactions between the three significant factors were also assessed, but no double interaction reached the significant threshold.
However, stenosis progression cannot be considered a ‘baseline' risk factor because a second evaluation (usually 6 months apart) should be performed for its assessment. For such a reason, we excluded such a variable in a further model and found that echogenicity has a role among baseline risk factors. More precisely, the hazard ratios of the other significant variables were strictly similar to those described above (e.g., hazard ratio of IMT changed from 1.25 to 1.26), while hypoechogenicity increased the risk up to 3.3 (95% CI: 1.4 to 8.0) with respect to hyperechogenic plaque.
A secondary analysis considered the first occurrence of stroke or TIA as the main event of interest and MI as competing event. In addition to the degree of stenosis, stenosis progression and IMT, plaque surface also represented an additional predictive factor. To briefly describe the effects of each of these four risk factors, we computed the annual risks for cerebrovascular event for each factor levels. Considering that for the whole sample the annual incidence rate was 4.7%, this rate changed from 2.7% in patients with a 60% to 70% stenosis to 8.6% for those with a 71% to 90% stenosis and to 10.4% for others for a 91% to 99% stenosis; from 2.2% for a regular surface to 10.1% for irregular surface and to 23.7% for ulcerated surface; from 2.1% for stable plaque to 15.2% for increasing plaque; from 2.6% for IMT<1.15 to 12.1% for IMT>1.15.
Discussion
The main finding of the present study is that carotid wall thickening and specific plaque characteristics are related to an increased risk of stroke ipsilateral to the ICA stenosis in asymptomatic subjects. In particular, an increase in IMT, a higher degree of stenosis, and its progression were able to qualify subjects at increased risk of stroke ipsilateral to severe asymptomatic carotid stenosis. Irregular plaque surface and ulceration emerged as further risk predictors for all ipsilateral neurovascular events. This apparent discrepancy was probably because of statistical reasons, as considering strokes and TIA together, the number of observed events increased, allowing the relationship between plaque surface alteration and increased risk to reach a significant level. Besides, we considered the presence of plaque ulceration or hemorrhage only at baseline, while stenosis progression represented the only parameter recordable longitudinally in all subjects.
Some results obtained in our study are not unexpected, as they have been previously suggested as additional findings by studies designed to explore other conditions. However, the relevant aspect of our investigation is that data were obtained in a prospective, ad-hoc-designed observation on a large cohort of subjects. Moreover, it is worth noting that we considered a study population in a real-life setting. In particular, the enrolled subjects were encouraged and checked for a careful adherence to treatment for vascular risk factors. In this respect, most prescriptions were followed for the main duration of the observation period, although we did not consider the effect on stroke risks of different classes of medications.
A significant carotid lumen narrowing is a condition frequently present in asymptomatic subjects, especially in elderly people.25 However, carotid endarterectomy does not seem to be indicated in most cases because there is evidence that pharmacological treatment is as effective as endarterectomy in reducing the risk of stroke in a large majority of subjects.4, 26 The potential risks of aggressive treatments urge us to identify those conditions that significantly increase the risk of stroke caused by carotid stenosis. This suggests the need to find accurate markers to identify subjects at the highest risk of becoming symptomatic in the attempt to make the risk/benefit ratio of surgery more favorable. The progression of the degree of carotid stenosis has been suggested as a general marker of increased susceptibility to vascular disease, probably because the evolution of carotid atherosclerosis may reflect that in other arterial beds.27 Conversely, as the correlation between severity of stenosis per se and ischemic events is imperfect,16, 28 other characteristics have been explored as potential markers of plaque vulnerability and stroke risk.29 Also increased carotid wall thickness has been associated with the risk of developing stroke in subjects with asymptomatic severe ICA stenosis, but most studies showed that carotid IMT provides reliable indications about the cumulative risk of all vascular events.30 Nevertheless, increased IMT was also related with larger brain infarction and clinical severity,12, 13 suggesting that carotid wall thickening may reflect the vulnerability of the atherosclerotic brain to ischemia. Despite an impressive number of investigations performed about this issue, the utility of morphologic, pathologic, and biochemical features of carotid plaque in predicting the occurrence of TIA and stroke has not been established prospectively. For this reason, we designed the present study to obtain prospective data about the possible correlations among vascular risk profiles, pharmacological treatment, carotid vessel characteristics, plaque morphology and surface, degree and progression of lumen narrowing. The obtained findings suggest to consider specific ultrasonographic markers to individuate asymptomatic subjects with an increased risk of cerebrovascular disease.
