Abstract
It has been proposed that prostaglandin E2 (PGE2) is released from astrocytic endfeet to dilate parenchymal arterioles through activation of prostanoid (EP4) receptors during neurovascular coupling. However, the direct effects of PGE2 on isolated parenchymal arterioles have not been tested. Here, we examined the effects of PGE2 on the diameter of isolated pressurized parenchymal arterioles from rat and mouse brain. Contrary to the prevailing assumption, we found that PGE2 (0.1, 1, and 5 μmol/L) constricted rather than dilated parenchymal arterioles. Vasoconstriction to PGE2 was prevented by inhibitors of EP1 receptors. These results strongly argue against a direct role of PGE2 on arterioles during neurovascular coupling.
Keywords: astrocytes, brain parenchymal arterioles, neurovascular coupling, prostaglandin E2
Introduction
Local blood flow is regulated by neuronal activity so as to match changes in the metabolic demand of brain tissue with the supply of oxygen and glucose, a process that is critical to maintain cerebral homeostasis and to prevent the development of ischemic conditions. This linkage between neuronal activity and cerebral blood flow, termed as neurovascular coupling or functional hyperemia,1 is rapid (seconds) and occurs at the level of the cerebral microcirculation within the brain parenchyma. Local increases in cerebral blood flow are mediated by dilation of parenchymal arterioles,2 which, unlike surface cerebral arteries (pial arteries), are encased by astrocytic processes (‘endfeet').1
Recent evidence indicates that information about neuronal activity is communicated to (1) surface arterioles via astrocytic processes comprising the glia limitans and to (2) parenchymal arterioles through elevation of astrocytic Ca2+ and the subsequent engagement of Ca2+-dependent vasodilatory pathways in astrocytic endfeet.1, 3 Two potential Ca2+-dependent astrocytic endfoot targets in particular have received considerable research attention: large-conductance, Ca2+-sensitive K+ (BK) channels, which deliver K+ into the restricted perivascular space and dilate vessels by activating strong inwardly rectifying K+ channels,4, 5, 6 and phospholipase A2,3, 7 which produces arachidonic acid through the hydrolysis of membrane lipids.
Prostaglandin E2 (PGE2), a metabolite of arachidonic acid produced by the action of cyclooxygenase (COX), has been proposed as a major mediator of neurovascular coupling.8 A role for PGE2 in neurovascular coupling has largely been inferred from studies on the effects of COX inhibitors on neurally evoked vasodilation in brain slices.8 However, some studies have found no effect of COX inhibition on neurovascular coupling in brain slices,6 and others have attributed the reduction of functional hyperemia by COX inhibition to effects on COX2 in neurons.9 Moreover, whether COX is even expressed in astrocytes has been called into question.10
In theory, PGE2 could act as a vasodilator or constrictor depending on the nature of the member of the G protein-coupled prostanoid (EP) receptor family. The Gs-coupled EP4 receptor signals through the cyclic adenosine monophosphate-dependent protein kinase (PKA) pathway, which is associated with dilation. Whereas the EP1 subtype is thought to couple to Gq-type α subunits and mediates an increase in intracellular Ca2+, consistent with a role in constriction. EP3, a subtype with promiscuous G protein-coupling propensity, has also been linked to elevation of intracellular Ca2+ and thus might mediate a contractile response to PGE2.
A critical test for the potential involvement of PGE2 in neurovascular coupling is the demonstration that PGE2 directly dilates isolated parenchymal arterioles. This evidence is currently lacking. In contrast, external K+, also posited as a mediator of neurovascular coupling, has been shown to be a potent, rapid, reversible dilator of parenchymal arterioles.4, 5, 6 Here, we tested the effects of PGE2 on the diameter of isolated pressurized parenchymal arterioles from rat and mouse brain. Contrary to expectation, we found that PGE2 constricted rather than dilated parenchymal arterioles.
Materials and methods
Animals
All experimental protocols used in this study were in accord with institutional guidelines approved by the Institutional Animal Care and Use Committee of the University of Vermont. Male C57BL6 mice and male Sprague–Dawley rats were used. Animals (aged 3 to 4 months) were euthanized by intraperitoneal injection of sodium pentobarbital (mouse: 100 mg/kg; rat: 150 mg/kg) followed by rapid decapitation.