Although in our study some ultrasonographic markers were able to predict stroke if considered individually, their coexistence did not additionally increase the risk. This observation may be explained by different, not mutually exclusive, hypotheses. One possibility is that as the physiopathology of stroke is multifactorial, even in presence of a severe carotid stenosis, the pathologic contribution of plaque morphology is partial, statistically reaching a ceiling effect in the case of a coexistence of ultrasound markers. Also, in our cohort, only few cases had more than one unfavorable marker making it difficult to statistically show an increased associated risk. In addition, atherosclerosis markers may be related to an increased stroke risk via different patophysiological mechanisms. In particular, ulcerated plaques are likely to be responsible for artery-to-artery embolic strokes, whereas a higher degree or progressive stenosis may be mainly linked to a hemodynamic failure or reduce plaque stability. In this view, a relevant interaction among ultrasound markers in increasing the stroke risk may be not expected. For this reason, based on our results, a full assessment of ultrasonographic characteristics of carotid wall and plaque, including stenosis progression, should be considered the most reliable approach to define patients' individual risk profile.
Some aspects of our study may deserve further consideration. The first one is that we did not evaluate cerebral hemodynamics to assess the risk of stroke related to carotid stenosis. However, the low availability of TCD would not allow a wider employment for the stratification of patients in clinical practice. In addition, as clinical assessment of patients did not include magnetic resonance angiography or other direct imaging evaluation of vessels morphology, we cannot rule out that some of the recorded cerebrovascular events could be determined by vulnerable nonhemodynamically significant plaques of intracranial vessels not detectable with TCD. Nevertheless, even in these patients, carotid stenosis may have a pathogenetic role impairing compensatory hemodynamic response. In fact, data from NASCET trial showed that carotid endarterectomy is more beneficial in patients with intracranial stenosis.18 Similarly, lacunar strokes were included as endpoint as they had been associated with a poor hemodynamic response in patients with carotid stenosis.11 Another point to discuss is the relatively high incidence of stroke in our cohort compared with more recent studies.31, 32 One explanation is that our study population might have been at particularly high risk, as we included subjects with a mean age above 70 years undergoing ultrasonographic evaluation of neck vessels for the presence of multiple vascular risk factors. Besides, compared with clinical trials, this study was performed in a real-life setting. Although treatment adherence was carefully controlled, it is possible that in everyday life, medical indications (including behavioral recommendations) may not be fully followed. Moreover, we considered as stroke events also clinical TIA with new vascular lesions detected by neuroimaging.19 Finally, many subjects developed heart ischemic disease. Nevertheless, carotid atherosclerosis should be considered as a threatening condition for the whole vascular tree: a disease striking one branch most likely affects the other branches too. Even if the surgical correction of carotid stenosis does not improve the risk for MI, this does negate the opportunity to better select subjects with the best prospective of benefitting from carotid endarterectomy. In addition, the finding confirms the necessity to consider all therapeutic options in subjects with particular ultrasonographic characteristics of carotid stenosis to offer them the most complete protection. Besides the surgical option, an aggressive pharmacological treatment of each vascular factor, associated with antiplatelet agents and a careful and aggressive correction of lifestyle risk conditions, such as overweight, smoking, and reduced physical activity, should be strongly encouraged.
The authors declare no conflict of interest.
References
- Chambers BR, Donnan GA.Carotid endarterectomy for asymptomatic carotid stenosis Cochrane Database Syst Rev 20054CD001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naylor AR. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol. 2011;9:116–124. doi: 10.1038/nrcardio.2011.151. [DOI] [PubMed] [Google Scholar]
- Naylor AR. What is the current status of invasive treatment of extracranial carotid artery disease. Stroke. 2011;42:2080–2085. doi: 10.1161/STROKEAHA.110.597708. [DOI] [PubMed] [Google Scholar]
- Woo K, Garg J, Hye RJ, Dilley RB. Contemporary results of carotid endarterectomy for asymptomatic carotid stenosis. Stroke. 2010;41:975–979. doi: 10.1161/STROKEAHA.110.578856. [DOI] [PubMed] [Google Scholar]
- Markus HS, King A, Shipley M, Topakian R, Cullinane M, Reihill S, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol. 2010;9:663–671. doi: 10.1016/S1474-4422(10)70120-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestrini M, Vernieri F, Pasqualetti P, Matteis M, Passarelli F, Troisi E, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA. 2000;283:2122–2127. doi: 10.1001/jama.283.16.2122. [DOI] [PubMed] [Google Scholar]
- Marshall RS, Rundek T, Sproule DM, Fitzsimmons BF, Schwartz S, Lazar RM. Monitoring of cerebral vasodilatory capacity with transcranial Doppler carbon dioxide inhalation in patients with severe carotid artery disease. Stroke. 2003;34:945–949. doi: 10.1161/01.STR.0000062351.66804.1C. [DOI] [PubMed] [Google Scholar]
- Tsivgoulis G, Alexandrov AV, Sloan MA. Advances in transcranial Doppler ultrasonography. Curr Neurol Neurosci Rep. 2009;9:46–54. doi: 10.1007/s11910-009-0008-7. [DOI] [PubMed] [Google Scholar]
- Palazzo P, Balucani C, Barlinn K, Tsivgoulis G, Zhang Y, Zhao L, et al. Association of reversed Robin Hood syndrome with risk of stroke recurrence. Neurology. 2010;75:2003–2008. doi: 10.1212/WNL.0b013e3181ffe4e4. [DOI] [PubMed] [Google Scholar]
- Nicolaides AN, Kakkos SK, Kyriacou E, Griffin M, Sabetai M, Thomas DJ, et al. Asymptomatic Carotid Stenosis and Risk of Stroke (ACSRS) Study Group. Asymptomatic internal carotid artery stenosis and cerebrovascular risk stratification. J Vasc Surg. 2010;52:1486–1496. doi: 10.1016/j.jvs.2010.07.021. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Pasqualetti P, Baruffaldi R, Catani S, Tibuzzi F, Altamura C, et al. Markers of lacunar stroke in patients with moderate internal carotid artery stenosis. J Neurol. 2006;253:321–327. doi: 10.1007/s00415-005-0989-3. [DOI] [PubMed] [Google Scholar]
- Heliopoulos I, Papaoiakim M, Tsivgoulis G, Chatzintounas T, Vadikolias K, Papanas N, et al. Common carotid intima media thickness as a marker of clinical severity in patients with symptomatic extracranial carotid artery stenosis. Clin Neurol Neurosurg. 2009;111:246–250. doi: 10.1016/j.clineuro.2008.10.007. [DOI] [PubMed] [Google Scholar]
- Silvestrini M, Cagnetti C, Pasqualetti P, Albanesi C, Altamura C, Lanciotti C, et al. Carotid wall thickness and stroke risk in patients with asymptomatic internal carotid stenosis. Atherosclerosis. 2010;210:452–457. doi: 10.1016/j.atherosclerosis.2009.12.033. [DOI] [PubMed] [Google Scholar]
- Goldstein LB, Bushnell CD, Adams RJ, Appel LJ, Braun LT, Chaturvedi S, American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Epidemiology and Prevention; Council for High Blood Pressure Research; Council on Peripheral Vascular Disease, and Interdisciplinary Council on Quality of Care and Outcomes Research et al. Guidelines for the primary prevention of stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:517–584. doi: 10.1161/STR.0b013e3181fcb238. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Alexandrov AV, Tegeler CH, Hobson RW, Dennis Baker J, Hopkins LN. Guidelines for screening of extracranial carotid artery disease: a statement for healthcare professionals from the multidisciplinary practice guidelines committee of the American Society of Neuroimaging; cosponsored by the Society of Vascular and Interventional Neurology. J Neuroimaging. 2007;17:19–47. doi: 10.1111/j.1552-6569.2006.00085.x. [DOI] [PubMed] [Google Scholar]