Pressurized Parenchymal Arterioles
After euthanasia, the brain was removed and placed into 4°C MOPS-buffered saline. Parenchymal arterioles, arising from the M1 region of the middle cerebral artery and perfusing the neocortex, were dissected. Precapillary arteriolar segments were then cannulated on glass micropipettes with one end occluded in an organ chamber (University of Vermont Instrumentation and Model Facility) and pressurized using an arteriograph system (Living Systems Instrumentation, Inc., Burlington, VT, USA). Parenchymal arterioles were pressurized to 40 mm Hg and superfused (4 mL/min) with prewarmed (35°C to 37°C), gassed (5% CO2, 20% O2, 75% N2) artificial cerebrospinal fluid (aCSF) for at least 1 hour. Only viable parenchymal arterioles, defined as those that developed pressure-induced myogenic tone greater than 20%, were used in subsequent experiments (Supplementary Figure 1A). The average percentage of tone was 36.6±3.4% (n=20) and 38.1±2.3% (n=23) in parenchymal arterioles from rat and mouse, respectively. Endothelial function was tested by assessing the vasodilator response to NS309 (1 μmol/L), an activator of endothelial small- (SK) and intermediate (IK)-conductance Ca2+-activated K+ channels. Drugs were applied by addition to the superfusate. Vessel internal diameter was continuously monitored using a CCD camera and edge-detection software (IonOptix, Milton, MA, USA). Maximal dilation was obtained in nominally Ca2+-free aCSF (0 μmol/L [Ca2+]o, 5 mmol/L EGTA).
Solutions
The composition of MOPS-buffered saline (in mmol/L) was 135 NaCl, 5 KCl, 1 KH2PO4, 1 MgSO4, 2.5 CaCl2, 5 𝒟-glucose, 3 MOPS, 0.02 EDTA, 2 pyruvate, bovine serum albumin (10 mg/mL), pH 7.3 at 4°C. The composition of aCSF (in mmol/L) was 125 NaCl, 3 KCl, 26 NaHCO3, 1.25 NaH2PO4, 1 MgCl2, 4 𝒟-glucose, 2 CaCl2; pH was 7.3 when aerated with 5% CO2.
Drugs
Stock solutions of PGE2 (10 mmol/L in dimethyl sulfoxide) were prepared from powder each day of the experiment or stored at −20°C for <1 month, and were protected from exposure to light in accordance with recommendations of the suppliers. During experiments, PGE2 was superfused immediately after dissolution in aCSF or dilution from dimethyl sulfoxide stock solutions to prevent PGA2 formation from PGE2 dehydration.
Prostaglandin E2 was purchased from different suppliers (Enzo Life Sciences, Farmingdale, NY, USA; Sigma-Aldrich, St Louis, MO, USA; and Tocris Bioscience, Ellisville, MO, USA). No significant differences were observed among the responses to PGE2 from the different vendors in parenchymal arteriolar diameter recording experiments. Prostacyclin (PGI2) was purchased from Enzo Life Sciences. ZM241385 and SC51322 were purchased from Tocris Bioscience. AH6809 and adenosine were purchased from Sigma-Aldrich.
Data Analysis and Statistics
Changes in arteriolar diameter were calculated as percent change from baseline (change in diameter/initial diameter). Data are expressed as means±SEMs. Differences between two groups were analyzed using Student's t-test. Statistical significance was tested at the 95% (P<0.05) confidence level.