- Executive Committee for the Asymptomatic Carotid Atherosclerosis Study Endarterectomy for asymptomatic carotid stenosis. JAMA. 1995;273:1421–1428. [PubMed] [Google Scholar]
- Leys D, Kwiecinski H, Bogousslavsky J, Bath P, Brainin M, Diener HC, EUSI Executive Committee et al. EUSI Writing Committee. Prevention. European Stroke Initiative. Cerebrovasc Dis. 2004;17 (Suppl. 2:15–29. doi: 10.1159/000074817. [DOI] [PubMed] [Google Scholar]
- Inzitari D, Eliasziw M, Gates P, Sharpe BL, Chan RK, Meldrum HE, et al. The causes and risk of stroke in patients with asymptomatic internal-carotid artery stenosis. N Engl J Med. 2000;342:1693–1700. doi: 10.1056/NEJM200006083422302. [DOI] [PubMed] [Google Scholar]
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2009;40:2276–2293. doi: 10.1161/STROKEAHA.108.192218. [DOI] [PubMed] [Google Scholar]
- De Bray JM, Glatt B. Quantification of atheromatous stenosis in the extracranial internal carotid artery. Cerebrovasc Dis. 1995;5:414–426. [Google Scholar]
- North American Symptomatic Carotid Endarterectomy Trial Collaborators Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, et al. Mannheim carotid intima–media thickness consensus (2004–2006). An update on behalf of the Advisory Board of the 3rd and 4th Watching the Risk Symposium, 13th and 15th European Stroke Conferences, Mannheim, Germany, 2004, and Brussels, Belgium, 2006. Cerebrovasc Dis. 2007;23:75–80. doi: 10.1159/000097034. [DOI] [PubMed] [Google Scholar]
- Gray-Weale AC, Graham JC, Burnett JR, Byrne K, Lusby RJ. Carotid artery atheroma: comparison of preoperative B-mode ultrasound appearance with carotid endarterectomy specimen pathology. J Cardiovasc Surg. 1988;29:676–681. [PubMed] [Google Scholar]
- Kern R, Szabo K, Hennerici M, Meairs S. Characterization of carotid artery plaques using real-time compound B-mode ultrasound. Stroke. 2004;35:870–875. doi: 10.1161/01.STR.0000120728.72958.4A. [DOI] [PubMed] [Google Scholar]
- de Weerd M, Greving JP, Hedblad B, Lorenz MW, Mathiesen EB, O'Leary DH, et al. Prevalence of asymptomatic carotid artery stenosis in the general population: an individual participant data meta-analysis. Stroke. 2010;41:1294–1297. doi: 10.1161/STROKEAHA.110.581058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selim MH, Molina CA. Medical versus surgical treatment of asymptomatic carotid stenosis: the ever-changing nature of evidence-based medicine. Stroke. 2011;42:1156–1157. doi: 10.1161/STROKEAHA.111.614156. [DOI] [PubMed] [Google Scholar]
- Sabeti S, Schlager O, Exner M, Mlekusch W, Amighi J, Dick P, et al. Progression of carotid stenosis detected by duplex ultrasonography predicts adverse outcomes in cardiovascular high-risk patients. Stroke. 2007;38:2887–2894. doi: 10.1161/STROKEAHA.107.488387. [DOI] [PubMed] [Google Scholar]
- Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, MRC Asymptomatic Carotid Surgery Trial (ACST) Collaborative Group et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- Prati P, Tosetto A, Casaroli M, Bignamini A, Canciani L, Bornstein N, et al. Carotid plaque morphology improves stroke risk prediction: usefulness of a new ultrasonographic score. Cerebrovasc Dis. 2011;31:300–304. doi: 10.1159/000320852. [DOI] [PubMed] [Google Scholar]
- Polak JF, Pencina MJ, Pencina KM, O'Donnell CJ, Wolf PA, D'Agostino RB. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med. 2011;365:213–221. doi: 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt L, Olivia MD, Geraghty C, Mehta OC, Rothwell Z, Low PM. Risk of ipsilateral stroke in patients with asymptomatic carotid stenosis on best medical treatment. A prospective, population-based study. Stroke. 2010;41:e11–e17. doi: 10.1161/STROKEAHA.109.561837. [DOI] [PubMed] [Google Scholar]
- Abbott AL. Medical (nonsurgical) intervention alone is now best for prevention of stroke associated with asymptomatic severe carotid stenosis: results of a systematic review and analysis. Stroke. 2009;40:e573–e583. doi: 10.1161/STROKEAHA.109.556068. [DOI] [PubMed] [Google Scholar]