Results
Prostaglandin E2 Constricts Pressurized Parenchymal Arterioles
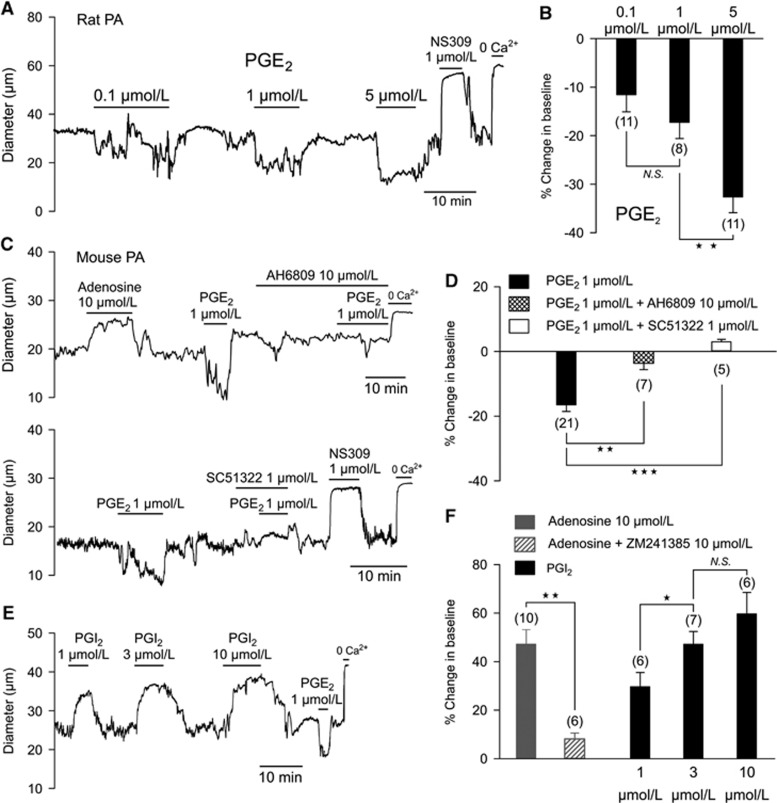
Although PGE2 released from astrocytes has been proposed as a major mediator of neurovascular coupling, whether PGE2 actually dilates parenchymal arterioles has not been experimentally determined. To test this directly, we assessed the effects of different concentrations of PGE2 on the diameter of isolated parenchymal arterioles, preconstricted by elevation of intravascular pressure to a physiologic level (40 mm Hg). Surprisingly, rather than inducing dilation, PGE2 constricted cerebral parenchymal arterioles from rat (Figures 1A and 1B) and mouse (Figures 1C to 1E) in a concentration-dependent manner. Prostaglandin E2 (1 μmol/L) constricted rat and mouse parenchymal arterioles by 18.6±3% (n=11) and 16.5±2% (n=21), respectively.
Figure 1.
(A–D) Rat and mouse parenchymal arterioles (PA) constrict in response to prostaglandin E2 (PGE2). (A) Recording of the internal diameter of a pressurized (40 mm Hg) rat parenchymal arteriole during the perfusion of PGE2 (0.1, 1, and 5 μmol/L), added noncumulatively. (B) Summary data (means±SEMs) showing changes in luminal diameter expressed as percentages relative to baseline diameter; the number of experiments is indicated in parentheses (**P<0.01, NS indicates nonsignificant). (C) Recordings of the internal diameter of pressurized (40 mm Hg) mouse parenchymal arterioles during the perfusion of PGE2 (1 μmol/L) with and without EP receptor inhibitors. Upper trace: nonselective EP1, EP2, and EP3 receptor antagonist AH6809 (10 μmol/L). Bottom trace: selective EP1 antagonist SC51322 (1 μmol/L). Both nonselective inhibition of EP1–3 receptors and specific inhibition of EP1 completely prevented PGE2-induced vasoconstriction in mouse parenchymal arterioles. Adenosine (10 μmol/L; top trace) and NS309 (1 μmol/L; bottom trace) were included as positive controls. (D) Summary data (means±SEMs) showing change in luminal diameter; the number of experiment is indicated in parentheses (**P<0.01, ***P<0.001). (E, F) Prostacyclin (PGI2) dilates mouse parenchymal arterioles. (E) Typical recordings of the internal diameter of pressurized (40 mm Hg) parenchymal arterioles during the perfusion of PGI2 (1, 3, and 10 μmol/L), added noncumulatively. (F) Summary data (means±SEMs) showing change in luminal diameter induced by adenosine with and without A2A receptor antagonist ZM241385 and PGI2; the number of experiments is indicated in parentheses (*P<0.05, **P<0.01, NS indicates nonsignificant).
In mouse, vasoconstriction induced by PGE2 (1 μmol/L) was significantly higher at an intraluminal pressure of 20 mm Hg, than at 80 mm Hg (25.8±2% versus 10.8±1%, n=7, Supplementary Figures 1B and C). Superfusing mouse parenchymal arterioles with aCSF gassed with 5% CO2, 10% O2, 85% N2 to lower the oxygen tension had no effect on the constriction to 1 μmol/L PGE2. The constriction measured in 10% O2 (17.1±2.6%, n=7) was very similar to the above-mentioned value in 20% oxygen. Thus, we have found that PGE2 invariably constricted parenchymal arterioles over a range of test conditions. Under these same test conditions, NS309, a synthetic activator of endothelial SK and IK channels, always dilated the parenchymal arterioles (Figures 1A and 1C), indicating that their dilator capacity was not impacted by the test conditions employed.
Prostaglandin E2-Induced Constriction of Pressurized Parenchymal Arterioles is Mediated by EP1 Receptors
In studies of newborn and adult pigs, an assessment of PGE2 receptor subtypes along with measurements of the effects of PGE2 on IP3 and cyclic adenosine monophosphate production suggests a predominant expression of EP1 with lesser levels of EP3 in adult brain microvessels.11 In mouse parenchymal arterioles, we found that vasoconstriction to PGE2 (1 μmol/L) was inhibited by the prostanoid receptor antagonist AH6809 (10 μmol/L), which does not discriminate among EP1, EP2, and EP3 receptors (Figures 1C and 1D).12 SC51322 (1 μmol/L), a selective antagonist of the EP1 receptor,12 completely inhibited the constriction to PGE2 (Figures 1C and 1D), indicating that PGE2 acts through EP1 receptors to constrict parenchymal arterioles. This is consistent with the likely coupling of EP1 receptors to G proteins of the Gq class, which typically mediate the contractile response to vasoconstrictor agonists. Prostaglandin E2 did not cause vasodilation in the presence of AH6809 or SC51322.
The Vasodilator Adenylyl Cyclase-Protein Kinase Pathway is Intact in Parenchymal Arterioles
It has been proposed that PGE2, acting through Gs-coupled receptors (e.g., EP4), stimulates the adenylyl cyclase-PKA pathway to dilate parenchymal arterioles.8 To confirm that the adenylyl cyclase-PKA pathway is operational in parenchymal arterioles, we tested the effects of the endogenous agents, adenosine and PGI2, which have been shown to relax smooth muscle through this mechanism. Both adenosine and prostacyclin caused rapid and reversible dilation of parenchymal arterioles (Figures 1C). Vasodilation to adenosine was prevented by preincubation with the adenosine A2A receptor antagonist ZM241385 (Figure 1D).
Prostaglandin E2 Constricts Pressurized Mouse Pial Arterioles
Previous studies have investigated the effects of PGE2 in isolated cerebral arteries from different species (Supplementary Table 1), the majority of which have shown vasoconstriction. Arteries from guinea pig, dog, pig, and baboon constrict in response to PGE2, whereas feline pial arteries display weak relaxation at lower concentrations and contraction at higher concentrations of PGE2. Studies using rat, rabbit, and postmortem human cerebral arteries have reached very divergent conclusions (Supplementary Table 1). In tests of PGE2 (0.1 and 1 μmol/L) on middle and posterior cerebral arteries from adult mice, we observed only vasoconstriction (Supplementary Figures 1D and E).
Discussion
A number of studies have proposed that astrocyte-derived PGE2 makes a major contribution to functional hyperemia.8 According to this model, PGE2, generated via a Ca2+-dependent mechanism through the action of astrocytic endfoot COX1 on phospholipase A2-derived arachidonic acid, is released from astrocytes and activates receptors on vascular smooth muscle cells, presumably Gs-coupled EP4 receptors, to promote arteriolar dilation. Much of the evidence for this mechanism comes from inhibitor-based studies using brain slice preparations in which Ca2+ elevation in astrocytes is induced directly through application of a metabotropic glutamate receptor agonist or indirectly through electrical field stimulation of neurons, after which the effects of COX inhibitors on arteriolar dilation are measured. These studies have yielded mixed results.6, 7
Several neurovascular coupling agents have been proposed including prostaglandins, epoxyeicosatrienoic acids, adenosine, oxygen, nitric oxide, H+, and K+.1, 8 Although mechanisms of functional hyperemia may vary substantially between regions of the brain, one absolute criterion that a putative ‘gliotransmitter' must satisfy, if it is to couple neuronal activation to vasodilation, is a demonstrated ability to dilate isolated arterioles. Adenosine, prostacyclin, and external H+ and K+4, 5, 13 are potent vasodilators that induce a rapid dilation when directly applied to isolated arterioles, and therefore fulfill this requirement. Prostaglandin E2 does not. Although the remote possibility that an unknown vasoconstrictor is released from remnant endfoot membranes in response to PGE2 cannot be formally excluded, we consider this highly unlikely given that pial arteries, which lack endfeet also constrict to PGE2 (Supplementary Figures 1D and E) and no dilation was observed in parenchymal arterioles in the presence of an EP1 blocker (Figure 1). Therefore, our results clearly indicate that PGE2 does not dilate cerebral parenchymal arterioles (Figure 1), which are critical to neurovascular coupling, but instead causes vasoconstriction through activation of EP1 receptors.
Interpretation of the effects of exogenous application of PGE2 on arteriolar diameter in brain slices or in vivo is potentially complicated by indirect effects on neurons and astrocytes. Our observations are consistent with studies reporting vasoconstriction of cerebral arteries in vivo14, 15 and reduced cerebral blood flow16, 17 in response to PGE2. Nevertheless, application of PGE2 has been shown to dilate cerebral arteries18, 19 and parenchymal arterioles in vivo,3 and in neonatal brain slices.7 The latter finding could be explained by the observation that EP2 and EP4 receptors, which mediate vasodilatory pathways, are predominantly expressed in the early stage of life.11, 20, 21 In the adult animal, it has been shown that trigeminal neurons, which innervate pial arteries, release the vasorelaxant calcitonin gene-related peptide in response to PGE2.22, 23 Depending on experimental conditions, perivascular nerves may or may not be functional—a possible cause of the discrepancies in both in vitro (Supplementary Table 1) and in vivo studies noted above. It should also be borne in mind that other studies have indicated a role for COX2 and PGE2 in modulating synaptic signaling in neurons.24, 25 Because astrocytes are recruited by synaptic release of glutamate,1 inhibition of such a mechanism would decrease neurovascular coupling efficiency, a result that could mistakenly be taken as evidence for a direct neurovascular-coupling function of PGE2. This hypothesis is consistent with the demonstrated contribution of COX2 to functional hyperemia9 despite the absence of COX2 expression in astrocytes.10 Moreover, ablation of the Cox1 gene in mice does not affect functional hyperemia,26 ruling out a contribution of this isoform to PGE2 production in this context. Accordingly, rather than providing evidence for a role for PGE2 as an astrocyte-derived agent, the effects of PGE2 or COX inhibitors in brain slices or in vivo3, 7 may reveal their actions in neurons and synaptic transmission.9, 27 In fact, it is possible that activation of cerebral arteriolar EP1 receptors and subsequent vasoconstriction may contribute to the neurotoxicity of PGE2.27
In conclusion, our results provide strong evidence that PGE2 is not directly involved in neurovascular coupling, and underscore the need to directly test putative neurovascular coupling transmitters on their target arterioles.
Acknowledgments
The authors would like to thank Dr Anne Joutel for her insightful comments on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by National Institutes of Health grants T32HL007647, T32HL0077944, R37DK053832, RO1HL44455, RO1HL58231, and PO1HL095488, the Fondation Leducq for the Transatlantic Network of Excellence on the Pathogenesis of Small Vessel Disease of the Brain, the Totman Medical Research Trust, and a postdoctoral fellowship from the American Heart Association (09POST2290090) to Fabrice Dabertrand.
Supplementary Material
References
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. doi: 10.1038/nn2003. [DOI] [PubMed] [Google Scholar]
- Fernandez-Klett F, Offenhauser N, Dirnagl U, Priller J, Lindauer U. Pericytes in capillaries are contractile in vivo, but arterioles mediate functional hyperemia in the mouse brain. Proc Natl Acad Sci USA. 2010;107:22290–22295. doi: 10.1073/pnas.1011321108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, et al. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci USA. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci USA. 2012;109:E1387–E1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, et al. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Araki E, Morham SG, Ross ME, Iadecola C. Cyclooxygenase-2 contributes to functional hyperemia in whisker-barrel cortex. J Neurosci. 2000;20:763–770. doi: 10.1523/JNEUROSCI.20-02-00763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrux C, Toussay X, Kocharyan A, Fernandes P, Neupane S, Levesque M, et al. Pyramidal neurons are ‘neurogenic hubs' in the neurovascular coupling response to whisker stimulation. J Neurosci. 2011;31:9836–9847. doi: 10.1523/JNEUROSCI.4943-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Varma DR, Chemtob S. Ontogenic increase in PGE2 and PGF2 alpha receptor density in brain microvessels of pigs. Br J Pharmacol. 1994;112:59–64. doi: 10.1111/j.1476-5381.1994.tb13029.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitz M, Adam M, Boie Y, Carriere M, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Dabertrand F, Nelson MT, Brayden JE. Acidosis dilates brain parenchymal arterioles by conversion of calcium waves to sparks to activate BK channels. Circ Res. 2012;110:285–294. doi: 10.1161/CIRCRESAHA.111.258145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda N, Kawakami M, Yoshida K. Constrictor action of oxyhemoglobin in monkey and dog basilar arteries in vivo and in vitro. Am J Physiol. 1991;260 (2 Pt 2:H420–H425. doi: 10.1152/ajpheart.1991.260.2.H420. [DOI] [PubMed] [Google Scholar]
- White RP, Hagen AA, Morgan H, Dawson WN, Robertson JT. Experimental study on the genesis of cerebral vasospasm. Stroke. 1975;6:52–57. doi: 10.1161/01.str.6.1.52. [DOI] [PubMed] [Google Scholar]
- Pickard JD, MacDonell LA, Mackenzie ET, Harper AM. Prostaglandin-induced effects in the primate cerebral circulation. Eur J Pharmacol. 1977;43:343–351. doi: 10.1016/0014-2999(77)90040-1. [DOI] [PubMed] [Google Scholar]
- White RP, Hagen AA. Cerebrovascular actions of prostaglandins. Pharmacol Ther. 1982;18:313–331. doi: 10.1016/0163-7258(82)90035-3. [DOI] [PubMed] [Google Scholar]
- Ellis EF, Wei EP, Kontos HA. Vasodilation of cat cerebral arterioles by prostaglandins D2, E2, G2, and I2. Am J Physiol. 1979;237:H381–H385. doi: 10.1152/ajpheart.1979.237.3.H381. [DOI] [PubMed] [Google Scholar]
- Armstead WM, Mirro R, Busija DW, Leffler CW. Vascular responses to vasopressin are tone-dependent in the cerebral circulation of the newborn pig. Circ Res. 1989;64:136–144. doi: 10.1161/01.res.64.1.136. [DOI] [PubMed] [Google Scholar]
- Hayashi S, Park MK, Kuehl TJ. Relaxant and contractile responses to prostaglandins in premature, newborn and adult baboon cerebral arteries. J Pharmacol Exp Ther. 1985;233:628–635. [PubMed] [Google Scholar]
- Wright DH, Abran D, Bhattacharya M, Hou X, Bernier SG, Bouayad A, et al. Prostanoid receptors: ontogeny and implications in vascular physiology. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1343–R1360. doi: 10.1152/ajpregu.2001.281.5.R1343. [DOI] [PubMed] [Google Scholar]
- Jenkins DW, Feniuk W, Humphrey PP. Characterization of the prostanoid receptor types involved in mediating calcitonin gene-related peptide release from cultured rat trigeminal neurones. Br J Pharmacol. 2001;134:1296–1302. doi: 10.1038/sj.bjp.0704357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeb L, Hellen P, Boehnke C, Hoffmann J, Schuh-Hofer S, Dirnagl U, et al. IL-1beta stimulates COX-2 dependent PGE(2) synthesis and CGRP release in rat trigeminal ganglia cells. PLoS ONE. 2011;6:e17360. doi: 10.1371/journal.pone.0017360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang N, Zhang J, Marcheselli V, Bazan NG, Chen C. Postsynaptically synthesized prostaglandin E2 (PGE2) modulates hippocampal synaptic transmission via a presynaptic PGE2 EP2 receptor. J Neurosci. 2005;25:9858–9870. doi: 10.1523/JNEUROSCI.2392-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clasadonte J, Poulain P, Hanchate NK, Corfas G, Ojeda SR, Prevot V. Prostaglandin E2 release from astrocytes triggers gonadotropin-releasing hormone (GnRH) neuron firing via EP2 receptor activation. Proc Natl Acad Sci USA. 2011;108:16104–16109. doi: 10.1073/pnas.1107533108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, Haensel C, Ross ME, Iadecola C. Cyclooxygenase-1 participates in selected vasodilator responses of the cerebral circulation. Circ Res. 2001;88:600–608. doi: 10.1161/01.res.88.6.600. [DOI] [PubMed] [Google Scholar]
- Andreasson K. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat. 2010;91:104–112. doi: 10.1016/j.prostaglandins.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.